Abstract
The mitochondrial inner membrane consists of the inner boundary membrane and invaginations called cristae, which differ in protein composition and likely have distinct functions. In this issue of The EMBO Journal, Wolf et al (2019) report that the cristae carry a higher membrane potential than the intervening boundary membranes. Their data suggest electro‐chemical discontinuity among segments of the inner membrane, implying that individual cristae may operate with some degree of independence.
Subject Categories: Membrane & Intracellular Transport
Energy coupling is one of the fundamental problems in biology. High‐energy bonds were thought to link substrate oxidation to ATP production until Mitchell proposed the idea of coupling through anisotropic proton expulsion (Mitchell, 1961). Although first viewed with skepticism, it was soon realized that the “proton gradient” is indeed the universal mechanism of energy conversion in all living organisms, coupling not only substrate oxidation of mitochondria (and bacteria) but also light harvesting of chloroplasts (and bacteria), to ATP formation. This led to the question as to the physical nature of the gradient, also called the proton‐motive force. Originally, this force was conceived as a pH difference between the intra‐ and the extra‐mitochondrial compartments, in which protons behave like diffusible solutes. However, the idea of a delocalized proton‐motive force is inconsistent with numerous experimental observations and has therefore been replaced by models, in which translocated protons remain closely attached to the surface of the membrane (Ferguson, 1995).
In the current work, Wolf et al (2019) further expand the notion of a localized proton‐motive force. Equipped with new high‐resolution fluorescence technology, the authors found that the mitochondrial inner membrane potential was not uniformly distributed because visible segments of the cristae had a higher potential than the surrounding boundary membrane. Membrane hyperpolarization induced by blocking ATP synthase increased the potential difference, whereas hypopolarization, resulting from uncoupling the proton‐motive force, did the opposite. Importantly, the difference in membrane potential between the cristae and the inner boundary membranes depended on normal cristae organization, as it was sensitive to the deletion of either Opa1 or the MICOS complex.
It is known that the respiratory chain, located within flat regions of the cristae, powers the proton‐motive force (Vogel et al, 2006). Hence, the observation that the proton‐motive force does not freely spread across the inner membrane suggests that individual cristae are electrically isolated from each other. In line with this finding, differences in membrane potential can be observed even between cristae, and local depolarization events require time to spread from one to the other end of a mitochondrion (Wolf et al, 2019).
The new findings rely on technical advances in the field of fluorescence microscopy, to break the diffraction limit, such as new detector designs and emission depletion. While the employed technology is promising, all dyes applied in the study bound stronger to cristae than to the inner boundary membrane. Thus, future work should verify that the measured membrane potentials are independent of the unique protein and lipid composition of the cristae.
Furthermore, how cristae become insulated from the inner boundary membrane remains an open question. One could imagine a physical barrier that restricts the movement of protons. Such barrier could be located at the crista junctions, the tubular structures that separate the cristae from the inner boundary membrane. However, crista junctions are formed by the large MICOS complex, therefore making it not straightforward to explain how a protein complex can restrict the movement of protons. Another potential explanation is that protons are pulled away from crista junctions by the ATP synthase, the principal consumer of the proton‐motive force (Fig 1). Since the ATP synthase assembles into dimer ribbons that run along crista rims (Strauss et al, 2008), it may direct proton diffusion toward the center of the cristae. As a result, random diffusion may become negligible, which would generate a difference in membrane potential between the cristae and the inner boundary membrane. The idea of vectorial proton movement on the membrane surface is consistent with the pH difference observed between the ATP synthase and the respiratory chain (Rieger et al, 2014). Thus, energy coupling in mitochondria may be realized by spatially confined proton loops running not only across but also along the cristae (Fig 1).
Figure 1. Spatial distribution of the proton‐motive force.
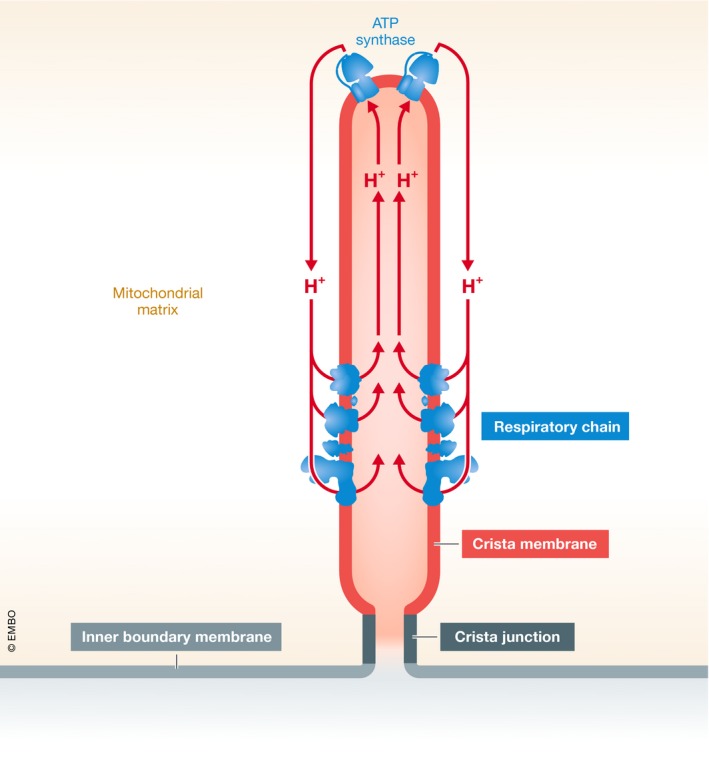
The mitochondrial inner membrane consists of membrane invaginations named cristae and the inner boundary membrane, which are joined by crista junctions. New data suggest that the proton‐motive force is largely confined to the crista membrane. The underlying mechanism may be the proton loop between the respiratory chain and the ATP synthase, which keeps the proton‐motive force away from the inner boundary membrane due to the specific localization of the proton pumps.
Cristae are compact supra‐molecular units, held together by strong protein–protein and protein–lipid interactions. Individual cristae are slow to mix with each other upon mitochondrial fusion (Wilkens et al, 2013) and show extraordinary stability as demonstrated by the long half‐life of crista membrane proteins (Kim et al, 2012) and lipids (Xu et al, 2016). Thus, the physical properties of the membranes may be a key factor ensuring the autonomy of the cristae.
In summary, Wolf et al (2019) propose a hitherto unrecognized element of spatial organization within the mitochondria. Their data suggest that cristae are the primary carrier of the proton‐motive force and possess functional autonomy. Perhaps the best example of crista autonomy can be found in MICOS mutants, where cristae become disconnected from the inner boundary membrane and yet retain a membrane potential (Wolf et al, 2019). In that case, cristae are indeed insulated transformers of metabolic energy.
The EMBO Journal (2019) 38: e103472
See also: DM Wolf et al (November 2019)
References
- Ferguson SJ (1995) Chemiosmotic coupling. Protons fast and slow. Curr Biol 5: 25–27 [DOI] [PubMed] [Google Scholar]
- Kim ST, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MPY, Ping P (2012) Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics 11: 1586–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi‐osmotic type of mechanism. Nature 191: 144–148 [DOI] [PubMed] [Google Scholar]
- Rieger B, Junge W, Busch KB (2014) Lateral pH gradient between OXPHOS complex IV and F(0)F(1) ATPsynthase in folded mitochondrial membranes. Nat Commun 5: 3103 [DOI] [PubMed] [Google Scholar]
- Strauss M, Hofhaus G, Schroder RR, Kuhlbrandt W (2008) Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 27: 1154–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F, Bornhovd C, Neupert W, Reichert AS (2006) Dynamic subcompartmentalization of the mitochondrial inner membrane. J Cell Biol 175: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens V, Kohl W, Busch K (2013) Restricted diffusion of OXPHOS complexes in dynamic mitochondria delays their exchange between cristae and engenders a transitory mosaic distribution. J Cell Sci 126: 103–116 [DOI] [PubMed] [Google Scholar]
- Wolf DM, Segawa M, Kondadi AK, Anand R, Bailey ST, Reichert AS, van der Bliek AM, Shackelford DB, Liesa M, Shirihai OS (2019) Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J 38: e101056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Phoon CKL, Berno B, D'Souza K, Hoedt E, Zhang G, Neubert TA, Epand RM, Ren M, Schlame M (2016) Loss of protein association causes cardiolipin degradation in Barth syndrome. Nature Chem Biol 12: 641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]


