Abstract
Various aryl‐ and heteroaryl‐substituted 2‐bromobiaryls are converted to cyclometalated lanthanum intermediates by reaction with nBu2LaCl⋅4 LiCl. These resulting lanthanum heterocycles are key intermediates for the facile preparation of functionalized 2,2′‐diiodobiaryls, silafluorenes, fluoren‐9‐ones, phenanthrenes, and their related heterocyclic analogues. X‐ray absorption fine structure (XAFS) spectroscopy was used to rationalize the proposed structures of the involved organolanthanum species.
Keywords: condensed aromatics, functionalized organometallics, halogen–lanthanum exchange, lanthanides, XAFS
One stone, four birds: Various 2‐bromobiaryl derivatives undergo a smooth cyclolanthanation upon reaction with nBu2LaCl⋅4 LiCl. The resulting cyclometalated lanthanum reagents are versatile intermediates for the facile preparation of valuable polyfunctional biaryl derivatives. X‐ray absorption fine structure (XAFS) measurements rationalize the proposed structures of the involved organolanthanum species.
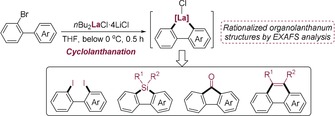
Organolanthanides are important organometallic intermediates in catalysis and organic synthesis.1 Functionalized aryl‐ and heteroaryllanthanum reagents, which are readily prepared via an iodine‐ or bromine‐lanthanum exchange, are of special interest.2 The low electronegativity of lanthanum (1.1)3 comparable to that of lithium (0.98) confers to the carbon–lanthanum bond a high ionic character and therefore high reactivity. Furthermore, the high valence of lanthanum(III) imparts this metal center with high oxophilicity and excellent Lewis acidity. Both of these properties have been extensively exploited for forming new carbon–carbon and carbon–heteroatom bonds.4 Herein, we report that readily prepared 2‐biaryllanthanum derivatives of type 1 obtained from the corresponding 2‐bromobiaryls of type 2 by reaction with nBu2LaCl⋅4 LiCl5a undergo a mild intramolecular C−H metalation6 leading to the cyclometalated biaryls of type 3 below room temperature within 0.5 h (Scheme 1 A). The organolanthanum species involved in this reaction process were detected by X‐ray absorption fine structure (XAFS) measurements (Scheme 1 B).5 The putative lanthanum–heterocycles 3 prove to be versatile intermediates that can be converted to a range of polyfunctional biaryl derivatives of types 4–7 under mild conditions (Scheme 1 C).
Scheme 1.
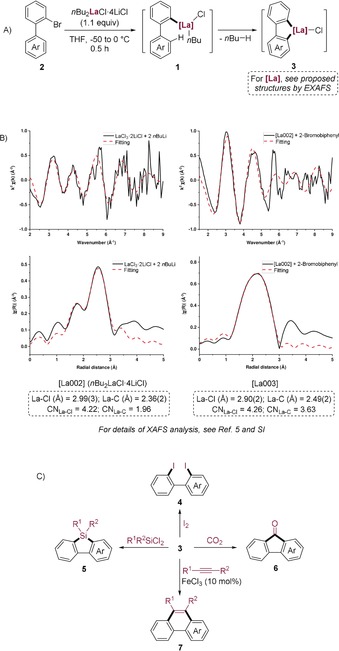
A) Cyclolanthanation of 2‐bromobiaryls of type 2. B) La L 3‐edge k 2‐weighted EXAFS spectra (top) and Fourier transforms (bottom) for [La002] (left) and [La003] (right). Solid black lines show the experimental data and dashed red lines are the best fitting results. C) Subsequent functionalization of 3 leading to products 4–7.
Compounds 4–7 are valuable precursors for functionalized organic materials with optical and electronic applications.7 Yoshikai has shown that 2‐iodobiaryls could be converted to 2,2′‐diiodobiaryls of type 4 via an oxidation–iodination process by a copper catalyst.8 Silafluorenes of type 5 were previously prepared by conventional nucleophilic substitution reactions9 or transition‐metal‐catalyzed couplings using 2,2′‐difunctionalized biaryl precursors such as halides, silanes, triflates, or boronic acids.10 Alternatively, intramolecular C−H silylation of 2‐biarylsilanes toward silafluorenes has been developed by a number of groups, using 1) a rhodium‐catalyzed synthesis as demonstrated by Kuninobu and Takai,11a, 11b He,11c, 11d and Mitsudo and Suga,11e 2) a sila‐Friedel–Crafts (SEAr) reaction as shown by Kobayashi and Kawashima,12a Ingleson,12b and Oestreich,12c, 12d and 3) a radical silylation reaction as reported by Studer,13a Li,13b and Jiang.13c Palladium‐catalyzed cyclocarbonylation of o‐halobiaryls with CO leading to fluoren‐9‐ones of type 6 has been reported by Larock,14 and other carbonyl sources such as furfural, formaldehyde, and phenyl formate have been employed in this cyclocarbonylation.15 Also, the dehydrogenative arylation of 2‐arylbenzaldehydes has been realized as an expedient method to synthesize benzocyclic ketones.16 Phenanthrenes of type 7 have been previously prepared by palladium‐catalyzed [4+2] annulation of alkynes with 2‐iodobiaryls as reported by Heck17a and Larock.17b Takahashi described a stoichiometric CrCl3‐mediated annulation of halobiaryls.18 Alternatively, the annulation of alkynes with 2‐magnesiated biaryls has been demonstrated by Nakamura19a and Yoshikai,19b under iron and chromium catalysis, respectively. The annulation of alkynes with other coupling reagents such as 2‐phenylbenzoic acid,20a 9‐chloro‐9‐borafluorene,20b and dibenzosilole20c has been achieved recently. The annulation of 1,2‐bis(pinacolatoboryl)alkenes with 2,2′‐dibromobiaryls under palladium catalysis also allowed the preparation of phenanthrenes of type 7.21
We began our study by optimizing the cyclometalation conditions of 2‐bromobiphenyl (2 a). As shown in Table 1, neither the lithiation nor magnesiation of 2 a led to any cyclometalated product (entries 1 and 2). Br/Sm exchange4c provided the cyclometalated intermediate in only moderate yields, as shown by the iodolysis leading to 2,2′‐diiodobiphenyl (4 a) in only 31–40 % yield (entries 3 and 4). However, the use of nBu2LaCl⋅4 LiCl gave satisfactory results. A reaction time of 30 min and a reaction temperature from −50 °C to 0 °C gave the optimal results (entries 5–8). Thus, the quenching of the reaction of 2 a with iodine under optimum conditions produced 4 a in 79 % isolated yield (entry 7).
Table 1.
Optimization of conditions for cyclometalation of 2‐bromobiphenyl (2 a).

|
Entry |
Reagent (1.1 equiv) |
T [°C] |
t [min] |
Conv. [%][b] |
Yield [%][b,c] |
|---|---|---|---|---|---|
|
1 |
nBuLi [a] |
−50 |
30 |
100 |
0 |
|
2 |
nBu2 Mg |
rt |
60 |
23 |
0 |
|
3 |
nBu2 SmMe⋅5LiCl |
−30 to rt |
30 |
100 |
31[d] |
|
4 |
nBu2 SmCl⋅4 LiCl |
−30 to rt |
30 |
100 |
40[d] |
|
5 |
nBu2 LaCl⋅4 LiCl |
−50 |
60 |
100 |
61[e] |
|
6 |
nBu2 LaCl⋅4 LiCl |
−50 to rt |
30 |
100 |
89[e] |
|
7 |
nBu2 LaCl⋅4 LiCl |
−50 to 0 |
30 |
100 |
90(79[f]) |
|
8 |
nBu2 LaCl⋅4 LiCl |
−50 to 0 |
60 |
100 |
80 |
[a] 2.2 equiv was used. [b] Determined by GC analysis. [c] The observed byproducts were mostly biphenyl, 2‐butylbiphenyl, and 2‐iodobiphenyl. [d] 2‐Butylbiphenyl was mainly generated. [e] 2‐Butylbiphenyl was observed in minor amounts. [f] Yield of isolated, analytically pure product.
The scope of this synthesis of 2,2′‐diiodobiaryl derivatives 4 was studied (Scheme 2). Thus, the presence of various substituents at position 4′ of the aromatic ring of 2‐bromobiaryls 2 b–2 f was possible (R=F, Cl, Br, OMe, SiMe3), which after iodolysis of the corresponding lanthanum heterocycles 3 b–3 f provided the diiodides 4 b–4 f in 61–84 % yield. Remarkably, an extra bromine atom in 2 d could be tolerated in the cyclolanthanation reaction. The presence of a substituent at a nonsymmetrical position in 2 as exemplified by 2‐bromo‐3′‐methoxybiphenyl (2 g) led to a mixture of two regioisomers 4 ga and 4 gb in a 4:1 ratio. Interestingly, in the case of 2‐bromo‐3′‐trifluoromethylbiphenyl (2 h) a fully regioselective C−H activation took place certainly due to the repulsive effect of the CF3 substituent (absence of attractive van der Waals interactions). Further annulated biaryls such as 1‐(2‐bromophenyl)naphthalene (2 i) provided the expected diiodide 4 i in 57 % yield. Various heterocyclic ring systems bearing a 2‐bromophenyl substituent underwent a related C−H metalation providing the corresponding diiodides 4 j–4 o in 66–83 % yield. Notably, 2‐bromo‐1,1,2‐triphenylethylene (2 p) was converted to the diiodide 4 p in 58 % yield via a modified procedure (see the Supporting Information). Interestingly, the diiodides of type 4 bearing two aryl rings with different electron densities could be regioselectively converted to their corresponding monomagnesium intermediate via a reaction with iPrMgCl⋅LiCl.22 Thus, the diiodide 4 b underwent preferentially (100 % selectivity) an I/Mg exchange on the more electron‐poor ring. A subsequent reaction with 1,2‐dibromotetrachloroethane produced the 2‐bromo‐4‐fluoro‐2′‐iodobiphenyl (8 a) in 84 % isolated yield. The 2,2′‐diiodo‐4‐methoxybiphenyl (4 e) underwent an I/Mg exchange with iPrMgCl⋅LiCl mostly at the less electron‐rich ring readily after bromolysis to give 8 b in 89 % yield (4:1 regioselectivity). Finally, the heterocyclic diiodide 4 m underwent an exclusive I/Mg exchange on the thienyl ring providing the 3‐bromo‐2‐(2‐iodophenyl)thiophene (8 c) in 78 % yield (Scheme 2).
Scheme 2.
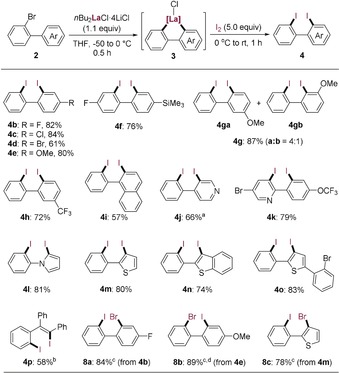
Synthesis of 2,2′‐diiodobiaryl derivatives 4 and 2‐bromo‐2′‐iodobiaryl derivatives 8. [a] The cyclometalated lanthanum intermediate of type 3 was generated in situ at −50 °C to −20 °C after 0.5 h. [b] The product was obtained by a modified method as shown in the Supporting Information. [c] The reaction was conducted with iPrMgCl⋅LiCl (1.0 equiv) at −40 °C for 1 h and then quenched by 1,2‐dibromotetrachloroethane. [d] NMR analysis reveals two isomers (4:1), and the major isomer is shown here.
Remarkably, silafluorenes of type 5 were directly prepared by cyclolanthanation of 2‐bromobiaryls. As shown in Scheme 3, the cyclometalated biaryls of type 3 reacted smoothly with dichlorodialkylsilanes (Me2SiCl2 and MePhSiCl2) to provide the corresponding silafluorenes (5 a–5 d) in 79–91 % yield. When substrates bearing a heterocyclic ring were used, the desired heterocyclic silafluorene derivatives (5 e–5 l) were obtained in 42–78 % yield. Interestingly, the silafluorene 5 d was submitted to a second cyclometalation using nBu2LaCl⋅4 LiCl and after treatment with Me2SiCl2 the disilylpentacyclic derivative 9 a was obtained in 82 % yield. Similarly, a second cyclolanthanation was performed on 5 l to provide a polycyclic molecule 9 b bearing seven contiguous annulated rings in 87 % yield. Such ladder π‐conjugated compounds are of interest for their photophysical properties.7b, 9c Besides, the lanthanum heterocycle 3 a was converted to spirosilabifluorene 9 c in 67 % yield and other heterofluorenes such as the stannafluorene 10 and phosphafluorene 11 in 81 % and 62 % yield, respectively.
Scheme 3.
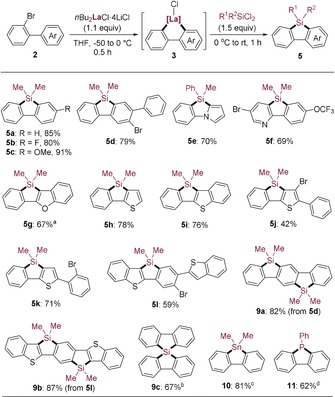
Synthesis of silafluorene derivatives (5 and 9) and other heterofluorenes (10 and 11). [a] The cyclometalated lanthanum intermediate of type 3 was generated in situ at −50 °C after 0.5 h. [b] SiCl4 (0.45 equiv) was used. [c] Me2SnCl2 (1.2 equiv) was used. [d] PhPCl2 (1.5 equiv) was used.
Interestingly, inspired by Xi's study on the reaction of 1,4‐dilithio‐1,3‐butadienes with CO2 producing cyclopentadiene derivatives,23 we examined the high reactivity of the lanthanum heterocycles of type 3, which underwent a direct cyclocarbonylation upon reaction with CO2 (1.0 atm). Thus, various 2‐bromobiphenyls were converted to fluoren‐9‐ones 6 a–6 c in 50–74 % yield (Scheme 4). Starting from heterocyclic 2‐bromobiaryl substrates provided the expected heterocyclic fluorenone derivatives (6 d–6 f) in 63–72 % yield (Scheme 4).
Scheme 4.
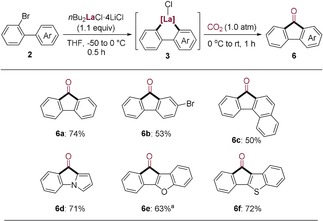
Synthesis of fluoren‐9‐one derivatives 6. [a] The cyclometalated lanthanum intermediate of type 3 was generated in situ at −50 °C after 0.5 h.
Finally, we used the 2‐bromobiaryl substrates to prepare various phenanthrene derivatives. An optimization study shows that the cyclometalated lanthanum species (3 a) did not react directly with diphenylacetylene (Table S2, entry 1) and that CrCl2 was not effective for this [4+2] annulation (entries 2 and 3). However, the use of CrCl3 in stoichiometric amounts as shown by Takahashi gave satisfactory results (entries 4 and 5).18 Nevertheless, it was found that a catalytic ring closure was possible using Fe(acac)3, FeCl3, or FeCl2 as a catalyst (10 mol %) (entries 6–9).24 The most convenient catalyst FeCl3 (10 mol %) produced the desired phenanthrene (7 a) in 79 % yield after workup. Similarly, several disubstituted alkynes were used to provide the corresponding phenanthrenes (7 b–7 d) in 51–67 % yield. Using heterocyclic substrates bearing a 2‐bromophenyl ring furnished the expected heterocyclic aromatic hydrocarbon derivatives (7 e–7 h) in 65–90 % yield. Finally, using 1,4‐diphenylbuta‐1,3‐diyne as substrate and reacting it with 3 a led to the atropisomeric product (7 i) in 54 % yield (Scheme 5).
Scheme 5.
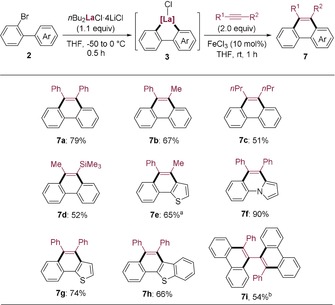
Synthesis of phenanthrene derivatives 7. [a] 1H NMR analysis reveals two regioisomers (10:1), one of which is shown here. [b] The yield was calculated based on the amount of 1,4‐diphenylbuta‐1,3‐diyne (0.45 equiv) used.
In summary, we have shown that various 2‐bromobiaryl derivatives underwent a smooth cyclolanthanation reaction below 0 °C within 0.5 h with nBu2LaCl⋅4 LiCl. The resulting cyclometalated lanthanum reagents proved to be versatile intermediates that could be readily converted to various polyfunctional 2,2′‐diiodobiaryls, silafluorenes, fluoren‐9‐ones, phenanthrenes, and their related heterocyclic analogues, showing the exceptional reactivity of aryl‐ and heteroaryllanthanum derivatives and the potential utility of this method for preparing condensed aromatics for new materials. Besides, structural investigation of the involved organolanthanum species was performed using XAFS analysis. Further exploration of the reactivity of organolanthanum reagents is underway in our laboratories.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) and the Cluster of Excellence e‐conversion for financial support. Y.H.C. appreciates the financial support from Wuhan University (young scholar start‐up funding). The technical services provided by beamline 44A at Taiwan Photon Source (TPS) of National Synchrotron Radiation Research Center (NSRRC), Taiwan, are appreciated. Miaomiao Zhu in the group of Prof. Wen‐Xiong Zhang and Prof. Zhenfeng Xi at Peking University is appreciated for her attempts to isolate intermediate 3. We also thank Albemarle (Frankfurt) and BASF (Ludwigshafen) for generous gifts of chemicals.
B. Wei, D. Zhang, Y.-H. Chen, A. Lei, P. Knochel, Angew. Chem. Int. Ed. 2019, 58, 15631.
Dedicated to Professor Zhenfeng Xi
Contributor Information
Prof. Dr. Yi‐Hung Chen, Email: yihungchen@whu.edu.cn.
Prof. Dr. Aiwen Lei, Email: aiwenlei@whu.edu.cn.
Prof. Dr. Paul Knochel, Email: paul.knochel@cup.uni-muenchen.de.
References
- 1.
- 1a. Molander G. A., Chem. Rev. 1992, 92, 29–68; [Google Scholar]
- 1b. Molander G. A., Romero J. A. C., Chem. Rev. 2002, 102, 2161–2185; [DOI] [PubMed] [Google Scholar]
- 1c. Zimmermann M., Anwander R., Chem. Rev. 2010, 110, 6194–6259; [DOI] [PubMed] [Google Scholar]
- 1d. Trifonov A. A., Lyubov D. M., Coord. Chem. Rev. 2017, 340, 10–61. [Google Scholar]
- 2.
- 2a. Benischke A. D., Anthore-Dalion L., Berionni G., Knochel P., Angew. Chem. Int. Ed. 2017, 56, 16390–16394; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16608–16612; [Google Scholar]
- 2b. Benischke A. D., Anthore-Dalion L., Kohl F., Knochel P., Chem. Eur. J. 2018, 24, 11103–11109. [DOI] [PubMed] [Google Scholar]
- 3. Pauling L., J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar]
- 4.
- 4a. Wunderlich S. H., Knochel P., Chem. Eur. J. 2010, 16, 3304–3307; [DOI] [PubMed] [Google Scholar]
- 4b. Xu L., Wang Y.-C., Wei J., Wang Y., Wang Z., Zhang W.-X., Xi Z., Chem. Eur. J. 2015, 21, 6686–6689; [DOI] [PubMed] [Google Scholar]
- 4c. Anthore-Dalion L., Benischke A. D., Wei B., Berionni G., Knochel P., Angew. Chem. Int. Ed. 2019, 58, 4046–4050; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 4086–4090. [Google Scholar]
- 5.
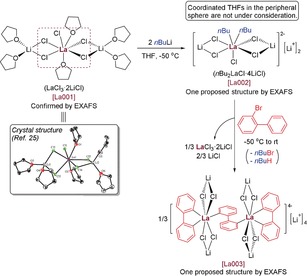
- 5a. nBu2LaCl⋅4 LiCl was freshly prepared by mixing LaCl3⋅2 LiCl in THF with 2.0 equiv of nBuLi at −50 °C for 0.5 h. Structural study: First, the structure of LaCl3⋅2 LiCl was examined using single-crystal X-ray diffraction analysis (Figure S1 and also Ref. [5b]) and its monomeric structure [La001] in solution was confirmed using extended X-ray absorption fine structure (EXAFS) analysis. Then, EXAFS study demonstrated that the transmetalation occurred upon the addition of nBuLi to LaCl3⋅2 LiCl, affording some new organolanthanum species [La002]. According to EXAFS results, it is reasonable to describe the title reagent [La002] with the tentative formula nBu2LaCl⋅4 LiCl. One possible structure of nBu2LaCl⋅4 LiCl was proposed as shown in Ref. [5b];
- 5b.EXAFS measurements can only detect the chemical environment around lanthanum while the peripheral sphere (including some coordinated THFs and Li atoms) is undetectable. The chemical structures were proposed based on the coordination number (CN) values determined from EXAFS refinements without calculation. It should be noted that the coordination number values are average values without taking into account the fraction of each La species. For example, after addition of 2-bromobiphenyl (2 a) to nBu2LaCl⋅4 LiCl, EXAFS results revealed that there were several proportional La species [La003] in the resulting solution. One representative structure of [La003] (3 a) was proposed as shown below. Please see the Supporting Information for experimental details and two other proposed structures of [La003];
-
5c.For literature data on La−C(alkyl) bond lengths and the proximate La−O(alkoxide) bond lengths, see: i) Atwood J. L., Lappert M. F., Smith R. G., Zhang H., J. Chem. Soc. Chem. Commun.
1988, 1308–1309; ii) [Google Scholar];
Barnhart D. M., Clark D. L., Gordon J. C., Huffman J. C., Watkin J. G., Zwick B. D., J. Am. Chem. Soc.
1993, 115, 8461–8462; iii) [Google Scholar];
Bambirra S., Meetsma A., Hessen B., Organometallics
2006, 25, 3454–3462; iv) [Google Scholar];
Cole M. L., Deacon G. B., Junk P. C., Wang J., Organometallics
2013, 32, 1370–1378.
 [Google Scholar]
[Google Scholar] - 6.
- 6a. Neugebauer W., Kos A. J., Schleyer P. v. R., J. Organomet. Chem. 1982, 228, 107–118; [Google Scholar]
- 6b. Snieckus V., Chem. Rev. 1990, 90, 879–933; [Google Scholar]
- 6c. Whisler M. C., MacNeil S., Snieckus V., Beak P., Angew. Chem. Int. Ed. 2004, 43, 2206–2225; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 2256–2276; [Google Scholar]
- 6d. Mulvey R. E., Mongin F., Uchiyama M., Kondo Y., Angew. Chem. Int. Ed. 2007, 46, 3802–3824; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 3876–3899; [Google Scholar]
- 6e. Mulvey R. E., Acc. Chem. Res. 2009, 42, 743–755; For rare-earth-metal-mediated C−H activation of adjacent aromatic rings, see: [DOI] [PubMed] [Google Scholar]
- 6f. Wang D., Cui D., Miao W., Li S., Huang B., Dalton Trans. 2007, 4576–4581; [DOI] [PubMed] [Google Scholar]
- 6g. Liu B., Cui D., Ma J., Chen X., Jing X., Chem. Eur. J. 2007, 13, 834–845; [DOI] [PubMed] [Google Scholar]
- 6h. Lyubov D. M., Fukin G. K., Cherkasov A. V., Shavyrin A. S., Trifonov A. A., Luconi L., Bianchini C., Meli A., Giambastiani G., Organometallics 2009, 28, 1227–1232; [Google Scholar]
- 6i. Zamora M. T., Johnson K. R. D., Hänninen M. M., Hayes P. G., Dalton Trans. 2014, 43, 10739–10750; [DOI] [PubMed] [Google Scholar]
- 6j. Zhu X., Li Y., Wei Y., Wang S., Zhou S., Zhang L., Organometallics 2016, 35, 1838–1846. [Google Scholar]
- 7.
- 7a. McQuade D. T., Pullen A. E., Swager T. M., Chem. Rev. 2000, 100, 2537–2574; [DOI] [PubMed] [Google Scholar]
- 7b. Fukazawa A., Yamaguchi S., Chem. Asian J. 2009, 4, 1386–1400; [DOI] [PubMed] [Google Scholar]
- 7c. Wu W., Liu Y., Zhu D., Chem. Soc. Rev. 2010, 39, 1489–1502. [DOI] [PubMed] [Google Scholar]
- 8. Wu B., Yoshikai N., Angew. Chem. Int. Ed. 2015, 54, 8736–8739; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 8860–8863. [Google Scholar]
- 9.
- 9a. Gilman H., Gorsich R. D., J. Am. Chem. Soc. 1955, 77, 6380–6381; [Google Scholar]
- 9b. Wang Z., Fang H., Xi Z., Tetrahedron Lett. 2005, 46, 499–501; [Google Scholar]
- 9c. Mouri K., Wakamiya A., Yamada H., Kajiwara T., Yamaguchi S., Org. Lett. 2007, 9, 93–96; [DOI] [PubMed] [Google Scholar]
- 9d. Wei B., Li H., Yin J., Zhang W.-X., Xi Z., J. Org. Chem. 2015, 80, 8758–8762. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Shimizu M., Mochida K., Hiyama T., Angew. Chem. Int. Ed. 2008, 47, 9760–9764; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 9906–9910; [Google Scholar]
- 10b. Tobisu M., Onoe M., Kita Y., Chatani N., J. Am. Chem. Soc. 2009, 131, 7506–7507; [DOI] [PubMed] [Google Scholar]
- 10c. Yabusaki Y., Ohshima N., Kondo H., Kusamoto T., Yamanoi Y., Nishihara H., Chem. Eur. J. 2010, 16, 5581–5585; [DOI] [PubMed] [Google Scholar]
- 10d. Liang Y., Zhang S., Xi Z., J. Am. Chem. Soc. 2011, 133, 9204–9207. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Ureshino T., Yoshida T., Kuninobu Y., Takai K., J. Am. Chem. Soc. 2010, 132, 14324–14326; [DOI] [PubMed] [Google Scholar]
- 11b. Murai M., Okada R., Asako S., Takai K., Chem. Eur. J. 2017, 23, 10861–10870; [DOI] [PubMed] [Google Scholar]
- 11c. Zhang Q.-W., An K., Liu L.-C., Yue Y., He W., Angew. Chem. Int. Ed. 2015, 54, 6918–6921; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 7022–7025; [Google Scholar]
- 11d. Zhang Q.-W., An K., Liu L.-C., Guo S., Jiang C., Guo H., He W., Angew. Chem. Int. Ed. 2016, 55, 6319–6323; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 6427–6431; [Google Scholar]
- 11e. Mitsudo K., Tanaka S., Isobuchi R., Inada T., Mandai H., Korenaga T., Wakamiya A., Murata Y., Suga S., Org. Lett. 2017, 19, 2564–2567. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Furukawa S., Kobayashi J., Kawashima T., J. Am. Chem. Soc. 2009, 131, 14192–14193; [DOI] [PubMed] [Google Scholar]
- 12b. Curless L. D., Ingleson M. J., Organometallics 2014, 33, 7241–7246; [Google Scholar]
- 12c. Omann L., Oestreich M., Angew. Chem. Int. Ed. 2015, 54, 10276–10279; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 10414–10418; [Google Scholar]
- 12d. Omann L., Oestreich M., Organometallics 2017, 36, 767–776. [Google Scholar]
- 13.
- 13a. Leifert D., Studer A., Org. Lett. 2015, 17, 386–389; [DOI] [PubMed] [Google Scholar]
- 13b. Xu L., Zhang S., Li P., Org. Chem. Front. 2015, 2, 459–463; [Google Scholar]
- 13c. Yang C., Wang J., Li J., Ma W., An K., He W., Jiang C., Adv. Synth. Catal. 2018, 360, 3049–3054. [Google Scholar]
- 14.
- 14a. Campo M. A., Larock R. C., Org. Lett. 2000, 2, 3675–3677; [DOI] [PubMed] [Google Scholar]
- 14b. Campo M. A., Larock R. C., J. Org. Chem. 2002, 67, 5616–5620. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Furusawa T., Morimoto T., Nishiyama Y., Tanimoto H., Kakiuchi K., Chem. Asian J. 2016, 11, 2312–2315; [DOI] [PubMed] [Google Scholar]
- 15b. Furusawa T., Morimoto T., Oka N., Tanimoto H., Nishiyama Y., Kakiuchi K., Chem. Lett. 2016, 45, 406–408; [DOI] [PubMed] [Google Scholar]
- 15c. Konishi H., Futamata S., Wang X., Manabe K., Adv. Synth. Catal. 2018, 360, 1805–1809. [Google Scholar]
- 16.
- 16a. Barluenga J., Trincado M., Rubio E., González J. M., Angew. Chem. Int. Ed. 2006, 45, 3140–3143; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 3212–3215; [Google Scholar]
- 16b. Wertz S., Leifert D., Studer A., Org. Lett. 2013, 15, 928–931; [DOI] [PubMed] [Google Scholar]
- 16c. Shi Z., Glorius F., Chem. Sci. 2013, 4, 829–833. [Google Scholar]
- 17.
- 17a. Wu G., Rheingold A. L., Geib S. J., Heck R. F., Organometallics 1987, 6, 1941–1946; [Google Scholar]
- 17b. Larock R. C., Doty M. J., Tian Q., Zenner J. M., J. Org. Chem. 1997, 62, 7536–7537. [Google Scholar]
- 18. Kanno K., Liu Y., Iesato A., Nakajima K., Takahashi T., Org. Lett. 2005, 7, 5453–5456. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Matsumoto A., Ilies L., Nakamura E., J. Am. Chem. Soc. 2011, 133, 6557–6559; [DOI] [PubMed] [Google Scholar]
- 19b. Yan J., Yoshikai N., Org. Lett. 2017, 19, 6630–6633. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Wang C., Rakshit S., Glorius F., J. Am. Chem. Soc. 2010, 132, 14006–14008; [DOI] [PubMed] [Google Scholar]
- 20b. Shoji Y., Tanaka N., Muranaka S., Shigeno N., Sugiyama H., Takenouchi K., Hajjaj F., Fukushima T., Nat. Commun. 2016, 7, 12704–12710; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20c. Ozaki K., Murai K., Matsuoka W., Kawasumi K., Ito H., Itami K., Angew. Chem. Int. Ed. 2017, 56, 1361–1364; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 1381–1384. [Google Scholar]
- 21. Shimizu M., Nagao I., Tomioka Y., Hiyama T., Angew. Chem. Int. Ed. 2008, 47, 8096–8099; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 8216–8219. [Google Scholar]
- 22.
- 22a. Krasovskiy A., Knochel P., Angew. Chem. Int. Ed. 2004, 43, 3333–3336; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 3396–3399; [Google Scholar]
- 22b. Stoll A. H., Krasovskiy A., Knochel P., Angew. Chem. Int. Ed. 2006, 45, 606–609; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 621–623. [Google Scholar]
- 23. Xi Z., Song Q., J. Org. Chem. 2000, 65, 9157–9159. [DOI] [PubMed] [Google Scholar]
- 24.For the proposed role of the Fe catalyst, see the Supporting Information (Scheme S1).
- 25.CCDC https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/anie.201908046 (LaCl3⋅2 LiCl) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


