Abstract
Objective
To evaluate the efficacy and safety profile of first-line bevacizumab (Bev)-containing pemetrexed-platinum chemotherapy in a real-world Chinese cohort with advanced non-squamous non-small cell lung cancer (NS-NSCLC).
Methods
A total of 415 eligible patients with NS-NSCLC who received first-line pemetrexed-platinum chemotherapy at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between February 2010 and September 2017 were reviewed retrospectively: 309 Bev(−) and 106 Bev(+) cases. Bev was administered at 7.5 mg/kg every 3 weeks in the Bev(+) group. To reduce the risk of a selection bias, a propensity score-matching (PSM) was conducted and 105 pairs of Bev(−) and Bev(+) cases were identified.
Results
The median duration of follow-up was 15.8 months. The median progression-free survival (PFS) was prolonged significantly in the Bev(+) group than in the Bev(−) group in overall (9.8vs. 7.8 months, P=0.006) and PSM pairs (9.8 vs. 6.6 months, P<0.001). Moreover, patients receiving maintenance therapy with pemetrexed plus Bev had longer PFS than those interrupted after induction chemotherapy, or those receiving mono-maintenance with pemetrexed (12.3vs. 4.8 vs. 8.6 months; P<0.001). Multivariate analyses revealed Bev to be one of the favorable prognostic factors for PFS, along with the predictor of maintenance therapy.
Conclusions
First-line induction and maintenance therapy with Bev (7.5 mg/kg every 3 weeks) combined with pemetrexed-platinum chemotherapy was efficacious and superior to non-Bev chemotherapy in Chinese patients with advanced NS-NSCLC.
Keywords: Bevacizumab, pemetrexed, non-squamous non-small cell lung cancer, maintenance treatment, propensity score matching
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80% of all cases of lung cancer worldwide and in China, and most patients are staged as “unresectable”, “advanced” or “metastatic” (1,2). Platinum-based chemotherapy regimens have, for a long time, been the standard first-line treatment for NSCLC with no sensitizing genetic mutations (3). However, their efficacy is limited with 5-year survival of 10%−15% and median survival of approximately 8−10 months (4-6).
Vascular endothelial growth factor (VEGF) is an important mediator in tumor-associated growth and angiogenesis (7). Bevacizumab (Bev; Avastin), a recombinant humanized monoclonal antibody, has been approved as a VEGF antagonist for treatment of several cancer types (8,9). Since 2004, it has been demonstrated that Bev-combined platinum-based standardized treatment can prolong progression-free survival (PFS) of patients with advanced non-squamous non-small cell lung cancer (NS-NSCLC) who can tolerate such treatment (10-12). Based on such findings, combination of Bev with chemotherapy was approved by the USA Food and Drug Administration for first-line treatment of NS-NSCLC. Subsequently, Bev-specific observational cohort studies were initiated to define the clinical outcomes among a broader population of patients receiving Bev-containing regimens in a real-world setting. Several observational cohort studies have compared the regimen of paclitaxel/carboplatin plus Bev and paclitaxel/carboplatin therapy in advanced NSCLC, and demonstrated the significant response and survival benefits of Bev (13,14). However, most available supporting data were from studies of paclitaxel/platinum in combination with Bev in Western countries, in which Bev was administered at 15 mg/kg every 3 weeks.
Compared with paclitaxel, pemetrexed-platinum chemotherapy combined with Bev at 7.5 mg/kg is used more widely in NS-NSCLC patients in China. Besides, The JMDB trial showed the noninferiority of pemetrexed/cisplatin over gemcitabine/cisplatin with regard to PFS in NSCLC patients [4.8 vs. 5.1 months; hazard ratio (HR)=1.04; 95% confidence interval (95% CI), 0.94−1.15] (15).
At present, the efficacy and safety of first-line Bev (7.5 mg/kg)-containing pemetrexed-platinum chemotherapy has not been evaluated specifically. Therefore, we conducted a retrospective propensity score-matched (PSM) real-world study in a Chinese population with advanced NS-NSCLC.
Materials and methods
Study design and patient enrollment
This retrospective, single-center study captured the clinical data from Chinese patients with advanced NS-NSCLC who received first-line paclitaxed-platinum chemotherapy with or without Bev between February 2010 and September 2017 at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The inclusion criteria were: 1) pathological diagnosis and radiographic examination confirmed local advanced or metastatic NS-NSCLC (stage IIIB−IV); 2) confirmation of written informed consent for chemotherapy; 3) receiving first-line pemetrexed-platinum chemotherapy with or without Bev; 4) receiving ≥2 cycles of chemotherapy; and 5) completeness of full medical records. Exclusion criteria were patients: 1) with squamous lung cancer; 2) who received maintenance treatment with epidermal growth factor receptor-tyrosine kinase inhibitors; 3) who received second- or more than second-line therapy with Bev after progression of first-line chemotherapy; or 4) lost to follow-up.
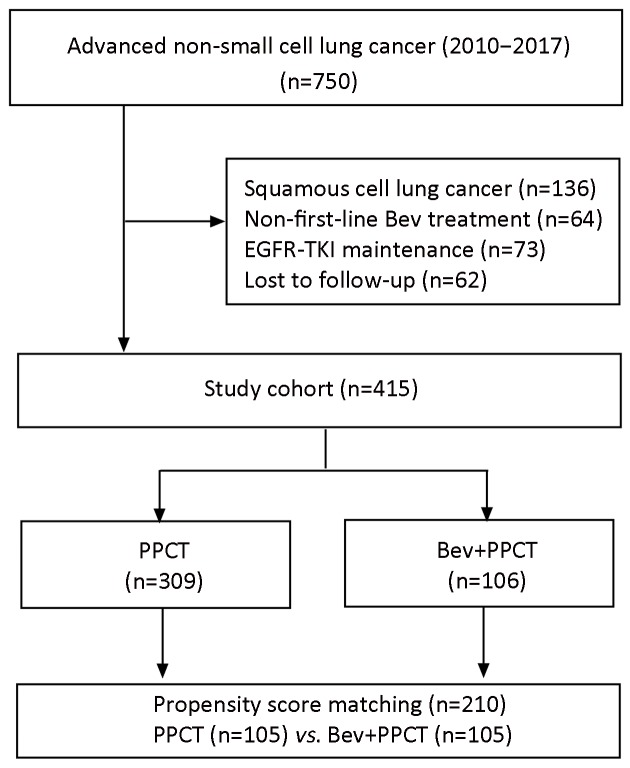
The patient flowchart is presented in Figure 1 . The study protocol was approved by the Independent Ethics Committee at the National Cancer Center (Beijing, China).
1.
Flowchart showing patient selection. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PPCT, platinum-pemetrexed-based chemotherapy; Bev, bevacizumab.
Data collection
Patient data (chief complaint, disease history, physical examination, imaging examinations and biochemical laboratory tests) were collected retrospectively from the electronic data system at our institute.
Treatment responses were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST1.1) (16,17). Complete response (CR) was defined as the disappearance of all target lesions and reduction in the short axis of any pathological lymph nodes (target or non-target) to <10 cm. Partial response (PR) was defined as a decrease of ≥30% in the sum of the diameters of target lesions. Progressive disease (PD) was defined as an increase of ≥20% in the sum of target lesions with an absolute increase of >5 mm, or the appearance of any one or more new lesions. Stable disease (SD) was defined as lesions whose shrinkage was not sufficient to qualify as PR or whose increase was not sufficient to qualify as PD. The objective response rate (ORR) is calculated as CR + PR. The disease control rate (DCR) is calculated as CR + PR + SD. The endpoint of primary efficacy, PFS, was defined as the time from treatment initiation until the first sign of disease progression or death from any cause.
Toxicities were evaluated according to the National Cancer Institute—Common Toxicity Criteria for Adverse Events version 4.0.
Statistical analysis
A one-to-one PSM method (18) was applied to reduce the selection bias when dealing with the large number of covariates among patients who received Bev treatment versus those who did not. Each variable was multiplied by a coefficient that was calculated by the logistic regression analysis, and the sum of these values was considered to be the propensity score for individual patients.
In the present study, the variables included in PSM were age, gender, smoking status, comorbidity, baseline histology, sensitizing driven mutations, brain metastases, pleural invasion, weight loss >5%, pemetrexed regimens, previous surgery, radiotherapy history, and history of hemoptysis. A description of categorical variables and the Chi-square test were used to check the balance between PSM subgroups.
Student’s t tests and Mann-Whitney U tests were used to establish the statistical significance of continuous categorical variables in all subgroups. The Kaplan-Meier method was used to estimate the impact of the duration of Bev administration upon survival, followed by the log-rank test to determine the statistical significance. Multivariate Cox analyses were used to estimate the effect of Bev treatment on PFS before and after match. As for matched variables, the Cox regression was performed among patients before PSM to explore their potential predictive values of survivals. The research goals mentioned above were achieved by analyses with IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA).
Results
Clinical characteristics
Between February 2010 and September 2017, 750 Chinese patients with advanced NS-NSCLC were investigated initially. Finally, 415 eligible patients were enrolled according to their treatment regimens: 309 patients in the Bev(−) group and 106 in the Bev(+) group.
The median age was 58 (range, 25−78) years and the ratio of males to females was 217 to 198. An extremely high prevalence of adenocarcinoma (404, 97.3%) was observed. Patients with an Eastern Cooperative Oncology Group Performance Score (ECOG PS) ≥2 (n=25; 6.0%), brain metastases (n=59; 14.2%), or a history of hemoptysis (n=33; 8.0%) were also included in our study. At baseline, 77 patients (18.6%) had a history of hypertension, 26 (6.3%) had diabetes mellitus, 15 (3.6%) had cardiovascular disease, 2 (0.5%) had a thrombosis disorder, and 7 (1.7%) had cerebrovascular disease. All these patients were receiving the relevant medications concomitantly. Baseline characteristics such as pleural invasion, ECOG PS, and history of surgery/radiotherapy were not distributed evenly across Bev(−) and Bev(+) groups before PSM (Table 1 ).
1.
Baseline characteristics before propensity-score-matched stratification by regimens
| Characteristics | PPCT (n=309) [n (%)] | Bev+PPCT (n=106) [n (%)] | P |
| ECOG PS, Eastern Cooperative Oncology Group Performance Score; EGFR, epidermal growth factor receptor; PPCT, platinum-pemetrexed-based chemotherapy; Bev, bevacizumab. | |||
Age (
 ) (year)
) (year)
|
56.3±10.3 | 56.2±10.2 | 0.989 |
| ≥60 years | 123 (39.8) | 43 (40.6) | 0.909 |
| Male | 168 (54.4) | 49 (46.2) | 0.176 |
| ECOG PS | 0.082 | ||
| 0−1 | 288 (93.2) | 102 (96.2) | |
| ≥2 | 21 (6.8) | 4 (3.8) | |
| Smoking history | 0.338 | ||
| No | 185 (59.9) | 72 (67.9) | |
| Yes | 124 (40.1) | 34 (32.1) | |
| Comorbidities | 0.082 | ||
| Hypertension | 61 (19.7) | 16 (15.1) | |
| Diabetes mellitus | 23 (7.4) | 3 (2.8) | |
| Cardiovascular disease | 12 (3.9) | 3 (2.8) | |
| Thrombotic disease | 1 (0.3) | 1 (0.9) | |
| Cerebrovascular disease | 4 (1.3) | 3 (2.8) | |
| Other | 57 (18.4) | 28 (26.4) | |
| Baseline histology | 0.914 | ||
| Adenocarcinoma | 301 (97.4) | 103 (97.2) | |
| Large-cell carcinoma | 1 (0.3) | 0 (0) | |
| Bronchoalveolar carcinoma | 1 (0.3) | 0 (0) | |
| Mixed | 6 (2.0) | 3 (2.8) | |
| Sensitizing driven mutation | |||
| EGFR positive | 99 (32.0) | 28 (26.4) | 0.285 |
| T790M positive | 6 (1.9) | 2 (1.9) | 0.668 |
| ALK positive | 19 (6.1) | 8 (7.5) | 0.380 |
| C-MET positive | 3 (0.9) | 1 (0.9) | 0.730 |
| ROS-1 positive | 2 (0.6) | 0 (0) | 0.554 |
| Pemetrexed regimens | 309 (100) | 106 (100) | − |
| Brain metastases | 46 (14.9) | 13 (12.3) | 0.312 |
| Pleural invasion | 92 (29.8) | 43 (40.6) | 0.028 |
| Weight loss >5% | 22 (7.1) | 5 (4.7) | 0.269 |
| History of hemoptysis | 26 (8.4) | 7 (6.6) | 0.359 |
| Maintenance therapy | 132 (42.7) | 68 (64.2) | 0.001 |
| Previous surgery | 33 (10.7) | 22 (20.8) | 0.008 |
| Radiotherapy history | 43 (13.9) | 22 (20.8) | 0.067 |
Treatment
All enrolled patients received pemetrexed plus platinum doublets with or without Bev. Chemotherapy was administered at 3-week intervals: pemetrexed (500 mg/m2, i.v., on d 1), platinum [cisplatin, 25 mg/m2, i.v., on d 1−3 or carboplatin (area under the curve, 4−5), i.v., on d 1], and Bev (7.5 mg/kg, i.v., on d 1). The median number of chemotherapy cycles was five. Two-hundred patients (48.2%) received a median of four cycles of pemetrexed maintenance treatment after induction chemotherapy. Patients in the Bev(+) group received 4−6 cycles (median, 4) of Bev plus pemetrexed-platinum induction chemotherapy followed by Bev-containing pemetrexed maintenance treatment.
To reduce the risk of a selection bias, one-to-one PSM was used for the selection of the Bev(−) group (n=309) and Bev(+) group (n=106) pairs, with a caliper width of 0.15 of standard deviation. One-hundred and five patients from each group were well matched without a significant difference at baseline (Table 2 ).
2.
Baseline characteristics after propensity score-matched stratification by regimens
| Characteristics | PPCT (n=105) [n (%)] | Bev+PPCT (n=105) [n (%)] | P |
| ECOG PS, Eastern Cooperative Oncology Group Performance Score; EGFR, epidermal growth factor receptor; PPCT, platinum-pemetrexed-based chemotherapy; Bev, bevacizumab. | |||
Age (
 ) (year)
) (year)
|
55.8±10.4 | 56.2±10.2 | 0.989 |
| ≥60 years | 39 (37.1) | 43 (41.0) | 0.336 |
| Male | 53 (50.5) | 48 (45.7) | 0.290 |
| ECOG PS | 0.169 | ||
| 0−1 | 94 (89.5) | 101 (96.2) | |
| ≥2 | 11 (10.5) | 4 (3.8) | |
| Smoking status | 0.667 | ||
| No | 71 (67.6) | 72 (68.6) | |
| Yes | 34 (32.4) | 33 (31.4) | |
| Comorbidities | 0.060 | ||
| Hypertension | 23 (21.9) | 16 (15.2) | |
| Diabetes | 8 (7.6) | 3 (2.9) | |
| Cardiovascular disease | 6 (5.7) | 3 (2.9) | |
| Thrombosis disease | 0 (0) | 1 (1.0) | |
| Cerebrovascular disease | 1 (1.0) | 3 (2.9) | |
| Other | 13 (12.4) | 28 (26.7) | |
| Baseline histology | 0.998 | ||
| Adenocarcinoma | 102 (97.1) | 102 (97.1) | |
| Large cell carcinoma | 1(1.0) | 1 (1.0) | |
| Bronchoalveolar carcinoma | 1 (1.0) | 1 (1.0) | |
| Mixed | 1 (1.0) | 1 (1.0) | |
| Sensitizing driven mutation | |||
| EGFR positive | 25 (23.8) | 28 (26.7) | 0.512 |
| T790M positive | 3 (2.9) | 2 (1.9) | 0.500 |
| ALK positive | 9 (8.6) | 8 (7.6) | 0.500 |
| C-MET positive | 2 (1.9) | 1 (1.0) | 0.500 |
| ROS-1 positive | 0 (0) | 0 (0) | − |
| Pemetrexed regimens | 105 (100) | 105 (100) | − |
| Brain metastases | 14 (13.3) | 13 (12.4) | 0.500 |
| Pleural invasion | 43 (41.0) | 42 (40.0) | 0.500 |
| Weight loss >5% | 8 (7.6) | 5 (4.8) | 0.284 |
| History of hemoptysis | 8 (7.6) | 7 (6.7) | 0.500 |
| Maintenance therapy | 46 (43.8) | 67 (63.8) | 0.001 |
| Previous surgery | 17 (16.2) | 21(20.0) | 0.296 |
| Radiotherapy history | 18 (17.1) | 22 (21.0) | 0.299 |
Bev administration is one of significant prognostic factors of PFS
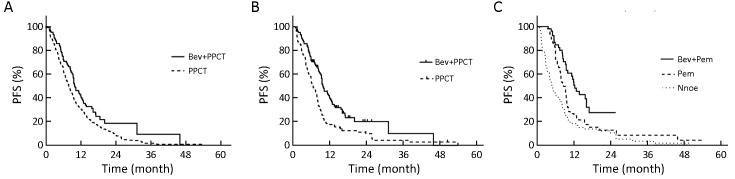
Patients in the Bev(+) group had a superior median PFS to those in the Bev(−) group (9.8vs. 7.8 months, P=0.006) (Figure 2A ) before PSM. Furthermore, the survival benefit of patients in the Bev(+) group was further confirmed after PSM (median PFS, 9.8 vs. 6.6 months, P<0.001) (Figure 2B ). Bev-containing regimens were proven to be one of the significant beneficial prognostic factors of PFS before (HR=0.70; 95% CI, 0.55−0.90; P=0.005) and after PSM (HR=0.66; 95% CI, 0.48−0.90; P=0.010). Besides, patients receiving maintenance therapy had more favorable PFS than those who did not. Median PFS was 4.8, 8.6 and 12.3 months for patients who did not receive maintenance therapy (n=98), received pemetrexed maintenance (n=58), or received maintenance therapy with pemetrexed plus Bev (n=54), respectively (HR=0.55; 95% CI, 0.40−0.76; P<0.001) (Figure 2C , Table 3 ).
2.
Progression-free survival (PFS) curves between different groups of patients before and after propensity score-matching (PSM). (A) Patients who received pemetrexed-platinum-based chemotherapy (PPCT) with bevacizumab (Bev) had higher PFS than those who received chemotherapy without Bev before PSM (median PFS, 9.8 vs. 7.8 months; HR=0.722, 95% CI, 0.57−0.92, P=0.006); (B) Patients who received PPCT with Bev had higher PFS than those who received chemotherapy without Bev after PSM (median PFS, 9.8vs. 6.6 months; HR=0.56, 95% CI, 0.41−0.76, P<0.001); (C) Patients receiving maintenance therapy with Bev plus pemetrexed (Pem) achieved superior PFS compared with those receiving non-maintenance treatment, or mono-Pem maintenance therapy (median PFS, 12.3vs. 4.8 vs. 8.6 months, HR=14.2, 95% CI, 9.27−13.19, P<0.001). HR, hazard ratio; 95% CI, 95% confidence interval.
3.
Baseline characteristics after propensity score-matched stratification by maintenance treatment
| Characteristics | Bev+Pem (n=54) [n (%)] | Pem (n=58) [n (%)] | None (n=98) [n (%)] | P |
| ECOG PS, Eastern Cooperative Oncology Group Performance Score; EGFR, epidermal growth factor receptor; Bev, bevacizumab; Pem, pemetrexed. | ||||
Age (
 ) (year)
) (year)
|
55.5±10.5 | 55.3±9.9 | 56.7±10.5 | 0.989 |
| ≥60 years | 21 (38.9) | 19 (32.8) | 42 (42.9) | 0.458 |
| Male | 24 (44.4) | 29 (50.0) | 48 (49.0) | 0.817 |
| ECOG PS | 0.086 | |||
| 0−1 | 53 (98.1) | 56 (96.6) | 86 (87.8) | |
| ≥2 | 1 (1.9) | 3 (5.2) | 12 (12.2) | |
| Smoking history | 14 (25.9) | 20 (34.5) | 33 (33.7) | 0.454 |
| Comorbidities | 28 (51.9) | 29 (50.0) | 48 (49.0) | 0.944 |
| Adenocarcinoma | 53 (98.1) | 58 (100) | 98 (100) | 0.982 |
| EGFR positive | 12 (22.2) | 9 (15.5) | 32 (32.7) | 0.292 |
| ALK positive | 7 (13.0) | 1 (1.7) | 9 (9.2) | 0.080 |
| Brain metastases | 10 (18.5) | 5 (8.6) | 12 (12.2) | 0.286 |
| Pleural invasion | 25 (46.3) | 24 (41.4) | 36 (36.7) | 0.510 |
| Weight loss >5% | 3 (5.6) | 1 (1.7) | 9 (9.2) | 0.170 |
| History of hemoptysis | 4 (7.4) | 2 (3.4) | 9 (9.2) | 0.404 |
| Previous surgery | 11 (20.4) | 12 (20.7) | 15 (15.3) | 0.617 |
| Radiotherapy history | 13 (24.1) | 7 (12.1) | 20 (20.4) | 0.242 |
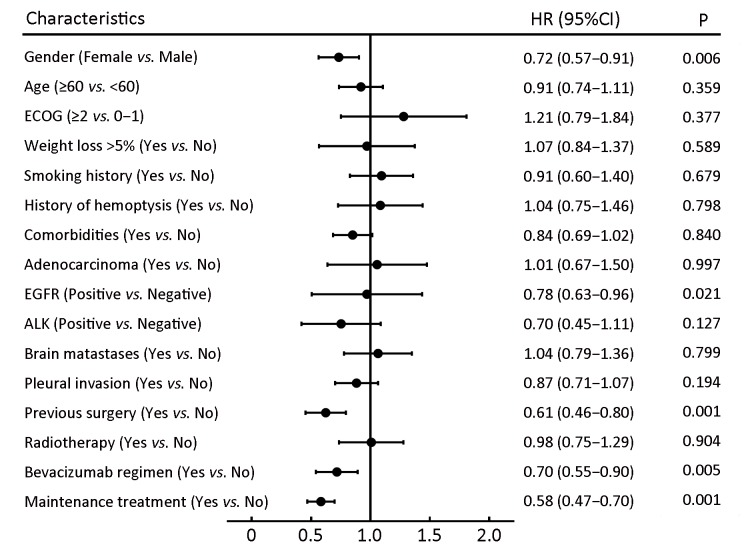
The relationship between the baseline characteristics of patients and PFS was explored via multivariate Cox analyses among patients before PSM (Figure 3 ): a history of surgery and epidermal growth factor receptor (EGFR) mutation were also significant factors of prolonged PFS in patients with NS-NSCLC.
3.
Multivariate Cox analyses for progression-free survival (PFS) in 415 patients before propensity score-matching (PSM). ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; HR, hazard ratio; 95% CI, 95% confidence interval.
Safety profile
The prevalence of Bev-associated serious adverse events (AEs), including hypertension, gastrointestinal perforations, arterial and venous thromboembolic events, hemoptysis, bleeding within the central nervous system, and other types of hemorrhage, were investigated. Consistent with the known profile of Bev at 7.5 mg/kg, the safety profile was acceptable in our study. Hemorrhagic events of grade ≥3 or new unexpected AEs in patients (including those with various complications) were not observed.
Discussion
Bev-containing chemotherapy has become standard first-line treatment for patients with advanced NS-NSCLC (19). Previous trials such as ECOG4599, AVAiL, SAiL, BEYOND and ARIES have demonstrated the superiority of Bev-containing chemotherapy with respect to ORR and PFS over chemotherapy alone against advanced NS-NSCLC (median PFS, 9.2−5.1vs. 6.5−4.5 months, P<0.001) (13,19-23). However, most of the chemotherapeutic regimens in those studies were paclitaxel/platinum, and the administration dose of Bev was 15 mg/kg. Interestingly, the AVAiL study showed that a lower dose (7.5 mg/kg) of Bev had comparable efficacy with a higher dose (15 mg/kg) in prolonging PFS (21). Most of the research data were generated from Western populations. The only Chinse study, BEYOUND, evaluated the efficacy of Bev at 15 mg/kg in combination with paclitaxel-platinum in a highly selective patient cohort, and showed a meaningful clinical benefit of Bev in Chinese patients with advanced NS-NSCLC (23).
However, three critical issues cannot be addressed by current research evidence. First, the optimal combination regimen (including the choice of maintenance treatment) has not been established (24-27). Second, the safety profile of Bev in patients with complications is not known. Third, the minimum effective and tolerable dose of Bev for Chinese population has not been determined. Therefore, we conducted a real-world study in Chinese patients with advanced NS-NSCLC to explore the efficacy of Bev-containing chemotherapy. Our results in 415 patients with NS-NSCLC further supported the notion that PFS can be improved significantly by employing a Bev-containing pemetrexed-platinum regimen. Bev administration was an independent beneficial prognostic factor for PFS (HR=1.51; P=0.036) in our study.
With regard to histology, gene mutation status and chemotherapy sensitivity, Chinese populations differ from those of Western people. The SAiL study showed a better ORR (68.8% vs. 50.8%), DCR (96.5% vs. 88.7%), median time to disease progression (TTP) (8.8 vs. 7.8 months) and overall survival (18.5 vs. 15.3 months) in 195 Chinese patients receiving first-line Bev combination therapy compared with the worldwide population (28). However, the survival benefits of the addition of Bev to chemotherapy have not been presented in older patients and those diagnosed with ECOG PS ≥2, brain metastases or comorbidities, even though such patients form a sizeable proportion of cancer sufferers (29). Although the ARIES study expanded the study of Bev into a broader real-world population (30), Asian populations were excluded.
Unlike ECOG4599 or AVAiL studies, our real-world study included assessment of older patients (age ≥60 years), pleural invasion, brain metastasis, ECOG PS ≥2, comorbidity medications and history of hemoptysis. With a median follow-up of 15.8 months, the median PFS was significantly different between Bev(+) and Bev(−) groups according to univariate analyses (9.8vs. 7.8 months, P=0.006). Among the 105 PSM-identified pairs, median PFS was significantly longer in Bev(+) patients (9.8 vs. 6.6 months, P<0.001), suggesting a result that was consistent before and after PSM correction. Taken together, our study confirmed that a Bev-containing pemetrexed-platinum regimen was superior to the pemetrexed-platinum regimen in terms of PFS in a real-world Chinese population with NS-NSCLC.
As reported by the PARAMOUNT study, the choice of receiving pemetrexed maintenance after induction therapy with pemetrexed plus cisplatin is a vital factor for achieving better efficacy and reducing side effects for NS-NSCLC patients (31-33). Furthermore, the AVAPERL trial (MO22089) confirmed the survival benefits of Bev-containing pemetrexed maintenance therapy after first-line Bev-containing cisplatin-pemetrexed induction treatment in NS-NSCLC patients (34). Consistently, our study demonstrated that PFS was prolonged significantly with Bev-containing pemetrexed maintenance therapy than pemetrexed maintenance alone (7.4 vs. 3.7 months; HR=0.57; 95% CI, 0.44−0.75; P<0.001) in overall and PSM-identified matched pairs (4.8vs. 8.6 vs. 12.3 months; HR=0.55; 95% CI, 0.40−0.76; P<0.001). Therefore, Bev-containing pemetrexed maintenance treatment was an effective regimen in the NS-NSCLC patients who received Bev-containing pemetrexed-platinum induction therapy.
Importantly, patients in the present study received a low dose of Bev (7.5 mg/kg), which is in accordance with the healthcare policy and related financial concerns in China. With this low dose, which is half of the dose reported previously (15 mg/kg), the benefits of Bev in improving PFS combined with pemetrexed-platinum therapy followed by maintenance treatment were observed. Moreover, the lower dose of Bev employed in the present study achieved similar favorable survival outcomes compared with those of studies using a higher administration dose of Bev, though making direct comparisons is difficult (20,23,34). In addition, hypertension of grade I−II, proteinuria, venous thrombosis, and nosebleeds were the only AEs observed at this dose, suggesting that 7.5 mg/kg is a safe and efficacious dose.
Our study had several limitations: retrospective nature, small sample size, and undertaken at a single institution. These factors affected the statistical power of our data and could have resulted in biases. Though PSM was used to match pairs to minimize the potential selection bias, it compromised the sample size. Besides, the predictive value of the balanced variables in this study may be further explored in prospective settings.
Conclusions
This study provided essential clinical insights into a real-world Chinese cohort with advanced NS-NSCLC. First-line Bev-containing pemetrexed-platinum chemotherapy benefited prolongation of PFS considerably, especially among patients receiving Bev-containing maintenance treatment with pemetrexed. Importantly, 7.5 mg/kg of Bev was a low-dose, inexpensive and efficacious option in Chinese patients with advanced NS-NSCLC.
Acknowledgements
This work was supported by Wu Jieping Fund (No. 320.6750.14266).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Chen Q, Guo L, et al Incidence and mortality of lung cancer in China, 2008-2012. Chin J Cancer Res. 2018;30:580–7. doi: 10.21147/j.issn.1000-9604.2018.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Akerley W, et al NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4. 2016. J Natl Compr Canc Netw. 2016;14:255–64. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilkington G, Boland A, Brown T, et al A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax. 2015;70:359–67. doi: 10.1136/thoraxjnl-2014-205914. [DOI] [PubMed] [Google Scholar]
- 5.Soon YY, Stockler MR, Askie LM, et al Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277–83. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 6.Xue C, Hu Z, Jiang W, et al National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–5. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Kang G, Wang T, et al Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett. 2018;16:687–702. doi: 10.3892/ol.2018.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assoun S, Brosseau S, Steinmetz C, et al Bevacizumab in advanced lung cancer: state of the art. Future Oncol. 2017;13:2515–35. doi: 10.2217/fon-2017-0302. [DOI] [PubMed] [Google Scholar]
- 9.Roviello G, Bachelot T, Hudis CA, et al The role of bevacizumab in solid tumors: A literature based meta-analysis of randomised trials. Eur J Cancer. 2017;75:245–58. doi: 10.1016/j.ejca.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, et al Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, Liu K, Jiang Z, et al The efficacy and safety of pemetrexed plus bevacizumab in previously treated patients with advanced non-squamous non-small cell lung cancer (NS-NSCLC) Tumour Biol. 2015;36:2491–9. doi: 10.1007/s13277-014-2862-4. [DOI] [PubMed] [Google Scholar]
- 12.Zahn MO, Linck D, Losem C, et al AVAiLABLE NIS - AVASTIN® in lung cancer treatment in routine oncology practice in Germany. BMC Cancer. 2019;19:433. doi: 10.1186/s12885-019-5618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Chavez A, Young T, Fages S, et al Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the Eastern Cooperative Oncology Group 4599 Study: results of an exploratory analysis. J Thorac Oncol. 2012;7:1707–12. doi: 10.1097/JTO.0b013e318265b500. [DOI] [PubMed] [Google Scholar]
- 14.Lynch TJ Jr, Spigel DR, Brahmer J, et al Safety and effectiveness of bevacizumab-containing treatment for non-small-cell lung cancer: final results of the ARIES observational cohort study. J Thorac Oncol. 2014;9:1332–9. doi: 10.1097/JTO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti GV, Parikh P, von Pawel J, et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 16.Nishino M, Jackman DM, Hatabu H, et al New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 2010;195:221–8. doi: 10.2214/AJR.09.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchida Y, Therasse P Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 18.Benedetto U, Head SJ, Angelini GD, et al Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112–7. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- 19.Crinò L, Dansin E, Garrido P, et al Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11:733–40. doi: 10.1016/S1470-2045(10)70151-0. [DOI] [PubMed] [Google Scholar]
- 20.Leighl NB, Zatloukal P, Mezger J, et al Efficacy and safety of bevacizumab-based therapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer in the phase III BO17704 study (AVAiL) J Thorac Oncol. 2010;5:1970–6. doi: 10.1097/JTO.0b013e3181f49c22. [DOI] [PubMed] [Google Scholar]
- 21.Reck M, von Pawel J, Zatloukal P, et al Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–9. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosty MP, Wozniak AJ, Jahanzeb M, et al Effectiveness and safety of post-induction phase bevacizumab treatment for patients with non-small-cell lung cancer: results from the ARIES observational cohort study. Target Oncol. 2015;10:509–16. doi: 10.1007/s11523-014-0355-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, Wu YL, Chen G, et al BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
- 24.Boye M, Wang X, Srimuninnimit V, et al First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never-smoker patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer: Quality of life results from a randomized phase iii trial. Clin Lung Cancer. 2016;17:150–60. doi: 10.1016/j.cllc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Karayama M, Inui N, Fujisawa T, et al Maintenance therapy with pemetrexed and bevacizumab versus pemetrexed monotherapy after induction therapy with carboplatin, pemetrexed, and bevacizumab in patients with advanced non-squamous non small cell lung cancer. Eur J Cancer. 2016;58:30–7. doi: 10.1016/j.ejca.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima T, Wakatsuki Y, Kobayashi T, et al Phase II study of cisplatin/pemetrexed combined with bevacizumab followed by pemetrexed/bevacizumab maintenance therapy in patients with EGFR-wild advanced non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol. 2018;81:1043–50. doi: 10.1007/s00280-018-3573-0. [DOI] [PubMed] [Google Scholar]
- 27.Soria JC, Mauguen A, Reck M, et al Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 28.Zhou CC, Bai CX, Guan ZZ, et al Safety and efficacy of first-line bevacizumab combination therapy in Chinese population with advanced non-squamous NSCLC: data of subgroup analyses from MO19390 (SAiL) study. Clin Transl Oncol. 2014;16:463–8. doi: 10.1007/s12094-013-1102-5. [DOI] [PubMed] [Google Scholar]
- 29.Keating GM Bevacizumab: a review of its use in advanced cancer. Drugs. 2014;74:1891–925. doi: 10.1007/s40265-014-0302-9. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak AJ, Kosty MP, Jahanzeb M, et al Clinical outcomes in elderly patients with advanced non-small cell lung cancer: results from ARIES, a bevacizumab observational cohort study. Clin Oncol (R Coll Radiol) 2015;27:187–96. doi: 10.1016/j.clon.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Pujol JL, Paz-Ares L, de Marinis F, et al Long-term and low-grade safety results of a phase III study (PARAMOUNT): maintenance pemetrexed plus best supportive care versus placebo plus best supportive care immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2014;15:418–25. doi: 10.1016/j.cllc.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares L, de Marinis F, Dediu M, et al Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 33.Cufer T, O’Brien ME The PARAMOUNT study and the re-challenge chemotherapy issue in advanced non-small cell lung cancer. Eur J Cancer. 2013;49:2269–70. doi: 10.1016/j.ejca.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Barlesi F, Scherpereel A, Gorbunova V, et al Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25:1044–52. doi: 10.1093/annonc/mdu098. [DOI] [PubMed] [Google Scholar]