Abstract
Background: Our previous studies showed that tetraspanin CD151 was implicated in the progression of hepatocellular carcinoma (HCC), mainly depending on the formation of functional complexes with molecular partners, including Mortalin. In this study, we investigate the role of mortalin in CD151-depedent progression of HCCs.
Methods: Immunofluorescent staining, western blot and quantitative real-time polymerase chain reaction (qRT-PCR) were used to investigate the expression and location of CD151 and Mortalin in four HCC cell lines with different metastatic ability. The relationship between Mortalin and CD151 was investigated in HCCLM3 cells using co-immunoprecipitation. CD151 or Mortalin expression in HCC cells were modified by transfection technology. Wound-healing assay and Transwell assay were used to assay the role of CD151 and Mortalin in cell migration and invasion. The expression and prognostic implication of CD151 and Mortalin in 187 cases of HCCs were analyzed.
Results: Expression of Mortalin in HCC cells was positive related to their metastatic ability and its tendency was in line with the expression of CD151. Immunofluorescent staining showed that Mortalin was located in cytoplasm, while positive staining for CD151 was observed in cytoplasm and membrane of HCC cells. co-IP revealed that Mortalin formed a complex with CD151. Down-regulation of Mortalin induced a moderate decreased CD151 protein, but not CD151 mRNA, while inhibition of CD151 did not influence the expression of Mortalin at the level of both protein and mRNA. Interference of Mortalin significantly inhibited the invasion and migration of HCC cells with high CD151 expression and partially restored the invasion and migration of HCC cells induced by CD151 over-expression. Clinically, high Mortalin expression correlated with malignant phenotype of HCC, such as microvascular invasion (p=0.017) and tumor diameter (p=0.001). HCC patients expressing high Mortalin were tend to have higher expression of CD151. HCC patients expressing high level of CD151 showed the poorer prognosis in a Mortalin-dependent manner.
Conclusions: Mortalin maybe stabilize of the structure of CD151-dependent tetraspanin-enriched microdomains and implicate in the progression of HCC.
Keywords: hepatocellular carcinoma, CD151, mortalin, tetraspanin-enriched microdomains, invasion
Introduction
As one of the most important member of the transmembrane 4 superfamily (TM4SF), it is well documented that tetraspanin CD151 is expressed in a wide range of normal cells such as endothelial cells and platelets, and involves in several physiological processes, such cell adhesion, motility, activation and proliferation 1. Recent evidences have recorded that CD151 frequently overexpresses in malignant tumor tissues, including hepatocellular carcinoma (HCC) 2, gallbladder carcinoma 3, breast cancer 4 and ovarian cancer 5, and acts as a “driver” in tumor progression through formation of tetraspanin CD151-enriched microdomains 2. Moreover, it is crucial for the function of CD151 to keep the structural stability of tetraspanin-enriched microdomains (TEM) 6. Our serial studies showed that CD151 involved in several pathological processes, including invasive ability, tumor neoangiogenesis and epithelial-mesenchymal transition (EMT), and then accelerated the invasion and metastasis in HCC 2, 7, 8. Based on the combination co-immunoprecipitation (Co-IP) with two-dimensional liquid chromatography coupled with tandem mass spectrometry (2D-LC-MS/MS), our results revealed that CD151 played the crucial role in the progression of HCC through formation of tetraspanin CD151 network with molecular partners, such as Mortalin, integrins α6β1 and c-Met 9. Moreover, the role of CD151 was influenced by its partner, and disassociation of tetraspanin CD151/integrins α6β1 using targeted monoclonal antibody (generated in our group) could inhibit the mobility and invasion of HCC cells in vitro 10, 11. Therefore, to explore the mechanism that keeps the structural stability of tetraspanin CD151 network is of significance for further disclosing the role of CD151 in HCC cells.
HCC is one of the most malignancies and ranks as the third most common cause of cancer-related mortality in the world 12, 13. The dismal outcome mainly contributes to highly metastatic ability of HCC cells 14. Therefore, it is considerable significance to disclose the role and molecular mechanism of key gene in the progression of HCC. Mortalin (also named as HSPA9, mthsp70, PBP74, Grp75) is a highly conserved molecular chaperone in the heat shock protein (HSP) 70 family 15. Mortalin is expressed in all cell types and tissues and performs cytoprotective functions by unfolding of proteins outside mitochondria and unidirectional translocation across mitochondrial membranes, and completes import by acting as an ATP-driven motor. Its function is induced by stress stimulus, such as ionizing radiation, glucose deprivation, calcium ionophore, ozone and hyperthyroidism 16. Recent studies reported that many of the human transformed cells and tumor cells including HCCs, had a high level of Mortalin expression 17. Overexpression of Mortalin was inclined to support the malignancy of carcinoma cells. However, the role and mechanism of Mortalin in HCC remain largely elusive.
In present study, we investigate the expression of Mortalin in HCCs, then analyze the interaction between Mortalin and CD151 in HCC cells. Finally, we evaluate the clinical implication of Mortalin and CD151 in HCCs.
Materials and Methods
Cell lines and culture
Four HCC cell lines Hep3B, HepG2 (purchased from ATCC), MHCC97L and HCCLM3 (established and preserved in Liver Cancer Institute of Fudan University) were used in present study and routinely raised 2, 18.
Immunofluorescent staining, Western blot and quantitative real-time polymerase chain reaction (qRT-PCR)
Immunofluorescent staining was used to detect the location of CD151 and Mortalin in Hep3B, HepG2, MHCC97L and HCCLM3 cells as described previously 7. Rabbit anti-human Mortalin monocolonal antibody (Cell Signaling Technology) and mouse anti-human CD151 monocolonal antibody (established by our team) were used at 1:200 dilutions. Western blot analysis was used to examine the expression of CD151 and Mortalin in HCC cells as described previously 7. Rabbit anti-human Mortalin monocolonal antibody (Cell Signaling Technology) and mouse anti-human CD151 monocolonal antibody (generated in our team) were used at 1:1000 dilutions. Goat anti-human β-actin antibody (1:2000) was used as control. Mortalin and CD151 mRNA expression in HCC cells were examined by qRT-PCR as described previously. For the PCR amplification, primers were used for Mortalin: 5-CCCCAAGTAAAGCTGTCAATCCT-3, 5-GACCATCAGCGGCAGTAGAGAAT-3; CD151: 5'-ACTTCATCCTGCTCCTCATCAT-3', 5'-TCCGTGTTCAGCTGCTGGTA-3'; GAPDH: 5-GGGGCTCTCCAGAACATCATCC-3, 5-ACGCCTGCTTCACCACCTTCTT-3. GAPDH was used as control.
Transfection and interference
Stable transfectant pGCSIL-GFP-shRNA-CD151 in HCC cells were constructed as previously described 8. The lentiviral-mediated pGCSIL-GFP-shRNA-mortalin was constructed as previously described (Shanghai Genechem Company Ltd., Shanghai, China). The shRNA targeting sequence for mortalin was identified high efficiency and used in subsequent experiment: 5'- ACATTGTGAAGGAGTTCAA-3'.
Immunoprecipitation assay
The protein interaction between Mortalin and CD151 was investigated in HCCLM3 cells using co-immunoprecipitation as described in our previous study 11. The primary antibodies rabbit anti-human Mortalin monocolonal antibody (Cell Signaling Technology) and mouse anti-human CD151 monoclonal antibody (produced by our team) were used at 1:1000 dilutions. IgG was used as control.
Wound healing and migration assay
A wound-healing assay was used to assay the role of CD151 and Mortalin in cell migration as described. Migration assay was performed as described elsewhere 10.
Patients and follow-up
187 cases of HCC tumor specimens were collected as previously described 2. Detailed clinicopathological characteristics were listed in Table 1. Follow up procedures were consistent with our previous study 8.
Table 1.
Correlation between CD151/Mortalin expression and clinicopathological features in 187 hepatocellular carcinoma patients
| Variables | CD151 staining | p value | Mortalin staining | p value | ||
|---|---|---|---|---|---|---|
| High | Low | High | Low | |||
| Sex (female vs. male) | ||||||
| Male | 91 | 69 | 0.627 | 88 | 72 | 0.680 |
| Female | 14 | 13 | 16 | 11 | ||
| Age(years) | ||||||
| ≥53 | 53 | 36 | 0.372 | 51 | 38 | 0.658 |
| <53 | 52 | 46 | 53 | 45 | ||
| HBsAg | ||||||
| Positive | 87 | 67 | 0.838 | 88 | 66 | 0.364 |
| Negative | 18 | 15 | 16 | 17 | ||
| Child-Pugh score | ||||||
| A | 102 | 80 | 0.607* | 101 | 81 | 0.827* |
| B | 3 | 2 | 3 | 2 | ||
| ALT(U/ml) | ||||||
| ≥75 | 89 | 75 | 0.166 | 88 | 76 | 0.150 |
| <75 | 16 | 7 | 16 | 7 | ||
| Serum AFP (ng/ml) | ||||||
| ≥20 | 64 | 60 | 0.079 | 68 | 56 | 0.764 |
| <20 | 41 | 22 | 36 | 27 | ||
| GGT(U/ml) | ||||||
| ≥75 | 35 | 20 | 0.183 | 32 | 23 | 0.648 |
| <75 | 70 | 62 | 72 | 60 | ||
| Cirrhosis | ||||||
| Yes | 96 | 73 | 0.580 | 95 | 74 | 0.614 |
| No | 9 | 9 | 9 | 9 | ||
| Diameter(cm) | ||||||
| ≥5 | 54 | 26 | 0.007 | 56 | 24 | 0.001 |
| <5 | 51 | 56 | 48 | 59 | ||
| Tumor number | ||||||
| Multiple | 19 | 11 | 0.387 | 16 | 14 | 0.784 |
| Solitary | 86 | 71 | 88 | 69 | ||
| Microvascular invasion (yes vs no) | ||||||
| Yes | 40 | 15 | 0.003 | 38 | 17 | 0.017 |
| No | 65 | 67 | 66 | 66 | ||
| Tumor Capsulation | ||||||
| Yes | 48 | 42 | 0.455 | 48 | 42 | 0.545 |
| None | 57 | 40 | 56 | 41 | ||
| Tumor differentiation | ||||||
| III/IV | 76 | 60 | 0.904 | 30 | 21 | 0.589 |
| I/II | 29 | 22 | 74 | 62 | ||
Abbreviations: HBsAg, Surface of antigen of Hepatitis B virus; ALT, Alanine transaminase; AFP, alpha-fetoprotein; GGT, Gamma-Glutamyltransferase. *Fisher's Exact Test
Tissue microarray (TMA) and immunohistochemistry
TMA was constructed as described previously 2, 19. The monoclonal mouse anti-human CD151 (1:200, established by our team) and rabbit anti-human Mortalin antibody (1:100, Cell Signaling Technology) antibodies were used to detect the expression of CD151 and Mortalin, respectively, based on a two-step protocol as previously described 8. The density of positive staining of CD151 and Mortalin was measured as described elsewhere 8, 20.
Immunofluorescence
The immunofluorescence was performed according to our previous study 2.
Statistical analysis
Statistical analysis was done with SPSS 16.0 software (SPSS, Chicago, IL). Student's t test was used for comparisons between groups. Categorical data were analyzed by the chi-square tests. Kaplan-Meier method and the log-rank test were used to assay the survival rates. p<0.05 was set as significant difference.
Results
High Mortalin expression play an important role in the progression of HCC cells
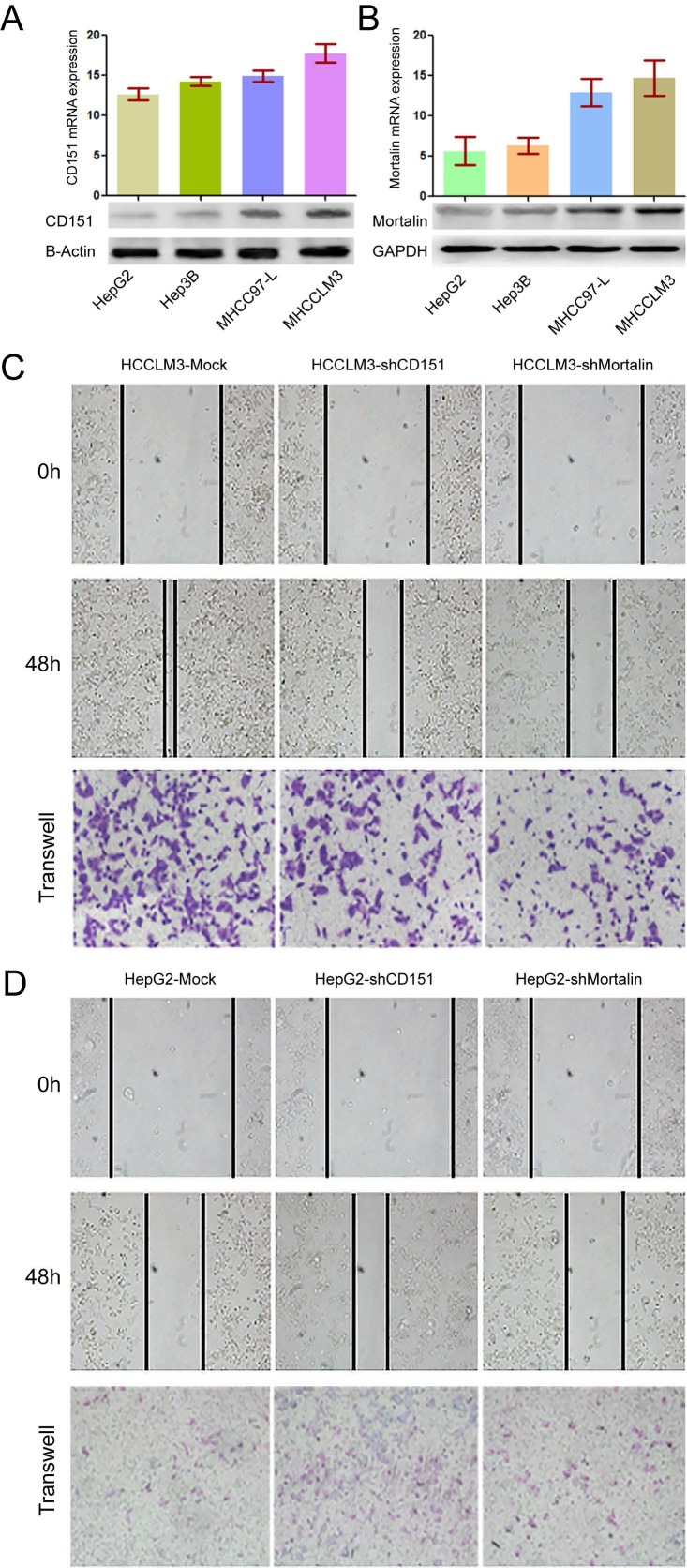
Previous study showed HCC patients with high CD151 expression were inclined to have poor prognosis 2. Here, we investigated the relationship between Mortalin and CD151 in HCC cells. Consistent with the results from our previous study 2, high metastatic HCCLM3 cells expressed the highest expression of CD151 at the protein and mRNA levels, while non-metastatic HepG2 presented the weak expression of CD151 (Figure 1A, p<0.05). In present study, the results showed that Mortalin expression in HCCLM3 cells was significantly higher than that in MHCC97-L (p<0.05) and HepG2 (p<0.01) cells at the protein and mRNA levels (Figure 1B). Next, we modified the expression of CD151 and/or Mortalin in HCC cells and assayed their roles in the invasion and migration of HCC cells. Expectedly, knockdown of CD151 expression in HCCLM3 cells reduced in a decreased migration and invasion (Figure 1C, p<0.05). Meanwhile, inhibition of Mortalin expression in HCCLM3 cells resulted in an impaired ability of migration and invasion as well (Figure 1C, p<0.05). Upregulation of CD151 in HepG2 cells resulted in an increased migration and invasion. When Mortalin expression in HepG2-CD151 cells were downregulated, their ability of migration and invasion were also impaired (Figure 1D, p<0.05). The results indicate that Mortalin play an important role in the progression of HCCs.
Figure 1.
Expression of CD151/Mortalin in HCCs and functional analysis. A. Expression of CD151 protein and mRNA in HCC cells with different metastatic potential. B. Expression of Mortalin protein and mRNA in HCC cells with different metastatic potential. C. Knockdown of CD151 or Mortalin expression in HCCLM3 cells resulted in a decreased migration and invasion. D. Upregulation of CD151 or Mortalin expression in HepG2 cells resulted in an increased migration and invasion.
Mortalin forms a complex with CD151 in HCC cells
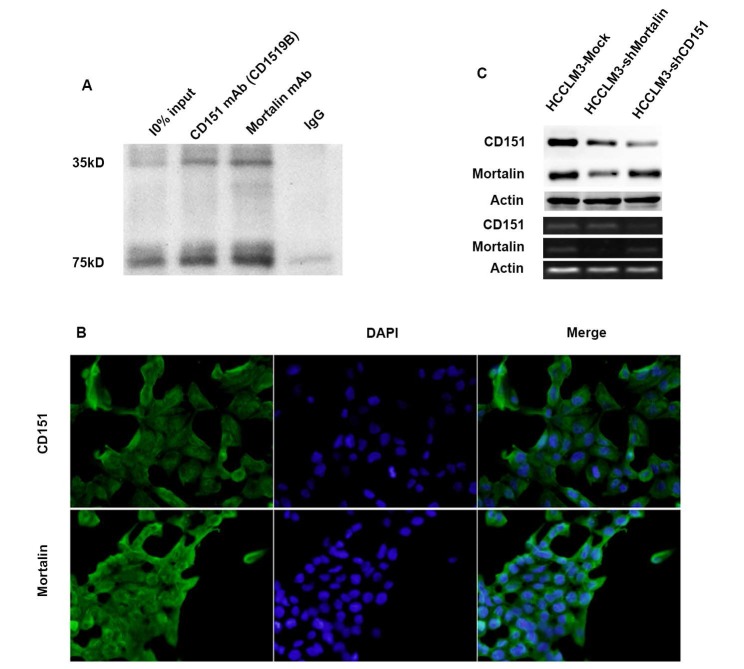
Previously, we used the combination co-IP with 2D-LC-MS/MS to show that Mortalin may be one of partners for CD151 in HCCLM3 cells 9. Here, we further examined the relation between Mortalin and CD151 in HCCM3 cells expressing high level of CD151 protein using co-IP. The result revealed that Mortalin forms a complex with CD151 in HCCLM3 cells (Figure 2A). Next, we performed the immunofluorescence assay to investigate the location of CD151 and Mortalin in HCC cells, and found that Mortalin was localized on the plasma in HCC cells, while CD151 in plasma and membrane (Figure 2B). Then, we modified the expression of CD151 and/or Mortalin in HCC cells and investigated their interaction. Interestingly, inhibition of Mortalin in HCC cells induced moderately decreased expression of CD151 protein, rather than CD151 mRNA. While downregulation of CD151 did not influence in the expression of Mortalin at the level of protein and mRNA (Figure 2C). Given the cytoprotective role of heat shock protein family, we suggest that Mortalin could protect CD151 protein from degradation.
Figure 2.
Mortalin formed a complex with CD151 in HCCs. A. Co-IP assay showed Mortalin formed a complex with CD151 in HCC cells. B. Immunofluorescence showed colocalization of CD151 and Mortalin in HCCLM3 cells (original magnification, ×400). C. Inhibition of Mortalin in HCC cells induced moderately decreased expression of CD151 protein, rather than CD151 mRNA. Downregulation of CD151 did not influence in the expression of Mortalin at the level of protein and mRNA.
CD151 involved in the progression of HCCs in a Mortalin-dependent manner
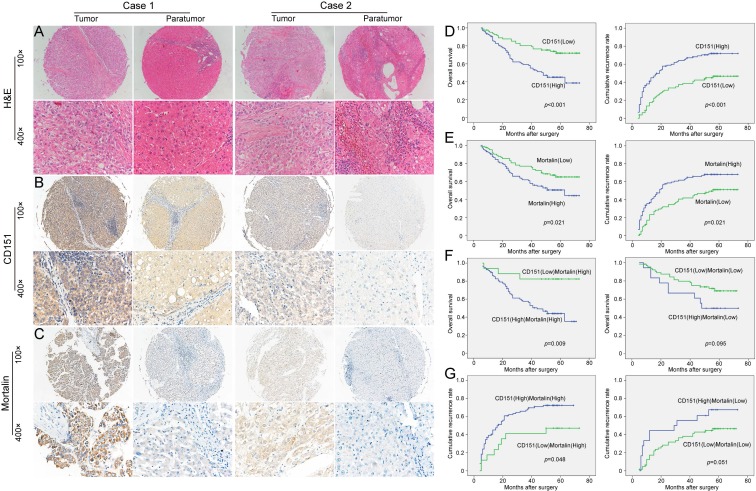
Here, we further investigated the implication of the CD151/Mortalin expression in a clinical setting including 187 cases of HCCs. After identification of primary HCC using hematoxylin-eosin staining (Figure 3A), we stained for CD151 and Mortalin in tissue microarray (TMA) slides using immunohistochemistry. Immunoreactivity of CD151 protein was located on the cell membranes and its intensity in tumor tissues was stronger than that in paratumoral tissues (Figure 3B). Positive Mortalin was expressed in the cytoplasm in a diffuse or granular pattern and its intensity in tumor samples was much stronger than that in paratumoral samples (Figure 3C). Next, we classified the whole cohort of patients into Mortalinhigh and Mortalinlow subgroup according to Mortalin expression and analyzed the relationship between Mortalin expression and clinical characteristics. Our results revealed that expression of Mortalin was positively correlated with microvascular invasion (p=0.017) and tumor diameter (p=0.001). However, other clinical characteristics, including age, sex, serum alpha fetoprotein, Child-Pugh score, tumor number, tumor capsulation and differentiation were not directly related to the expression of Mortalin. As described in our previous study, high level of CD151 was significantly related to tumor size (>5cm) (p=0.007) and vascular invasion (p=0.003) (Table 1).
Figure 3.
The expression and prognostic implication of CD151/Mortalin in HCC. A. Diagnosis by Hematoxylin and eosin (H&E) staining B. Expression of CD151 in tumor tissues was stronger than that in paratumoral tissues. Moreover, the expression of CD151 in different tumor sample had evidently variation. C. Tumor tissues had higher expression of Mortalin than that of paratumoral tissues. Moreover, the expression of Mortalin in tumor tissues had evidently variation. D. Prognostic implication of CD151 expression in HCC patients E. Prognostic implication of Mortalin expression in HCC patients F and G. Among Mortalinhigh subgroup of HCC patients, those with CD151high had shorter OS and higher cumulative recurrence rates than those with CD151low. However, in Mortalinlow subgroup of HCC patients, CD151 expression did not exert on the prognosis.
Then, we investigated the clinical implication of Mortalin/CD151 in the prognosis of HCC patients. In line with our previous results, present study also showed that CD151 expression was inversely related to the poor prognosis of HCC (Figure 3D). The OS rate of the Mortalinlow group was higher than those in the Mortalinhigh group (66.3% versus 51%). The cumulative recurrence rates of the Mortalinhigh group was significantly higher than that of the low level of Mortalin group (66.3% versus 49.4%, Figure 3E). Given the role of Mortalin in the stability of CD151 protein, we investigated the clinical implication of CD151 expression in the subgroup with different level of Mortalin. Interestingly, among Mortalinhigh subgroup of HCC patients, HCC patients with CD151high had shorter OS and higher cumulative recurrence rates than those with CD151low (Figure 3F and G). However, in Mortalinlow subgroup of HCC patients, CD151 expression did not exert on the prognosis (Figure 3F and G). These data also support the notion that Mortalin probably prevent from degradation of CD151 protein and involve in the progression of HCCs. A Cox proportional hazards model showed CD151 was an independent prognostic indicator for OS (p=0.011) and cumulative recurrence (p=0.002) (Table 2 and 3). While Mortalin was an independent prognostic indicator for cumulative recurrence (p=0.046) (Table 3). When CD151/Mortalin was adopted as a covariate, we found that CD151/Mortalin was also an independent prognostic predictor for cumulative recurrence (p=0.021) (Table 3).
Table 2.
Univariate and multivariate analyses of factors associated with overall survival.
| Factors | Univariate, p | Multivariate | |||
|---|---|---|---|---|---|
| HR | 95%Cl | p Value | |||
| Sex (female vs. male) | 0.203 | NA | |||
| Age (years) (≥53 vs. <53) | 0.217 | NA | |||
| HBsAg (positive vs. negative) | 0.087 | NA | |||
| Child-Pugh classification (A vs. B) | 0.149 | NA | |||
| Serum ALT, U/L (≥75 vs. <75) | 0.260 | NA | |||
| Serum AFP, ng/L (≥20 vs. <20) | 0.298 | NA | |||
| Liver cirrhosis (yes vs. no) | 0.593 | NA | |||
| Tumor size (diameter, cm) (<5 vs. ≥5) | <0.001 | 0.510 | 0.317-0.823 | 0.006 | |
| Tumor number (multiple vs. single) | 0.048 | NS | |||
| Tumor capsulation (yes vs. no) | 0.168 | NA | |||
| Tumor differentiation (III/IV vs. I/II.) | 0.084 | NA | |||
| Microvascular invasion (yes vs. no) | <0.001 | 2.231 | 1.383-3.597 | 0.001 | |
| TNM stage (I/II vs. III/IV) | 0.001 | NA | |||
| CD151 expression (high vs. low) | <0.001 | 0.515 | 0.310-0.858 | 0.011 | |
| Mortalin expression (high vs. low) | 0.023 | NS | |||
| CD151/Mortalin expression (low vs. high) | <0.001 | NS | |||
Abbreviation: 95% CI: 95% confidence interval; AFP, alpha-fetoprotein; TNM, tumor-node-metastasis; HBsAg, hepatitis B surface antigen; HR, hazard ratio; NA, not adopted; NS, not significant; OS, overall survival. Cox proportional hazards regression model.
Table 3.
Univariate and multivariate analyses of factors associated with cumulative recurrence rate.
| Factors | Univariate, p | Multivariate | p value | |
|---|---|---|---|---|
| HR | 95%Cl | |||
| Sex (female vs. male) | 0.245 | NA | ||
| Age (years) (≥53 vs. <53) | 0.285 | NA | ||
| HBsAg (positive vs. negative) | 0.483 | NA | ||
| Child-Pugh classification (A vs. B) | 0.879 | NA | ||
| Serum ALT, U/L (≥75 vs. <75) | 0.838 | NA | ||
| Serum AFP, ng/L (≥20 vs. <20) | 0.099 | NA | ||
| Liver cirrhosis (yes vs. no) | 0.176 | NA | ||
| Tumor size (diameter, cm) (≥5 vs. <5) | <0.001 | 0.549 | 0.368-0.819 | 0.003 |
| Tumor number (multiple vs. single) | 0.005 | NS | ||
| Tumor Capsulation (yes vs. no) | 0.051 | NA | ||
| Tumor differentiation (III/IV vs. I/II.) | 0.078 | NA | ||
| Microvascular invasion (yes vs. no) | <0.001 | 1.736 | 1.140-2.644 | 0.010 |
| TNM stage (I/II vs. III/IV) | 0.002 | NA | ||
| CD151 expression (low vs. high) | <0.001 | 0.532 | 0.354-0.799 | 0.002 |
| Mortalin expression (low vs. high) | 0.003 | 0.664 | 0.444-0.992 | 0.046 |
| CD151/Mortalin expression (low vs. high) | <0.001 | 0.626 | 0.421-0.932 | 0.021 |
Abbreviation: 95% CI, 95% confidence interval; AFP, alpha-fetoprotein; TNM, tumor-node-metastasis; HBsAg, hepatitis B surface antigen; HR, hazard ratio; NA, not adopted; NS, not significant; OS, overall survival. Cox proportional hazards regression model.
Discussion
In present study, our results revealed that high metastatic HCC cells tend to express high level of Mortalin. Interference of Mortalin significantly inhibited the invasion and migration of HCC cells. Clinically, HCC patients with Mortalin overexpression had poor prognosis. Therefore, we conclude that Mortalin does play an important role in in the progression of HCC.
A more interesting result from our study is that Mortalin could form a complex with CD151 and prevent from destabilization of CD151-depedent TEM and involve in the progression of HCC. TEM was considered as a function unit for tetraspanin family, and its stability is a requisite for the role of tetraspanin CD151 related to the invasion and metastasis in malignant tumors, including HCC 2. In our study, high metastatic HCC cells express high level of Mortalin and CD151. Mortalin formed a complex with CD151 in HCC cells. Importantly, down-regulation of Mortalin induced a moderate decreased CD151 protein, but not CD151 mRNA, while inhibition of CD151 did not influence the expression of Mortalin. Moreover, upregulation of CD151 expression in HCC cells partially restored the ability of invasion and migration of HCC cells induced by interference of Mortalin. More importantly, HCC patients with CD151 overexpression had poor prognosis, to a large extent, depending on high Mortalin expression in tumor tissues. These data support our notion that Mortalin stabilize CD151-depedent TEM and involve in the progression of HCC. Mortalin exists in multiple subcellular sites of cell, including the mitochondrion, plasma membrane, endoplasmic reticulum, cytosol, and nucleus. It may serve as safeguards to maintain homeostasis and integrity of protein interactions and play a vital role in multiple processes of cell, such as stress response, intracellular trafficking, cell proliferation, and differentiation 21. Recent studies have focused on the role of Mortalin in carcinogenesis and tumor progression. Mortalin could efficiently protect cancer cells from endogenous and exogenous oxidative stress 22. Mortalin also inactivated tumor suppressor protein p53 functions and activated telomerase and heterogeneous ribonucleoprotein K (hnRNP-K) proteins, thus promoting carcinogenesis and tumor metastasis 23. Starenki D et al 24 reported that mortalin was upregulated in human medullary thyroid carcinoma (MTC) tissues and its depletion robustly induces cell death and growth arrest by inducing transient extracellular signal-regulated kinase (MEK/ERK) activation and altering mitochondrial bioenergetics. Chen J et al 17 also found that the overexpression of Mortalin was correlated with the metastatic phenotype of HCC cells and promoted the progression by induction of the EMT. In our serial studies, CD151 was validated as a key gene related to the invasiveness and metastasis of HCC. CD151 could form a complex with integrin α6β1 and c-Met and involved in several pathological processes, such invasiveness, neoangiogenesis and EMT 2, 8. The function of CD151 depends on the stability of TEM. Our study also confirmed that CD151 antibody targeting the CD151-integrin α6β1 binding domain could disassociate the TEM and inhibit the function of CD151 9. Therefore, as one of the molecular chaperones of CD151, Mortalin efficiently stabilized the CD151-depended TEM. Certainly, our study has some limitation as well. For example, the binding site of CD151/Mortalin need to be uncovered.
Acknowledgments
The authors thank Mei-Yu Hu for her technical help. This study was supported by the National Natural Science Foundation of China (81972232, 81472840, 81602513, 81502028, and 81672825) and the Shanghai Municipal Natural Science Foundation (18410720700, 17411951200, and 17ZR1405400).
Author Contributions
Concept and design: Guo-Ming Shi, Ai-Wu Ke, Xiao-Wen Zhang; Experiments: Li-Xin Liu, Hai-Ying Zeng, Peng-Fei Zhang, Xiao-Jun Guo, Xiao-Yong Huang, Rui-Zhao Dong; Chi Zhang, Qiang Kang, Hao Zou; Data collection: Xin-Yu Zhang, Lu Zhang; Data analysis and visualization: Li-Xin Liu, Jia-Bing Cai, Jia-Cheng Lu; Writing article: Li-Xin Liu, Jia-Cheng Lu, Jia-Bing Cai.
Abbreviations
- HCC
hepatocellular carcinoma
- AFP
α-fetoprotein
- PI3K
phosphatidylinositol 3-kinase
- Co-IP
co-immunoprecipitation
- MS
mass spectrometry
- TMA
tissue microarray
- TNM
tumor node metastasis
- shRNA
short hairpin RNA
- qRT-PCR
quantitative real-time polymerase chain reaction
- 2D-LC-MS/MS
two-dimensional liquid chromatography coupled with tandem mass spectrometry
- DMEM
Dulbecco's modified Eagle medium
- FBS
fetal bovine serum
- PBS
phosphate buffer solution
- DAPI
4',6-Diamidino-2-phenylindole
- BSA
bovine serum albumin
- SDS
sodium dodecyl sulfate
- OS
overall survival
References
- 1.Hwang S, Takimoto T, Hemler ME. Integrin-independent support of cancer drug resistance by tetraspanin CD151. Cell Mol Life Sci. 2019;76:1595–604. doi: 10.1007/s00018-019-03014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY. et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503. doi: 10.1002/hep.22639. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto N, Morine Y, Utsunomiya T, Imura S, Ikemoto T, Arakawa Y. et al. Role of CD151 expression in gallbladder carcinoma. Surgery. 2014;156:1212–7. doi: 10.1016/j.surg.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 4.Kwon MJ, Park S, Choi JY, Oh E, Kim YJ, Park YH. et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer. 2012;106:923–30. doi: 10.1038/bjc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medrano M, Communal L, Brown KR, Iwanicki M, Normand J, Paterson J. et al. Interrogation of Functional Cell-Surface Markers Identifies CD151 Dependency in High-Grade Serous Ovarian Cancer. Cell Rep. 2017;18:2343–58. doi: 10.1016/j.celrep.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Kumari S, Devi Gt, Badana A, Dasari VR, Malla RR. CD151-A Striking Marker for Cancer Therapy. Biomark Cancer. 2015;7:7–11. doi: 10.4137/BIC.S21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke AW, Shi GM, Zhou J, Huang XY, Shi YH, Ding ZB. et al. CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140:1629–41.e15. doi: 10.1053/j.gastro.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Shi GM, Ke AW, Zhou J, Wang XY, Xu Y, Ding ZB. et al. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52:183–96. doi: 10.1002/hep.23661. [DOI] [PubMed] [Google Scholar]
- 9.Devbhandari RP, Shi GM, Ke AW, Wu FZ, Huang XY, Wang XY. et al. Profiling of the tetraspanin CD151 web and conspiracy of CD151/integrin beta1 complex in the progression of hepatocellular carcinoma. PLoS One. 2011;6:e24901. doi: 10.1371/journal.pone.0024901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke AW, Zhang PF, Shen YH, Gao PT, Dong ZR, Zhang C. et al. Generation and characterization of a tetraspanin CD151/integrin alpha6beta1-binding domain competitively binding monoclonal antibody for inhibition of tumor progression in HCC. Oncotarget. 2016;7:6314–22. doi: 10.18632/oncotarget.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang XY, Ke AW, Shi GM, Zhang X, Zhang C, Shi YH. et al. alphaB-crystallin complexes with 14-3-3zeta to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:2235–47. doi: 10.1002/hep.26255. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS. et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) Liver Cancer. 2018;7:235–60. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 15.Na Y, Kaul SC, Ryu J, Lee JS, Ahn HM, Kaul Z. et al. Stress chaperone mortalin contributes to epithelial-mesenchymal transition and cancer metastasis. Cancer Res. 2016;76:2754–65. doi: 10.1158/0008-5472.CAN-15-2704. [DOI] [PubMed] [Google Scholar]
- 16.Tai-Nagara I, Matsuoka S, Ariga H, Suda T. Mortalin and DJ-1 coordinately regulate hematopoietic stem cell function through the control of oxidative stress. Blood. 2014;123:41–50. doi: 10.1182/blood-2013-06-508333. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Liu WB, Jia WD, Xu GL, Ma JL, Huang M. et al. Overexpression of Mortalin in hepatocellular carcinoma and its relationship with angiogenesis and epithelial to mesenchymal transition. Int J Oncol. 2014;44:247–55. doi: 10.3892/ijo.2013.2161. [DOI] [PubMed] [Google Scholar]
- 18.Zhang PF, Wei CY, Huang XY, Peng R, Yang X, Lu JC. et al. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol Cancer. 2019;18:105. doi: 10.1186/s12943-019-1031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF. et al. Distinct PD-L1/PD1 Profiles and Clinical Implications in Intrahepatic Cholangiocarcinoma Patients with Different Risk Factors. Theranostics. 2019;9:4678–87. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang Q, Cai JB, Dong RZ, Liu LX, Zhang C, Zhang PF. et al. Mortalin promotes cell proliferation and epithelial mesenchymal transition of intrahepatic cholangiocarcinoma cells in vitro. J Clin Pathol. 2017;70:677–83. doi: 10.1136/jclinpath-2016-204251. [DOI] [PubMed] [Google Scholar]
- 21.Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002;7:309–16. doi: 10.1379/1466-1268(2002)007<0309:ahfcmm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadhwa R, Ryu J, Ahn HM, Saxena N, Chaudhary A, Yun CO. et al. Functional significance of point mutations in stress chaperone mortalin and their relevance to Parkinson disease. J Biol Chem. 2015;290:8447–56. doi: 10.1074/jbc.M114.627463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu J, Kaul Z, Yoon AR, Liu Y, Yaguchi T, Na Y. et al. Identification and functional characterization of nuclear mortalin in human carcinogenesis. J Biol Chem. 2014;289:24832–44. doi: 10.1074/jbc.M114.565929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starenki D, Hong SK, Lloyd RV, Park JI. Mortalin (GRP75/HSPA9) upregulation promotes survival and proliferation of medullary thyroid carcinoma cells. Oncogene. 2015;34:4624–34. doi: 10.1038/onc.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]