Summary
Tumours can escape T‐cell responses by losing major histocompatibility complex (MHC)/ human leucocyte antigen (HLA) class I molecules. In the early stages of cancer development, primary tumours are composed of homogeneous HLA class I‐positive cancer cells. Subsequently, infiltration of the tumour by T cells generates a vast diversity of tumour clones with different MHC class I expressions. A Darwinian type of T‐cell‐mediated immune selection results in a tumour composed solely of MHC class I‐negative cells. Metastatic colonization is a highly complex phenomenon in which T lymphocytes and natural killer cells play a major role. We have obtained evidence that the MHC class I phenotype of metastatic colonies can be highly diverse and is not necessarily the same as that of the primary tumour. The molecular mechanisms responsible for MHC/HLA class I alterations are an important determinant of the clinical response to cancer immunotherapy. Hence, immunotherapy can successfully up‐regulate MHC/HLA class I expression if the alteration is reversible (‘soft’), leading to T‐cell‐mediated tumour regression. In contrast, it cannot recover this expression if the alteration is irreversible (‘hard’), when tumour cells escape T‐cell‐mediated destruction with subsequent cancer progression. This review summarizes clinical and experimental data on the complexity of immune escape mechanisms used by tumour cells to avoid T and natural killer cell responses. We also provide in‐depth analysis of the nature of MHC/HLA class I changes during metastatic colonization and contribute evidence of the enormous diversity of MHC/HLA class I phenotypes that can be produced by tumour cells during this process.
Keywords: antigen presentation/processing, cancer, CD8 cell, cytotoxicity, immunotherapy
Introduction
Immunotherapy has become one of the most efficient tools in cancer treatment with the successful clinical application of monoclonal antibodies that target molecules involved in regulating T‐cell cytotoxicity, including antibodies against programmed cell death protein 1 and its ligand, and cytotoxic T‐lymphocyte antigen 4 (CTLA‐4).1, 2, 3, 4, 5 These treatments are applied to patients at late stages of their disease, when the cancer has already progressed and metastasized to distant organs and tissues. The anti‐CTLA‐4 antibody ipilimumab has been reported to double the survival of patients with metastatic melanoma, but only a subset of patients objectively demonstrates clinical benefit.6 There is solid evidence of a major role for T lymphocytes in the recognition and destruction of virus‐infected cells and cancer cells through interaction of the T‐cell receptor with mutated antigens presented as peptides by the corresponding major histocompatibility complex (MHC) class I allele.6, 7, 8, 9 We discovered that T‐lymphocyte‐mediated or natural killer (NK) cell‐mediated immune selection determines the fate of a tumour (its rejection or escape) and the final MHC/ human leucocyte antigen (HLA) class I phenotype of a given metastatic colony. A Darwinian type of T‐cell‐mediated immune selection of MHC class I‐deficient tumour cells takes place,10, 11, 12, 13 starting with MHC class I‐positive primary tumours in early stages and resulting in the formation of a homogeneously MHC class I‐negative tumour (Figs 1 and 2). During this immune selection process, tumour infiltration by cytotoxic T cells coincides with the transition to a heterogeneous MHC class I phenotype, when the tumour is composed of both MHC class I‐positive and class I‐negative cells. These T‐lymphocyte‐mediated changes in tumour MHC class I expression are associated with a substantial structural transformation in the tumour tissue nest.14 We have defined two different stages of this process: a ‘permissive phase’, when T lymphocytes are infiltrating the tumour niche and killing cancer cells; and an ‘encapsulated phase’, when the tumour is composed solely of MHC class I‐negative cells and T lymphocytes are retained only in the surrounding stroma of a granuloma‐like structure.15 It was recently reported that that interferon (IFN) released by tumour‐infiltrating lymphocytes can induce DNA damage and mutations, promoting the MHC/HLA class I heterogeneity observed in primary tumours.16 These data suggest that tumour‐infiltrating lymphocytes per se may possibly promote the Generation of Diversity in the primary tumour and that the genetic instability observed in some tumours may not be the main driving force inducing MHC/HLA changes.
Figure 1.
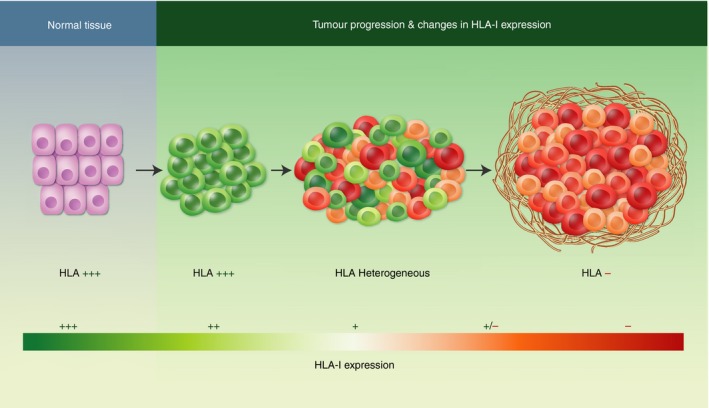
Evolution of major histocompatibility complex (MHC)/ human leucocyte antigen (HLA) class I expression in the primary tumour. Normal tissues are HLA class I‐positive. The primary tumour is also positive in early malignant stages. When T lymphocytes infiltrate the tumour, different tumour clones appear with distinct HLA‐I expression phenotypes. An explosion of MHC diversity results from T‐cell‐mediated immune selection, when cytotoxic tumour‐infiltrating lymphocytes destroy HLA class I‐positive cancer cells and leave behind negative cells, generating a tumour that is homogeneously negative for HLA class I expression.
Figure 2.
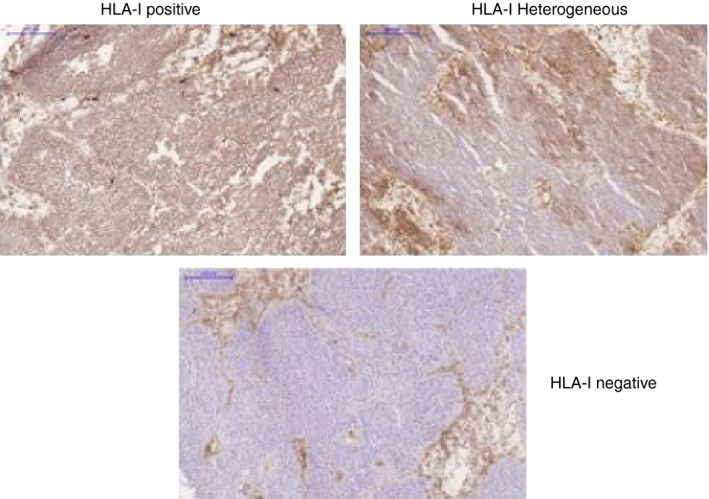
Human leucocyte antigen (HLA) class I‐positive, ‐heterogeneous and ‐negative bladder cancer tissues. Bladder cancer tissues obtained from three different patients were stained with an antibody that recognizes a monomorphic determinant of HLA class I antigens. Each example represents a different stage of HLA class I expression during the natural history of tumour development: early stage (HLA class I‐positive), intermediate stage (HLA class I‐heterogeneous) and late stage (HLA class I‐negative).
HLA class I loss is a frequent and well‐characterized mechanism of immune evasion from tumour‐specific cytotoxic T lymphocytes (CTLs).17, 18, 19 However, there has been a lack of comparative analyses of HLA class I expression during cancer progression between primary tumours and autologous metastatic lesions.20, 21 The analysis of HLA class I expression in metastases is important to understand why some patients respond to T‐cell‐mediated immunotherapies and others do not, and why some metastatic lesions regress and others progress in the same patient. Unfortunately, there are limitations to this type of study, given the difficulty in obtaining tumour tissue samples of metastatic lesions from patients undergoing immunotherapy.22
We have been investigating the evolution of HLA expression in primary tumours and in autologous metastases during cancer progression. HLA class I‐altered phenotypes in metastases can be highly diverse and not always associated with total HLA class I loss. The vast polymorphism of the HLA system contributes to a wide variety of different altered tumour phenotypes, which hampers identification of a specific phenotype in a given patient. These phenotypes were described some time ago23 and have recently been revisited.15 They can range from a total loss of expression to partial alterations, including HLA haplotype loss or absence of a single locus or single HLA allele. Each of these aberrations may translate into a lack of activation of anti‐tumour T‐cell cytotoxicity in a peptide‐specific HLA‐restricted manner. Identification of each of these phenotypes requires both immunohistological and molecular analysis of tumour tissue specimens and is important for the selection of personalized cancer treatment.
We also discuss here the potential capacity of metastatic lesions to recover MHC/HLA class I expression after immunotherapy, which depends on the reversible/soft or irreversible/hard nature of the molecular mechanism responsible for the altered HLA class I phenotypes, and which determines the progression or regression of metastatic lesions in response to treatment.24, 25, 26
MHC/HLA class I expression during the natural history of tumour development
The evolution of a cancer frequently begins with the development of a benign lesion that is followed by a multistep process in which the accumulation of genetic mutations leads to the formation of a malignant cancer able to invade and metastasize, i.e. the primary tumour.27 The immune system has developed efficient mechanisms to detect these genetic changes and destroy aberrant tumour cells through the immunosurveillance mediated by adaptive immunity.28 It is now known that T lymphocytes play a major role in eliminating tumour cells by recognizing neoantigens produced by multiple mutations accumulated in malignant cells, which are presented as peptides to T‐cell receptors via MHC class I molecules.7, 8, 29 At the same time, NK cells can control tumour growth, as part of the innate immune response, by eliminating cancer cells that lack classical MHC class I molecules.
MHC/HLA class I expression in primary tumours
Tumours are MHC/HLA class I‐positive during early phases of their development.10 T lymphocytes infiltrate various types of tumour, including melanoma,30 and lung,31, 32 pancreas33 and colorectal carcinomas.34 The degree of tumour infiltration by T cells and the T‐cell receptor repertoire are correlated with the mutational burden of the tumour tissue in question, among other factors.35 Importantly, the level of HLA class I expression in the primary tumour niche also directly correlates with the number of tumour‐infiltrating lymphocytes, which are more numerous with higher HLA expression.11 We have obtained evidence of an ‘explosion of diversity’ in MHC/HLA class I expression phenotypes in the primary tumour (Fig. 1). Various tumour clones with different HLA class I expressions are selected by T lymphocytes,36 and this selection of HLA class I‐negative tumour cells can be monitored using immunohistology or flow cytometry to analyse HLA class I expression in human solid tumours.20, 37 We also observed this wide diversity of MHC class I expression patterns in a mouse model of methylcholanthrene‐induced sarcoma, in which multiple tumour clones originated from the primary tumour were all found to have different MHC class I profiles.38 This MHC/HLA class I heterogeneity on the tumour cell surface yields tumour cells with different HLA class I expression patterns, ranging from a positive or heterogeneous phenotype to a completely negative phenotype (Fig. 2). The immune selection process leads to tumour tissue reorganization, with T cells localized in the stroma at the margin of the HLA class I‐negative tumour.11 Cancer cells create an immunosuppressive environment, with the involvement of myeloid‐derived suppressor cells, regulatory T cells and M2 macrophages. At the same time, the host attempts to build a barrier around the tumour mass and isolate it.32
Natrual killer cells are also critical for cancer immunosurveillance, and their activation is determined by the balance of signals delivered by activating receptors and inhibitory receptors. However, it remains poorly understood how NK cells specifically recognize transformed cells and dominant negative feedback pathways and how tumours escape NK cell control. Numerous reports have indicated that NK cells are dysfunctional in cancer and are characterized by either anergy or reduced cytotoxicity. This may explain why NK cells rarely infiltrate solid tumours and are mostly restricted to the stroma at the tumour invasive margin, even in cases of HLA class I‐negative tumours.14
MHC/HLA class I expression in metastases
Cancer becomes a systemic disease when tumour cells migrate to secondary sites. Metastatic colonization is highly complex, beginning with invasion of the surrounding tissues through rupture of the basal membrane and the dissemination of single tumour cells via the lymph nodes and blood.39 This biological phenomenon ends with the colonization of distant organs, which can often kill the host. The colonization is not random, and the ‘seed and soil’ concept proposed by Fidler and Kripke was confirmed by demonstration of the involvement of specific adhesion molecules that are expressed in different tissues that direct metastatic colonization.40 The immune system, particularly T and NK cells, plays a key role in controlling metastatic disease and destroying metastatic colonies that are already present. NK cells are particularly important in the control of circulating tumour cells in metastatic disease or haematological cancers, and it is believed that the efficacy of this control can vary according to the route of metastatic dissemination. For instance, the destruction of HLA class I‐negative metastatic cells is known to be more efficient if the route of colonization is the blood circulation.
The primary tumour contains clones with diverse MHC phenotypes, as noted above, but an understanding of this heterogeneity is incomplete without analysis of the MHC/HLA diversity in distant metastases. There is accumulating evidence that the MHC class I phenotype of metastatic lesions can be highly diverse and that different metastatic colonies acquire new alterations, generating novel phenotypes and increasing the cancer heterogeneity.36 It is possible to find metastatic lesions with the same MHC class I phenotype as the tumour clones already present in the primary tumour; however, some metastases can generate novel MHC phenotypes. It is also possible to observe metastases with different HLA class I phenotypes that coexist in the same patient. There is also clear evidence of changes in the MHC class I phenotype of tumour cells during metastatic colonization to avoid T‐cell responses. As already mentioned, this is followed by a new round of ‘Generation of Diversity’ and a Darwinian type of T‐cell‐mediated immune selection similar to those described in the primary tumour (Fig. 3). These different possibilities are highly relevant for cancer immunotherapy and should be investigated in various types of cancer.
Figure 3.
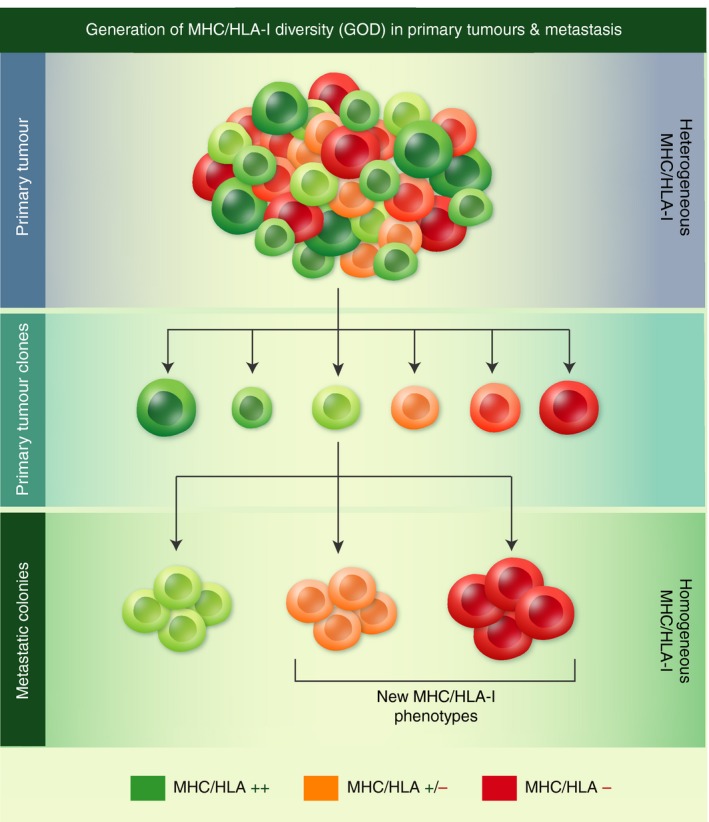
Diversity of human leucocyte antigen (HLA) class I phenotypes of metastatic colonies. The HLA class I phenotype of metastatic colonies can already be present in the primary tumour or can be different and can also be generated by the accumulation of new mutations during metastatic colonization. In this figure, the primary tumour is HLA class I heterogeneous with a wide diversity of tumour clones expressing different levels of HLA class I molecules. In contrast, the metastatic lesions are homogeneous for HLA class I expression, with different altered HLA class I phenotypes.
Generation of HLA class I‐negative metastatic lesions
During metastatic dissemination, the T‐cell‐mediated killing of HLA class I‐positive cancer cells generates a metastatic colony that is homogeneously HLA class I‐negative. Our study of a subcutaneous melanoma sample (obtained from our collaboration with Dr Gustav Gaudernak from the Radium Hospital in Oslo) showed that the primary melanoma was heterogeneous for HLA class I expression, with positive and negative areas. Laser capture microdissection allowed us to analyse the molecular mechanism of total HLA class I loss in negative areas, detecting loss of heterozygosity (LOH) at chromosomes 6 and 15 and a point mutation in codon 67 of the β 2‐microglobulin gene41 (see Fig. 4). Interestingly, we detected the same alterations in a metastatic lesion obtained 10 months later from the same melanoma patient, which was homogeneously HLA class I‐negative and had the same molecular lesion responsible for HLA class I loss (LOH in chromosome 6 and 15 and point mutation in codon 67 of β 2‐microglobulin gene). These results strongly suggest the T‐cell‐mediated elimination of HLA class I‐positive cells and the escape from the primary tumour of melanoma cells with genetic aberrations that subsequently disseminate and produce the HLA class I‐negative metastatic lesion.
Figure 4.
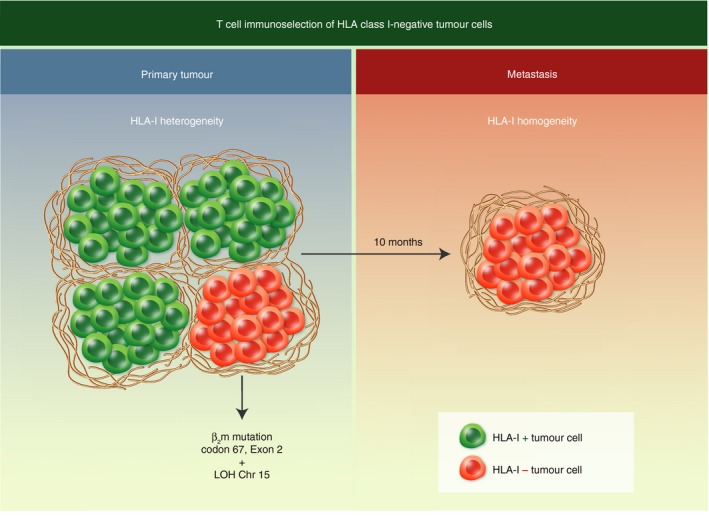
Selection of human leucocyte antigen (HLA) class I‐negative tumour cells from a heterogeneous primary tumour. The HLA class I‐negative metastases are derived from a primary tumour clone carrying the same point mutation in codon 67 of the β 2‐microglobulin gene and loss of heterozygosity at chromosome 15.
In 1998, in cooperation with Thierry Boon from the Ludwig Institute in Brussels, we described HLA class I‐negative metastases in two melanoma patients immunized with MAGE peptides presented by the HLA‐A1 allele. The metastatic tissues and autologous tumour cell lines lost HLA class I expression as the result of a point mutation in the β 2‐microglobulin gene, which abolished the start codon in one patient and introduced a premature stop codon in the other.42 The second β 2‐microglobulin allele was absent in both patients because of LOH in chromosome 15, which affected the β 2‐microglobulin region. The end result was that the melanoma tumour cells were completely HLA class I‐negative, preventing the presentation of a tumour antigenic peptide to T cells, which may explain the lack of response to peptide immunotherapy in these patients. It is often asked why metastatic lesions are frequently HLA class I‐negative and why NK cells do not eliminate these HLA class I‐negative cancer cells during metastatic dissemination. As mentioned earlier, NK cells seem to be more efficient in killing HLA‐negative metastatic cells when they disseminate through the blood circulation but less efficient in controlling their spread via the lymphatic system, as demonstrated in the example above.
HLA class I‐positive metastatic lesions
The generation of HLA‐positive liver metastases derived from microsatellite unstable (MSI‐H) colorectal cancer represents an example of the NK cell‐mediated elimination of metastatic cells that lack HLA class I expression. The primary tumour was HLA class I heterogeneous or negative when analysed by immunohistological techniques43, 44 but produced homogeneously HLA class I‐positive liver metastases. Dr Matthias Kloor in Heidelberg obtained data indicating that the liver tissue was colonized by colorectal tumour cells that disseminated via the portal vein and were controlled by NK cells.45 The NK cells can destroy HLA class I‐negative cells but not HLA class I‐positive tumour cells, thereby selecting HLA class I‐positive metastases in the liver (see Fig. 5).
Figure 5.
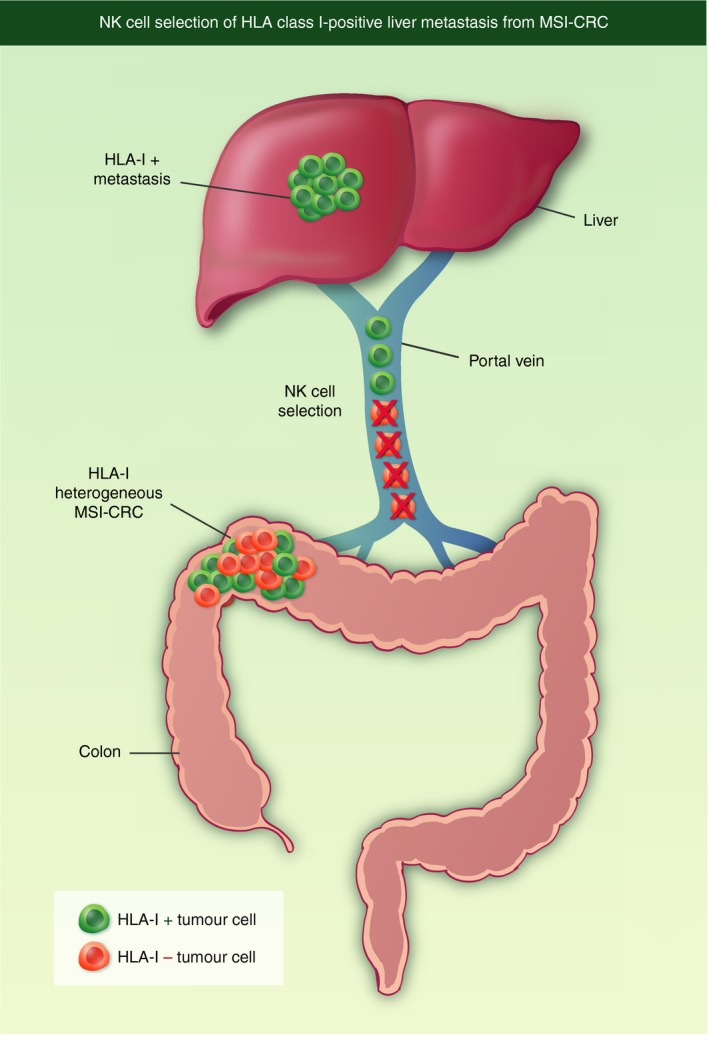
Selection of human leucocyte antigen (HLA) class I‐positive metastases from a heterogeneous primary tumour. Liver metastases derived from microsatellite unstable colorectal cancer (MSI‐H‐CRC) are HLA class I‐positive. Metastatic cells travel via the portal vein to the liver, and natural killer (NK) cells select HLA class I‐positive cells by destroying HLA class I‐negative cells.
Other independent observations also point to the role of NK cells in controlling the spread of metastatic cells to different tissues of the body via the blood. It was reported that uveal melanoma, which disseminates via the blood, has a better prognosis when it is HLA class I‐negative than when it is HLA class I‐positive, suggesting that NK cells can kill HLA class I‐negative tumour cells when they start to metastasize.46 Likewise, analysis of a large series of primary colorectal tumours found an association between HLA class I‐negative phenotype and an improved prognosis.47
These observations indicate an important role for NK cells in selecting HLA class I‐positive metastatic cells during dissemination through the blood. In this context, it is well known that HLA‐B alleles carrying the Bw6 epitope can interact with NK inhibitory receptors controlling NK cell cytotoxicity. In one study, HLA class I expression at allelic level was compared between leukaemia cells and autologous normal cells, and the down‐regulation of HLA‐A and/or HLA‐B allospecificities was detected in the majority of patients; interestingly, this down‐regulation affected HLA alleles of the HLA‐Bw4 subgroup, which do not interact with NK cell receptors.48 The authors proposed that the selective down‐regulation of HLA‐A and HLA‐Bw6 specificities and the preservation of HLA‐Bw4 provide leukaemic cells with a combined escape mechanism from T lymphocytes and NK cells.48
Metastatic lesions with different HLA class I phenotypes in the same patient
We have reported clinical cases of cancer patients with two distinct HLA class I phenotypes in different metastases.24 This comes to light when the response to immunotherapy differs between metastatic nodes, indicating their behaviour as distinct entities, defining these patients as ‘mixed responders’. In collaboration with Francesco Marincola at the National Institutes of Health (Bethesda, MD), we analysed the gene expressions of 15 melanoma metastases (10 regressing and 5 progressing) from two ‘mixed responders’ treated with different types of immunotherapy.30 Whole‐genome transcriptional analysis revealed an association between the regression of melanoma metastases and immune rejection mediated by the up‐regulation of genes involved in antigen presentation and in the IFN‐mediated response. Among the 30 000 genes in the metastases under study, the up‐regulation of genes related to immune rejection was detected in the regressing metastases, with a major enrichment of transcripts located in chromosome 6, where antigen presentation and numerous inflammatory‐related genes are localized. In contrast, progressing metastases showed low transcription levels of the genes involved in these pathways. Quantitative expression analysis of HLA‐A, ‐B and ‐C genes in microdissected tumour nests indicated higher HLA expression in regressing than in progressing metastases. This suggests that efficient tumour cell recognition and elimination in regressing metastases is associated with activation of the immune rejection mechanisms triggered by HLA class I up‐regulation (Fig. 6). In progressing lesions, however, cancer cells are not recognized by immune cells because of irreversible alterations in HLA class I, β 2‐microglobulin and/or IFN genes. In this regard, it has been proposed that there is an ‘immunological constant of rejection’ common to alloimmune interactions, graft‐versus‐host disease and solid tumours.49
Figure 6.
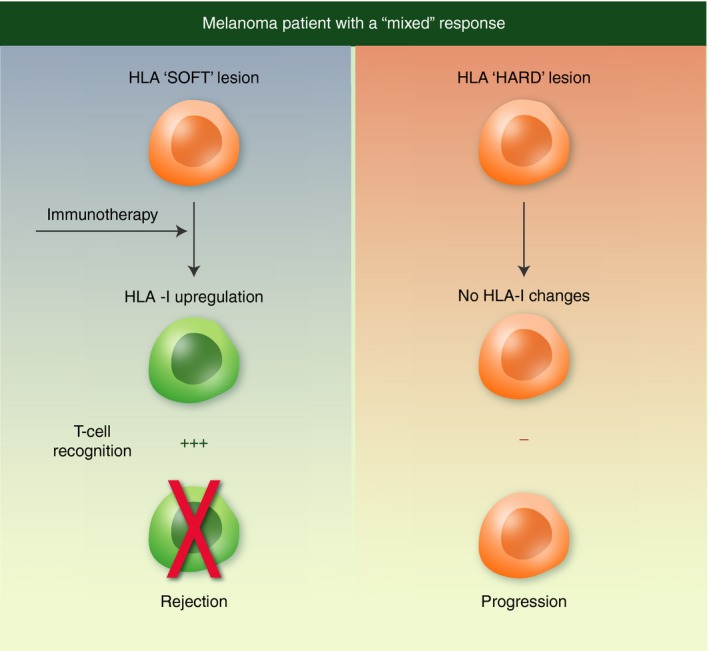
Different human leucocyte antigen (HLA) class I expression in metastases from the same patient. This figure shows two metastatic lesions analysed in a ‘mixed responder’ to immunotherapy (a). One lesion is capable of up‐regulating HLA class I to induce a T‐cell response and regresses. The other lesion cannot up‐regulate HLA class I and eventually progresses. The type of molecular alteration (hard or soft) responsible for the HLA class I down‐regulation plays a pivotal role in these different responses.
In 2001, in cooperation with Alex Knuth in Mainz, we analysed the HLA class I expression of different metastases from two patients with melanoma who were undergoing peptide immunotherapy with MelanA/MART‐1, tyrosinase and gp100.50 All three metastases analysed in the first patient showed a similar HLA class I alteration, with two populations of melanoma cells, one of which was HLA class I‐positive and the other had LOH in the short arm of chromosome 6, leading to an HLA haplotype loss. The second patient had two metastatic lesions with an identical HLA class I molecular defect, i.e. HLA‐B locus down‐regulation, but one expressed the tumour antigen (MelanA/MART‐1) and the other did not. We highlight that the antigen‐positive metastasis regressed after immunotherapy whereas the other progressed rapidly.50
Metastases resistant to HLA up‐regulation because of mutations in IFN pathways
Different types of IFNs (IFN‐α, IFN‐β and IFN‐γ) play an important role in cellular defence against viral infections.51 HLA class I and II up‐regulation in virally infected and tumour cells is a well‐established biological function of IFNs, leading to increased antigen presentation to T lymphocytes. Any molecular defect that blocks this function can contribute to an escape route from T‐cell cytotoxicity and favours virus dissemination and tumour progression. We have demonstrated that tumour cell lines can have molecular defects in IFN‐α or IFN‐γ signalling pathways, preventing HLA class I up‐regulation through the mutation of signal transducer and activator of transcription 1 (STAT‐1) genes. In AGS gastric carcinoma cells, a lack of response to either type of IFN was associated with a low level of transcriptional factor binding to an IFN responsive sequence element.52, 53 In Caki‐2 renal cell carcinoma cells, resistance to IFNs resulted from the absence of DNA binding activity for IFN regulatory factor‐1 and for STAT‐1.54 Dr Annette Paschen and co‐workers at the University of Essen recently reported genetic defects in the IFN signalling pathway in a large number of melanoma cell lines derived from metastatic lesions and showed that HLA class I expression was impaired by these alterations.55
Changes in HLA class I expression during metastatic colonization
Metastatic cells change the HLA class I phenotype to avoid T‐cell and NK cell recognition during metastatic colonization. However, the monitoring of these changes is hampered by the difficulty in obtaining cell lines and samples during the course of cancer progression. Nevertheless, these alterations are well documented in melanoma. In 1997, Ikeda and co‐workers reported distinct HLA class I patterns in two melanoma cell lines (MEL.A and MEL.B) derived from metastases in the same patient at different time‐points of cancer progression.56 MEL.A cell line expressed several antigens recognized by autologous CTLs through HLA class I molecules, whereas MEL.B cells lost the expression of all class I molecules except for HLA‐A24. HLA class I‐restricted CTLs were obtained and proved able to lyse MEL.B cells. Surprisingly, however, these CTLs failed to lyse MEL.A cells, even though they expressed the tumour‐specific antigen. The explanation is that the new CTLs expressed the NK inhibitory receptor, which interacts with the HLA‐Cw7 present in MEL.A cells but not in MEL.B cells.56
In 1998, we collaborated with Jesper Zeuten in Copenhagen in a study that evidenced different HLA class I expression patterns in metastases obtained from two melanoma patients at different time‐points.57, 58 Melanoma cell lines derived from two subcutaneous metastases in patient FM55 (FM55M1 and FM55M2) showed that the expression of both HLA‐B alleles was significantly reduced but could be completely recovered by IFN‐γ treatment. A new cell line was obtained from FM55 after in vitro treatment with a heterologous HLA‐A2 restricted CTL clone. This cell line (R22.2) expressed a single HLA class I allele as the result of an additional HLA haplotype loss. The second melanoma patient (FM37) presented the same HLA class I changes (expression of single HLA allele), now obtained in an in vivo lesion (see Fig. 7).
Figure 7.
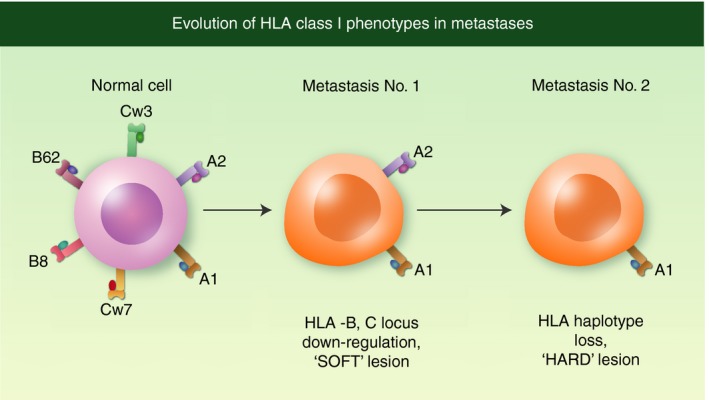
Evolution of human leucocyte antigen (HLA) class I changes during metastatic colonization. At the beginning of mestastatic dissemination, tumour cells down‐regulate HLA‐B and HLA‐C locus products in a coordinated manner by decreasing their transcription. At later stages, these cells acquire an additional alteration, loss of HLA haplotype, producing tumour cells that express a single HLA class I allele.
Cancer immunotherapy: impact of ‘soft’ and ‘hard’ HLA class I molecular lesions
Cancer immunotherapy has attracted considerable attention since the successful introduction of immune checkpoint inhibitors involved in the regulation of T lymphocyte cytotoxicity.3, 5 The use of these checkpoint antibodies, e.g. anti‐programmed cell death protein 1 and anti‐CTLA‐4, has achieved metastatic regression in many patients, especially in metastases derived from melanoma and lung tumours (Fig. 8).
Figure 8.
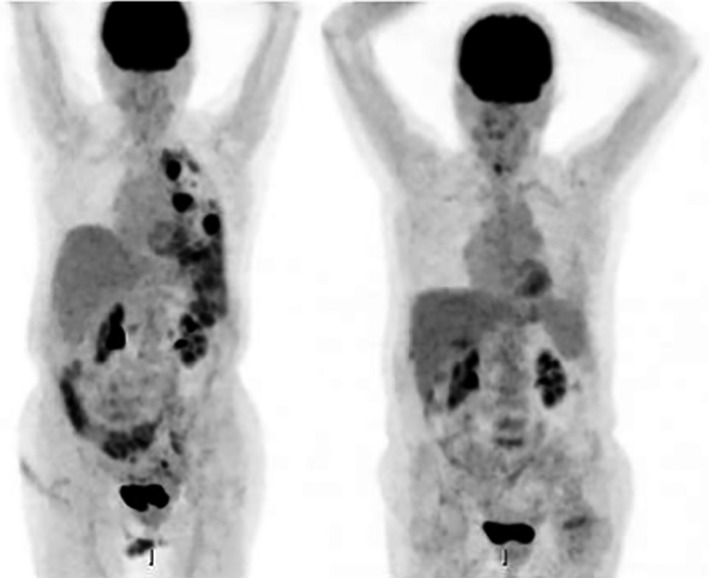
A complete response obtained in a patient with lung metastases of a lung carcinoma treated with anti‐programmed cell death protein 1 antibody (Ipilimumab): left side, before treatment; right side, after treatment. The lung metastatic nodes disappeared after the treatment. Images are courtesy of Dr Antonio Rodriguez from the Department of Nuclear Medicine of the Hospital Virgen de las Nieves, Granada, Spain.
However, a significant proportion of patients do not respond to this treatment for reasons that are poorly understood. We believe that HLA class I loss may be involved in this resistance. Although there is considerable information on the prevalence of various patterns of MHC class I defects and underlying molecular mechanisms in different types of cancer,12, 13, 15, 19, 23, 59 few data are available on changes in HLA class I expression during the course of cancer immunotherapy. We propose that the progression or regression of a metastatic lesion in cancer patients undergoing immunotherapy would be determined by the molecular mechanism responsible for MHC class I alterations and not by the type of immunotherapy.25, 26 These alterations can be reversible (‘soft’ lesion) or irreversible (‘hard’ lesion) by treatment with cytokines. Hard lesions include LOH at chromosome 6 in the HLA region with the simultaneous loss of three HLA class I alleles: HLA‐A, HLA‐B and HLA‐C.60, 61, 62 This is a frequent mechanism that generates HLA haplotype loss in approximately 35% of tumours with different histotypes. Another mechanism leading to irreversible HLA defects is LOH in chromosome 15, which carries the β 2‐microglobulin gene, together with a point mutation in the other homologous gene. This genetic lesion is associated with the total loss of HLA class I expression and has been described in B‐cell lymphomas, melanomas, microsatellite unstable colorectal carcinomas and other types of cancer.37, 43, 63, 64 Further details are published in the review by Bernal et al.65 Mutations, deletions and somatic recombinations in a single HLA class I gene have also been described66, 67, 68 but are not frequent events. Resistance to IFN‐mediated up‐regulation of HLA class I expression is a common mechanism of tumour escape through mutations affecting the JAK‐STAT component of the IFN‐mediated signalling pathway. These tumour cells express low levels of HLA class I molecules that cannot be up‐regulated. Another example of this irreversible molecular mechanism was recently reported in melanoma.55 Soft lesions include the down‐regulation of HLA class I antigens by defects in the gene regulation of HLA class I heavy chain genes, β 2‐microgobulin gene and components of the antigen‐processing machinery. These abnormal HLA class I phenotypes have low mRNA levels of these genes, which appear to be co‐ordinately down‐regulated and can be corrected in vitro and in vivo by treatment with T helper type 1 cytokines.69
Immunotherapies modify the tumour microenvironment and induce the release of Th1 cytokines, such as IFN or tumour necrosis factor,70 which can up‐regulate HLA class I and II molecules and recover the antigen presentation capability of tumour cells. Primed T cells can again recognize tumour antigens and different T‐cell clonal populations can be identified. This phenomenon has been designated ‘epitope spreading’ and is a clear indication that immunotherapy can boost tumour cell recognition by T cells.71, 72 HLA class I expression can only be recovered when the molecular mechanism responsible for HLA class I down‐regulation is reversible (soft). However, the tumour will maintain the HLA class I‐negative phenotype and will progress when the molecular mechanism is irreversible (hard).73 Indeed, it was recently observed in melanoma that metastatic lesions progressing after immune checkpoint‐blocking immunotherapy harboured mutations in the β 2‐microglobulin gene and LOH at chromosomes 6 and 15.74 In‐depth analysis of tissues from immunotherapy‐resistant metastatic lesions will help to achieve major advances in this important area of research.
Conclusions
MHC/HLA class I loss should be viewed as a crucial step during the natural history of tumour development rather than as an obstacle to successful cancer immunotherapy. This loss also provides indirect evidence that immune surveillance is effective in the host until tumour immune escape prevails. Immune evasion associated with defects in antigen presentation to T lymphocytes is a common phenomenon and underlines the relevance of T cells in the rejection of solid tumours. Information available on MHC/HLA class I loss or down‐regulation in metastatic lesions remains limited because of the difficulties in obtaining tissues for HLA analysis. Most data on these phenomena derive from the analysis of subcutaneous or lymph node metastases in melanoma patients. It is becoming clear that the generation of MHC class I diversity is not only produced in the primary tumour but also continues during the process of metastatic dissemination. We provide evidence that a metastatic HLA class I altered phenotype can already be present in the primary tumour, but that de novo HLA class I alterations can also be generated during metastatic colonization. Cancer immunotherapy has achieved a breakthrough with the improved response of metastatic cancers to newly designed treatments with monoclonal antibodies targeting immune check‐points. However, the reason why some patients respond and others do not remains poorly understood. We believe that tumour HLA class I up‐regulation is an essential step but can only be achieved if the molecular lesion is reversible (soft). Nevertheless, despite the important past and present observations described in this review, HLA analysis of primary tumour tissues and metastatic lesions is still not included in the majority of cancer treatment protocols, a situation that is surely ripe for change.75
Disclosure
The authors declare no competing financial interest.
Acknowledgements
The authors thank Dr Monica Bernal from the Department of Haematology for providing the figures for this review, Dr Antonio Rodriguez from the Department of Nuclear Medicine, Hospital Virgen de las Nieves in Granada for the positron emission tomography/computed tomography images and Dr Francisco Perea from the Department of Clinical Analysis for the immunohistological images. This study was supported by grants from the Spanish Institute of Health Carlos III (ISCIII, Instituto Carlos III) co‐financed by the European Union (FEDER‐Fondo Europeo de Desarrollo Regional) (PI14/01978, PI16/00752, Q2827015E, PI17/00197, PT17/0015/0041), the Junta de Andalucía (Group CTS‐143) and the Beckman‐Coulter and Abbott Foundations.
References
- 1. Allison JP, McIntyre BW, Bloch D. Tumor specific antigen of murine T‐lymphoma defined with monoclonal antibody. J Immunol 1982; 129:2293–300. [PubMed] [Google Scholar]
- 2. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11:3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allison JP, Hurwitz AA, Leach D. Manipulation of costimulatory signals to enhance anti‐tumor T‐cell responses. Curr Opin Immunol 1995; 7:682–6. [DOI] [PubMed] [Google Scholar]
- 4. Iwai Y, Terawaki S, Honjo T. PD‐1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol 2005; 17:133–44. [DOI] [PubMed] [Google Scholar]
- 5. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science 1996; 271:1734–6. [DOI] [PubMed] [Google Scholar]
- 6. Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O et al Pooled analysis of long term survival data from phase II and phase III from Ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994; 12:337–65. [DOI] [PubMed] [Google Scholar]
- 8. Boesen M, Svane IM, Engel AM, Rygaard J, Thomsen AR, Werdelin O. CD8+ T cells are crucial for the ability of congenic normal mice to reject highly immunogenic sarcomas induced in nude mice with 3‐methylcholantrene. Clin Exp Immunol 2000; 121:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romero P, Coulie P. Adaptive T‐cell immunity and tumor antigen recognition In: Rees RC, ed. Tumor Immunology and Immunotherapy. Oxford: Oxford University Press, 2014: 1–14. [Google Scholar]
- 10. Garrido F, Cabrera T, Concha A, Glew S, Ruiz‐Cabello F, Stern P. Natural history of HLA antigens during tumour development. Immunol Today 1993; 14:491–9. [DOI] [PubMed] [Google Scholar]
- 11. Aptsiauri N, Ruiz‐Cabello F, Garrido F. The transition from HLA‐I positive to HLA‐I negative primary tumors: the road to escape from T cell responses. Curr Opin Immunol 2018; 51:123–32. [DOI] [PubMed] [Google Scholar]
- 12. Bodmer WF, Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG. Tumour escape from immune response by variation in HLA expression and other mechanisms. Ann NY Acad Sci 1993; 690:422–49. [DOI] [PubMed] [Google Scholar]
- 13. Campoli M, Chang G, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine 2002; 20(Suppl. 4):A40–5. [DOI] [PubMed] [Google Scholar]
- 14. Garrido F, Perea F, Bernal M, Sanchez‐Palencia A, Aptsiauri N, Ruiz‐Cabello F. The escape of cancer from T cell mediated immune surveillance: HLA class I loss and tumour tissue architecture. Vaccine 2017; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrido F, Ruiz‐Cabello F, Aptsiauri N. Rejection versus escape: the tumour MHC dilema. Cancer Immunol Immunother 2017; 66:259–71. 10.1007/s00262-016-1947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeda K, Nakayama M, Hayakawa Y, Koyima Y, Ikeda H, Imai N et al IFN‐γ is required for cytotoxic T cell dependent cancer genome immunoediting. Nat Commun 2017; 8:14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aptsiauri N, Cabrera T, Garcia‐Lora A, Lopez‐Nevot MA, Ruiz‐Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol 2007; 256:139–89. [DOI] [PubMed] [Google Scholar]
- 18. Garrido F, Klein E. MHC antigen expression I. Human tumors In: Klein E, Garrido F, eds. Seminars in Cancer Biology, Vol 2‐1 Netherlands: Elsevier, 1991:1–2. [Google Scholar]
- 19. Marincola FM, Jafee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T‐cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74:181–273. [DOI] [PubMed] [Google Scholar]
- 20. Cabrera T, Lopez‐Nevot MA, Gaforio JJ, Ruiz‐Cabello F, Garrido F. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother 2003; 52:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aptsiauri N, Carretero R, Garcia-Lora A, Real LM, Cabrera T, Garrido F. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol Immunother 2008; 57:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez‐Nevot MA, Esteban F, Ferron A, Gutierrez J, Oliva MR, Romero C et al HLA class I gene expression on human primary tumours and autologous metastases: demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br J Cancer 1989; 59:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garrido F, Ruiz‐Cabello F, Cabrera T, Perez‐Villar JJ, Lopez‐Botet M, Duggan‐Keen M et al Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 1997; 18:89–95. [DOI] [PubMed] [Google Scholar]
- 24. Carretero R, Romero JM, Ruiz‐Cabello F, Maleno I, Rodriguez F, Camacho FM et al Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics 2008; 60:439–47. [DOI] [PubMed] [Google Scholar]
- 25. Garrido F, Cabrera T, Aptsiauri N. “Hard” and “Soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int J Cancer 2010; 127:249–56. [DOI] [PubMed] [Google Scholar]
- 26. Garrido F, Algarra I, García‐Lora AM. The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural‐irreversible “hard” lesions. Cancer Immunol Immunother 2010; 59:1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759–67. [DOI] [PubMed] [Google Scholar]
- 28. Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970; 13:1–27. [DOI] [PubMed] [Google Scholar]
- 29. Zinkernagel RM, Doherty PC. Restriction of in vitro T cell‐mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 1974; 248:701–2. [DOI] [PubMed] [Google Scholar]
- 30. Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, Engle AM et al Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon‐mediated rejection genes. Int J Cancer 2012; 131:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S et al HLA class I antigen expression is associated with a favorable prognosis in early stage non‐small cell lung cancer. Cancer Sci 2007; 98:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perea F, Bernal M, Sanchez‐Palencia A, Carretero J, Torres C, Bayarri C et al The absence of HLA class I expression in non‐small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer 2017; 140:888–99. [DOI] [PubMed] [Google Scholar]
- 33. Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I et al Control of T cell mediated immune response by HLA class I in human pancreatic carcinoma. Clin Can Res 2005; 11:498–504. [PubMed] [Google Scholar]
- 34. Galon J, Costes A, Sanchez Cabo F, Kirilovsky A, Mlenick B, Lagorde‐Pages C et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960–4. [DOI] [PubMed] [Google Scholar]
- 35. Gröbner S, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva V et al The landscape of genomic alterations across childhood cancers. Nature 2018; 555:E10. [DOI] [PubMed] [Google Scholar]
- 36. Garrido F, Romero I, Aptsiauri N, Garcia‐Lora AM. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer 2016; 138:271–80. [DOI] [PubMed] [Google Scholar]
- 37. Koopman LA, Corver WE, van der Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med 2000; 191:961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Algarra I, Gaforio JJ, Garrido A, Mialdea MJ, Perez M, Garrido F. Heterogeneity of MHC‐class‐I antigens in clones of methylcholanthrene‐induced tumors. Implications for local growth and metastasis. Int J Cancer Suppl 1991; 6:73–81. [DOI] [PubMed] [Google Scholar]
- 39. Fidler I, Kripke M. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977; 197:893–5. [DOI] [PubMed] [Google Scholar]
- 40. Sökeland G, Schumacher U. The functional role of integrins during intra‐ and extravasation within the metastatic cascade. Mol Cancer 2019; 18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. del Campo AB, Kyte JA, Carretero J, Zinchencko S, Mendez R, Gonzalez‐Aseguinolaza G et al Immune escape of cancer cells with β2 microglobulin loss over the course of metastatic melanoma. Int J Cancer 2014; 134:102–13. [DOI] [PubMed] [Google Scholar]
- 42. Benitez R, Godelaine D, Lopez‐Nevot MA, Brasseur F, Jimenez P, Marchand M et al Mutations of the β2‐microglobulin gene result in a lack of HLA class I molecules on melanoma cells of two patients immunized with MAGE peptides. Tissue Antigens 1998; 52:520–9. [DOI] [PubMed] [Google Scholar]
- 43. Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz‐Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: β2‐microglobulin inactivation in MSI‐positive tumors and LMP7/TAP2 downregulation in MSI‐negative tumors. Tissue Antigens 2003; 61:211–9. [DOI] [PubMed] [Google Scholar]
- 44. Menon AG, Tollenaar RA, van de Velde CJ, Putter H, Janssen‐van Rhijn CM, Keijzer R et al P53 and HLA class I expression are not down‐regulated in colorectal cancer liver metastases. Clin Exp Metastasis 2004; 21:79–85. [DOI] [PubMed] [Google Scholar]
- 45. Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer 2010; 127:1001–10. [DOI] [PubMed] [Google Scholar]
- 46. Jager MJ, Hurks HM, Levitskaya J, Kiessling R. HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol 2002; 63:444–51. [DOI] [PubMed] [Google Scholar]
- 47. Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH et al Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer 2006; 118:6–10. [DOI] [PubMed] [Google Scholar]
- 48. Demanet C, Mulder A, Deneys V, Worsham MJ, Maes P, Claas FH et al Down‐regulation of HLA‐A and HLA‐Bw6 but not HLA‐Bw4 allospecificities in leukemic cells: an escape mechanism from CTLs and NK attack. Blood 2004; 103:3122–30. [DOI] [PubMed] [Google Scholar]
- 49. Wang E, Worschech A, Marincola F. The immunological constant of rejection. Trends Immunol 2008; 29:256–62. [DOI] [PubMed] [Google Scholar]
- 50. Mendez R, Serrano A, Jager E, Maleno I, Ruiz‐Cabello F, Knuth A et al Analysis of HLA class I expression in different metastases from two melanoma patients undergoing peptide immunotherapy. Tissue Antigens 2001; 57:508–19. [DOI] [PubMed] [Google Scholar]
- 51. Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol 2017; 38:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abril E, Mendez R, Garcia A, Serrano A, Cabrera T, Garrido F et al Characterization of a gastric tumor cell line defective in MHC class I inducibility by both α and γ interferon. Tissue Antigens 1996; 47:391–8. [DOI] [PubMed] [Google Scholar]
- 53. Abril E, Real LM, Serrano A, Jimenez P, Garcia A, Canton J et al Unresponsiveness to interferon associated with STAT1 protein deficiency in a gastric adenocarcinoma cell line. Cancer Immunol Immunother 1998; 47:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dovhey SE, Ghosh NS, Wright KL. Loss of interferon‐γ inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res 2000; 60:5789–96. [PubMed] [Google Scholar]
- 55. Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N et al Acquired IFNγ resistance impairs anti‐tumor immunity and gives rise to T‐cell‐resistance melanoma lesions. Nat Commun 2017; 8:15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C et al Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 1997; 6:199–208. [DOI] [PubMed] [Google Scholar]
- 57. Real LM, Jimenez P, Canton J, Kirkin A, Garcia A, Abril E et al In vivo and in vitro generation of a new altered HLA phenotype in melanoma tumor cell variants expressing a single HLA class I allele. Int J Cancer 1998; 75:317–23. [DOI] [PubMed] [Google Scholar]
- 58. Real LM, Jimenez P, Kirkin A, Serrano A, Garcia A, Canton J et al Multiple mechanisms of immune evasion can coexist in melanoma tumor cell lines derived from the same patient. Cancer Immunol Immunother 2001; 49:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. MacGranahan N, Rosenthal R, Hiley C, Rowan A, Watkins T, Wilson G et al Allele‐specific HLA loss and immune escape in lung cancer evolution. Cell 2017; 171:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jimenez P, Canton J, Collado A, Cabrera T, Serrano A, Real LM et al Chromosome loss is the most frequent mechanism contributing to HLA haplotype loss in human tumors. Int J Cancer 1999; 83:91–7. [DOI] [PubMed] [Google Scholar]
- 61. Maleno I, Lopez‐Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother 2002; 51:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maleno I, Cabrera CM, Cabrera T, Paco L, Lopez‐Nevot MA, Collado A et al Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics 2004; 56:244–53. [DOI] [PubMed] [Google Scholar]
- 63. Challa‐Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV et al Combine genetic inactivation of β2 microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 2011; 20:728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. D'Urso CM, Wang CG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured FO‐1 melanoma cells due to a defect in β2 microglobulin expression. J Clin Invest 1991; 87:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bernal M, Ruiz‐Cabello F, Concha A, Paschen A, Garrido F. Implication of the β2‐microglobulin gene in the generation of tumour escape phenotypes. Cancer Immunol Immunother 2012; 61:1359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Z, Seliger B, Mike N, Momburg F, Knuth A, Ferrone S. Molecular analysis of the HLA‐A2 antigen loss by melanoma cells SK‐MEL‐29.1.22 and SK‐MEL‐29.1.29. Cancer Res 1998; 58:2149–57. [PubMed] [Google Scholar]
- 67. Jimenez P, Cabrera T, Mendez R, Esparza C, Cozar JM, Tallada MA et al Nucleotide insertion in exon 4 is responsible for the absence of expression of an HLA‐A*0301 allele in a prostate carcinoma cell line. Immunogenetics 2001; 53:606–10. [DOI] [PubMed] [Google Scholar]
- 68. Serrano A, Brady CS, Jimenez P, Duggan‐Keen MF, Mendez R, Stern P et al A mutation determinig the loss of HLA‐A2 antigen expression in a cervical carcinoma reveals novel splicing of human MHC class I classical transcripts in both tumoral and normal cells. Immunogenetics 2000; 51:1047–52. [DOI] [PubMed] [Google Scholar]
- 69. Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M et al Coordinated downregulation of the antigen presentation machinery and HLA class I/β2‐microglobulin complex is responsible for HLA‐ABC loss in bladder cancer. Int J Cancer 2005; 113:605–10. [DOI] [PubMed] [Google Scholar]
- 70. del Campo AB, Carretero J, Aptsiauri N, Garrido F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue Antigens 2012; 79:147–54. [DOI] [PubMed] [Google Scholar]
- 71. Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joshep‐Pietras D et al Anti CTLA‐4 broadens the melanoma‐reactive CD8+ T cell response. Sci Transl Med 2014; 6:1–9. [DOI] [PubMed] [Google Scholar]
- 72. Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T et al CTLA4 blockade broadens the peripheral T cell receptor repertoire. Clin Cancer Res 2014; 20:2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garrido F, Aptsiauri N, Doorduijn E, Garcia‐Lora A, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol 2016; 39:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zaretsky JM, Garcia‐Diaz A, Shin DS, Escuin‐Ordinas H, Hugo W, Hu‐Lieskovan S et al Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016; 375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Garrido F. MHC class‐I loss and cancer immune escape. Adv Exp Med Biol 2019; 1151:101. [DOI] [PubMed] [Google Scholar]


