Abstract
Objectives: The aim of this study was to validate an ultrasound protocol for evaluating the anterolateral ligament of the knee. Methods: A Thiel technique cadaveric specimen was used to validate an optimal scanning position and develop an ultrasound protocol to evaluate the anterolateral ligament. Three musculoskeletal sonographers acquired short- and long-axis images of the anterolateral ligament in 36 knees from 18 healthy volunteers. Anterolateral ligament length, thickness, width, and distance between anterolateral ligament insertion and lateral tibia plateau were measured. Intraclass Correlation Coefficient (ICC) was calculated. Results: The inter-rater reliability for anterolateral ligament thickness was poor, ICC = 0.35 (95% CI: –0.06–0.63). The inter-rater reliability for anterolateral ligament length and width was good, ICC = 0.80 (95% CI 0.64–0.89), ICC = 0.88 (95% CI 0.79–0.94), respectively; and the inter-rater reliability for the distance between insertion and lateral tibia plateau was excellent, ICC = 0.96 (95% CI 0.93–0.98). Conclusions: Ultrasonography is a reliable method for evaluating the anterolateral ligament. There is an excellent reliability for the distal part of the anterolateral ligament. As injuries usually occur in this part of the ligament, this protocol may be used to evaluate the anterolateral ligament in patients with suspected anterior cruciate ligament tears in clinical practice.
Keywords: anterolateral ligament, musculoskeletal ultrasound, knee instability, anterior cruciate ligament, knee
Introduction
The publication of Claes et al.(1) in 2013 brought the anterolateral ligament (ALL) of the knee to the attention of researchers and clinicians. These authors investigated cadaveric knees based on the description of Paul Segond(2), who saw a “pearly fibrous resistant band” associated with an avulsion fracture at the anterolateral proximal tibia (Segond fracture) in 1879. Claes et al. reported that 97% of 41 unpaired cadaveric knees had a well-defined ligamentous structure, which they designated the anterolateral ligament (ALL). Since then, the anatomical characteristics of the ALL have been further defined by many authors(3–8). Among these, there is a consensus that the ALL is a triangular, anterolateral ligamentous structure, underneath the iliotibial band (ITB)(9) (Fig. 1).
Fig. 1.
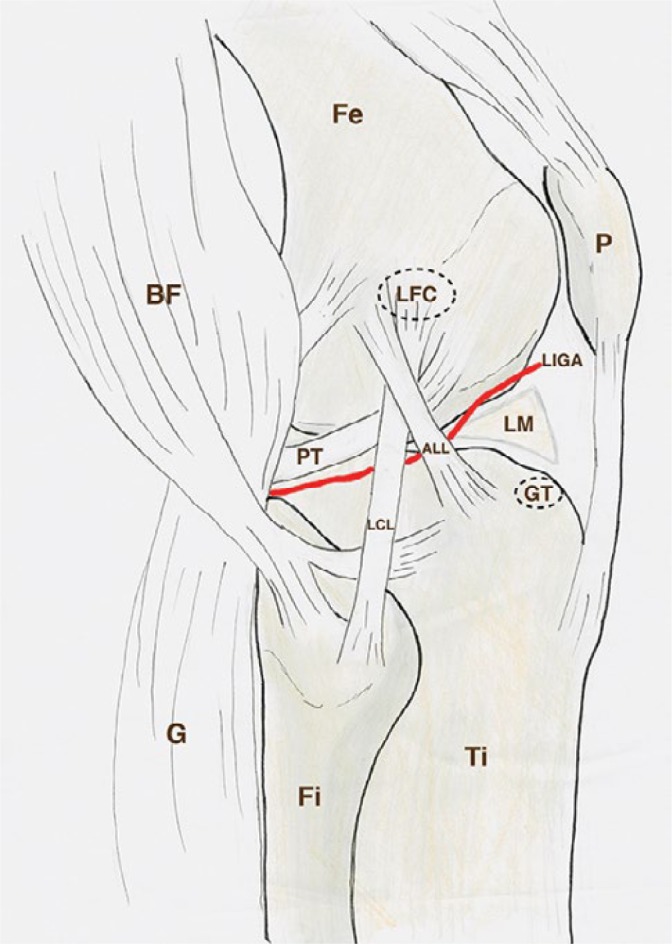
Schematic drawing of the lateral side of the knee, deep to the iliotibial band. Fe, femur; Ti, tiba; Fi, fibula; P, patella; G, lateral head of gastrocnemius; BF, biceps femoris; LFC, lateral femur condyle; GT, Gerdy’s tubercle; PT, popliteus tendon; LCL, lateral collateral ligament; ALL, anterolateral ligament; LM, lateral meniscus; LIGA, lateral inferior genicular artery
The function of this ligament is to provide anterolateral knee stability by preventing the lateral tibia from subluxation anteriorly relative to the femur(9,10). Studies have documented ALL injuries in 46% to 79% in combination with ACL injuries(11,12). Persisting rotational instability after ACL reconstruction (prevalence 25%)(9) seems to be due to insufficiency of these lateral structures(13). Combined reconstruction of the ALL and ACL should be considered depending on patient history, clinical signs, imaging, and patient profile(9,14).
Musculoskeletal ultrasonography (US) is a non-invasive, cost-effective, and valid method to visualize extraarticular structures in real time. Only a few studies investigated the visualization of the ALL with US(15–19). These studies were unable to determine how to reliably visualize the ALL with US(20). It is hypothesized that point-of-care ultrasound (POCUS) of the ALL may be useful if an ACL tear is suspected. However, a standardized protocol in how to reliably visualize the ALL with US is needed.
The purpose of this study is to present a standardized protocol to visualize the ALL with US and to determine interrater reliability.
Materials and methods
Neri et al.(21) reported that the maximum length of the ALL was reached in 30° flexion with internal rotation. More flexion resulted in a decrease in tension on the ALL. In order to be able to dynamically exam a patient’s knee without the help of an assistant, the patient can be placed in a lateral position. In this position the examiner is able to scan and control the position of the knee with more or less tension on the ALL by himself. To evaluate the use of US to visualize the ALL in this position, a Thiel embalmed cadaver specimen was used. A Thiel embalmed specimen was chosen due to its acoustic, mechanical, and elastic properties(22,23). First, a dynamic ultrasound examination of the cadaveric knee was performed and images were stored on the ultrasound device (Fig. 2). Short and long axis images were acquired and measurements (length, width, thickness and distance between insertion and lateral tibia plateau) were performed by consensus. Following consensus by two experienced US examiners (MdM, MK: MDM 20 years of experience, MK 5 years of experience) surgical needles were placed at the origin and insertion of the ALL. Second, the knee was dissected to evaluate the position of the needles. The ALL could be identified between the needles, as it ran slightly proximal and posterior from the lateral femur condyle over the lateral collateral ligament, attached to the lateral meniscus and then to the tibia, halfway between Gerdy’s tubercle and the fibula head (Fig. 3).
Fig. 2.
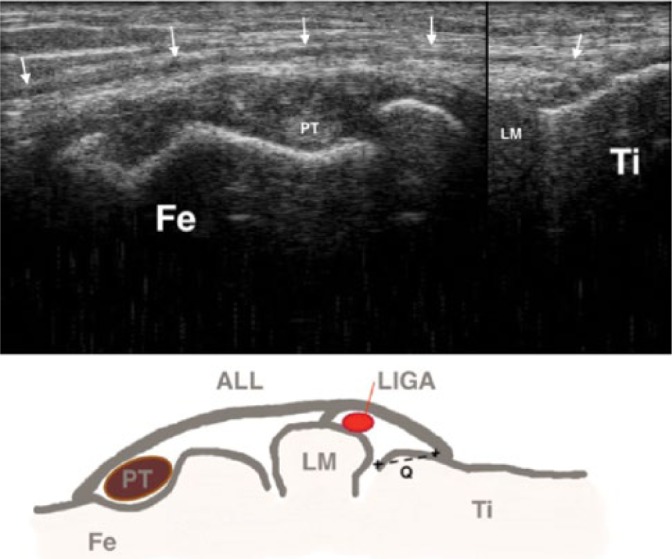
Ultrasound image of the cadaveric knee showing the anterolateral ligament (arrows) attached to the tibia (Ti) running over the popliteus tendon (PT) to the femur (Fe) condyle. Schematic drawing of the structures (B): Fe, femur; Ti, tibia; LM, lateral meniscus; arrows, anterolateral ligament; LIGA, lateral inferior genicular artery; Q, measurement distance between anterolateral ligament insertion and tibia plateau
Fig. 3.
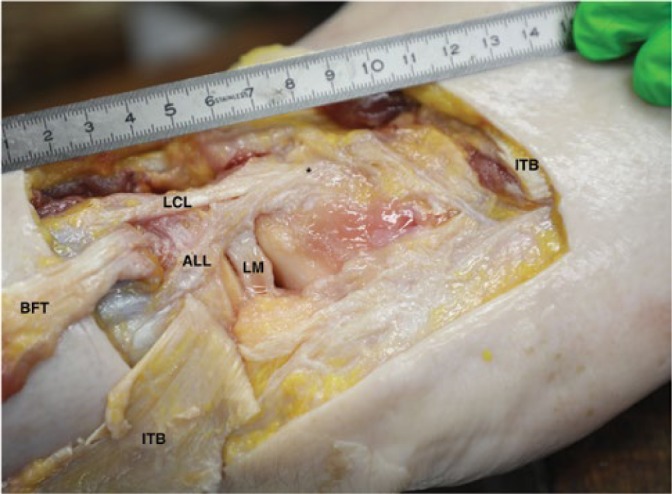
Anatomical dissection of the lateral knee showing the anterolateral ligament (ALL), lateral femur condyle (*), lateral meniscus (LM), lateral collateral ligament (LCL) with the iliotibial band (ITB) and biceps femoris tendon (BFT) reflected
To consider a target population at risk, healthy active people between 18 and 55 years were included, by non-probability sampling. Informed consent was obtained from each participant, and the rights of human subjects were protected. Three raters, all physical therapists, MSc, with at least 3 years of experience in US (MAP, ML, MK) performed all measurements. Each sonographer completed a 4 hour training session in order to get familiar with the ALL protocol prior to this study. The volunteer underwent a clinical examination by an experienced physical therapist to exclude clinical anterolateral instability.
To visualize the ALL in vivo a standardized protocol based on the validation of the scanning position was defined with the participant lying on the ipsilateral side with the upper leg in 30° flexion and his foot hanging off the examination table. A pillow was placed under his knee. The examiner sat behind the participant in order to easily adjust US settings and be able to flex and rotate the leg, if necessary. The ITB was located in its long axis at its insertion on Gerdy’s tubercle. Then, the probe was slowly rotated towards the fibular head. Halfway between Gerdy’s tubercle and head of the fibula, the distal insertion of the ALL can be found. In this position, the popliteus tendon and the lateral inferior genicular artery (LIGA) were used as landmarks to identify the ALL running to the lateral femur condyle. In this position (Fig. 4), length, thickness (just above the LIGA) and distance from insertion to tibia plateau were measured. The width of the ALL was measured in a short axis view above the LIGA. Images and measurements were recorded and stored on the US system.
Fig. 4.
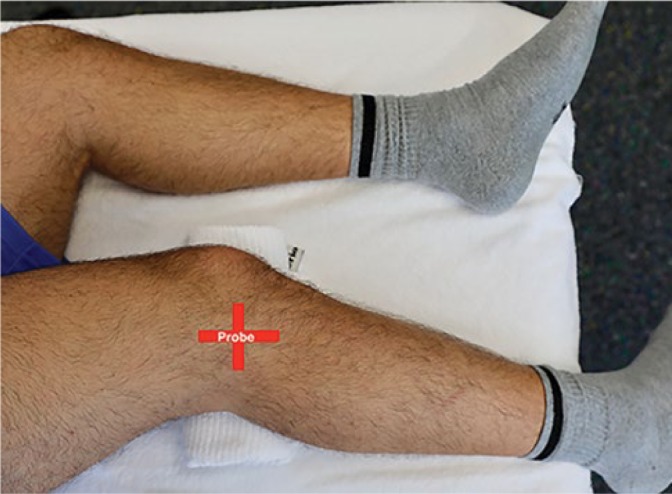
The participants were placed in a lateral position with the knee on a pillow in 30° flexion. The foot was hanging over the table. The positions of the probe are indicated by the red lines
For the three raters (k = 3) a good reliability was set at 0.75 (R1), whereas a poor reliability was set below 0.5 (R0)(24). The value of α is pre-specified to be 0.05 (which represents the probability of a Type I error). The power was set on 85% (Type II error), thus β = 1-0.85 = 0.15. Out of the equation, the minimal sample size required was n = 26.7 measurements.
The three raters performed measurements of the ALL with a Philips Affiniti 50G (Royal Philips, Amsterdam, The Netherlands) ultrasound device and an 18-5 MHz, linear 5 cm transducer on both legs of the participant.
Data analyses were performed using IBM SPSS Version 21 (SPSS Inc. Chicago, Il, USA). All data was checked for assumptions. Descriptive statistics were calculated, and the Intraclass Correlation Coefficient (ICC) was used to assess inter-rater reliability (ICC2.1; two-way random effects model, single measurement type defined in absolute agreement) reported with 95% confidence interval (CI) of the estimated ICC. The level of reliability was based on the general guideline according to Koo, 2016(25).
Results
In the Thiel embalmed specimen the needle markings placed with US corresponded exactly to the ALL position observed at subsequent dissection and confirmed by an experienced anatomist (EC, 20 years of experience). Eighteen healthy subjects (12 male and 6 female) participated in this study. Mean (± standard deviation) of age, height, and weight were 41.2 (±10.3) years, 179.2 (±8.5) cm and 84.7 (±13.1) kg. From 36 knees, two were excluded due to prior surgery. There were no signs of clinical anterolateral instability in the remaining 34 knees. The three raters were able to localize the ALL in 33 knees (97%). Characteristics of the participants and ALL are shown in Table 1. The ICC was calculated as the data was normally distributed. The inter-rater reliability of ALL thickness was poor, ICC = 0.35 (95% CI: -0.6-0.63). The inter-rater reliability was good for of ALL length and width, ICC 0.80 (95% CI: 0.64-0.89) and 0.88 (95% CI: 0.79–0.94), respectively; and excellent for the distance between insertion of the ALL and lateral tibia plateau, ICC 0.96 (95% CI: 0.93-0.98), as seen in Tab. 2.
Tab. 1.
Descriptive statistics of participants (age, height, weight, BMI) and ALL measurements (length of ALL, thickness of ALL, width of ALL, distance to tibial plateau). All measurements are given in mm. ALL – anterolateral ligament
| n | Lower | Higher | Mean | SD | |
|---|---|---|---|---|---|
| Age (year) | 33 | 23 | 54 | 41.2 | 10.3 |
| Height (cm) | 33 | 164 | 188 | 179.2 | 8.5 |
| Weight (kg) | 33 | 63.3 | 104 | 84.7 | 13.1 |
| BMI | 33 | 21.5 | 31.1 | 26.3 | 2.9 |
| ALL length | 33 | 38.7 | 53.3 | 46.9 | 4.2 |
| ALL thickness | 33 | 0.52 | 1.24 | 0.94 | 0.16 |
| ALL width | 33 | 4.4 | 11.7 | 8.4 | 2.3 |
| Distance to tibia plateau | 33 | 2.2 | 9.2 | 5.7 | 1.8 |
Tab. 2.
Intraclass correlation coefficients for ALL length, thickness, width, and distance to tibia. Also shown 95% confidence interval and significance
| Intraclass correlation | 95% confidence interval | Significance | ||
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| ALL length | 0.799 | 0.643 | 0.894 | <0.001 |
| ALL thickness | 0.346 | -0.058 | 0.0632 | 0.038 |
| ALL width | 0.884 | 0.794 | 0.939 | <0.001 |
| Distance to tibia plateau | 0.959 | 0.927 | 0.978 | <0.001 |
Discussion
US has been used to visualize the ALL in five previous studies. Cianca et al.(16) were the first to describe a short and long axis view of the ALL in a single knee of a healthy male subject. They reported that the ligament was easiest to identify when the knee was in 90° flexion with slight internal rotation. However, they had no anatomical correlation to prove that the visualized structure corresponded to the ALL. Cavaignac et al.(18) reported a 100% sensitivity in visualizing the ALL in 18 unembalmed cadaveric knees by placing ultrasound guided metal needles in the proximal and distal ends of the ALL, using a 12-MHz linear transducer by a single radiologist. They concluded US to be a suitable tool to identify the ALL. In a technical note, the same authors described a supine scanning position with the knee in 90° flexion and internally rotated by an assistant in an operative setting(26). Capo et al.(15) reported that US was not able to reliably identify the ALL at its femoral and tibial attachment sides and to distinguish it from the deep fibres of the ITB. They used a 14-MHz linear transducer and placed the knee in 30° to 60° flexion and internal rotation. Oshima et al.(17) reported that US could be used to confirm the integrity of the ALL as they localized the ligament using real-time virtual sonography in 18 knees of 9 healthy male volunteers (28–37 years old). Thickness, length, and the distance between tibial insertion and the lateral tibial plateau were compared between MRI and US (in 30° knee flexion).
After evaluating US in a cadaveric observation, we determined the inter-rater reliability in 34 healthy knees. Mean length and width of the ALL in our study was 46.9 (±4.2) mm, resp. 8.4 (±2.3) mm, which corresponded well to with Neri et al.(21). They reported ALL length of 45.29 (±4.1) mm in 30° knee flexion and a width of 8.36 (±0.69) mm in 84 fresh frozen cadaveric knees. Faruch et al.(19) reported an ALL thickness of 0.97 (±0.13) mm using US in 30 healthy subjects. Taneja et al.(27) reported a distance of 5.7 mm in MRI of 36 knees; Claes et al.(1) reported a mean distance of 6.5 (±1.4) mm in 41 cadaveric knees.
In our study the Intraclass Correlation Coefficient (ICC) was poor for ALL thickness, good for ALL length and width and excellent for the distance between insertion and lateral tibia plateau. Our findings are consistent with prior studies(3,17), that reported variability in the femoral attachment and a strong connection between the structures around the lateral femoral condyle, making the ALL more difficult to identify in this area. The poor consistency in the ALL thickness measurement may be due to the accuracy of US measuring beyond millimetres. A small measurement error of 0.1 mm, indicates a difference of 11% to the mean thickness (0.9 mm). Faruch et al.(19) reported the difference between ALL thickness in healthy- (0.97 ± 0.13 mm) and ALL injured subjects (1.46 ± 0.27 mm) measured with US. This means an increase in ALL thickness of more than 50%. Due to the small standard deviation and despite a poor reliability it can be assumed that this protocol would be able to detect such an increase. The good ICC of the ALL width measurement indicates the possibility to reliably evaluate the ALL in short axis, between the ITB and the LCL around the joint line.
Nearly perfect reliability of the measurement of the distal insertion of the ALL has important implications for patient care. Almost all injuries of the ALL are located in the distal insertion of the ligament. Faruch et al.(19) found that 100% of ALL injuries that occur in combination with ACL tears are located at the tibial attachment. Visualisation with US has the advantage of not only being able to show bony avulsion, the Segond fracture, but also show a pure ligamentous injury, which may lead to similar mechanical instability.
Our study has several limitations. Healthy active participants were included in our study. Our results may not hold up in patients suspected of having an ACL tear, where there might be pathological changes of the ALL. Integrity instead of morphologic characteristics should be evaluated in this situation. Studies in pathological cases are needed. Validation of the ultrasound protocol was done on a single Thiel embalmed cadaveric knee. The advantage of this embalming method is that the specimens retain tissue characteristic as in a fresh specimen in contradistinction to classic embalming where tissues harden and tissue planes become inseparable. Despite a good agreement between US and dissection, this can only be considered as a minimal validation. The mean age of the participants (41 years) did not entirely match the mean age of people at risk for an ACL tear (18 to 25 years)(28).
Conclusion
The US protocol presented is reliable for evaluating the anterolateral ligament of the knee. There is an excellent reliability for the distal part of the ALL. As injuries typically occur in this part of the ligament, our protocol is promising for evaluating the ALL in patients with suspected ACL tears in clinical practice and on the playfield.
Acknowledgment
We thank the Anatomical Research Training and Education (ARTE) of the Vrije Universiteit Brussel, Belgium to perform the cadaveric evaluation and the SOMT University, Amersfoort, the Netherlands for the use of the facilities and equipment in this study.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J: Anatomy of the anterolateral ligament of the knee. J Anat 2013; 223: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segond P: Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Prog Med 1879; 7: 297–299. [Google Scholar]
- 3.Daggett M, Ockuly AC, Cullen M, Busch K, Lutz C, Imbert P. et al. : Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy 2016; 32: 835–841. [DOI] [PubMed] [Google Scholar]
- 4.Dodds AL, Halewood C, Gupte C, Williams A, Amis AA: The anterolateral ligament: anatomy, length changes and association with the segond fracture. Bone Joint 2014; 96-B: 325–331. [DOI] [PubMed] [Google Scholar]
- 5.Helito CP, Demange MK, Bonadio MB, Tírico LE, Gobbi RG, Pécora JR. et al. : Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med 2013; 1: 2325967113513546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz C, Sonnery-Cottet B, Niglis L, Freychet B, Clavert P, Imbert P: Behavior of the anterolateral structures of the knee during internal rotation. Orthop Traumatol Surg Res 2015; 101: 523–528. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JP, Magnussen RA, Gezmez F, Uguen A, Jacobi M, Weppe F. et al. : The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc 2012; 20: 147–152. [DOI] [PubMed] [Google Scholar]
- 8.De Maeseneer M, Boulet C, Willekens I, Lenchik L, De Mey J, Cattrysse E. et al. : Segond fracture: involvement of the iliotibial band, anterolateral ligament, and anterior arm of the biceps femoris in knee trauma. Skeletal Radiol 2015; 44: 413–421. [DOI] [PubMed] [Google Scholar]
- 9.Sonnery-Cottet B, Daggett M, Fayard JM, Ferretti A, Helito CP, Lind M. et al. : Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament – deficient knee. J Orthop Traumatol 2017; 18: 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Watt L, Khan M, Rothrauff BB Ayeni OR, Musahl V, Getgood A. et al. : The structure and function of the anterolateral ligament of the knee: a systematic review. Arthroscopy 2015; 31: 569–582. [DOI] [PubMed] [Google Scholar]
- 11.Claes S, Luyckx T, Vereecke E, Bellemans J: The Segond fracture: a bony injury of the anterolateral ligament of the knee. Arthroscopy 2014; 30: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 12.Van Dyck P, Clockaerts S, Vanhoenacker FM, Lambrecht V, Wouters K, De Smet E. et al. : Anterolateral ligament abnormalities in patients with acute anterior cruciate ligament rupture are associated with lateral meniscal and osseous injuries. Eur Radiol 2016; 26: 3383–3391. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Vyas D, Moloney G, Bedi A, Pearle AD, Musahl V: What does it take to have a high-grade pivot shift? Knee Surg Sports Traumatol Arthrosc 2012; 20: 737–742. [DOI] [PubMed] [Google Scholar]
- 14.Roessler PP, Schüttler KF, Heyse TJ, Wirtz DC, Efe T: The anterolateral ligament (ALL) and its role in rotational extra-articular stability of the knee joint: a review of anatomy and surgical concepts. Arch Orthop Trauma Surg 2016; 136: 305–313. [DOI] [PubMed] [Google Scholar]
- 15.Capo J, Kaplan DJ, Fralinger DJ, Adler RS, Campbell KA, Jazrawi LM. et al. : Ultrasonographic visualization and assessment of the anterolateral ligament. Knee Surg Sports Traumatol Arthrosc 2017; 25: 3134–3139. [DOI] [PubMed] [Google Scholar]
- 16.Cianca J, John J, Pandit S, Chiou-Tan FY: Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil 2014; 93: 186. [DOI] [PubMed] [Google Scholar]
- 17.Oshima T, Nakase J, Numata H, Takata Y, Tsuchiya H: Ultrasonography imaging of the anterolateral ligament using real-time virtual sonography. Knee 2016; 23: 198–202. [DOI] [PubMed] [Google Scholar]
- 18.Cavaignac E, Wytrykowski K, Reina N, Pailhé R, Murgier J, Faruch M. et al. : Ultrasonographic identification of the anterolateral ligament of the knee. Arthroscopy 2016; 32: 120–126. [DOI] [PubMed] [Google Scholar]
- 19.Faruch Bilfeld M, Cavaignac E, Wytrykowski K, Constans O, Lapègue F, Chiavassa Gandois H. et al. : Anterolateral ligament injuries in knees with an anterior cruciate ligament tear: contribution of ultrasonography and MRI. Eur Radiol 2018; 28: 58–65. [DOI] [PubMed] [Google Scholar]
- 20.Bottene Villa Albers M, Yoshida M, Fu FH, Onishi K: Ultrasonographic visualization of anterolateral complex of the knee. Oper Tech Orthop 2017; 27: 121–125. [Google Scholar]
- 21.Neri T, Palpacuer F, Testa R, Bergandi F, Boyer B, Farizon F. et al. : The anterolateral ligament: anatomic implications for its reconstruction. Knee 2017; 24: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 22.Munirama S, Eisma R, Columb M, Corner GA, McLeod GA: Physical properties and functional alignment of soft-embalmed Thiel human cadaver when used as a simulator for ultrasound-guided regional anaesthesia. Br J Anaesth 2016; 116: 699–707. [DOI] [PubMed] [Google Scholar]
- 23.Sawhney C, Lalwani S, Ranjan Ray B, Sinha S, Kumar A: Benefits and pitfalls of cadavers as learning tool for ultrasound-guided regional anesthesia. Anesth Essays Res 2017; 11: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter SD, Eliasziw M, Donner A: Sample size and optimal designs for reliability studies. Stat Med 1998; 17: 101–110. [DOI] [PubMed] [Google Scholar]
- 25.Koo TK, Li MY: A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavaignac E, Laumond, Reina GN, Wytrykowski K, Murgier J, Faruch M. et al. : How to test the anterolateral ligament with ultrasound. Arthrosc Tech 2017; 7: e29–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taneja AK, Miranda FC, Braga CAP, Gill CM, Hartmann LG, Santos DC. et al. : MRI features of the anterolateral ligament of the knee. Skeletal Radiol 2015; 44: 403–410. [DOI] [PubMed] [Google Scholar]
- 28.Sanders TL, Maradit Kremers H, Bryan AJ, Larson DR, Dahm DL, Levy BA. et al. : Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med 2016; 44: 1502–1507. [DOI] [PubMed] [Google Scholar]


