Abstract
Purpose: The purpose of this study was to evaluate melanoma-targeting property of 90Y-DOTA-GGNle-CycMSHhex to facilitate its potential therapeutic application.
Materials and Methods: DOTA-GGNle-CycMSHhex was synthesized and readily labeled with 90Y in 0.25 M NH4Ac-buffered solution to generate 90Y-DOTA-GGNle-CycMSHhex. The specific receptor binding, internalization, and efflux of 90Y-DOTA-GGNle-CycMSHhex were determined on B16/F10 murine melanoma cells. The biodistribution property of 90Y-DOTA-GGNle-CycMSHhex was examined on B16/F10 melanoma-bearing C57 mice.
Results: 90Y-DOTA-GGNle-CycMSHhex displayed receptor-specific binding, rapid internalization, and prolonged efflux on B16/F10 melanoma cells. 90Y-DOTA-GGNle-CycMSHhex exhibited high uptake and prolonged retention in melanoma, and fast urinary clearance on B16/F10 melanoma-bearing C57 mice. The B16/F10 tumor uptake was 20.73% ± 7.99%, 19.93% ± 5.73%, 14.8% ± 4.61%, and 6.69% ± 1.85% ID/g at 0.5, 2, 4, and 24 h postinjection, respectively.
Conclusions: 90Y-DOTA-GGNle-CycMSHhex displayed melanocortin-1 receptor (MC1R) targeting and specificity on B16/F10 melanoma cells and tumors. The favorable melanoma-targeting property and fast urinary clearance of 90Y-DOTA-GGNle-CycMSHhex warranted its evaluation for melanoma therapy in future studies.
Keywords: melanocortin-1 receptor, melanoma therapy, 90Y-DOTA-GGNle-CycMSHhex
Introduction
Malignant melanoma is the most lethal skin cancer and fifth most commonly diagnosed cancer in the United States with ∼96,480 new cases in 2019.1 Extreme aggressiveness of metastatic melanoma leads to high mortality among metastatic melanoma patients. Despite the promising result that the median overall survival of metastatic melanoma patients has been improved by months through new treatments such as Vemurafenib (BRAF inhibitor), ipilimumab (targeting CTLA-4), and Nivolumab (PD-1 inhibitor),2–6 the treatments are far from satisfactory due to the low long-term survival (<10%) for metastatic melanoma patients. There is an urgent need to develop new therapeutic agents for melanoma.
Melanocortin-1 receptor (MC1R) is an attractive G protein-coupled receptor (GPCR), which overexpresses on both murine and human melanoma cells.7–12 Importantly, >80% of amelanotic and melanotic human metastatic melanoma samples exhibit MC1Rs.7 Recently, we have demonstrated the clinical relevance of MC1R for melanoma imaging through the first-in-human study of 68Ga-DOTA-GGNle-CycMSHhex (1,4,7,10-tetraazacyclononane-1,4,7,10-tetraacetic acid-Gly-Gly-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-CONH2), which targets MC1Rs.13 Remarkably, 68Ga-DOTA-GGNle-CycMSHhex positron emission tomography (PET) could clearly visualize the melanoma metastases in brain, lung, connective tissue, and small intestine of melanoma patients.13 These exciting first-in-human images of melanoma metastases highlighted the potential of MC1R as a melanoma target for targeted radionuclide therapy.
We have been interested in utilizing therapeutic radionuclides to target MC1Rs for melanoma therapy. In our previous report, we determined the melanoma-targeting property of 177Lu-DOTA-GGNle-CycMSHhex on B16/F1 melanoma-bearing C57 mice.14 Interestingly, 177Lu-DOTA-GGNle-CycMSHhex displayed high B16/F1 melanoma uptake of 20.25% ± 4.59% and 21.63% ± 6.27% ID/g at 0.5 and 2 h postinjection, respectively. Meanwhile, the melanoma lesions were clearly visualized using 177Lu-DOTA-GGNle-CycMSHhex as an imaging probe.14 In this study, we are interested in replacing 177Lu with 90Y to examine whether the change of therapeutic radionuclide could still maintain favorable melanoma-targeting property of 90Y-DOTA-GGNle-CycMSHhex. 90Y is a high-energy β-particle emitter with a maximum β energy of 2.3 MeV and a half-life of 2.7 days. Specifically, we prepared 90Y-DOTA-GGNle-CycMSHhex and determined its MC1R-targeting property on B16/F10 melanoma cells and tumor-bearing mice.
Materials and Methods
Chemicals and reagents
Amino acids, DOTA-tri-tert-butyl ester, and resin were purchased from Advanced ChemTech, Inc. (Louisville, KY), Macrocyclics, Inc. (Richardson, TX) and Novabiochem (San Diego, CA) for peptide synthesis, respectively. 90YCl3 was purchased from PerkinElmer Health Sciences, Inc. (Waltham, MA) for radiolabeling and biodistribution. MC1R antibody (Rabbit/IgG) and FITC-conjugated antirabbit secondary antibody were purchased from Thermo Scientific (Rockford, IL) for MC1R staining on melanoma cells and tumors. All other chemicals used in this study were purchased from Thermo Fisher Scientific (Waltham, MA) and used without further purification. Four percent paraformaldehyde (PFA) in phosphate-buffered saline (PBS) was obtained from Alfa Aesar (Ward Hill, MA), xylene was obtained from Fisher Chemical (Fair Lawn, NJ), 4′,6-diamidino-2-phenylindole (DAPI) Fluoromount-G mounting medium was obtained from SouthernBiotech (Birmingham, AL), and Prolong Diamond antifade mounting reagent with DAPI was obtained from Life Technologies (Eugene, OR). B16/F10 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Preparation, serum stability, and specific binding of 90Y-DOTA-GGNle-CycMSHhex
DOTA-GGNle-CycMSHhex was synthesized using standard fluorenylmethyloxycarbonyl (Fmoc) chemistry and characterized by liquid chromatography-mass spectrometry.15 90Y-DOTA-GGNle-CycMSHhex was prepared in a 0.25 M NH4OAc-buffered solution (pH 4.5). In brief, 30 μL of 90YCl3 (37–74 MBq in 0.05 M HCl aqueous solution), 10 μL of 1 mg/mL peptide aqueous solution, and 200 μL of 0.25 M NH4OAc were added to a reaction vial and incubated at 75°C for 30 min. After the incubation, 10 μL of 0.5% EDTA (ethylenediaminetetraacetic acid) aqueous solution was added to the reaction vial to scavenge potential unbound 90Y3+ ions. The radiolabeled complexes were purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse-phase analytical column (Deerfield, IL) using the following gradient at a 1 mL/min flowrate. The mobile phase consisted of solvent A (20 mM HCl aqueous solution) and solvent B (100% CH3CN). The gradient was initiated and kept at 82:18 A/B for 3 min followed by a linear gradient of 82:18 A/B to 72:28 A/B over 20 min. Then, the gradient was changed from 72:28 A/B to 10:90 A/B over 3 min followed by an additional 5 min at 10:90 A/B. Thereafter, the gradient was changed from 10:90 A/B to 82:18 A/B over 3 min. The purified peptide was purged with N2 gas for 15 min to remove the acetonitrile. The pH of the final solution was adjusted to 7.4 with 0.1 N NaOH and sterile saline for animal studies.
In vitro serum stability of 90Y-DOTA-GGNle-CycMSHhex was determined by incubation in mouse serum at 37°C for 4 h and monitored for degradation by Reversed-phase high-performance liquid chromatography (RP-HPLC). The specific binding of 90Y-DOTA-GGNle-CycMSHhex was determined on B16/F10 melanoma cells. In brief, the B16/F10 cells (1 × 106 cells/tube, n = 3) were incubated at 25°C for 2 h with ∼0.037 MBq of 90Y-DOTA-GGNle-CycMSHhex with or without 10 μg (6.07 nmol) of unlabeled [Nle4, D-Phe7]-α-MSH (NDP-MSH) in 0.3 mL of binding medium [Modified Eagle's medium with 25 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline]. After the incubation, the cells were rinsed three times with 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M PBS and measured in a Wallac 1480 automated γ counter (PerkinElmer, NJ).
Internalization and efflux of 90Y-DOTA-GGNle-CycMSHhex
Cellular internalization and efflux of 90Y-DOTA-GGNle-CycMSHhex were evaluated on B16/F10 melanoma cells. The B16/F10 cells (3 × 105/well) were seeded onto a 24-well cell culture plate and incubated at 37°C overnight. After being washed once with binding medium, the cells were incubated at 25°C for 20, 40, 60, 90, and 120 min (n = 3) in the presence of ∼130,000 counts per minute of HPLC purified 90Y-DOTA-GGNle-CycMSHhex. After incubation, the cells were rinsed with 2 × 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M PBS. Cellular internalization of 90Y-DOTA-GGNle-CycMSHhex was assessed by washing the cells with acidic buffer (40 mM sodium acetate [pH 4.5] containing 0.9% NaCl and 0.2% BSA) to remove the membrane-bound radioactivity. The remaining internalized radioactivity was obtained by lyzing the cells with 0.5 mL of 1 N NaOH for 5 min. Membrane-bound and internalized 90Y activity was counted in a γ counter. Cellular efflux of 90Y-DOTA-GGNle-CycMSHhex was determined by incubating cells with 90Y-DOTA-GGNle-CycMSHhex at 25°C for 2 h, removing nonspecific bound activity with 2 × 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M PBS rinse, and monitoring radioactivity released into cell culture medium. At time points of 20, 40, 60, 90, and 120 min, the radioactivity on cell surface and in cells was separately collected and counted in a γ counter.
MC1R staining on B16/F10 melanoma cells and lesions
The B16/F10 cells (1 × 105 cells/well) were seeded onto a 4-well Lab-Tek Chamber Glass Slide System (Thermo Scientific, MA) and incubated at 37° overnight. After 24 h, the cells were fixed with 4% PFA in PBS and incubated at room temperature for 15 min, washed with PBS three times, treated with 0.5% Triton X-100 at room temperature for 15 min, and washed with PBS three times. The cells were incubated with MC1R antibody (1:300 dilution) at room temperature for 1 h followed by PBS wash three times, then incubated with FITC-conjugated antirabbit secondary antibody (1:100 dilution) at room temperature for 30 min followed by PBS wash three times. The cells were stained for nuclei and mounted with DAPI Fluoromount-G mounting medium (SouthernBiotech) and stayed in the dark at room temperature for 24 h. The fluorescent signal was observed and recorded at 100 × magnification under an Olympus FV1000 confocal microscope.
All animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. B16/F10 flank melanoma-bearing mice were generated for MC1R staining and biodistribution studies. In brief, each C57 mouse was subcutaneously inoculated with 1 × 106 B16/F10 cells on the right flank. Ten days postinoculation, the tumor weights reached ∼0.2 g. The B16/F10 tumor was used to generate paraffin-embedded tumor sections (5 μm thickness) for MC1R staining. The paraffin-embedded tumor sections were deparaffinized with xylene first, and incubated with MC1R antibody (1:300 dilution) at room temperature for 1 h followed by PBS wash three times, then incubated with FITC-conjugated antirabbit secondary antibody (1:100 dilution) at room temperature for 30 min followed by PBS wash three times. Tissue samples were stained and mounted with Prolong Diamond antifade mounting reagent with DAPI (Life Technologies). The fluorescent signal was observed and recorded at 100 × magnification under an Olympus FV1000 confocal microscope.
Biodistribution and bremsstrahlung imaging of 90Y-DOTA-GGNle-CycMSHhex
The biodistribution property of 90Y-DOTA-GGNle-CycMSHhex was determined on B16/F10 flank melanoma-bearing C57 mice (Charles River, Wilmington, MA). Each melanoma-bearing mouse was injected with 0.037 MBq of 90Y-DOTA-GGNle-CycMSHhex through the tail vein. Mice were sacrificed at 0.5, 2, 4, and 24 h postinjection, and tumors and organs of interest were harvested, weighed, and counted. Blood values were taken as 6.5% of the whole-body weight. The specificity of the tumor uptake of 90Y-DOTA-GGNle-CycMSHhex was determined by coinjecting 10 μg (6.07 nmol) of unlabeled NDP-MSH, which is a linear α-MSH peptide analog with subnanomolar MC1R binding affinity.
We were interested whether B16/F10 melanoma lesions could be imaged by single photon emission computed tomography (SPECT) by collecting 90Y bremsstrahlung photons. As an exploratory effort, we examined the bremsstrahlung imaging property of 90Y-DOTA-GGNle-CycMSHhex on B16/F10 flank melanoma-bearing C57 mice using a small energy window of 126.5–155.7 keV. Each melanoma-bearing mouse was injected with 7.4 MBq of 90Y-DOTA-GGNle-CycMSHhex through the tail vein. SPECT imaging study was performed at 2 h postinjection. CT data were collected followed by SPECT data acquisition. Reconstructed SPECT/CT data were visualized using Vivoquant (Invicro, Boston, MA).
Results
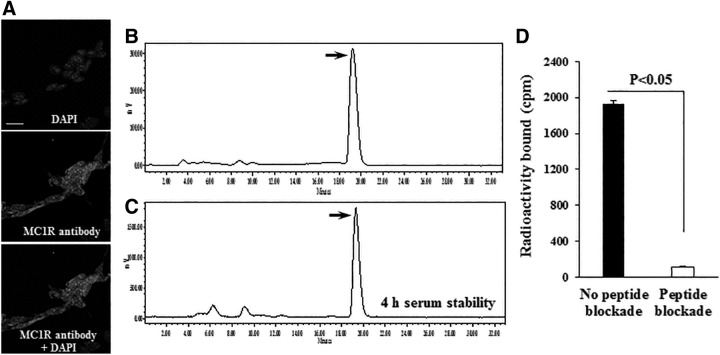
90Y-DOTA-GGNle-CycMSHhex (Fig. 1) was readily prepared with >95% radiolabeling yield, and was completely separated from its excess nonlabeled peptide by RP-HPLC. The retention time of 90Y-DOTA-GGNle-CycMSHhex and DOTA-GGNle-CycMSHhex was 19.3 and 17.1 min, respectively. The specific activity of 90Y-DOTA-GGNle-CycMSHhex was ∼4.8962 × 104 mCi/μmol. 90Y-DOTA-GGNle-CycMSHhex was stable in mouse serum at 37°C for 4 h (Fig. 2). 90Y-DOTA-GGNle-CycMSHhex displayed receptor-mediated binding on B16/F10 cells. Approximately 94% of 90Y-DOTA-GGNle-CycMSHhex uptake was blocked on B16/F10 cells (p < 0.05) (Fig. 2). The MC1R expression on B16/F10 cells was examined through fluorescence staining using MC1R antibody. As shown in Figure 2, MC1R-binding antibody showed substantial binding on B16/F10 cells.
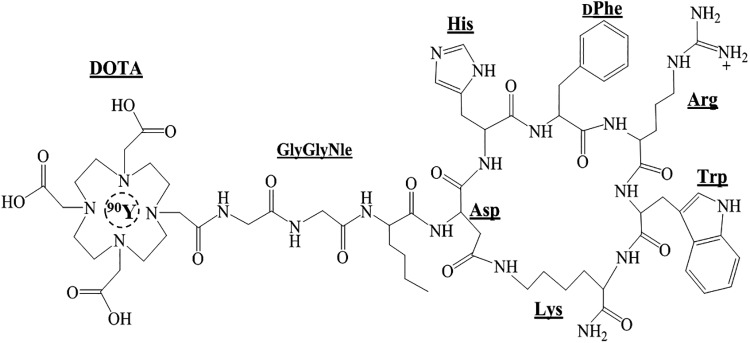
FIG. 1.
Schematic structure of 90Y-DOTA-GGNle-CycMSHhex.
FIG. 2.
Fluorescence staining of MC1Rs on B16/F10 melanoma cells (A) using MC1R antibody (white). The nuclei were stained with DAPI (grey). The microscopic images were acquired by confocal laser microscopy at 100 × magnification. Scale bar, 20 μm. Radioactive HPLC profile of 90Y-DOTA-GGNle-CycMSHhex (B, TR = 19.3 min) and its mouse serum stability (C) after 4 h incubation at 37°C. Arrows indicate the original compound of 90Y-DOTA-GGNle-CycMSHhex. Specific binding of 90Y-DOTA-GGNle-CycMSHhex on B16/F10 (D) cells with or without peptide blockade. DAPI, 4′,6-diamidino-2-phenylindole; HPLC, high-performance liquid chromatography; MC1R, melanocortin-1 receptor.
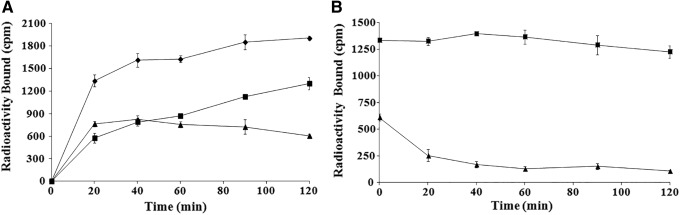
The cellular internalization and efflux of 90Y-DOTA-GGNle-CycMSHhex on 16/F10 cells are presented in Figure 3. 90Y-DOTA-GGNle-CycMSHhex exhibited rapid cellular internalization on B16/F1 cells. Approximately 49% and 68% of 90Y-DOTA-GGNle-CycMSHhex activity were internalized in the B16/F10 cells after 40 min and 2 h incubation, respectively. Cellular efflux of 90Y-DOTA-GGNle-CycMSHhex demonstrated that 92% of the 90Y activity remained inside the B16/F10 cells 2 h after incubating cells in culture medium at 25°C.
FIG. 3.
Cellular internalization (A) and efflux (B) of 90Y-DOTA-GGNle-CycMSHhex on B16/F10 melanoma cells. Total bound radioactivity (♦), internalized radioactivity (▪), and cell membrane radioactivity (▴) are presented as counts per minute.
The biodistribution result of 90Y-DOTA-GGNle-CycMSHhex on B16/F10 melanoma-bearing mice is presented in Table 1. The B16/F10 tumor uptake was 20.73% ± 7.99% and 19.93% ± 5.73% ID/g at 0.5 and 2 h postinjection, respectively. 90Y-DOTA-GGNle-CycMSHhex exhibited prolonged retention in B16/F10 tumor, with 14.8% ± 4.61% and 6.69% ± 1.85% ID/g at 4 and 24 h postinjection, respectively. The coinjection of nonradioactive NDP-MSH blocked 94% of the tumor uptake at 2 h postinjection, demonstrating that the tumor uptake was MC1R mediated. As shown in Figure 4, MC1R-binding antibody displayed substantial binding on B16/F10 tumor sections. 90Y-DOTA-GGNle-CycMSHhex displayed a rapid urinary clearance, with ∼91% of the injected activity being washed out of the body by 2 h postinjection. The accumulation of 90Y-DOTA-GGNle-CycMSHhex in normal organs was <1% ID/g except in kidneys. The renal uptake was 12.68% ± 5.2%, 7.44% ± 1.85%, and 7.75% ± 1.59% ID/g at 0.5, 2, and 4 h postinjection, respectively, and decreased to 5.23% ± 1.76% ID/g at 24 h postinjection. The coinjection of NDP-MSH did not significantly reduce the renal uptake (p > 0.05), indicating that the renal uptake of 90Y-DOTA-GGNle-CycMSHhex was not receptor mediated. 90Y-DOTA-GGNle-CycMSHhex exhibited high tumor/blood and tumor/normal organ uptake ratios were demonstrated as early as 0.5 h postinjection.
Table 1.
Biodistribution of 90Y-DOTA-GGNle-CyCMSHhex on B16/F10 Murine Melanoma-Bearing C57 Mice
| Tissues | 0.5 h | 2 h | 2 h blockade | 4 h | 24 h |
|---|---|---|---|---|---|
| Percent injected dose/gram (% ID/g) | |||||
| Tumor | 20.73 ± 7.99 | 19.93 ± 5.73 | 1.20 ± 1.04a | 14.8 ± 4.61 | 6.69 ± 1.85 |
| Brain | 0.10 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.03 ± 0.02 |
| Blood | 2.76 ± 0.69 | 0.36 ± 0.15 | 0.25 ± 0.12 | 0.22 ± 0.12 | 0.06 ± 0.07 |
| Heart | 1.28 ± 0.51 | 0.14 ± 0.10 | 0.13 ± 0.05 | 0.16 ± 0.09 | 0.11 ± 0.07 |
| Lung | 3.06 ± 1.05 | 0.42 ± 0.08 | 0.19 ± 0.12 | 0.27 ± 0.05 | 0.13 ± 0.15 |
| Liver | 0.99 ± 0.19 | 0.63 ± 0.14 | 0.39 ± 0.12 | 1.20 ± 1.34 | 0.60 ± 0.20 |
| Skin | 2.48 ± 1.05 | 0.78 ± 0.29 | 0.10 ± 0.12 | 0.53 ± 0.29 | 0.37 ± 0.18 |
| Spleen | 1.4 ± 1.36 | 0.24 ± 0.17 | 0.15 ± 0.12 | 0.31 ± 0.17 | 0.53 ± 0.42 |
| Stomach | 1.36 ± 0.4 | 0.87 ± 0.47 | 0.15 ± 0.06 | 0.61 ± 0.27 | 0.76 ± 0.52 |
| Kidneys | 12.68 ± 5.2 | 7.44 ± 1.85 | 4.99 ± 0.69 | 7.75 ± 1.59 | 5.23 ± 1.76 |
| Muscle | 0.73 ± 0.51 | 0.19 ± 0.13 | 0.10 ± 0.10 | 0.22 ± 0.31 | 0.15 ± 0.14 |
| Pancreas | 0.61 ± 0.43 | 0.18 ± 0.05 | 0.06 ± 0.07 | 0.14 ± 0.09 | 0.16 ± 0.14 |
| Bone | 1.37 ± 0.44 | 0.41 ± 0.18 | 0.15 ± 0.14 | 0.44 ± 0.34 | 0.2 ± 0.04 |
| Percent injected dose (% ID) | |||||
| Intestines | 1.42 ± 0.38 | 0.57 ± 0.22 | 0.33 ± 0.15 | 1.36 ± 1.16 | 1.92 ± 3.51 |
| Urine | 75.42 ± 6.72 | 90.62 ± 1.79 | 96.77 ± 0.37 | 89.45 ± 4.25 | 93.01 ± 4.43 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/blood | 7.51 | 55.36 | 4.8 | 67.27 | 111.5 |
| Tumor/kidney | 1.63 | 2.68 | 0.24 | 1.91 | 1.28 |
| Tumor/lung | 6.77 | 47.45 | 6.32 | 54.81 | 51.46 |
| Tumor/liver | 20.94 | 31.63 | 3.08 | 12.33 | 11.15 |
| Tumor/muscle | 28.4 | 104.89 | 12.0 | 67.27 | 44.6 |
The data were presented as percentage injected dose/gram or as percentage injected dose (mean ± SD, n = 5).
p < 0.05 for determining significance of differences in tumor and kidney uptake between 90Y-DOTA-GGNle-CyCMSHhex with or without peptide blockade at 2 h postinjection.
FIG. 4.
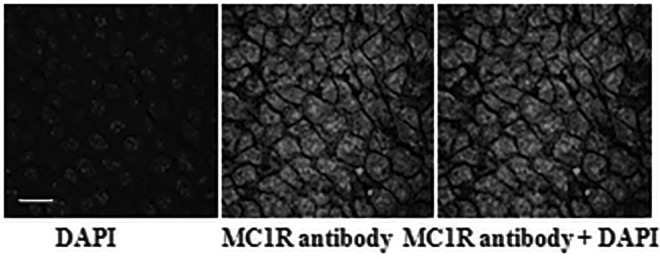
Fluorescence staining of MC1Rs on B16/F10 tumor section using MC1R antibody (white). The nuclei were stained with DAPI (grey). The microscopic images were acquired by confocal laser microscopy at 100 × magnification. Scale bar, 20 μm. DAPI, 4′,6-diamidino-2-phenylindole; MC1R, melanocortin-1 receptor.
The representative maximum intensity projection bremsstrahlung SPECT image of B16/F10 melanoma-bearing mouse is presented in Figure 5. The B16/F10 flank melanoma lesions could be visualized by collecting bremsstrahlung photons from 90Y-DOTA-GGNle-CycMSHhex at 2 h postinjection. However, as shown in Figure 5, the scattered photons over the body were substantially collected by SPECT, thus decreasing the contrast of tumor to normal organ.
FIG. 5.
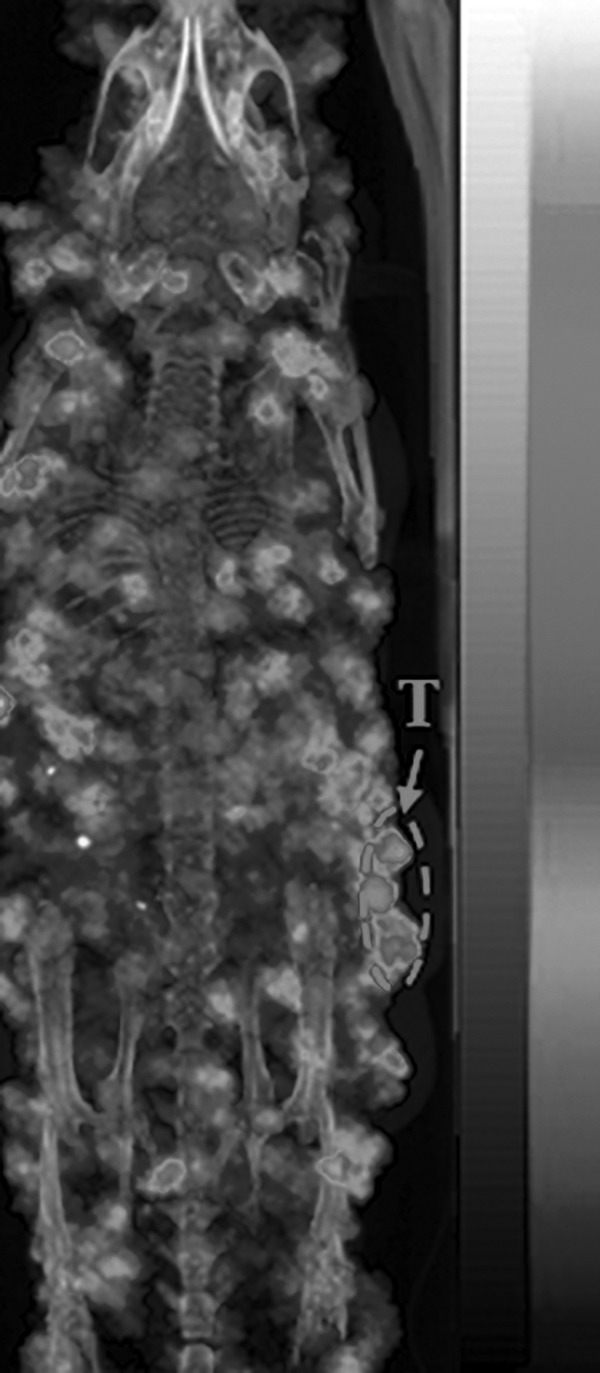
Representative maximum intensity projection Bremsstralung SPECT/CT image of a B16/F10 flank melanoma-bearing C57 mouse using 90Y-DOTA-GGNle-CycMSHhex as an imaging probe at 2 h postinjection. Melanoma lesions are highlighted with an arrow on the image. white and black indicate high and low activity on the scale bar, respectively. SPECT, single photon emission computed tomography.
Discussion
The remarkable first-in-human images of patients with metastatic melanomas highlighted the clinical relevance of MC1R as a molecular target for melanoma imaging and therapy.13 We have been interested in developing MC1R-targeted therapeutic peptides for melanoma therapy.14,16 Both 177Lu and 90Y are attractive β-emitters with different half-lives and β-energy levels. 177Lu has a half-life of 6.7 days with low-energy β-particles (0.479 MeV), whereas 90Y has a half-life of 2.7 days with high-energy β-particles (2.3 MeV). Meanwhile, 177Lu also emits γ-rays (113 and 208 keV) that can be used for imaging, while 90Y is a pure β-emitter. We previously reported promising melanoma-targeting and imaging properties of 177Lu-DOTA-GGNle-CycMSHhex.14 In this study, we examined the biodistribution of 90Y-DOTA-GGNle-CycMSHhex on B16/F10 melanoma-bearing C57 mice. We selected B16/F10 melanoma cells for this study because they are highly metastatic and can readily form pulmonary melanoma metastases when injected into the tail veins of C57 mice.17 Favorable melanoma-targeting property of 90Y-DOTA-GGNle-CycMSHhex will underscore the potential of utilizing DOTA-GGNle-CycMSHhex to deliver both 177Lu and 90Y to address the tumor size and burden when needed.
90Y-DOTA-GGNle-CycMSHhex displayed MC1R-specific binding, and exhibited rapid cellular internalization and prolonged efflux on B16/F10 melanoma cells. The change of radionuclide from 177Lu to 90Y maintained similar melanoma-targeting property. For instance, the tumor uptake of 90Y-DOTA-GGNle-CycMSHhex was 20.73% ± 7.99% and 19.93% ± 5.73% ID/g at 0.5 and 2 h postinjection, respectively, whereas the tumor uptake of 177Lu-DOTA-GGNle-CycMSHhex was 20.25% ± 4.59% and 20.63% ± 6.27% ID/g at 0.5 and 2 h postinjection, respectively. Meanwhile, 90Y-DOTA-GGNle-CycMSHhex and 177Lu-DOTA-GGNle-CycMSHhex displayed similar fast urinary clearance. The accumulation of both peptides was low in normal organs except in kidneys that could be potential dose-limiting organs in melanoma therapy studies. Nonetheless, the rapid accumulation and prolonged retention of 90Y-DOTA-GGNle-CycMSHhex highlighted its potential for melanoma therapy in future studies.
Both 90Y-DOTA-Re(Arg11)CCMSH and 90Y-DOTA-Re(Glu2, Arg11)CCMSH were reported to target MC1Rs for potential melanoma therapy.18,19 Their B16/F1 melanoma uptake was 25.7% ± 4.64% and 11.71% ± 1.32% ID/g at 2 h postinjection, respectively. Although the tumor uptake of 90Y-DOTA-Re(Glu2, Arg11)CCMSH was lower than that of 90Y-DOTA-Re(Arg11)CCMSH, 90Y-DOTA-Re(Glu2, Arg11)CCMSH displayed higher tumor to kidney uptake ratio than 90Y-DOTA-Re(Arg11)CCMSH. Interestingly, 90Y-DOTA-GGNle-CycMSHhex exhibited the highest tumor to kidney uptake ratio (2.68 at 2 h postinjection) among these peptides because of its high tumor uptake and low renal uptake. The improved tumor to kidney uptake ratio of 90Y-DOTA-GGNle-CycMSHhex would potentially deliver more radiation to tumor without increasing the radiation to kidneys.
The melanoma lesions could be clearly visualized by SPECT using 177Lu-DOTA-GGNle-CycMSHhex as an imaging agent due to the γ-rays from 177Lu.14 Although 90Y is a pure β-emitter, Bremsstrahlung photons could be produced by interaction of the β-particles of 90Y with tissue. Thus, both Bremsstrahlung SPECT and PET of 90Y have been investigated and reported in the literature.20–23 In this study, we examined the Bremsstrahlung SPECT of 90Y-DOTA-GGNle-CycMSHhex on melanoma-bearing mice using a small energy window of 126.5–155.7 keV. Although the flank melanoma lesions could be visualized by collecting bremsstrahlung photons from 90Y-DOTA-GGNle-CycMSHhex, the presence of scattered photons in the image resulted in degradation in contrast to tumor to normal organ. The challenge in imaging 90Y using SPECT is due to its continuous Bremsstrahlung spectrum with energies up to 2.3 MeV and the absence of a photopeak, making the traditional small energy window-based scatter rejection ineffective.
Yttrium-86 is an attractive PET radionuclide that can be produced by a cyclotron through the 86Sr (p,n) 86Y reaction. It has a half-life of 14.7 h and can serve as an imaging surrogate for 90Y to form a true matched-pair theranostic radionuclide. 86Y-DOTA-ReCCMSH(Arg11) has been developed to target MC1Rs for melanoma imaging. It exhibited rapid high B16/F1 melanoma uptake (11.87% ± 3.31% ID/g at 0.5 h postinjection) and low accumulation in normal organs expect in kidneys.24 The PET images of melanoma-bearing mice using 86Y-DOTA-ReCCMSH(Arg11) clearly demonstrated its melanoma imaging potential due to its high tumor concentration and low nontarget tissue accumulation. However, the availability of 86Y can potentially be a limiting factor since it is not a commercial radionuclide. Alternatively, 68Ga-DOTA-GGNle-CycMSHhex can be used to monitor the response when examining the therapeutic efficacy of 90Y-DOTA-GGNle-CycMSHhex in the future.
Conclusions
90Y-DOTA-GGNle-CycMSHhex displayed MC1R targeting and specificity on B16/F10 melanoma cells and tumors. The favorable melanoma-targeting property and fast urinary clearance of 90Y-DOTA-GGNle-CycMSHhex warranted its evaluation for melanoma therapy in future studies.
Acknowledgment
We thank Dr. Fabio Gallazzi for his technical assistance.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported in part by the NIH grant R01CA225837 and University of Colorado Denver start-up fund. Microscopy imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin 2019;69:7. [DOI] [PubMed] [Google Scholar]
- 2. Chapman PB, Hauschild A, Robert C, et al. ; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950. [DOI] [PubMed] [Google Scholar]
- 6. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatro JB, Wen Z, Entwistle ML, et al. Interaction on an α-melanocyte stimulating hormone-diptheria toxin fusion protein with melanotropin receptors in human metastases. Cancer Res 1992;52:2545. [PubMed] [Google Scholar]
- 8. Siegrist W, Solca F, Stutz S, et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res 1989;49:6352. [PubMed] [Google Scholar]
- 9. Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology 1987;121:1900. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Cheng Z, Hoffman TJ, et al. Melanoma-targeting properties of 99mTechnetium-labeled cyclic α-melanocyte-stimulating hormone peptide analogues. Cancer Res 2000;60:5649. [PubMed] [Google Scholar]
- 11. Miao Y, Whitener D, Feng W, et al. Evaluation of the human melanoma targeting properties of radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Bioconjug Chem 2003;14:1177. [DOI] [PubMed] [Google Scholar]
- 12. Guo H, Shenoy N, Gershman BM, et al. Metastatic melanoma imaging with an 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide. Nucl Med Biol 2009;36:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Xu J, Gonzalez R, et al. 68Ga-DOTA-GGNle-CycMSHhex targets the melanocortin-1 receptor for melanoma imaging. Sci Transl Med 2018;10:eaau4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo H, Miao Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg Med Chem Lett 2013;23:2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo H, Yang J, Gallazzi F, et al. Effects of the amino acid linkers on melanoma-targeting and pharmacokinetic properties of indium-111-labeled lactam bridge-cyclized α-MSH peptides. J Nucl Med 2011;52:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miao Y, Shelton T, Quinn TP. Therapeutic efficacy of a 177Lu labeled DOTA conjugated α-melanocyte stimulating hormone peptide in a murine melanoma-bearing mouse model. Cancer Biother Radiopharm 2007;22:333. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Xu J, Cheuy L, et al. Novel Pb-203-labeled lactam-cyclized alpha-melanocyte-stimulating hormone peptide for melanoma imaging. Mol Pharm 2019;16:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miao Y, Hoffman TJ, Quinn TP. Tumor targeting properties of 90Y and 177Lu labeled alpha-melanocyte stimulating hormone peptide analogues in a murine melanoma model. Nucl Med Biol 2005;32:485. [DOI] [PubMed] [Google Scholar]
- 19. Miao Y, Fisher DR, Quinn TP. Reducing renal uptake of 90Y and 177Lu labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl Med Biol 2006;33:723. [DOI] [PubMed] [Google Scholar]
- 20. Elschot M, Vermolen BJ, Lam M, et al. Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS One 2013;8:e55742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porter CA, Bradley KM, Hippeläinen ET, et al. Phantom and clinical evaluation of the effect of full Monte Carlo collimator modelling in post-SIRT yttrium-90 Bremsstrahlung SPECT imaging. EJNMMI Res 2018;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yue J, Mauxion T, Reyes DK, et al. Comparison of quantitative Y-90 SPECT and non-time-of-flight PET imaging in post-therapy radioembolization of liver cancer. Med Phys 2016;43:5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barber TW, Cherk MH, Powell A, et al. Correlation of clinical outcomes with bremsstrahlung and Y-90 PET/CT imaging findings following Y-90 radiosynoviorthesis: A prospective study. EJNMMI Res 2016;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McQuade P, Miao Y, Yoo J, et al. Imaging of melanoma using 64Cu and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of (-MSH. J Med Chem 2005;48:2985. [DOI] [PubMed] [Google Scholar]