Abstract
Objective: To determine quantitative parameters of dermal wound healing senescence in aged BALB/cByJ mice (an important animal model of aging) and to evaluate the potential for therapeutic intervention by fibroblast growth factor-1 (FGF-1).
Approach: Utilize a novel noninvasive fine-sampled photographic methodology to quantify wound healing parameters for healing phases from wounding through to wound closure.
Results: Parameters associated with key healing phases were quantified and compared between nonaged and aged cohorts of both genders. The results identify a sexual dimorphism in dermal wound healing, with nonaged females exhibiting a greater overall healing efficiency than males. This enhanced healing in females, however, senesces with age such that healing parameters for aged males and females are statistically indistinguishable. Topical application of FGF-1 was identified as an effective therapeutic intervention to treat dermal healing senescence in aged females.
Innovation: The FGF intervention is being analyzed using a new recently published model. This approach significantly increases the amount of preclinical animal data obtainable in wound healing studies, minimizes cohort number compared with (lethal) histological studies, and permits a direct statistical comparison between different healing studies.
Conclusion: Quantitative parameters of dermal wound healing, obtained from noninvasive fine-sampled photographic data, identify topical FGF-1 as an effective therapeutic to treat the senescence of dermal healing present in aged female BALB/cByJ mice.
Keywords: animal model, inflammatory phase, proliferative phase, sexual dimorphism, splinted excision
Michael Blaber, PhD.

Introduction
Aging is associated with a general impairment of wound healing,1–5 and chronic nonhealing dermal ulcers in the growing elderly population are a major clinical and economic burden of increasing magnitude.6–10 The development of novel therapeutics to effectively promote dermal wound healing in aged patients is of vital importance to the improvement of health and quality of life, and to address the increasing economic burden associated with nonhealing dermal wounds in the aged population. Gender and age comprise two important risk factors for altered drug exposure and response,11 and male and female sex hormones have diverse and potentially opposite effects upon dermal wound healing.12–15 Age- and gender-related differences in wound healing, and the response to therapeutic intervention, are, therefore, important to elucidate early in preclinical animal models of efficacy in the therapeutic development pipeline.
We report quantitative parameters of wound healing for cohorts of nonaged (2–3 months) and aged (10–14 months), male and female, BALB/cByJ mice. BALB/cByJ is a commonly utilized inbred mouse strain for aging studies; however, detailed and quantitative analyses of dermal wound healing have yet to be reported. The surgical wound procedure utilized is the splinted excisional wound, which minimizes the skin contraction property of “loose-skinned” rodents, and more faithfully represents the primacy of re-epithelialization characteristic of human dermal healing.16,17 The quantitation of wound healing utilizes a recently developed mathematical model of healing,18 utilizing fine-sampled (i.e., daily) photographic wound healing data, and extracts substantially greater quantitative information compared with previous methodologies. This analysis permits quantitative determination and comparison of key wound healing parameters, including duration of the initial latent phase of healing (associated with hemostasis and inflammatory phases), wound radius during the stasis phase, duration of the subsequent active proliferative phase (Tp) of healing, rate of wound healing during the active phase, and overall time to wound closure (Tc).
We report the effects of aging upon wound healing for male and female 10- to 14-month-old “retired breeders” compared with 2- to 3-month-old mature nonaged controls. In human terms, 10- to 14-month-old mice are considered “middle aged,”19 and demonstrate reproductive senescence reducing both litter frequency and size. The results demonstrate an age-related impairment of dermal wound healing in female mice, whereas male mice show little if any age-related impairment. In this regard, the nonaged male, aged male, and aged female cohorts exhibit similar overall wound healing parameters, whereas nonaged females exhibit uniquely enhanced healing parameters. Also characterized are the effects of topical fibroblast growth factor-1 (FGF-1) upon aged male and female dermal healing parameters. The specific cell types required for healing of skin, and accompanying growth of new blood vessels, include dermal fibroblasts, vascular endothelial cells, and epidermal keratinocytes. FGF-1 has been demonstrated to stimulate mitosis and migration of each of these cell types20–22; thus, FGF-1 is potentially an effective single therapeutic agent for stimulating dermal healing.23 The results identify a significant response to topical FGF-1 in aged females but not in aged males. This response in aged females effectively reverses the age-related impairment in dermal healing and recovers the enhanced time to wound closure exhibited by nonaged females.
Clinical Problem Addressed
Animal studies of efficacy are a key component in the development pipeline of human therapeutics. One guiding principle for such studies is the maximization of quantitative data obtained, thereby enabling the smallest effective cohort. In addition, establishment of a standard methodology for quantitation permits wound healing parameters from different studies to be directly compared. The present report describes the application of a novel mathematical model of dermal wound healing, utilizing noninvasive fine-sampled photographic data, to robustly determine key wound healing parameters to evaluate the effects of both age and gender in BALB/cByJ mice (a “standard” rodent strain for aging studies). In addition, therapeutic intervention of FGF-1 in aged mice is also quantified. The methodology enables a robust statistical comparison of wound healing parameters between these cohorts using a limited cohort of animals.
Materials and Methods
Animal Care and Use Committee approval
All procedures involving animals were approved by the Florida State University Animal Care and Use Committee (Protocol No. 1637) and the welfare of all animals was supervised by a staff veterinarian.
Mouse strain selection
The National Institute of Aging (NIA) maintains aged rodent colonies for aging studies, including C57BL/6 and BALB/cByJ mice. These two strains are thus de facto “standard” mice for aging studies; however, detailed dermal wound healing data are lacking. In this regard, C57BL/6 exhibits a spontaneous, genetically based, and age-related severely pruritic ulcerative dermatitis, which can confound dermal healing studies.24 The BALB/cByJ strain does not develop such dermatitis, and is, therefore, a more appropriate strain for dermal studies in aging. BALB/cByJ mice were purchased from The Jackson Laboratory (JAX), the sole commercial source for this strain. BALB/cByJ male mice reach puberty by 5 weeks of age and females by 8 weeks; males have an average lifespan of 664 ± 202 days and females 734 ± 154 days.25 Controls included sexually mature male and female mice between 2 and 3 months of age (referred throughout as “nonaged”). Although JAX does not maintain an aged BALB/cByJ colony, male and female retired breeders of 7–9 months of age are available. Retired breeders were purchased and housed until they were between 10 and 14 months of age (300–420 days). Ten- to 14-month-old mice are considered “middle-aged,” with an approximate human equivalent age of 38–47 years.19 This age range exhibits an onset of senescent changes including increases in WBC count, monocyte differential, eosinophil count, plasma albumin, as well as a general reduction in plasma lipase, serum iron, serum thyroxine, whole body bone area, and body mass index.25–27 Furthermore, as retired breeders, these mice exhibit a reproductive senescence associated with both reduced frequency and size of litters. The 10- to 14-month-old male and female BALB/cByJ mice are thus referred throughout as “aged.” Mice were housed in a temperature-controlled facility (23°C) with a 12 h light/dark cycle, with water and food provided ad libitum, and nesting material provided. Mice were fed LabDiet® 5001 standard rodent chow containing 4.5% fat by weight. Identifying numbers were tattooed on the tail.
FGF-1 protein
A recombinant 141 amino acid form of human FGF-1 protein was expressed from Escherichia coli and purified to apparent homogeneity as previously described.28 This protein was buffer exchanged against phosphate-buffered saline (PBS) pH 7.4 and sterile filtered through a 0.2 μm syringe filter. This purified protein was subsequently formulated with 3 × mass heparin sulfate (HS) (also suspended in sterile filtered PBS) and diluted with sterile PBS to a protein concentration of 0.1 mg/mL (and heparin concentration of 0.3 mg/mL).
Splinted excisional wound model and dosing of FGF-1
The splinted excisional surgical model17,29 followed a previously published methodology.30 In brief, two 6 mm diameter full-thickness excisional wounds were introduced by biopsy punch on the upper dorsal region. These wounds were splinted using 7.92 mm inside diameter (ID) 14.27 mm outside diameter (OD) silicone washers affixed with eight radial sutures. After suturing, wounds were treated with 5.6 μL of sterile vehicle (PBS buffer) or sterile filtered recombinant FGF-1 formulated with 3 × HS (referred to as “FGF/HS” hereafter) at 2 μg/cm2 wound area (i.e., 5.6 μL of 0.1 mg/mL FGF/HS for a nominal wound area of 0.28 cm2). This dose approaches a practical maximum due to excessive bleeding being caused at higher dosing by the added HS.30 Wounds were covered using Tegaderm® transparent bioclusive dressing (3M Healthcare, St. Paul, MN). A novel mouse jacket specifically developed to protect the wound region was utilized.31 Mice were redosed on days 3, 7, and 10 postsurgery (necessitating bandage replacement). Redosing on day 10 utilized 2.5 μL FGF/HS or PBS vehicle in an effort to maintain an approximately constant volume/wound area ratio with typically decreased wound size at this day.
Fine-sampled photographic data of wound healing
A daily photographic record of wound healing followed a previously published methodology.30 In the workflow, daily photographic data were analyzed to obtain wound area, and from this the wound radius. These data (i.e., wound radius as a function of time) were then fit to a mathematical model18 (see Equation 1 below) by a nonlinear least squares routine—generating the fitted parameters. Macrophotography with a DSLR camera was utilized to achieve a full-frame composition of the wound onto the 6 MP CCD sensor (i.e., permitting the maximum countable pixels for the wound). Thus, wound sizes down to 0.1% of the original area (i.e., constituting ∼600 measurable pixels) could be accurately measured. Quantification of the wound area utilized ImageJ software.32 Horizontal and vertical size references to the nominal 7.92 mm ID of the splint were used to calibrate the image dimensions and account for potential skew. The wound epithelial border is readily delineated against the underlying fascia/granulation tissue with such imaging (for detailed examples of such photographic analyses, Blaber et al.30). The vertical and horizontal normalized area calculations were averaged to define the individual wound area. An equivalent wound radius was calculated as (area/π)0.5. Wound measurement was performed by three independent analysts and the results were averaged (analysts were not blinded to the particular cohort). Mice were photographed daily until postoperative day 18, and then every other day until wound closure.
Wound healing model and statistical analysis
Wound radius as a function of time R(t) was fit to a piecewise linear function as described by Blaber and colleagues.18 This model parameterizes the initial wound radius R0, the initial period of wound stasis (covering the hemostasis and inflammatory phases) Ti, and subsequent active proliferative phase of healing ending in the time to wound closure Tc:
 |
Derived parameters of this model include the duration of the active proliferative phase Tp = Tc − Ti, as well as the healing rate (HR) during the proliferative phase HR = R0/Tp (Fig. 1). The s parameter is a scaling parameter describing the curvature of the intersection of the initial wound stasis and active proliferative healing phases. The model parameters are largely insensitive to the value of s, which is fixed at 0.05 for all analyses. The initial period of wound stasis identified by photographic data is consistent with the hemostasis and inflammatory phases of healing; the subsequent proliferative phase identified by photographic data is consistent with the proliferative phase of healing.18 Fitting of experimental photographic data (i.e., measured wound radius) to this function was accomplished by an independent analyst using the nonlinear least squares fitting software DataFit (Oakdale Engineering, Oakdale, PA). Coefficients of determination R2 for the fit were also calculated using DataFit. Statistical p-values for the comparison of wound healing parameters between cohorts were calculated using the statistical analysis server at www.socscistatistics.com using a two-tailed model for two independent means.
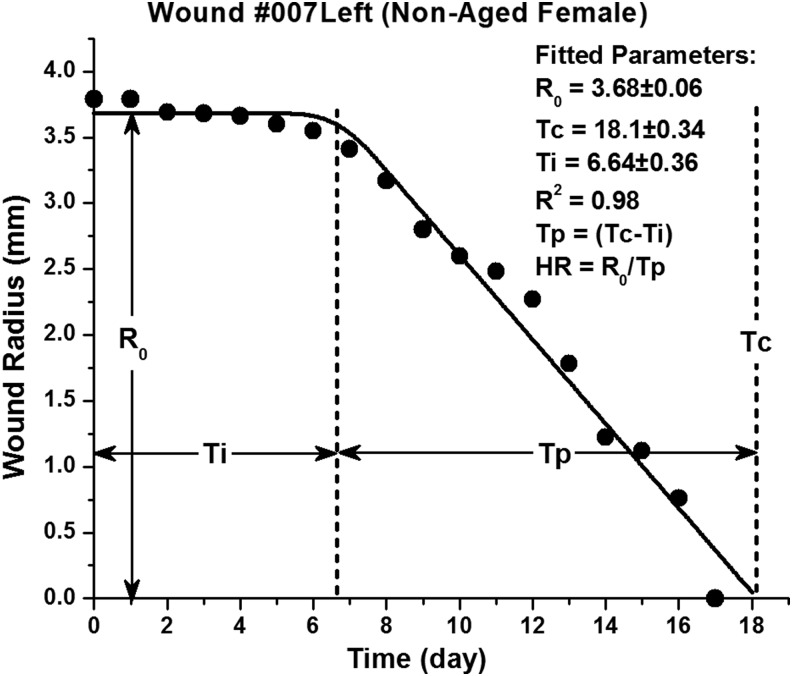
Figure 1.
Example fitting of a single wound data set to the wound model. The wound healing model is a piece-wise linear function describing an initial wound stasis phase Ti corresponding to the hemostasis and inflammatory phases, having constant wound radius R0, followed by an active proliferative phase Tp with constant HR until wound closure Tc.18 HR, healing rate.
Study cohort
Overall, the study included six cohorts: (1) mature nonaged males, (2) mature nonaged females, (3) aged males, (4) aged females, (5) aged males with FGF-1 intervention, and (6) aged females with FGF-1 intervention. Each cohort comprised 10 individuals on day 0 (surgery), and 2 wounds were introduced per animal (for 20 wounds total per cohort, day 0). Animal losses, due to death or infection, led to a modest reduction in the final number of wounds per cohort (see the Results section below).
Results
The overall animal survival to wound closure, for all cohorts, was ∼75%. Three mice in the aged male cohort died before surgery, and were replaced. Animal survival of surgery was excellent, with no loss of animals on day 0. Deaths before wound closure comprised ∼15% of each cohort, with a higher apparent rate among aged males. A total of 10 animals (∼8% of the total and approximately equally distributed among all cohorts) exhibited wounds that were nonhealing, with a copious yellow exudate. These were interpreted as infection, and animals were euthanized accordingly; overall, however, aseptic surgical technique was largely achieved. Two animals exhibited signs of pain, and were euthanized accordingly. In a few cases, visual inspection at the time of bandage replacement (i.e., during dosing on days 3, 7, or 10) identified loss of suture integrity for one wound; such wounds were omitted from analysis due to wound contraction. One animal escaped from the protective jacket and damaged the splint sutures. Overall, a wound sample number of n ≥ 14 was achieved for each cohort (Table 1).
Table 1.
BALB/cByJ mice cohort and average wound healing parameters
| Cohort | Numbera(n) | Age (day) | R0(mm) | Tc(day) | Ti(day) | HR (mm/day) | Tp(day) | R2b |
|---|---|---|---|---|---|---|---|---|
| Nonaged malec | 15 | 101 ± 3 | 3.48 ± 0.19 | 22.3 ± 3.3 | 6.0 ± 1.8 | 0.224 ± 0.055 | 16.4 ± 3.7 | 0.98 ± 0.01 |
| Nonaged femalec | 18 | 112 ± 4 | 3.24 ± 0.25 | 17.0 ± 2.3 | 5.1 ± 1.4 | 0.278 ± 0.046 | 11.9 ± 1.7 | 0.97 ± 0.02 |
| Aged male | 15 | 309 ± 3 | 3.48 ± 0.16 | 24.1 ± 7.1 | 7.3 ± 3.8 | 0.236 ± 0.092 | 16.8 ± 6.4 | 0.97 ± 0.01 |
| Aged female | 14 | 307 ± 1 | 3.62 ± 0.21 | 22.4 ± 5.5 | 6.9 ± 2.5 | 0.249 ± 0.060 | 15.4 ± 4.1 | 0.98 ± 0.02 |
| Aged male+FGFd | 15 | 383 ± 36 | 3.61 ± 0.14 | 27.4 ± 2.5 | 6.2 ± 2.3 | 0.172 ± 0.020 | 21.2 ± 2.6 | 0.98 ± 0.01 |
| Aged female+FGFd | 17 | 408 ± 2 | 3.63 ± 0.21 | 18.2 ± 3.6 | 6.6 ± 1.4 | 0.336 ± 0.090 | 11.6 ± 3.4 | 0.98 ± 0.02 |
Error values are reported as standard deviation.
Total number of wounds in cohort.
Average coefficient of determination R2 for model fitting to individual data sets.
Data from Cogan et al.18
FGF-1 formulated with 3 × mass heparin sulfate, dosed on days 0, 3, 7, and 10 at 2 μg/cm2.
FGF-1, fibroblast growth factor-1; HR, healing rate; R0, initial wound radius; Tc, time to wound closure; Ti, initial wound stasis phase; Tp, active proliferative phase.
Fitting of the wound data to the wound healing model yielded excellent results in all cases: the coefficient of determination R2 for the fitted model averaged ∼0.97 for all data sets, and the standard error associated with fitted parameters R0, Tc, and Ti were ≤10% in all cases. A typical fit (involving a wound from a nonaged female) is illustrated in Fig. 1. A summary of experimental cohorts and derived wound healing parameters is provided in Table 1. Age, gender, and FGF-1 treatment cohort pair-wise comparisons, with corresponding statistical p-values, are provided in Table 2, and the results are described hereunder in terms of these pair-wise comparisons.
Table 2.
Wound healing parameters and p-values for age- and gender-related cohort comparisons in BALB/cByJ mice
| Cohort | R0(mm) | Tc(day) | Ti(day) | HR (mm/day) | Tp(day) |
|---|---|---|---|---|---|
| Gender differences in dermal healing | |||||
| Nonaged | |||||
| Nonaged male | 3.48 ± 0.19 | 22.3 ± 3.3 | 6.0 ± 1.8 | 0.224 ± 0.055 | 16.4 ± 3.7 |
| Nonaged female | 3.24 ± 0.25 | 17.0 ± 2.3 | 5.1 ± 1.4 | 0.278 ± 0.046 | 11.9 ± 1.7 |
| p | 0.004 | <0.00001 | 0.148 | 0.007 | 0.00009 |
| Aged | |||||
| Aged male | 3.48 ± 0.16 | 24.1 ± 7.1 | 7.3 ± 3.8 | 0.236 ± 0.092 | 16.8 ± 6.4 |
| Aged female | 3.62 ± 0.21 | 22.4 ± 5.5 | 6.9 ± 2.5 | 0.249 ± 0.060 | 15.4 ± 4.1 |
| p | 0.038 | 0.517 | 0.267 | 0.621 | 0.318 |
| Age differences in dermal healing | |||||
| Male | |||||
| Nonaged male | 3.48 ± 0.19 | 22.3 ± 3.3 | 6.0 ± 1.8 | 0.224 ± 0.055 | 16.4 ± 3.7 |
| Aged male | 3.48 ± 0.16 | 24.1 ± 7.1 | 7.3 ± 3.8 | 0.236 ± 0.092 | 16.8 ± 6.4 |
| p | 0.750 | 0.196 | 0.424 | 0.873 | 0.335 |
| Female | |||||
| Nonaged female | 3.24 ± 0.25 | 17.0 ± 2.3 | 5.1 ± 1.4 | 0.278 ± 0.046 | 11.9 ± 1.7 |
| Aged female | 3.62 ± 0.21 | 22.4 ± 5.5 | 6.9 ± 2.5 | 0.249 ± 0.060 | 15.4 ± 4.1 |
| p | 0.00002 | 0.0009 | 0.003 | 0.134 | 0.004 |
| Effects of FGF/HS on aged mice dermal healing | |||||
| Aged male | |||||
| Aged male | 3.48 ± 0.16 | 24.1 ± 7.1 | 7.3 ± 3.8 | 0.236 ± 0.092 | 16.8 ± 6.4 |
| Aged male+FGF | 3.61 ± 0.14 | 27.4 ± 2.5 | 6.2 ± 2.3 | 0.172 ± 0.020 | 21.2 ± 2.6 |
| p | 0.064 | 0.282 | 0.719 | 0.073 | 0.268 |
| Aged female | |||||
| Aged female | 3.62 ± 0.21 | 22.4 ± 5.5 | 6.9 ± 2.5 | 0.249 ± 0.060 | 15.4 ± 4.1 |
| Aged female+FGF | 3.63 ± 0.21 | 18.2 ± 3.6 | 6.6 ± 1.4 | 0.336 ± 0.090 | 11.6 ± 3.4 |
| p | 0.682 | 0.011 | 0.266 | 0.004 | 0.008 |
| Nonaged females vs. aged females+FGF/HS | |||||
| Nonaged female | 3.24 ± 0.25 | 17.0 ± 2.3 | 5.1 ± 1.4 | 0.278 ± 0.046 | 11.9 ± 1.7 |
| Aged female+FGF | 3.63 ± 0.21 | 18.2 ± 3.6 | 6.6 ± 1.4 | 0.336 ± 0.090 | 11.6 ± 3.4 |
| p | 0.00002 | 0.274 | 0.006 | 0.025 | 0.730 |
Two-tailed hypothesis for two independent means.
HS, heparin sulfate.
Nonaged male versus nonaged female
A comparison of nonaged male and female cohorts shows that females exhibit an active proliferative phase Tp that is 4.5 days shorter than males, as well as a time to wound closure Tc that is 5.3 days shorter than males (both with p < 0.0001), whereas the initial wound stasis phase Ti is statistically indistinguishable between these cohorts. The shorter proliferative phase in nonaged females corresponds to a ∼24% faster HR compared with nonaged males (p = 0.007). There is also a statistically significant smaller initial wound radius R0 for females (p = 0.004; Discussion section).
Aged male versus aged female
A comparison of aged male and aged female cohorts indicates no statistically significant difference for any wound healing parameter except for wound radius R0. The aged female cohort exhibits a 0.14 mm larger average wound radius (p = 0.038) than aged males.
Nonaged male versus aged male
A comparison of nonaged and aged male cohorts indicates no statistically significant difference (i.e., p ≤ 0.05) for any wound healing parameter.
Nonaged female versus aged female
A comparison of nonaged female and aged female cohorts indicates a statistically significant difference in all wound healing parameters except for HR. Aging in females is associated with a 5.4-day longer time to wound closure Tc (p = 0.0009), a 1.8-day longer initial stasis phase Ti (p = 0.003), and a 3.5-day longer proliferative phase Tp (p = 0.004). Aged females also exhibit a 0.38 mm larger R0 wound radius (p = 0.00002; Discussion section). A graphical comparison of data sets for the nonaged and aged female cohorts is shown in Fig. 2.
Figure 2.
Effect of age and gender on wound healing and FGF/HS upon aged female wound healing. Left panel: Nonaged females have enhanced dermal wound healing compared with aged females, nonaged males, or aged males (which as a group heal similarly) (Tables 1 and 2). Right panel: In aged female mice topical FGF-1 at 2 μg/cm2 (formulated with 3 × mass HS and dosed on days 0, 3, 7, and 10) results in an increase in HR during the active proliferative phase Tp such that the overall time to wound closure Tc is essentially indistinguishable from that of nonaged females. Error bars in both graphs are SEM. FGF-1, fibroblast growth factor-1; HS, heparin sulfate; SEM, standard error of the measurement.
Effect of 2 μg/cm2 topical FGF/HS on aged males
Topical application of FGF/HS on aged males had no statistically significant effect on any wound healing parameter (i.e., p ≤ 0.05).
Effect of 2 μg/cm2 topical FGF/HS on aged females
Topical application of FGF/HS on aged females had no statistically significant effect upon either wound radius R0 or initial stasis phase Ti but did result in a 4.2-day reduction in time to wound closure Tc (p = 0.011), a 3.8-day reduction in the duration of the active proliferative phase of healing Tp (p = 0.008), and a ∼35% increase in HR (p = 0.004). A graphical representation of the aged female+FGF/HS cohort data is shown in Fig. 2.
Discussion
Two principal methodologies are commonly utilized to quantify the progression of dermal wound healing: photography and histology. These two methodologies provide complementary information regarding the progression of wound healing. Photographic data quantify changes in the visible morphology of healing based upon the underlying histological events during the hemostasis, inflammatory, and proliferative phases (the wound remodeling phase subsequent to wound closure is not readily discernible with visible photography). Mukai et al. reported the analysis of both a photographic and sparse-sampled (i.e., days 3, 7, 11, and 14) histological study of excisional wound healing in C57BL/6 mice.33 This report demonstrated a correlation between the initial stasis phase identified by photographic data and the inflammatory healing phase characterized by histology; furthermore, the active healing phase identified by photographic data corresponded to the proliferative healing phase characterized by histology. The mathematical analysis of fine-sampled photographic data reported by Blaber and colleagues18 provides for a robust parameterization of healing phases (which is not possible with sparse-sampled histological data); furthermore, such analyses permit robust statistical analyses for cohort comparisons. Of significant additional benefit, photographic data are noninvasive, whereas histological sampling is a lethal event in mice (necessitating a correspondingly larger number of animals per cohort).
This study demonstrates an age-related senescence in dermal wound healing in female mice, characterized by a ∼12% increase in initial wound radius R0 (p = 0.00001), a ∼43% increase in time to wound closure Tc (p = 0.0002), a ∼35% increase in the duration of the inflammatory phase Ti (p = 0.009), a ∼16% reduction in the HR (p = 0.016), and a ∼46% increase in the duration of the proliferative phase Tp (p = 0.001). Thus, the shorter time to wound closure in nonaged females is due to the combination of greater effectiveness for several healing parameters (Fig. 2). Conversely, male mice exhibit no statistically significant difference in wound healing parameters in response to aging. However, an apparent consequence of aging, affecting both male and female cohorts, is a ∼100–400% increase in the standard deviation of wound healing parameters, Tc, Tp, and HR (parameters associated with the active proliferative phase of healing) (Fig. 3). Thus, both male and female aged mice exhibit a general dysregulation of the healing process as evidenced by an increased standard deviation for healing parameters.
Figure 3.
Standard deviation of Tp parameter in response to aging. The standard deviation of several parameters of wound healing is increased in response to aging for both male and female cohorts (although the average value for cohorts may be statistically unchanged). The Tp parameter is shown here, but similar deviations are observed for HR and Tc (Table 1).
Aging in females is associated with a ∼12% (0.39 mm) increase in the wound radius R0 (p = <0.00001; Table 2 and Fig. 2). The silicone splint (which fixes the wound radius) is sutured immediately subsequent to the excisional wound; thus, this wound expansion is occurring essentially concurrent with excision. Skin is naturally under tension, and such tension is opposed by elastic fibers of the skin (principally collagen, elastin, and matrix proteoglycan).34 Reduced elasticity results in a reduced ability to oppose the intrinsic tension of skin, leading to thinning of the skin and greater wound gape. Thus, the increase in R0 for the aged female mice is consistent with an age-associated reduction in skin elasticity. With regard to R0, aged females exhibit similar values to both nonaged and aged males, thus, the young female mice appear to have a uniquely enhanced skin elasticity as determined from the R0 parameter. This conclusion is consistent with the known effects of estrogen upon skin elasticity and reduced levels of estrogen in aged mice. Decreased levels of estrogen lead to atrophy and thinning of the skin, a decrease in skin elasticity and strength, and these effects are considered part of the aging senescence of female skin associated with decreased collagen and blood flow to this organ.35,36 Studies of plasma levels of sex hormones in 12- to 15-month-old reproductively senescent female C57BL/6 mice reported a reduced level of estrogen and a dysfunction of the hypothalamic–pituitary complex.37 The fine-sampled photographic analysis thus also appears capable of providing some basic quantitative information related to skin elasticity.
The topical application of FGF-1 as a therapeutic in wound healing has been investigated in both normal and diabetic nonaged rats and mice, although a detailed age- and gender-related study has not been reported.23,30,38–41 FGF-1 is of interest as a therapeutic in wound healing since it is chemotactic for inflammatory cells including macrophages42 and is also mitogenic/chemotactic for dermal fibroblasts,20,22,43 vascular endothelial cells,21,44,45 and epidermal keratinocytes.20,46,47 Thus, FGF-1 as a single therapeutic agent has the potential to promote dermal healing. In aged females, FGF-1 treatment resulted in a 24% reduction in wound time to closure Tc (p = 0.003), a 41% increase in HR (p = 0.0009), and a 32% reduction in duration of the proliferative phase Tp (p = 0.002) (parameters associated with the active proliferative phase), but had no effect upon initial wound radius R0 or duration of the initial wound stasis phase Ti. The dosage regimen of FGF/HS on days 0, 3, 7, and 10 results in two applications during the initial wound stasis phase and two applications during the active proliferative phase. The wound stasis phase Ti is statistically unaffected by FGF/HS treatment; thus, the results indicate that the improvement in dermal healing in aged females by FGF/HS is driven principally by effects upon the active proliferative phase of healing. This result is consistent with the mitogenic and chemotactic properties of FGF-1 upon fibroblasts, keratinocytes, and endothelial cells. In contrast to the effect in aged females, FGF/HS had little apparent effect on the wound healing in aged males.
The presence of estrogen receptor α and β has been reported in cultured human fibroblasts,48 keratinocytes,49 and vascular endothelial cells.50 Estrogen treatment can accelerate human dermal wound healing in both aged males and females.51 Estrogen activates the ERK1/2 signaling pathways, induces the expression of c-fos and c-jun, and upregulates cyclin D1 in keratinocytes.49 FGF-1 has also been shown to activate the ERK1/2, c-fos, and c-jun cyclin D1 and p38 MAPK pathways in chrondrocytes (although the response is inhibitory and not proliferative for these cells).52,53 Possible functional cross-talk between the sex hormone and FGF axes is indicated by FGF-1 administration, preventing bone loss and stimulating bone formation in ovarectomized rats54 and estrogen-induced expression of FGF-1 in endometrial cancer cells.55 Further work is needed to understand whether the observed gender difference in response to FGF/HS in BALB/cByJ mice is a dose–response difference in males (i.e., necessitating a higher effective dose for males), or whether there may be intersection of FGF-1 and estrogen signaling activity in females. As regards the outcome of FGF/HS therapeutic intervention in dermal healing of aged females, the time to wound closure Tc and active proliferative phase Tp are statistically indistinguishable with the nonaged female cohort, and the HR of the aged females treated with FGF is enhanced compared with that of the nonaged females (Fig. 2). Therapeutic intervention of FGF/HS in the aged female cohort can, therefore, be described as effectively recovering the more efficient wound healing observed in nonaged female cohort (with the exception of R0 and intrinsic skin elasticity).
In summary, a novel mathematical analysis of fine-sampled (i.e., daily) photographic data of dermal wound healing in BALB/cByJ mice provides quantitative parameters for the initial stasis phase and subsequent active proliferative phase of healing, and may also provide information regarding intrinsic skin elasticity. The results identify a senescence-related impairment in dermal wound healing in aged female mice. Furthermore, topical application of 2 μg/cm2 FGF/HS results in a time to wound closure equivalent to that of nonaged females. The results suggest possible cross-talk between the estrogen and FGF-1 functional axes in dermal wound healing.
Innovation
Preclinical animal efficacy studies of wound healing can benefit from new methods to maximize data with limited cohort size, and to define standard methodologies that permit comparison of healing parameters from different studies. A novel mathematical analysis of fine-sampled photographic data is used to quantify key parameters of healing in mouse excisional wounds. The results permit robust statistical comparison between cohorts and with limited cohort size. An age-related senescence of dermal healing parameters in aged female BALB/cByJ mice is demonstrated, and topical application of FGF-1 is shown to restore dermal healing similar to nonaged females (through enhanced proliferative phase parameters).
Key Findings.
A novel mathematical analysis of fine-sampled photographic data permits the quantitation of detailed parameters of dermal healing in preclinical animal studies.
Female BALB/cByJ mice exhibit an age-related senescence of dermal wound healing.
Topical application of FGF-1, in a single-dosage study, effectively restores youthful dermal healing in aged BALB/cByJ females through enhanced proliferative phase parameters.
Acknowledgments and Funding Sources
This study was supported by a research support agreement from Trefoil Therapeutics, Inc. to M.B. and a grant from the FSU Institute for Successful Longevity to M.B. and K.M.H. B.N.P. was supported by an FSU College of Medicine Summer Fellowship. C.A.T. was supported by a McKnight Fellowship and the Department of Biomedical Sciences.
Abbreviations and Acronyms
- FGF-1
fibroblast growth factor-1
- FGF/HS
FGF-1 formulated with 3 × mass heparin sulfate
- HS
heparin sulfate
- HR
healing rate
- ID
inside diameter
- JAX
The Jackson Laboratories
- PBS
phosphate-buffered saline
- R0
initial wound radius
- Tc
time to wound closure
- Ti
initial wound stasis phase
- Tp
active proliferative phrase
Author Disclosure and Ghostwriting
M.B. acknowledges equity ownership in Trefoil Therapeutics, Inc. The content of this article was expressly written by the authors listed. No ghostwriters were used in the preparation of this article.
About the Authors
Michael Blaber, PhD, is a professor of biomedical sciences in the FSU College of Medicine. His research includes studies of FGF-1 in regenerative medicine. He is also cofounder of Trefoil Therapeutics, Inc. a development stage company focused on the application of novel engineered FGF-1 compounds in underserved disease areas. Alana P. Mellers, BS, and Bhavi N. Patel, BS, are medical school students in the FSU College of Medicine. Connie A. Tenorio, BS, is a graduate student in the department of biomedical sciences in the FSU College of Medicine. Diana A. Lacatusu, BS, and Brett D. Powell, BS, are undergraduate research assistants at FSU. Kathleen M. Harper, DVM, PhD, is attending veterinarian in the FSU Laboratory Animal Resources facility.
References
- 1. Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev 2001;122:1203–1220 [DOI] [PubMed] [Google Scholar]
- 2. Reed MJ, Karres N, Eyman D, Vernon RB, Edelberg JM. Age-related differences in repair of dermal wounds and myocardial infarcts attenuate during the later stages of healing. In Vivo 2006;20:801–806 [PubMed] [Google Scholar]
- 3. Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology 2002;3:337–345 [DOI] [PubMed] [Google Scholar]
- 4. Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen 2015;23:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim DJ, Mustoe T, Clark RA. Cutaneous wound healing in aging small mammals: a systematic review. Wound Repair Regen 2015;23:318–339 [DOI] [PubMed] [Google Scholar]
- 6. Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med 2007;13:30–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 2014;11:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. In: U.S. Census Bureau Report P25-1140. ed. 2014:1–28 [Google Scholar]
- 10. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018;21:27–32 [DOI] [PubMed] [Google Scholar]
- 11. Tannenbaum C, Day D. Age and sex in drug development and testing for adults. Pharmacol Res 2017;121:83–93 [DOI] [PubMed] [Google Scholar]
- 12. Ashcroft GS, Mills SJ. Androgen receptor–mediated inhibition of cutaneous wound healing. J Clin Invest 2002;110:615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell L, Emmerson E, Davies F, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med 2010;207:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toraldo G, Bhasin S, Bakhit M, et al. Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes. Wound Repair Regen 2012;20:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crompton R, Williams H, Ansell D, et al. Oestrogen promotes healing in a bacterial LPS model of delayed cutaneous wound repair. Lab Invest 2016;96:439–449 [DOI] [PubMed] [Google Scholar]
- 16. Michaels J, Churgin SS, Blechman KM, et al. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen 2007;15:665–670 [DOI] [PubMed] [Google Scholar]
- 17. Davidson JM, Yu F, Opalenik SR. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care 2013;2:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cogan NG, Mellers A, Patel B, et al. A mathematical model for the determination of mouse excisional wound healing parameters from photographic data. Wound Repair Regen 2018. DOI 10.1111/wrr.12634 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19. Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE. and Smith AL, ed. The Mouse in Biomedical Research, 2nd ed. Burlington, MA: Elsevier, 2007:637–672 [Google Scholar]
- 20. Shipley GD, Keeble WW, Hendrickson JE, Coffey RJ, Jr., Pittelkow MR. Growth of normal human keratinocytes and fibroblasts in serum-free medium is stimulated by acidic and basic fibroblast growth factor. J Cell Physiol 1989;138:511–518 [DOI] [PubMed] [Google Scholar]
- 21. Thomas KA, Rios-Candelore M, Gimenez-Gallego G, et al. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A 1985;82:6409–6413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rozengurt E. The Mitogenic Response of Cultured 3T3 Cells: Integration of Early Signals and Synergistic Effects in a Unified Framework, vol 4 Amsterdam: Elsevier, 1985 [Google Scholar]
- 23. Mellin TN, Mennie RJ, Cashen DE, et al. Acidic fibroblast growth factor accelerates dermal wound healing. Growth Factors 1992;7:1–14 [DOI] [PubMed] [Google Scholar]
- 24. Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol Online 1994;31:293–300 [DOI] [PubMed] [Google Scholar]
- 25. Yuan R, Meng Q, Nautiyal J, et al. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci U S A 2012;109:8224–8229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogue MA, Grubb SC. The Mouse Phenome Project. Genetica 2004;122:71–74 [DOI] [PubMed] [Google Scholar]
- 27. Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 2016;152:244–248 [DOI] [PubMed] [Google Scholar]
- 28. Brych SR, Blaber SI, Logan TM, Blaber M. Structure and stability effects of mutations designed to increase the primary sequence symmetry within the core region of a β-trefoil. Protein Sci 2001;10:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galiano RD, Michaels J 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 2004;12:485–492 [DOI] [PubMed] [Google Scholar]
- 30. Blaber SI, Diaz J, Blaber M. Accelerated healing in NONcNZO10/LtJ type 2 diabetic mice by FGF-1. Wound Repair Regen 2015;23:538–549 [DOI] [PubMed] [Google Scholar]
- 31. Blaber M, Blaber SI. Small Animal Restraining Harness or Jacket. USA: Florida State University Research Foundation, 2017 [Google Scholar]
- 32. Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoto Int 2004;11:36–42 [Google Scholar]
- 33. Mukai K, Nakajima Y, Urai T, et al. 17 beta-Estradiol administration promotes delayed cutaneous wound healing in 40-week ovariectomised female mice. Int Wound J 2016;13:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gahagnon S, Mofid Y, Josse G, Ossant F. Skin anisotropy in vivo and initial natural stress effect: a quantitative study using high-frequency static elastography. J Biomech 2012;45:2860–2865 [DOI] [PubMed] [Google Scholar]
- 35. Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. Skin aging and menopause: implications for treatment. Am J Clin Dermatol 2003;4:371–378 [DOI] [PubMed] [Google Scholar]
- 36. Holzer G, Riegler E, Honigsmann H, Farokhnia S, Schmidt JB, Schmidt B. Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study. Br J Dermatol 2005;153:626–634 [DOI] [PubMed] [Google Scholar]
- 37. Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH, FSH and prolactin in aged female C57BL/6 mice. J Reprod Fertil 1980;58:377–386 [DOI] [PubMed] [Google Scholar]
- 38. Mellin TN, Cashen DE, Ronan JJ, Murphy BS, DiSalvo J, Thomas KA. Acidic fibroblast growth factor accelerates dermal wound healing in diabetic mice. J Invest Dermatol 1995;104:850–855 [DOI] [PubMed] [Google Scholar]
- 39. Matuszewska B, Keogan M, Fisher DM, et al. Acidic fibroblast growth factor: evaluation of topical formulations in a diabetic mouse wound healing model. Pharm Res 1994;11:65–71 [DOI] [PubMed] [Google Scholar]
- 40. Tan Y, Xiao J, Huang Z, et al. Comparison of the therapeutic effects of recombinant human acidic and basic fibroblast growth factors in wound healing in diabetic patients. J Health Sci 2008;54:432–440 [Google Scholar]
- 41. Wang W, Lin S, Xiao Y, et al. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci 2008;82:190–204 [DOI] [PubMed] [Google Scholar]
- 42. Rossini M, Cheunsuchon B, Donnert E, et al. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int 2005;68:2621–2628 [DOI] [PubMed] [Google Scholar]
- 43. Senior RM, Huang SS, Griffin GL, Huang JS. Brain-derived growth factor is a chemoattractant for fibroblasts and astroglial cells. Biochem Biophys Res Comm 1986;141:67–72 [DOI] [PubMed] [Google Scholar]
- 44. Terranova VP, DiFlorio R, Lyall RM, Hic S, Friesel R, Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol 1985;101:2330–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stokes CL, Rupnick MA, Williams SK, Lauffenburger DA. Chemotaxis of human microvessel endothelial cells in response to acidic fibroblast growth factor. Lab Invest 1990;63:657–668 [PubMed] [Google Scholar]
- 46. Tsuboi R, Sato C, Shi C-M, Ogawa H. Stimulation of keratinocyte migration by growth factors. J Dermatol 1992;19:652–653 [DOI] [PubMed] [Google Scholar]
- 47. Meyer M, Müller A-K, Yang J, et al. FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin. J Cell Sci 2012;125:5690–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haczynski J, Tarkowski R, Jarzabek K, et al. Human cultured skin fibroblasts express estrogen receptor α and β. Int J Mol Med 2002;10:149–153 [PubMed] [Google Scholar]
- 49. Verdier-Sevrain S, Yaar M, Cantatore J, Traish A, Gilchrest BA. Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. FASEB J 2004;18:1252–1254 [DOI] [PubMed] [Google Scholar]
- 50. Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci 2002;966:143–157 [DOI] [PubMed] [Google Scholar]
- 51. Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MWJ. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999;155:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dailey L, Laplantine E, Priore R, Basilico C. A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol 2003;161:1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raucci A, Laplantine E, Mansukhani A, Basilico C. Activation of the ERK1/2 and p38 mitogen-activated protein kinase pathways mediates fibroblast growth factor-induced growth arrest of chondrocytes. J Biol Chem 2004;279:1747–1756 [DOI] [PubMed] [Google Scholar]
- 54. Dunstan CR, Boyce R, Boyce BF, et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J Bone Miner Res 1999;14:953–959 [DOI] [PubMed] [Google Scholar]
- 55. Fujimoto J, Hori M, Ichigo S, Tamaya T. Antiestrogenic compounds inhibit estrogen-induced expression of fibroblast growth factor family (FGF-1, 2, and 4) mRNA in well-differentiated endometrial cancer cells. Eur J Gynaecol Oncol 1997;18:497–501 [PubMed] [Google Scholar]