Abstract
Objective
Spinal Cord Stimulation (SCS) overlaps painful areas with paresthesia to alleviate pain. Ten kHz High‐Frequency SCS (HF10 cSCS) constitutes a treatment option that can provide pain relief without inducing paresthesia. In this retrospective, open‐label study, we evaluated the efficacy of HF10 cSCS in chronic neck and/or upper limb pain.
Methods
Between May 2015 and August 2017, 24 consecutive patients with neck and/or upper limb pain were treated with HF10 cSCS. The patients’ mean age was 61.4 years (range: 40.1–82.6 years). The mean neck and upper limb pain at baseline was 8.8 (range: 7.0–10) and 7.5 (range: 6.0–9.0) according to the visual analog scale (VAS). Functionality was evaluated using the Oswestry Disability Index (ODI). To assess health‐related psychological impairment, we used the Global Assessment of Functioning questionnaire.
Results
Twenty‐three patients responded to treatment. Pain intensity reduced significantly to a mean score of VAS 2.5 (range: 2.0–4.0) for neck and 2.0 (range: 1.0–3.0) for upper limb pain after 6 months. At 12 months, VAS scores for neck and upper limb pain reduced to 2.2 (range: 1.0–3.0) and 1.7 (range: 1.0–3.0), respectively. Mean ODI scores decreased from 31 (range: 21–42) at baseline to 19.9 (range: 8–26) after 12 months. In three patients, infection of the IPG pocket occurred r and 8.7 months after surgery. One patient has had lead migration resulting in a surgical revision.
Interpretation
HF10 cSCS therapy has proven to be effective in reducing neck and upper limb pain significantly and increasing functional capacity. These results warrant further studies with larger patient series and longer follow‐ups.
Introduction
Rates of chronic pain are increasing due to an aging population, improved survival rates after traumatic injuries as well as an increasing numbers of patients seeking new treatment options.1 High‐frequency (10 kHz) Spinal Cord Stimulation (HF10 cSCS) is a paresthesia‐free option for patients suffering from chronic back and leg pain refractory to pharmacological therapy or other treatment modalities.2, 3, 4, 5, 6 While HF10 cSCS in the thoracolumbar area is widely reported in the literature, only few reports exist for HF10 cSCS for the treatment of chronic pain in the neck and upper extremities.7, 8
In this single‐center, retrospective, open‐label study, we report our knowledge of HF10 cSCS for the treatment of chronic neck and upper limb pain.
Methods
In this study, we retrospectively evaluated the efficacy of HF10 cSCS therapy (NEVRO Corp., Menlo Park, USA) in patients suffering from neuropathic, chronic neck, and upper limb pain refractory to conservative and surgical treatment. It runs in accordance with the Declaration of Helsinki as well as data protection regulations, good clinical practice guidelines, and German Ethic Guidelines where a separate ethics committee approval is not required for retrospective studies. Furthermore, written informed consent was obtained from all patients.
Patients were eligible when (1) aged over 18 years; (2) had a predominant neck and/or upper limb pain for over 6 months and a minimum intensity of 5 out of 10 on the visual analogue scale (VAS); (3) no response to conventional or surgical treatments and (4) at least 6 months on analgesic medications (morphine, opioids, antineuropathic drugs).
Procedure
All patients underwent a trial of HF10 cSCS. Under general anesthesia, a small skin incision was performed and a Tuohy needle was introduced at the upper lumbar level to the epidural space according to the “loss of resistance” technique. One eight‐contact lead was advanced cranially in the dorsal epidural space under fluoroscopic guidance, and placed with the distal tip at the C2 vertebral level at the anatomic midline (Fig. 1). The lead was anchored and sutured to the supraspinal ligament or paravertebral muscle fascia, connected to temporary extensions, diverted subcutaneously to the left flank and connected to an external stimulator for the duration of the trial period (1–3 weeks).
Figure 1.
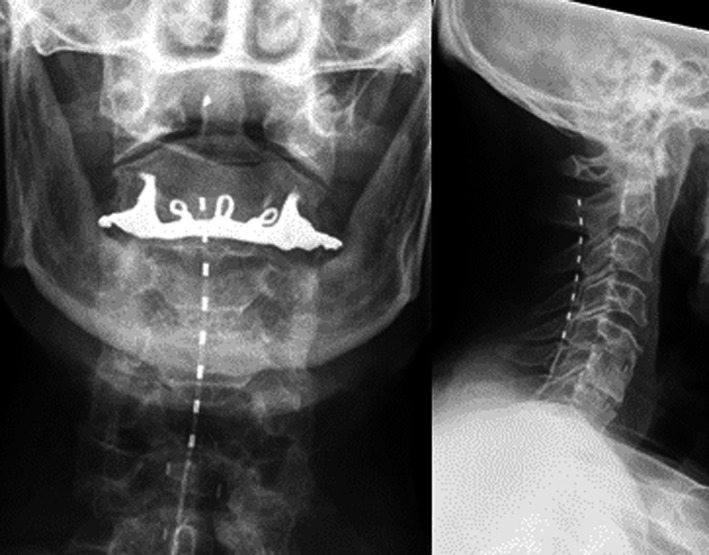
Placement of an octrode lead on X‐ray (p.a. left, lat. right) ranging from the level of C2–C5.
Various stimulation programs were provided to target the dorsal columns of C2‐C5 (frequency: 10 kHz, pulse width: 30 µsec, amplitude: 0.6–2.1 mA (mean: 0.9 mA)). In all patients, the stimulation was paresthesia‐free and kept for 24 h a day.
Patients who reported a pain reduction of ≥50% from baseline on the VAS after the trial period were implanted with an internal pulse generator (IPG; Senza™ system, NEVRO Corp., Menlo Park). The IPG was implanted subcutaneously either in the left abdominal wall or in the left gluteal region and connected to the epidural leads.
Data collection and follow‐up
Baseline measures were collected before the trial and 1 week thereafter; then at 3, 6 and 12 months after permanent implantation.
Follow‐up data were collected retrospectively from the patients’ records and questionnaires. Pain intensity including neck and upper limb pain was measured using the VAS. Although validated for patients with lower back and leg pain, we evaluated functionality using the Oswestry Disability Index (ODI) which we transferred for neck and upper limb with scores ranging from 0 to 100 whereas higher scores were associated with increased disability.8 For evaluation of health‐related psychological impairment, we used the Global Assessment of Functioning (GAF) questionnaire ranging from 0 to 100% in 10% intervals with higher scores meaning less disturbances.9 We also collected data on the patients’ experience as global impression and satisfaction as well as use of analgesic medication.
Adverse events (AEs) were defined as hardware‐related (lead migration, fracture, disconnection) or procedure‐related (infections, IPG pocket pain, new neurologic symptoms).
Statistical analysis
Descriptive statistics were reported as counts and percentages, mean, and range. AEs were reported descriptively. For statistical analysis, we used SPSS Statistics™ Version 23 (IBM Corp., Armonk, NY, USA). Differences from baseline were compared using a paired samples t‐test. A P‐value of ≤0.05 was considered significant.
Results
Between May 2015 and August 2017, 24 consecutive patients with neck and/or upper limb pain were treated with HF10 cSCS at our department. Patients’ characteristics are summarized in Table 1. One patient did not respond to the trial (response rate 96%). All others received an IPG (Senza™ system, NEVRO Corp., Menlo Park, USA) after a mean time of 4.8 weeks (range: 3.3–6.8 weeks) after successful trial.
Table 1.
Patients’ characteristics at baselinea (n = 24).
| n | Mean (Range) | |
|---|---|---|
| Male/female ratio | 9/15 | |
| Age (years) | 61.4 (40.1–82.6) | |
| Neck pain | 2 | |
| Upper limb pain | 1 | |
| Mixed neck/upper limb pain | 21 | |
| VAS (Neck) | 8.8 (7.0–10) | |
| VAS (Upper Limb) | 7.5 (6.0–9.0) | |
| ODI | 31 (21–42) | |
| GAFb | 50–41 (50–41 to 80–71) | |
| Surgery prior HF10 | 8 | 1 (1–4) |
VAS, visual analogue scale; ODI, Oswestry Disability Index; GAF, Global assessment of functioning.
baseline scores prior to any trial.
Median value.
Neck pain
At 3 months postoperatively (n = 22), mean relief of neck pain was 71.6% (from VAS 8.8 at baseline to 2.5 after 3 months). At 6 months (n = 21), the mean pain relief was 70.9% (from VAS 8.6 at baseline to 2.5 after 6 months). For 20 patients, data were available after a follow‐up of 12 months. These patients reported a mean pain relief of 74.1% (from VAS 8.5 at baseline to 2.2 (P < 0.01), table and Fig. 2). The different VAS scores at baseline resulted in excluding the explanted patients.
Figure 2.
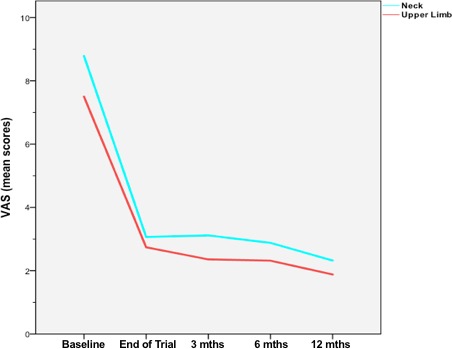
VAS scores for neck (blue line) and upper limb pain (red line) over the complete follow‐up time.
Upper limb pain
With a mean VAS of 7.5, upper limb pain scores were lower than neck pain scores at baseline. After 3 months (n = 22), we achieved a mean pain relief of 72% (from VAS 7.5 at baseline to 2.1). At 6 months (n = 21), the mean pain relief was 72.2% (from VAS 7.2 at baseline to 2.0). For 20 patients, data were available after a follow‐up of 12 months showing a mean pain relief of 76.7% from VAS 7.3 at baseline to 1.7 after 12 months (P < 0.05, table and Fig. 2). Also here, the different VAS scores at baseline resulted in excluding the explanted patients.
Improvements
To measure the patients’ functionality and health‐related psychological impairment, we used the ODI and GAF.8, 9 Regarding the ODI, mean baseline score was 31 (range: 21–42). After 3 months, the score decreased to 20 (range: 6–28) reflecting an improvement of 35.5%. At 6 and 12 months, the score remained stable showing values of 20.1 (range: 8–25) and 19.8 (8–26), respectively (Fig. 3).
Figure 3.
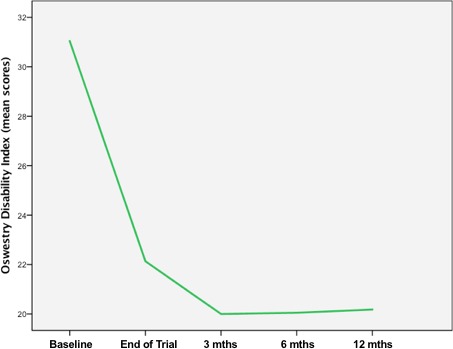
ODI scores over the complete follow‐up time.
At baseline, a median GAF interval of 50–41% (range: 50–41 to 80–71%) was documented. During the 3‐, 6‐ and 12 months follow‐up, the median GAF interval increased to 80–71% (range: 70–61 to 100–91%), 70–61% (range: 70–61 to 100–91%), and 70–61% (range: 70–61 to 100–91%), respectively (Table 2).
Table 2.
Results.
| End of trial (n = 23a,22b) | 3 months (n = 22) | 6 months (n = 21) | 12 months (n = 20) | |
|---|---|---|---|---|
| VAS (Neck) | 3.1 (2.0–5.0) | 2.5 (2.0–4.0) | 2.5 (2.0–4.0) | 2.2 (1.0–3.0) |
| VAS (Upper Limb) | 2.3 (1.0–3.0) | 2.1 (1.0–3.0) | 2.0 (1.0–3.0) | 1.7 (1.0–3.0) |
| ODI | 22.1 (15–29) | 20 (6–28) | 20.1 (8–25) | 19.9 (8–26) |
| GAFc | 80–71 (60–51 to 100–91) | 80–71 (70–61 to 100–91) | 70–61 (70–61 to 100–91) | 70–61 (60–51 to 100–91) |
VAS, visual analogue scale; ODI, Oswestry Disability Index; GAF, Global Assessment of Functioning.
For neck pain.
For upper limb pain.
Median values.
Medication intake
All patients took some form of analgesic (opioids n = 23, NSAID n = 10) at baseline (Table 3). The total dosage of oral morphine equivalent decreased from 1020 mg/day at baseline to 450 mg/day (55.9%) at 12 months. The total dosage of NSAID (n = 10) decreased from 6750 mg/day at baseline to 1425 mg/day (78.9%) after 12 months.
Table 3.
Medication intake before and 12 months after treatment.
| Drug | Intake before treatment (n = 23) | Morphine equivalent dose (mg) | Intake 12 months after treatment (n = 23) | Morphine equivalent dose (mg) | Reduction (%) |
|---|---|---|---|---|---|
| Morphinea (mg) | 210b | – | 160b | – | 23.8 |
| Oxycodona (mg) | 440b | 220 | 220b | 110 | 50 |
| Tramadola (mg) | 2650b | 530 | 900b | 180 | 66 |
| Ibuprofenc (mg) | 6000 | – | 1200 | – | 80 |
| Voltarenc (mg) | 750 | – | 225 | – | 70 |
Opioids.
Patients’ total dose per day (n = 23).
NSAID.
Patient satisfaction
After the deadline of follow‐up (31 October 2017, median follow‐up 12.2 months; range 5.1–14.5 months, explanted patients excluded), the patients were asked to rate their level of satisfaction with the HF10 cSCS device. Seventeen of 20 patients reported to be “very satisfied” (n = 15, 75%) or “satisfied” (n = 2, 10%) with the device. Three patients (15%) reported a significant improvement in functionality and daily living and to be glad to have this device. However, some patients complained about the time‐consuming processes for self‐programming (n = 2), or annoying recharging intervals (n = 1), rating their level of satisfaction as “undecided.”
Adverse events (AE)
In three of 23 patients, an infection of the IPG pocket occurred 2.3, 3.3 and 8.7 months after surgery. In one case, MRSA and in two cases Staphylococcus epidermidis were detected. In all cases of infection, the SCS device was explanted.
One patient (3.7%) reported renewed neck pain. Several attempts of reprogramming failed. An X‐ray of the cervical spine revealed significant lead migration leading to surgical revision. Thereafter, pain decreased again to the previously reported level.
Discussion
In our retrospectively designed study, we showed the efficacy of HF10 cSCS therapy for patients suffering from neuropathic, chronic neck, and/or upper limb pain. A significant and consistent pain relief was achieved for both, neck and upper limb pain. Furthermore, there was a significant improvement of functional capacity. Also a reduction in opioid use over 50% was an important finding due to the fact that in the long‐term, high dosages of opioids for chronic pain were associated with negative health impacts and societal costs.12, 13, 14 The observed AEs were infection of the IPG pocket (n = 3) and lead migration (n = 1). We observed no iatrogenic neurologic symptoms.
The results achieved with HF10 cSCS are well comparable with studies dealing with traditional cervical spinal cord stimulation.1, 15
Vallejo et al. treated five patients harboring neck and/or upper limb pain with cervical SCS.16 All patients had previously undergone ventral cervical fusion without successful pain reduction. After cervical SCS, four patients reported a pain reduction of approximately 70% after 1–9 months. Based on this experience, Vallejo et al. concluded that cervical SCS could be an effective option for patients with failed cervical fusion surgery.
Moens et al. treated seven patients with neuropathic neck and/or upper limb pain with cervical SCS using a plate electrode.17 Analogue to the study of Vallejo et al. all patients underwent unsuccessful ventral cervical fusion surgery. After 10 months, all patients reported significant pain reduction greater than 75%.
Besides two studies concerning HF10 cSCS for headache and migraine,10, 11 two more studies reported about their experience in treating chronic pain patients with HF10 cSCS.2, 7
Al‐Kaisy et al. treated 15 patients with chronic limb pain in 2014. Nine of them with HF10 cSCS. Six had upper limb neuropathic pain and three patients suffered from hand CRPS. One patient failed during the trial phase while eight patients received the IPG. After 6 months, seven patients had a pain relief between 30 to>90% rating their satisfaction as good to excellent. One patient was not satisfied due to a pain relief <10%.2
In 2016, Russo et al. presented a retrospective investigation of 256 patients suffering from chronic pain who were treated with HF10 SCS. In 36 patients, cervical electrodes were implanted for head with or without neck pain (n = 21, group 1) and for neck with or without arm/shoulder pain (n = 15, group 2). However, 10 patients in group 1 (47.6%) and four patients in group 2 (26.7%) had a failed trial. Those patients whose trial was successful had a mean pain reduction of 56.3% (group 1, NPRS 8.0 at baseline to 3.5 at 6 months) and 44.7% (group 2, NPRS 7.6 at baseline to 4.2 at 6 months), respectively.7
Adverse events reported in the literature were mostly hardware malfunctions, lead migrations and breakages, infections, and over‐/under stimulation. The surgical risk was equal as for thoracic SCS.1
The advantages of cervical SCS are its minimally invasive and reversible nature. Moreover, it has been shown to provide significant pain relief. Due to paresthesia mapping during surgery, the patient had to be in mild sedation to fully cover the pain area with paresthesia. This could be disturbing in some patients during the course of the therapy, especially at night. HF10 cervical SCS has the same advantages as mentioned above but is completely paresthesia‐free. Patients undergo surgery in general anesthesia because paresthesia mapping is not necessary. In experienced hands, it could reduce surgery time significantly.
Study limitation
This study is a single‐center retrospective study with a short follow‐up time of only 1 year and lack of a control group. The low dropout rate, however, reduces an overestimation of the excellent treatment effect.
These preliminary results show that HF10 cSCS therapy reduces neck and upper limb pain significantly and increases functional capacity, while the procedure‐related complications are low. The results are encouraging and HF10 cSCS therapy should be regarded as a therapeutic option for patients with chronic neuropathic pain. These results warrant further studies with larger patient series and longer follow‐ups.
Author Contribution
Faycal El Majdoub and Mohammad Maarouf participated in conception and design of the study. They also participated in data collection and analysis and wrote the manuscript. Clemens Neudorfer, Ronald Richter and Simon Schieferdecker participated in data collection and analysis. All authors approved the final draft of the paper.
Conflict of Interest
Faycal El Majdoub and Mohammad Maarouf received honoraria for consulting and lecturing from NEVRO; Ronald Richter received financial support for congresses from NEVRO. Clemens Neudorfer and Simon Schieferdecker have no conflict of interest.
Funding information
None declared.
References
- 1. Deer TR, Skaribas IM, Haider N, et al. Effectiveness of cervical spinal cord stimulation for the management of chronic pain. Neuromodulation 2014;17:265–271. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Kaisy A, Palmisani S, Smith T, et al. The use of 10‐kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation 2015;18:18–23. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Kaisy A, Van Buyten JP, Smet I, et al. Sustained effectiveness of 10 kHz high‐frequency spinal cord stimulation for patients with chronic, low back pain: 24‐month results of a prospective multicenter study. Pain Med 2014;15:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapural L, Yu C, Doust MW, et al. Novel 10‐kHz high‐frequency therapy (HF10 Therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA‐RCT randomized controlled trial. Anesthesiology 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 5. Kapural L, Yu C, Doust MW, et al. Comparison of 10‐kHz high‐frequency and traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain. Neurosurgery 2016;79:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Buyten J‐P, Al‐Kaisy A, Smet I, et al. High‐frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter european clinical study. Neuromodulation 2013;16:59–66. [DOI] [PubMed] [Google Scholar]
- 7. Russo M, Verrills P, Mitchell B, et al. High frequency spinal cord stimulation at 10 khz for the treatment of chronic pain: 6‐month australian clinical experience. Pain Phys 2016;19:267–280. [PubMed] [Google Scholar]
- 8. Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–273. [PubMed] [Google Scholar]
- 9. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976;33:766–71. [DOI] [PubMed] [Google Scholar]
- 10. Lambru G, Trimboli M, Palmisani S, et al. Safety and efficacy of cervical 10 kHz spinal cord stimulation in chronic refractory primary headaches: a retrospective case series. J Headache Pain 2016;17:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arcioni R, Palmisani S, Mercieri M, et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: A prospective, open‐label, exploratory study. Eur J Pain 2016;20:70–78. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Kaisy A, Palmisani S, Smith TE, et al. 10 kHz high‐frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof‐of‐concept study. Neuromodulation 2017;20:63–70. [DOI] [PubMed] [Google Scholar]
- 13. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ 2015;5:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015;162:276–286. [DOI] [PubMed] [Google Scholar]
- 15. Chivukula S, Tempel ZJ, Weiner GM, et al. Cervical and cervicomedullary spinal cord stimulation for chronic pain: efficacy and outcomes. Clin Neurol Neurosurg 2014;127:33–41. [DOI] [PubMed] [Google Scholar]
- 16. Vallejo R, Kramer J, Benyamin R. Neuromodulation of the cervical spinal cord in the treatment of chronic intractable neck and upper extremity pain: a case series and review of the literature. Pain Phys 2007;10:305–311. [PubMed] [Google Scholar]
- 17. Moens M, De Smedt A, Brouns R, et al. Retrograde C0‐C1 Insertion of cervical plate electrode for chronic intractable neck and arm pain. World Neurosurg 2011;76:352–354. [DOI] [PubMed] [Google Scholar]


