Abstract
Background: This meta-analysis aimed to explore if immunotherapy or chemotherapy alone or in combination is a better first line treatment strategy for advanced non-small cell lung cancer (NSCLC) patients.
Methods: Electronic databases including Google Scholar, PMC, PubMed, EMBASE, Scopus and the major conference proceedings were searched for relevant randomized controlled trials (RCTs) comparing outcomes of immune-checkpoint inhibitor combined with chemotherapy or immune-checkpoint inhibitor alone over chemotherapy alone in patients with advanced NSCLC without previous treatment. Study heterogeneity was assessed using the I2 test.
Results: A total of 14 RCTs including 8,081 treatment naïve advanced NSCLC patients were enrolled in this study. Our results showed that in comparison to chemotherapy alone, introducing immunotherapy into first-line chemotherapy has significant benefit in tumor response (RR, 1.27; 95% CI, 1.09 to 1.48), progression-free survival (PFS) (HR, -0.43; 95% CI, -0.56 to -0.31), and overall survival (OS) (HR, -0.30; 95% CI, -0.45 to -0.14) but with an increased risk of grade3 - 5 toxicity (RR, 1.11; 95% CI, 1.04 to 1.18). The pooled results of comparison of immune therapy alone with chemotherapy alone in selected patients with positive expression of Programmed Death-ligament (PD-L1) or with a high tumor mutational burden, demonstrated similar tumor response (RR, 1.13; 95% CI, 0.88 to 1.46), 3 - 5 grade toxicity (RR, 0.69; 95% CI, 0.40 to 1.19) and long-term outcomes, including OS (HR, -0.20; 95% CI, -0.43 to 0.03) and PFS (HR, -0.24; 95% CI, -0.61 to 0.14).
Conclusions: Our meta-analysis showed the superiority of combination therapy over monotherapy with chemotherapeutic agents in terms of tumor response, and long-term survival, but with an increased the 3 - 5 grade toxicity. And immune-checkpoint inhibitors alone showed similar tumor response, toxicity and long-term outcomes compared to platinum-based chemotherapy in selected patients.
Introduction
Lung cancer is one of the most lethal diseases and has become the leading cause of cancer related deaths 1, 2. Non-small cell lung cancer (NSCLC) is the largest subtype of lung cancer, comprising approximately 85% cases 3, 4. The first-line treatment strategy for advanced NSCLC is based on the expression of oncogenic aberrations, such as epidermal growth factor receptor gene (EGFR), anaplastic lymphoma kinase gene (ALK), and orphan receptor tyrosine kinase (ROS) 1, 5. However, most patients with NSCLC do not harbor these genetic aberrations; thus, cytotoxic chemotherapy is still the first-line treatment for such patients 6, 7. Chemotherapy alone is associated with a median overall survival (OS) of 8 - 10 months, progression-free survival (PFS) of 4 - 6 months, and objective tumor response rates of 25 - 35% 8. Moreover, the toxic effects of platinum-based chemotherapy are a concern for both clinicians and patients, as these severely impair quality of life 9. Therefore, developing new agents with better effectiveness and less toxicity is crucial.
Drugs interrupting immune checkpoints, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), anti-programmed cell death protein 1 (PD-1), anti-programmed cell death-ligand 1 (PD-L1), and others, can enhance anti-tumor immunity and mediate durable cancer regressions 10, 11. Previous studies have demonstrated promising therapeutic value of immune checkpoint inhibitors as these lead to improved tumor responses, prolonged long-term survival, and less toxicity for patients with metastatic NSCLC who had progressed during or after platinum-based chemotherapy 11-16. However, patients with advanced NSCLC usually undergo rapid deterioration during the first course of treatment, and less than half of these patients receive second-line therapy 17.
Over time several studies exploring the safety and efficacy of immunotherapy as the first-line treatment strategy for advanced NSCLC have been published 18-32. Due to the availability of a wide range of immunotherapeutic agents and distinct treatment strategies, there is no unanimous conclusion about the therapeutic status of immunotherapy in the management of naïve NSCLC patients. Thus, we performed this meta-analysis of randomized controlled clinical trials (RCTs), which included patients with locally advanced NSCLC with metastasis, to ascertain whether immune checkpoint agents alone or in combination with chemotherapy improve survival outcomes in NSCLC patients who received chemotherapy alone as a first-line treatment.
Material and methods
Study selection
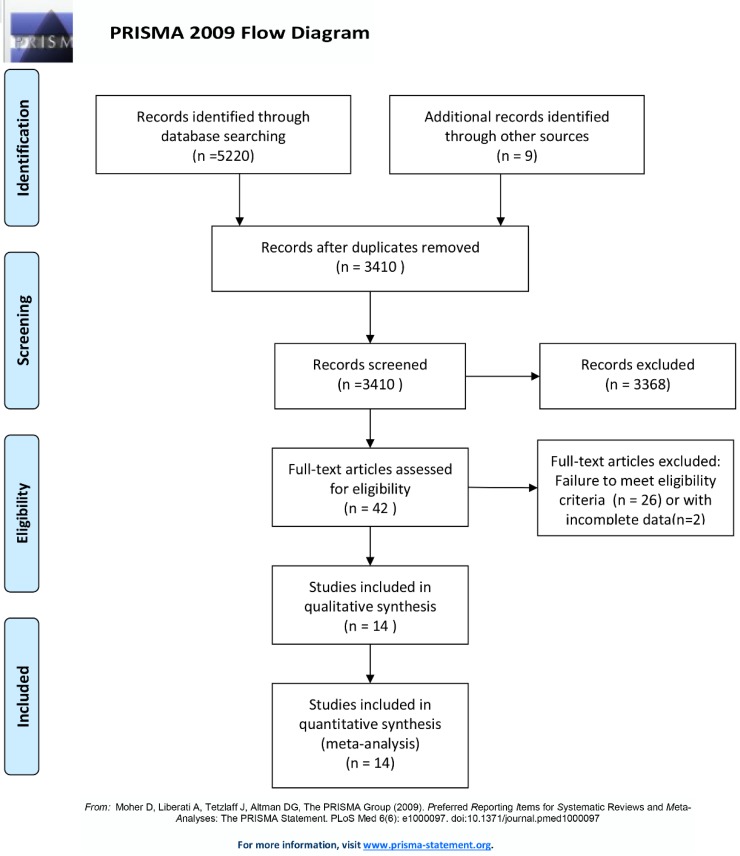
Electronic databases including Google Scholar, PMC, PubMed, EMBASE, Scopus, and the major conference proceedings (the American Society of Clinical Oncology and the European Society for Medical Oncology) were searched by two authors (Chen and Zhou) independently for RCTs published between 1st January, 2010 and 1st June, 2019. The following medical subject heading (MeSH) terms were used: (1) "non-small cell lung cancer or NSCLC"; (2) "nivolumab or pembrolizumab or atezolizumab or ipilimumab or durvalumab"; (3) "PD-1 or PD-L1 or CTLA-4 or immune checkpoint inhibitor"; (4) "Randomized Controlled Trial or RCT". All potentially eligible and relevant clinical studies were manually retrieved and examined. Studies that met the following criteria were included in this meta-analysis: (a) RCTs; (b) studies comparing the combination of immune therapy and chemotherapy with chemotherapy alone in the treatment of advanced treatment-naive NSCLC patients; and (c) studies comparing immune therapy alone with chemotherapy alone in the treatment of advanced treatment-naive NSCLC patients. Non-randomized controlled trials, or studies unrelated to the first-line immune therapy, were excluded. Ultimately, 14 RCTs were included for quantitative analysis (Fig. 1). Any disagreements about the processes of study selection, data extraction, and methodological quality assessment were resolved by discussion and consensus with an independent expert (Zhuang).
Figure 1.
Flow chart depicting the selection algorithm and screening process.
Data Extraction
The following information from the eligible studies were extracted by two authors (Tang and Chen) independently: year of publication, number of included patients, treatment regimen, and clinical outcomes. Clinical outcome measures included tumor response, long-term survival [progression-free survival (PFS) and overall survival (OS)], and the toxicity (3-5 grade toxicity and toxicity leading to discontinuation of treatment). Tumor response was stratified as objective responders who obtained a complete or partial response and as non-responders who experienced a stable or progressive disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 33.
Assessment of methodological quality
Two authors (Chen and Zhou) independently assessed the methodological quality of the eligible studies according to the Cochrane Collaboration guidelines v5.1.0 34.
Data analysis
All statistical analyses were performed using Stata12.0 software (Stata Corporation, College Station, TX, USA). Risk ratios (RRs) and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for dichotomous data. Heterogeneity among these included studies was evaluated using I2 statistics, where an I2 value > 50% was defined as substantial heterogeneity according to the Cochrane Collaboration guidelines v5.1.0 34. When I2 was < 50%, the fixed-effects model was used to assess outcomes; otherwise, the random-effects model were preferred. Sensitivity analysis using both fixed and random-effect models for the same data was conducted to confirm the robustness and reliability of the results.
Results
1.1. Search strategy
The database search retrieved 5220 records. After deleting duplicate results, a total of 3410 abstracts were screened for eligibility, and 42 clinical trials were considered potentially eligible for inclusion based on titles and abstract review. After retrieving and further analyzing the full-text of these studies, another 28 studies were excluded; the remaining 14 studies 18-32 were finally included in the meta-analysis (Fig. 1). These 14 RCTs included a total of 8,081 patients: 4,391 patients had been administered immunotherapy alone or combination of chemotherapy and immunotherapy, and the remaining the 3,690 patients had been administered platinum-based chemotherapy alone. The characteristics of all 14 included studies are shown in Table 1. Among these 14 included studies, ten RCTs had compared combination of checkpoint inhibitor and chemotherapy with chemotherapy alone 18-26, 31, 32. The other four RCTs had compared checkpoint inhibitor alone with chemotherapy alone 27-30.
Table 1.
Summary of 14 randomized controlled trials included in the meta-analysis.
| Study | Author | Year | Study Group (regime and no. of Pts.) |
Control Group (regime and no. of Pts.) |
Inclusion criteria | ||
|---|---|---|---|---|---|---|---|
| CA184-041 | Lynch et al. 25 | 2012 | Ipi+Chemo | 70 | Chemo alone | 66 | Stage IIIB or IV NSCLC |
| KEYNOTE 021 | Langer et al. 19 | 2016 | Pembro+Chemo | 60 | Chemo alone | 63 | Stage IIIB or IV, non-squamous NSCLC without targetable genetic aberration |
| NCT01285609 | Govindan et al. 26 | 2017 | Ipi+Chemo | 388 | Chemo alone | 361 | stage IV or recurrent squamous NSCLC |
| IMpower 150 | Socinski et al. 20 | 2018 | Atezo+Chemo | 400 | Chemo alone | 400 | Stage IIIB or IV, non-squamous NSCLC without targetable genetic aberration |
| IMpower 131 | Jotte et al. 22 | 2018 | Atezo+Chemo | 343 | Chemo alone | 340 | Stage IV, squamous NSCLC without |
| IMpower 132 | Papadimitrakopoulou et al. 23 | 2018 | Atezo+Chemo | 292 | Chemo alone | 286 | Stage IV non-squamous NSCLC without targetable genetic aberration |
| KEYNOTE 407 | Paz-Ares et al. 21 | 2018 | Pembro+Chemo | 278 | Chemo alone | 281 | Stage IV, squamous NSCLC |
| KEYNOTE 189 | Gandhi et al. 18 | 2018 | Pembro+Chemo | 410 | Chemo alone | 206 | Stage IV non-squamous NSCLC without targetable genetic aberration |
| IMpower 130 | West et al. 24 | 2019 | Atezo+Chemo | 473 | Chemo alone | 232 | Stage IV, non-squamous NSCLC without targetable genetic aberration |
| PACIFIC | Antonia et al. 31, 32 | 2018 | Durva+Chemo | 476 | Chemo alone | 237 | stage III, unresectable NSCLC |
| KEYNOTE 024 | Reck et al. 29 | 2016 | Pembro alone | 154 | Chemo alone | 151 | Stage IIIB or IV, NSCLC without targetable genetic aberration but with a PD-L1 tumor-expression level of 50% or more |
| CheckMate 026 | Carbone et al. 27 | 2017 | Nivo alone | 271 | Chemo alone | 270 | Stage IV or recurrent NSCLC without targetable genetic aberration but with a PD-L1 tumor-expression level of 5% or more |
| CheckMate 227 | Hellmann et al. 28 | 2018 | Nivo+Ipi | 139 | Chemo alone | 160 | Stage IV or recurrent NSCLC without targetable genetic aberration, with a high tumor mutational burden (≥10 mutations per megabase) |
| KEYNOTE 042 | Mok et al. 30 | 2018 | Pembro alone | 637 | Chemo alone | 637 | Stage IV or recurrent NSCLC without targetable genetic aberration but with a PD-L1 tumor-expression level of 1% or more |
Abbreviations: Pts -patients, Pembro- Pembrolizumab, Atezo -Atezolizumab, Nivo- Nivolumab, Ipi- Ipilimumab, Durva-Durvalumab, Chemo- Chemotherapy
1.2. Outcome assessments
1.2.1 Tumor response
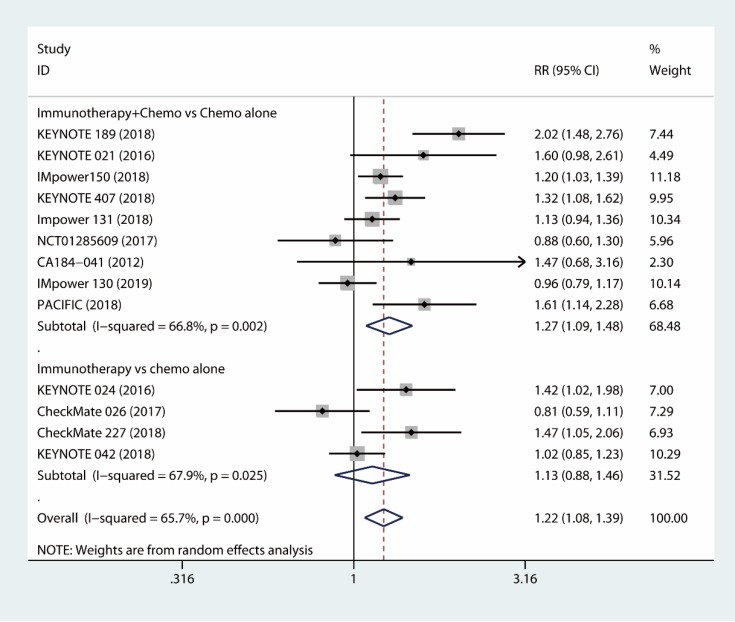
The results based on ten RCTs 18-26, 31, 32 showed that combined immunotherapy and chemotherapy had significant benefit compared to chemotherapy alone with respect to tumor response (RR, 1.27; 95% CI, 1.09 to 1.48; I2 = 66.8%) (Fig. 2). Additionally, the immune-checkpoint inhibitor alone was not inferior to chemotherapy alone as the first-line therapy with respect to tumor response rate (RR, 1.13; 95% CI, 0.88 to 1.46; I2 = 67.9%) 27-30 (Fig. 2). As a whole, the use of the immunotherapy as the first-line therapy increased the objective tumor response (RR, 1.22; 95% CI, 1.08 to 1.39; I2 = 65.7%) (Fig. 2).
Figure 2.
Forest plots of tumor response comparing combination therapy or immunotherapy alone versus chemotherapy alone.
1.3. Toxicity
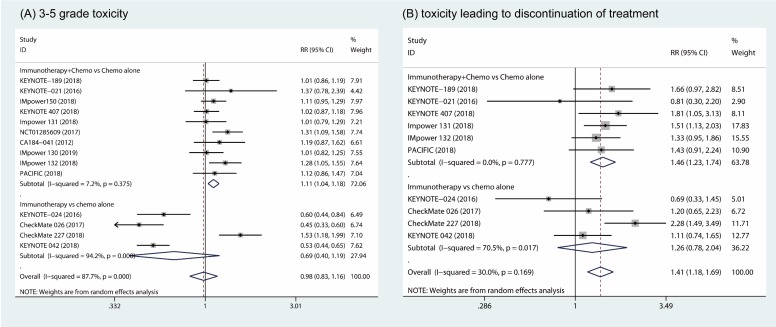
The pooled results showed that the combination of immunotherapy and chemotherapy significantly increased toxicity compared to chemotherapy alone (RR, 1.11; 95% CI, 1.04 to 1.18; I2 = 7.2%) 18-26, 31, 32. However, no significant difference in 3 - 5 grade toxicity was found between patients in the monotherapy arms (RR, 0.69; 95% CI, 0.40 to 1.19; I2 = 94.2%) (Fig. 3A) 27-30. Furthermore, more patients who underwent the combination of immunotherapy and chemotherapy discontinued their treatment due to the toxicity in combination of immunotherapy and chemotherapy group compared to chemotherapy alone (RR, 1.46; 95% CI, 1.23 to 1.74; I2 = 0%) 18, 19, 21-23, 31, 32. However, patients who discontinued their treatment due to toxicity was comparable between groups of immune therapy alone and chemotherapy alone (RR, 1.26; 95% CI, 0.78 to 2.04; I2 = 70.5%) (Fig. 3B) 27-30.
Figure 3.
Forest plots of 3 - 5 grade toxicity comparing combination therapy or immunotherapy alone versus chemotherapy alone (A). Forest plots of toxicity leading to discontinue of treatment comparing combination therapy or immunotherapy alone versus chemotherapy alone (B).
1.4. Progression-free survival and overall survival
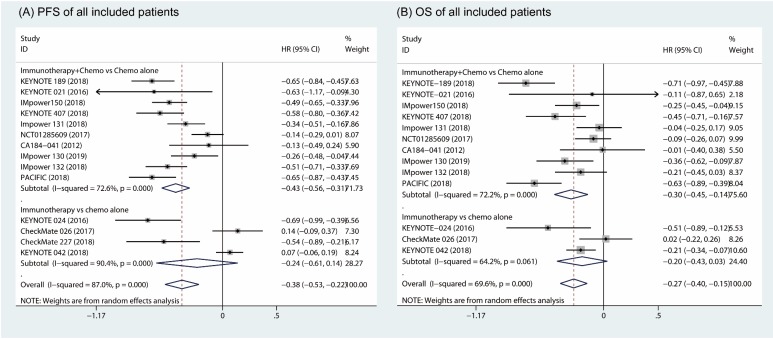
Based on random effects model analysis, a statistically significant benefit of combination of immune therapy and chemotherapy over chemotherapy alone was observed in term of PFS (HR, -0.43; 95% CI, -0.56 to -0.31; I2 = 72.6%) (Fig. 4A) 18-26, 31, 32. The OS also improved upon addition of an immune checkpoint inhibitor with chemotherapy as the first-line therapy (HR, -0.30; 95% CI, -0.45 to -0.14; I2 = 72.2%) (Fig. 4B). However, there was no significant difference between patients who received immunotherapy compared to those who took platinum-based chemotherapy in terms of PFS (HR, -0.24; 95% CI, -0.61 to 0.14, I2 = 90.4%; Fig. 4A) and OS (HR, -0.20; 95% CI, -0.43 to 0.03; I2 = 64.2%; Fig. 4B) 27-30.
Figure 4.
Forest plots of progress free survival comparing combination therapy or immunotherapy alone versus chemotherapy alone (A). Forest plots of overall survival comparing combination therapy or immunotherapy alone versus chemotherapy alone (B).
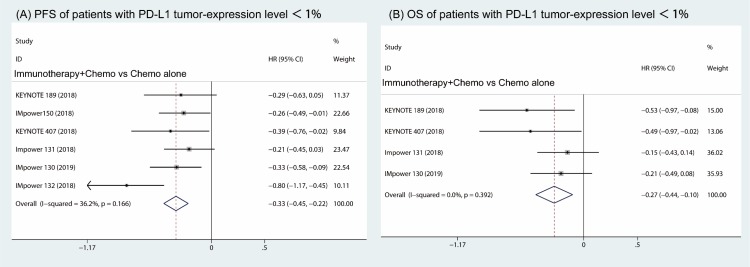
Furthermore, the subgroup analysis of patients with PD-L1 expression less than 1% revealed that both the PFS and OS were prolonged in the combination of immune therapy and chemotherapy compared with chemotherapy alone (HR, -0.33; 95% CI, -0.45 to -0.22; I2 = 36.2% (Fig 5A) and HR, -0.27; 95% CI, -0.44 to -0.10; I2 = 0.0% (Fig 5B)).
Figure 5.
Subgroup analysis of patients with PD-L1 tumor-expression level < 1% in combination therapy versus chemotherapy alone. Forest plots of progress free survival (A) and overall survival (B).
1.5. Methodological quality and sensitivity analyses
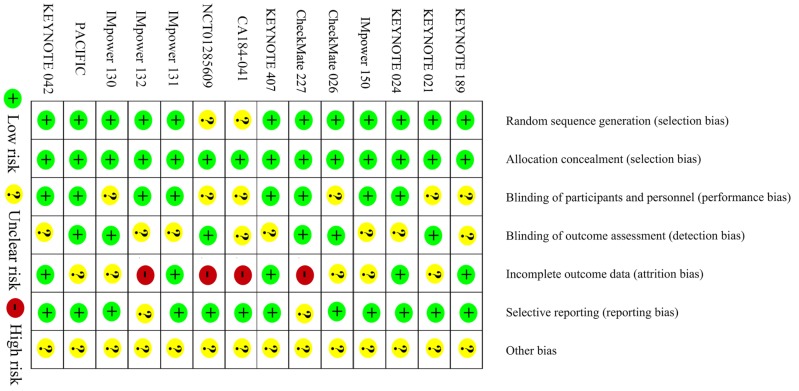
The methodological quality of the eligible studies is shown in Figure 6. All studies were assessed as level A. The sensitivity analyses showed robustness and reliability of our results.
Figure 6.
Risk of bias of the included trials.
Discussion
Our meta-analysis aimed to compare the treatment regimes of immunotherapy alone or in combination with chemotherapy with chemotherapy alone in patients with advanced treatment-naive NSCLC.
Based on all the available information extracted from the included trials, we found that combination of immunotherapy and platinum-based chemotherapy as a first-line therapy has a favorable long-term effect. It is important to note that the studies, which compared the combination of chemotherapy and immunotherapy with chemotherapy alone, included all treatment-naive patients regardless of PD-L1 expression on tumor cells. Due to the functional mechanism of the immune checkpoint inhibitor, expression levels of PD-L1 on tumor cells assessed by immunohistochemistry have been regarded as a potential responsive biomarker to these agents 14, 35. The previous study showed that the treatment efficiency in patients with a higher PD-L1 tumor-expression level was significantly better than those with a lower PD-L1 tumor-expression level, when treated by the immune checkpoints inhibitor 36, 37. But, the subgroup analysis of this study showed that patients who had low or negative expression of PD-L1(≤1%) also benefitted from combined immunotherapy and chemotherapy as a first-line treatment in both PFS and OS. Although favorable long-term survival was observed in combined immunotherapy and chemotherapy group, the initial 3-6 month survival curve often overlaps or even crosses 38, which means that the efficacy of immunotherapy combined with chemotherapy in the early stage of treatment may not be superior to chemotherapy18-26. The higher rate of toxicity might offset the therapeutic effect of the combination of immunotherapy and chemotherapy at the onset of treatment 38.
Comparison of monotherapy arms of immune-checkpoint inhibitors and chemotherapy shows that selected patients with positive PD-L1 tumors 27, 29, 30 or a high mutation burden 28, who were administered immunotherapy alone, experienced longer PFS and OS. However, the difference was not significant with high heterogeneity among the studies. The results of the KEYNOTE-024 study demonstrated an advantage of pembrolizumab over chemotherapy in patients with a PD-L1 tumor-expression level ≥50% with regards to long-term survival. PFS improvement of 4.3 months was observed in the pembrolizumab group, despite the finding that 43% of patients who had undergone chemotherapy crossed over to the pembrolizumab group 29. The results of KEYNOTE-042 30 which recruited treatment-naïve stage IIIB-IV NSCLC patients with a PD-L1 tumor-expression level ≥1% demonstrated patients in the pembrolizumab group enduring a prolonged OS compared with chemotherapy group. The subgroup analysis of patients with a PD-L1 tumor-expression level ≥ 50% showed that the PFS and OS were significantly longer in pembrolizumab group compared with chemotherapy group. However, in patients with a PD-L1 tumor-expression level <50%, there was no significant difference in PFS and OS between the two groups 30. Based on these observations, we proposed that pembrolizumab alone is effective in patients a PD-L1 tumor-expression level ≥50%. However, in the CheckMate 026 study, no significant advantage of nivolumab over chemotherapy was observed in terms of objective tumor response and long-term survival among patients with PD-L1 expression >5% 27. The subgroup analysis demonstrated that even in patients with PD-L1 expression >50%, there is no significant difference between nivolumab monotherapy group and chemotherapy group. Positive detection of PD-L1 on tumor cells alone may not be sufficient to predict outcomes among patients who receive immunotherapy. Owing to the complexity of the immune system, patients with low- PD-L-1 expression may also benefit from immunotherapy. Thus, new biomarkers, such as tumor mutational burden for response to immune-oncological agent, beyond PD-L1 expression levels, may be the most critical markers for selecting patients for immunotherapy. The CheckMate 026 trial demonstrated that among patients with a high tumor-mutation burden, nivolumab monotherapy achieved a higher response rate compared to chemotherapy alone (47% vs. 28%). The median PFS of patients receiving nivolumab monotherapy (9.7 months) has been reported to be much longer than those receiving chemotherapy alone (5.8 months) 27. Furthermore, the CheckMate 227 trial demonstrated that PFS was significantly longer with first-line nivolumab plus ipilimumab (7.2 months) compared to chemotherapy (5.5 months) among NSCLC patients with a high tumor mutational burden (≥10 mutations per megabase), irrespective of PD-L1 expression levels.
Numerous limitations of these trials may hinder the fairness of this meta-analysis. Since only 14 RCTs were included in this meta-analysis, the results were underpowered. Furthermore, the HRs and corresponding 95% CIs were mainly extracted from the original studies without access to individualized data, which might have contributed to reporting bias. High heterogeneity was observed among the included studies, which may have decreased the strength of our meta-analysis. The high heterogeneity may be explained by the following: First, different immune checkpoint inhibitor regimens were used in different studies. Second, inclusion criteria differed among the included studies. Third, different treatment strategies had been used among studies.
The results of this meta-analysis demonstrated the superiority of combined immunotherapy with chemotherapy over chemotherapy alone in terms of tumor response and long-term survival as a first-line treatment strategy for advanced NSCLC patients but with higher rate of 3 - 5 grade toxicity. And immune-checkpoint inhibitors alone showed similar tumor response, toxicity and long-term outcomes compared to platinum-based chemotherapy in selected patients.
Acknowledgments
The present study was funded by the Human Provincial Science and Technology Department (2017JJ2345) and the National Natural Science Foundation of China (No. 81673516).
References
- 1.Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Hong QY, Wu GM, Qian GS, Hu CP, Zhou JY, Chen LA. et al. Prevention and management of lung cancer in China. Cancer. 2015;121(Suppl 17):3080–8. doi: 10.1002/cncr.29584. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Peng X, Zhou Y, Xia K, Zhuang W. Comparing the benefits of chemoradiotherapy and chemotherapy for resectable stage III A/N2 non-small cell lung cancer: a meta-analysis. World J Surg Oncol. 2018;16:8. doi: 10.1186/s12957-018-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N. et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 6.Xia N, An J, Jiang QQ, Li M, Tan J, Hu CP. Analysis of EGFR, EML4-ALK, KRAS, and c-MET mutations in Chinese lung adenocarcinoma patients. Exp Lung Res. 2013;39:328–35. doi: 10.3109/01902148.2013.819535. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C. et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26:1877–83. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 8.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE. et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–8. doi: 10.2147/OTT.S100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Lee KH, Cho EK, Kim DW, Kim SW, Kim JH. et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer. 2018;122:234–42. doi: 10.1016/j.lungcan.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. New Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. New Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordoni R, Ciardiello F, von Pawel J, Cortinovis D, Karagiannis T, Ballinger M, Patient-Reported Outcomes in OAK: A Phase III Study of Atezolizumab Versus Docetaxel in Advanced Non-Small-cell Lung Cancer. Clin Lung Cancer; 2018. [DOI] [PubMed] [Google Scholar]
- 17.Lazzari C, Bulotta A, Ducceschi M, Viganò MG, Brioschi E, Corti F. et al. Historical evolution of second-line therapy in non-small cell lung cancer. Frontiers in medicine. 2017;4:4. doi: 10.3389/fmed.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. New Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 19.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17:1497–508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. New Engl J Med. 2018;378:2288–301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. New Engl J Med; 2018. [DOI] [PubMed] [Google Scholar]
- 22.Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein MA. et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab+ carboplatin+ paclitaxel or nab-paclitaxel vs carboplatin+ nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36:LBA9000. [Google Scholar]
- 23.Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, IMPOWER132: PFS and safety results with 1L atezolizumab+ carboplatin/cisplatin+ pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol; 2018. p. 13. [Google Scholar]
- 24.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology; 2019. [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 26.Govindan R, Szczesna A, Ahn M-J, Schneider C-P, Gonzalez Mella PF, Barlesi F. et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35:3449–57. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 27.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M. et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. New Engl J Med. 2017;376:2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C. et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. New Engl J Med. 2018;378:2093–104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. New Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 30.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ. et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 31.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. New Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 32.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. New Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J. Green S. Cochrane handbook for systematic reviews of interventions Version 5.1. 0. The Cochrane Collaboration. Confidence intervals; 2011. [Google Scholar]
- 35.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–68. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 38.Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC. et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–62. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]