Abstract
Several studies suggest that peripheral blood and lymph node micrometastases may be a causative factor for gastric cancer recurrence. Cytokeratin 20 shows enriched expression in intestinal epithelial cells. This study aimed to evaluate the clinical utility of monitoring cytokeratin 20 levels in peripheral blood and lymph nodes of patients with gastric cancer for detecting micrometastasis and predicting prognosis. We detected messenger RNA levels of cytokeratin 20 in gastric cancer cell lines and in the peripheral blood of 125 patients (85 patients with gastric cancer and 40 patients with benign neoplasm) by fluorescence quantitative real-time polymerase chain reaction both before and after radical resection. In all, 1586 lymph node samples from 85 patients with gastric cancer were evaluated for cytokeratin 20 expression using real-time polymerase chain reaction, as well as by immunohistochemistry staining with anti-pan-keratin and anti-cytokeratin 20 antibodies. All patients underwent follow-up until cancer-related death or for more than 3 years after tumor resection. We found that elevated cytokeratin 20 expression in peripheral blood as detected by quantitative real-time polymerase chain reaction closely correlates with poor clinicopathological characteristics. Detecting cytokeratin 20 messenger RNA in the lymph nodes by quantitative real-time polymerase chain reaction enabled more accurate determination of the clinicopathological staging of gastric cancer, best treatment approach, and prognosis. Our findings show that patients with increased cytokeratin 20 messenger RNA expression in the peripheral blood or lymph nodes have a shorter time to recurrence and poorer overall survival.
Keywords: gastric cancer, cytokeratin, micrometastasis, prognosis, lymph nodes, peripheral blood
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer death in both sexes.1,2 Despite curative gastrectomy, extended lymph node dissection,3-6 and adjuvant chemotherapy, GC can recur in both regional and distant sites in the majority of patients, including those without lymph node metastasis based on conventional histological hematoxylin and eosin (H&E) staining.7-9 Several studies have concluded that peripheral blood and lymph node micrometastasis might be a key causative factor for GC recurrence.10-14
Lymph node micrometastases of 0.2 to 2.0 mm in size have been detected in lymph nodes by immunohistochemical (IHC)-based evaluation of cytokeratin (CK) expression, a component of the cell skeleton involved in cytomorphology.15 More recently, fluorescence quantitative real-time polymerase chain reaction (qRT-PCR) technology (which is a sensitive, specific, and rapid method) has been widely used to detect the presence of circulating cancer cells in the peripheral blood and lymph nodes.16 However, there are few reports on the detection and clinical significance of pre- and postoperative CK20 messenger RNA (mRNA) levels in the peripheral blood of patients with GC. Furthermore, the clinical impact of peripheral blood and lymph node micrometastasis on GC prognosis remains controversial.17-19 Several studies report that peripheral blood and lymph node micrometastasis in GC is a strong indicator of overt metastatic disease or poor prognosis.20,21 However, others have reported no significant correlation between micrometastasis and other clinic pathological characteristics and that the presence of micrometastasis does not influence patient prognosis.22-26
In the present study, we detected pre- and postoperative CK20 mRNA levels in the peripheral blood of patients with GC using qRT-PCR. We also detected CK20 expression in lymph node samples from 85 patients with GC using qRT-PCR as well as by IHC staining with anti-pan-keratin and anti-CK20 antibodies and estimated the clinical and prognostic value of these biomarkers in patients with GC.
Materials and Methods
Cell Lines and Culture Conditions
The GC lines AGS (moderately differentiated), SGC-7901 (moderately differentiated), N87 (well differentiated), BGC-823 (poorly differentiated), and MKN-45 (poorly differentiated) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and maintained with Roswell Park Memorial Institute medium (RPMI HyClone, Logan, Utah) supplemented with 10% fetal bovine serum (Thermo Scientific HyClone, Logan, Utah). All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Patients
From January 2013 to June 2013, a total of 85 patients with GC who underwent gastrectomy at the Department of General Surgery of People’s Hospital of Taizhou City were enrolled in this study. The inclusion criteria of the study were as follows: (1) radical gastrectomy for the primary tumor and D2 lymphadenectomy following the Japanese Research Society for Gastric Cancer guidelines,27 (2) complete clinicopathological and follow-up data, (3) no prior chemotherapy or radiotherapy before surgery, (4) no gross or microscopic residual or recurrent gastric tumor, (5) no distant metastases prior to surgery, and (6) no other synchronous malignancies or serious disease in other systems.
The histological diagnosis and tumor–node–metastasis (TNM) staging were based on the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system.28 The study protocol was approved by the Medical Ethics Committee of Taizhou People’s Hospital of Jiangsu Province, the Hospital Affiliated to Medical of Yangzhou University (TZRY-EC-13-0167), and all patients provided written informed consent.
Patient clinicopathologic parameters were collected, including gender, age, tumor location, tumor diameter, histological grade, Borrmann type, invasive depth, lymph node status, peripheral blood concentrations of cancer-related antigens (ie, carcino-embryonic antigen(CEA), carbohydrate antigen(CA)724, and CA199), and tumor emboli in the microvessels. A total of 40 patients with benign neoplasm were used as negative controls.
Specimens
Before and after surgical resection, a 10 mL sample of peripheral blood was obtained from each patient through a catheter inserted into a peripheral vessel and collected into EDTA tubes. The blood samples were anticoagulated with heparin (5 U/mL), stored at 4°C, and processed within 4 hours after blood collection.
Half of each resected lymph node was fixed in 10% formalin and embedded in paraffin for routine histopathological and IHC examination. The other half was stored in a clean tube at −80°C for RNA extraction.
Total RNA Isolation and Complementary DNA Synthesis
Mononuclear cells were isolated from 5 mL heparinized whole blood by density gradient centrifugation using a lymphocyte separating medium (Huajing Corp, Shanghai, China). Mononuclear cells and GC cell lines were washed 3 times with phosphate-buffered saline, then centrifuged at 2000 rpm for 5 minutes. Total RNA was then extracted from cell lysates using TRIzol reagent (Life Technologies, Gaithersburg, Maryland), according to the manufacturer’s instructions. For the lymph node samples, 50 to 100 mg of tissues were homogenized in 1 mL of TRIzol reagent using a power homogenizer prior to RNA extraction. The concentration and purity of RNA was determined using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, Delaware): A260 to A280 ratios in the range of 1.8 to 2.0 were considered pure. First-strand complementary DNA (cDNA) was synthesized using the SuperScript First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, California), according to the manufacturer’s instructions, and then stored at −20°C for subsequent qPCR experiments.
Fluorescence qRT-PCR
The mRNA expression levels of CK20 were detected by qRT-PCR using the ABI 7700 sequence detector (Perkin Elmer/Applied Biosystems, Foster City, California). β-Actin was used as an internal control to normalize mRNA levels. The primers targeting CK20 and β-actin were as follows: CK20 upstream primer: 5′-CAGACACACGGTGAACTATGG-3′ and downstream primer: 5′-GATCAGCTTCCACTGTTAGACG-3′; β-actin upstream primer: 5′-CACGAAACTACCTTCAACTCC-3′ and downstream primer: 5′-CATACTCCTGCTTGCTGATC-3′. All primers were synthesized by Life Technologies (Shanghai, China).
The amplification reaction mixture consisted of 4 μL cDNA, 25 μL 10× buffer, 1 μL dNTP, 0.4 μL Taq DNA polymerase, 1 μL each of sense and antisense primers (including CK20 and β-actin), and distilled H2O to a final volume of 50 μL. The amplification reaction mixture was amplified through 40 cycles. The amplification reaction mixture was first heated at 95°C for 5 minutes to terminate the reverse transcription reaction, and thermal cycling was carried out at 95°C for 30 seconds, 62°C for 30 seconds, and 72°C for 30 seconds, followed by final extension at 72°C for 10 minutes. The sample was then slowly cooled to 4°C. All reactions were run in triplicate. The same procedure was performed with the standard agent and a positive or negative control. The relative levels of normalized gene expression were calculated with the equation 2−ΔΔCT, in which ΔCT = CT gene − CT control. Receiver operating characteristic (ROC) statistics were employed to estimate the cut points to distinguish between high and low expression of CK20 in peripheral blood and lymph nodes from GC samples. The cutoff values for CK20 were determined in the blood samples of healthy donors and GC tissue using the maximal χ2 method.
Immunohistochemistry
The IHC was performed as described previously,29 using anti-CK20 monoclonal antibody (Abcam, Cambridge, United Kingdom) and primary pan-CK antibody A45-B/B3 detecting CK8, CK18, and CK19 (AS Diagnostik, Hückeswagen, Germany). Negative controls were treated identically, though the primary antibodies were omitted, and positive controls were provided by the kit supplier. The results were assessed by 2 independent pathologists without knowledge of the patient clinical status. Positive staining was defined as moderate or strong brown or dark brown staining in cells. No visible staining or light brown staining in cells was defined as negative; detection of at least 0.2 mm foci combined with amplification H&E staining to confirm the configuration of cells was regarded as a positive case.
Follow-Up
After curative resection, all patients were followed regularly every 3 months during the first 2 years and every 6 months during the third to fifth years. The final follow-up date was June 30, 2016. No patients were lost to follow-up. The median follow-up duration was 39.2 months (range: 36-43 months) after surgery. A total of 36 (42.4%) cases were metastasis or recurrence, of which 29 (34.1%) had tumor-related deaths.
Statistical Analysis
Statistical analyses were performed with SPSS 16.0 for Windows (SPSS, Chicago, Illinois). The correlations between CK20 mRNA expression and clinicopathological features were analyzed using the χ2 test and the correlation of the 3 methods (H&E staining, IHC, and qRT-PCR) was calculated. Univariate analysis was performed to identify variables associated with prognosis. Cox regression analysis was used to identify independent predictors of prognosis in patients with GC. The survival rate curves were compared using the log-rank test. P values <.05 were considered statistically significant.
Results
Cytokeratin 20 mRNA Expressed in GC Cell Lines
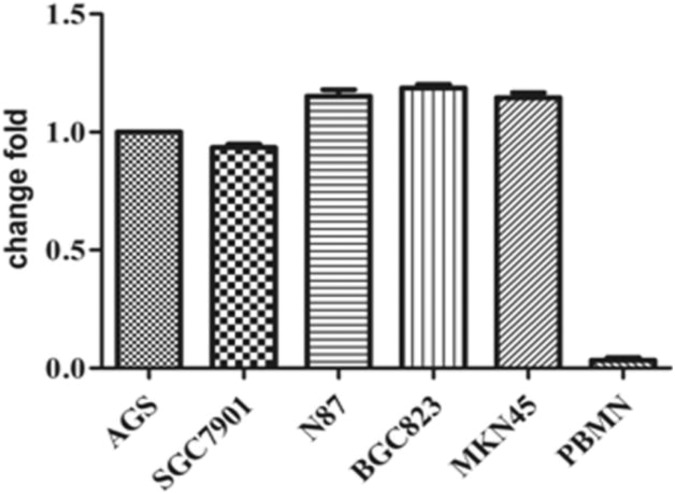
The CK20 mRNA was found to be expressed in GC cell lines, and there was no significant difference in CK20 expression among the 5 cell lines examined (t = 1.734, P = .181; Figure 1). Cell line SGC-7901 with the lowest expression of CK20 was selected for subsequent in vitro experiments. To validate the sensitivity of the qRT-PCR, different counts of SGC-7901 cells (105, 104, 103, 102, 101, and 1) were added to a suspension containing 108 peripheral blood mononuclear cells (PBMNs), and corresponding mRNA levels of CK20 were evaluated. We found the sensitivity for CK20 detection to be 10−7 in this in vitro model of gastric micrometastases.
Figure 1.
CK20 mRNA is expressed in multiple GC cell lines. There was no significant difference in CK20 expression among the 5 cell lines examined (t = 1.734, P = .181). CK20 indicates cytokeratin 20; mRNA, messenger RNA.
Patients and Characteristics
A total of 85 patients with GC were included in our study. There were 26 females (30.6%) and 59 males (69.4%), and the median age was 65.0 years (range: 35-80 years). All patients were histologically confirmed to have adenocarcinoma following pathological assessment of resected tumors. Participant characteristics and baseline measures are shown in Table 1. A total of 40 patients with benign tumors were used as negative controls, including 4 cases of gastric stromal tumor, 11 cases of thyroid adenoma, 7 cases of nodular goiter, 10 cases of benign breast tumor, and 8 cases of uterine fibroids.
Table 1.
Correlations Between Preoperative CK20 mRNA Expression in Peripheral Blood and Clinicopathological Features of 85 Patients With Gastric Cancer.
| Clinicopathological Feature | Negative, n (%) | Positive, n (%) | χ2 Test | P Value |
|---|---|---|---|---|
| 50 Cases (58.8%) | 35 Cases (41.2%) | |||
| Gender | 0.666 | .415 | ||
| Male | 33 (66.0) | 26 (74.3) | ||
| Female | 17 (34.0) | 9 (25.7) | ||
| Age (years) | 0.387 | .543 | ||
| ≥60 | 40 (80.0) | 26 (74.3) | ||
| <60 | 10 (20.0) | 9 (25.7) | ||
| Tumor location | 2.219 | .528 | ||
| Upper | 16 (32.0) | 16 (45.7) | ||
| Middle | 15 (30.0) | 8 (22.9) | ||
| Lower | 16 (32.0) | 8 (22.9) | ||
| Diffuse/multiple lesions | 3 (6.0) | 3 (8.5) | ||
| Tumor diameter (cm) | 22.012 | 1.661 × 10−5 | ||
| <3 | 24 (48.0) | 1 (2.9) | ||
| 3-5 | 12 (24.0) | 10 (28.6) | ||
| >5 | 14 (28.0) | 24 (68.5) | ||
| Histological grade | 5.873 | .015 | ||
| Well/moderately differentiated | 26 (52.0) | 9 (25.7) | ||
| Poorly differentiated/undifferentiated | 24 (48.0) | 26 (74.3) | ||
| Borrmann type | 30.506 | 1.079 × 10−6 | ||
| I | 5 (10.0) | 0 (0.0) | ||
| II | 26 (52.0) | 3 (8.6) | ||
| III | 12 (24.0) | 9 (25.7) | ||
| IV | 7 (14.0) | 23 (65.7) | ||
| Invasive depth | 18.286 | 1.901 × 10−5 | ||
| T1-T2 | 32 (64.0) | 6 (17.1) | ||
| T3-T4 | 18 (36.0) | 29 (82.9) | ||
| Lymph node status | 58.564 | 1.968 × 10−14 | ||
| N0 | 43 (86.0) | 0 (0.0) | ||
| N1 | 51 (0.0) | 5 (14.3) | ||
| N2 | 2 (4.0) | 10 (28.6) | ||
| N3 | 0 (0.0) | 20 (57.1) | ||
| CEA | 30.310 | 3.683 × 10−8 | ||
| Normal | 48 (96.0) | 15 (42.9) | ||
| High | 2 (4.0) | 20 (57.1) | ||
| CA724 | 29.628 | 5.234 × 10−8 | ||
| Normal | 47 (94.0) | 14 (40.0) | ||
| High | 3 (6.0) | 21 (60.0) | ||
| CA199 | 25.739 | 3.908 × 10−7 | ||
| Normal | 48 (96.0) | 17 (48.6) | ||
| High | 2 (4.0) | 18 (51.4) | ||
| Tumor emboli in the microvessels | 27.811 | 1.338 × 10−7 | ||
| Negative | 33 (66.0) | 3 (8.6) | ||
| Positive | 17 (34.0) | 32 (91.4) |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CK20, cytokeratin 20; mRNA, messenger RNA.
Correlation Between Preoperative CK20 mRNA Levels and Clinicopathological Factors
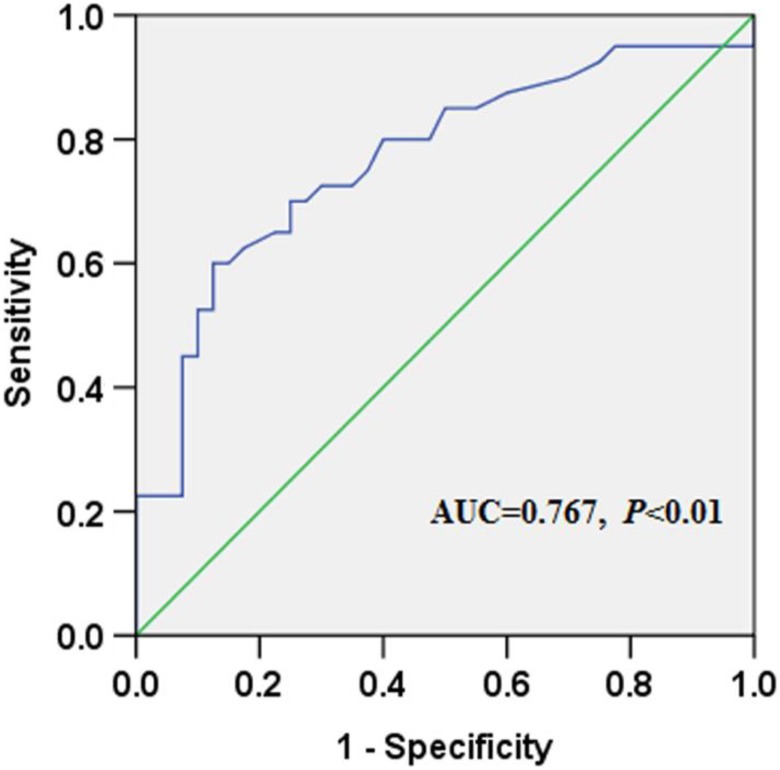
In the blood samples of healthy donors, the median CK20 mRNA level was 0.43 (range: 0.27-2.15) and the median CK20 level in GC tissues was 0.93 (range: 0.23-4.17). The ROC statistics were used to estimate the cutoff scores to distinguish positive and negative CK20 expression. Scores ≥0.83 were considered to indicate positive expression (Figure 2). Based on these results, none of the negative controls showed positive CK20 expression preoperatively, while 35 of 85 patients with primary GC showed positive CK20 expression (positive ratio: 41.2%). A significant difference between the 2 groups was found (χ2 = 22.876, P = 1.72 × 10−6). The associations between the pre-CK20 mRNA levels and the clinicopathological characteristics are shown in Table 1.
Figure 2.
The ROC statistics were used to estimate the cutoff scores to distinguish between positive and negative CK20 mRNA expression. CK20 indicates cytokeratin 20; mRNA, messenger RNA; ROC, receiver operating characteristic.
Preoperative expression levels of CK20 in peripheral blood from patients with GC correlated with tumor diameter (P = 1.661 × 10−5), invasive depth (P = 1.901 × 10−5), differentiation grade (P = .015), Borrmann type (P = 1.079 × 10−6), lymphatic metastasis (P = 1.968 × 10−14), tumor emboli in the microvessels (P = 1.338 × 10−7), and concentration of cancer-related antigens in peripheral blood, including CEA (P = 3.683 × 10−8), CA724 (P = 5.234 × 10−8), and CA199 (P = 3.908 × 10−7). However, expression of CK20 in peripheral blood did not correlate with the demographic characteristics or tumor location (P > .05).
Correlation Between Postoperative CK20 mRNA Levels and Clinicopathological Factors
On the first day after resection, CK20 levels as detected by qPCR were still negative among the 40 patients with benign tumors. In contrast, 49 cases (57.6%) in the patient with GC group showed positive CK20 expression. There was a significant difference between the malignant and benign tumor groups (χ2 = 37.926, P = 7.34 × 10−10). The associations between the post-CK20 mRNA levels and the clinicopathological characteristics are shown in Table 2.
Table 2.
Correlations Between Postoperative CK20 mRNA Expression in Peripheral Blood and Clinicopathological Features of 85 Patients With Gastric Cancer.
| Clinicopathological Feature | Negative, n (%) | Positive, n (%) | χ2 Test | P Value |
|---|---|---|---|---|
| 36 Cases (42.4%) | 49 Cases (57.6%) | |||
| Gender | 0.232 | .630 | ||
| Male | 26 (72.2) | 33 (67.3) | ||
| Female | 10 (27.8) | 16 (32.7) | ||
| Age (years) | 4.547 | .053 | ||
| ≥60 | 32 (88.9) | 34 (69.4) | ||
| <60 | 4 (11.1) | 15 (30.6) | ||
| Tumor location | 3.655 | .301 | ||
| Upper | 10 (27.8) | 22 (44.9) | ||
| Middle | 13 (36.1) | 10 (20.4) | ||
| Lower | 10 (27.8) | 14 (28.6) | ||
| Diffuse/multiple lesions | 3 (8.3) | 3 (6.1) | ||
| Tumor diameter (cm) | 32.221 | 8.271 × 10−9 | ||
| <3 | 23 (63.9) | 2 (4.1) | ||
| 3-5 | 7 (19.4) | 15 (30.6) | ||
| >5 | 6 (16.7) | 32 (65.3) | ||
| Histological grade | 3.470 | .062 | ||
| Well/moderately differentiated | 19 (52.8) | 16 (32.7) | ||
| Poorly differentiated/undifferentiated | 17 (47.2) | 33 (67.3) | ||
| Borrmann type | 20.178 | 1.559 × 10−4 | ||
| I | 3 (8.3) | 2 (4.1) | ||
| II | 21 (58.4) | 8 (16.3) | ||
| III | 7 (19.4) | 14 (28.6) | ||
| IV | 5 (13.9) | 25 (51.0) | ||
| Invasive depth | 32.468 | 1.212 × 10−8 | ||
| T1-T2 | 29 (80.6) | 9 (18.4) | ||
| T3-T4 | 7 (19.4) | 40 (81.6) | ||
| Lymph node status | 48.412 | 1.739 × 10−10 | ||
| N0 | 34 (94.4) | 9 (18.4) | ||
| N1 | 1 (2.8) | 9 (18.4) | ||
| N2 | 1 (2.8) | 11 (22.4) | ||
| N3 | 0 (0.0) | 20 (40.8) | ||
| CEA | 17.378 | 3.064 × 10−5 | ||
| Normal | 35 (97.2) | 28 (57.1) | ||
| High | 1 (2.8) | 21 (42.9) | ||
| CA724 | 19.974 | 7.852 × 10−6 | ||
| Normal | 35 (97.2) | 26 (53.1) | ||
| High | 1 (2.8) | 23 (46.9) | ||
| CA199 | 19.215 | 2.805 × 10−6 | ||
| Normal | 36 (100.0) | 29 (59.2) | ||
| High | 0 (0.0) | 20 (40.8) | ||
| Tumor emboli in the microvessels | 42.955 | 5.600 × 10−11 | ||
| Negative | 30 (83.3) | 6 (12.2) | ||
| Positive | 6 (16.7) | 43 (87.8) |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CK20, cytokeratin 20; mRNA, messenger RNA.
Positive CK20 expression in peripheral blood postoperatively was closely related to tumor diameter (P = 8.271 × 10−9), Borrmann type (P = 1.559 × 10−4), invasive depth (P = 1.212 × 10−8), lymphatic metastasis (P = 1.739 × 10−10), microvessel invasion (P = 5.600 × 10−11), and cancer-related antigens, including CEA (P = 3.064 × 10−5), CA724 (P = 7.852 × 10−6), and CA199 (P = 2.805 × 10−6). Similar to findings for preoperative CK20 expression, there was no association between CK20 and demographic factors and tumor location (P > .05). Additionally, there was no association between CK20 and the degree of differentiation (P = .062).
Cytokeratin 20 mRNA Levels in Postoperative Peripheral Blood Compared With Preoperative Levels
Among the 85 patients with GC, 35 (41.2%) showed positive CK20 expression in preoperative peripheral blood, while 49 (57.6%) had increased CK20 expression in postoperative peripheral blood. There was a significant increase in CK20 expression in postoperative peripheral blood compared with preoperative levels (χ2 = 4.612, P = .032).
We analyzed the clinicopathological features of patients with positive CK20 mRNA expression in postoperative blood alone compared to 50 patients with negative preoperative expression of CK20 mRNA. We found that expression of CK20 mRNA in postoperative blood alone was related to age (P = .012), tumor diameter (P = .001), invasive depth (P = .031), lymph node status (P = .016), and tumor emboli in the microvessels (P = 3.339 × 10−5). The associations between postoperative positive CK20 mRNA expression in peripheral blood alone and clinicopathological characteristics are shown in Table 3.
Table 3.
Correlations Between Postoperative Positive CK20 mRNA Expression in Blood Alone and Clinicopathological Features of 50 Patients With Preoperative Negative CK20 mRNA Expression.
| Clinicopathological Feature | Negative, n (%) | Positive, n (%) | χ2 Test | P Value |
|---|---|---|---|---|
| 36 Cases (72.0%) | 14 Cases (28.0%) | |||
| Gender | 0.22 | .187 | ||
| Male | 26 (72.2) | 7 (50.0) | ||
| Female | 10 (27.8) | 7 (50.0) | ||
| Age (years) | 6.34 | .012 | ||
| ≥60 | 32 (88.9) | 8 (57.1) | ||
| <60 | 4 (11.1) | 6 (42.9) | ||
| Tumor location | 4.20 | .241 | ||
| Upper | 10 (27.8) | 6 (42.9) | ||
| Middle | 13 (36.1) | 2 (14.2) | ||
| Lower | 10 (27.8) | 6 (42.9) | ||
| Diffuse/multiple lesions | 3 (8.3) | 0 (6.1) | ||
| Tumor diameter (cm) | 13.77 | .001 | ||
| <3 | 23 (63.9) | 1 (7.1) | ||
| 3-5 | 7 (19.4) | 5 (35.7) | ||
| >5 | 6 (16.7) | 8 (57.2) | ||
| Histological grade | 0.031 | .860 | ||
| Well/moderately differentiated | 19 (52.8) | 7 (50.0) | ||
| Poorly differentiated/undifferentiated | 17 (47.2) | 7 (50.0) | ||
| Borrmann type | 2.46 | .482 | ||
| I | 3 (8.3) | 2 (14.3) | ||
| II | 21 (58.4) | 5 (35.7) | ||
| III | 7 (19.4) | 5 (35.7) | ||
| IV | 5 (13.9) | 2 (14.3) | ||
| Invasive depth | 4.66 | .031 | ||
| T1-T2 | 29 (80.6) | 7 (50.0) | ||
| T3-T4 | 7 (19.4) | 7 (50.0) | ||
| Lymph node status | 8.25 | .016 | ||
| N0 | 34 (94.4) | 9 (64.3) | ||
| N1 | 1 (2.8) | 4 (28.6) | ||
| N2 | 1 (2.8) | 1 (7.1) | ||
| CEA | 0.50 | .479 | ||
| Normal | 35 (97.2) | 13 (57.1) | ||
| High | 1 (2.8) | 1 (42.9) | ||
| CA724 | 0.50 | .479 | ||
| Normal | 35 (97.2) | 13 (53.1) | ||
| High | 1 (2.8) | 1 (46.9) | ||
| CA199 | 5.37 | .074 | ||
| Normal | 36 (100.0) | 12 (59.2) | ||
| High | 0 (0.0) | 2 (40.8) | ||
| Tumor emboli in the microvessels | 17.25 | 3.339 × 10−5 | ||
| Negative | 30 (83.3) | 3 (12.2) | ||
| Positive | 6 (16.7) | 11 (87.8) |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CK20, cytokeratin 20; mRNA, messenger RNA.
Correlation Between Peripheral Blood CK20 mRNA Levels and Patient Survival
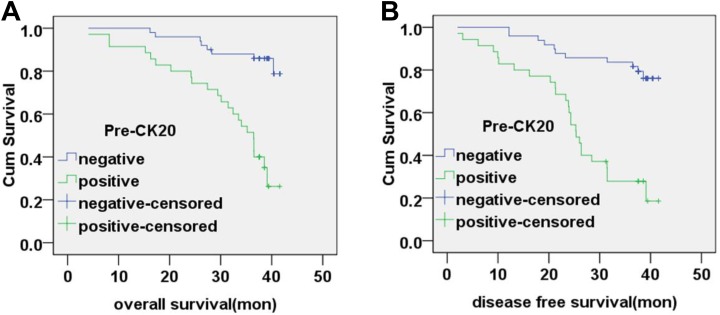
All patients underwent follow-up until cancer-related death or more than 3 years after tumor resection. The median follow-up interval was 39.2 months. The overall survival (OS) time was 39.20 ± 0.87 months (95% confidence interval [CI]: 37.51-40.90) for preoperative CK20-negative patients and 31.62 ± 1.84 months (95% CI: 28.01-35.23) for preoperative CK20-positive patients. The disease-free survival (DFS) time was 37.72 ± 1.19 months (95% CI: 35.38-40.05) for preoperative CK20-negative patients and 25.99 ± 2.01 months (95% CI: 22.06-29.03) for preoperative CK20-positive patients. The OS and DFS time for preoperative CK20-positive patients was significantly poorer than that of CK20-negative patients (χ2 = 24.13, P < .01; χ2 = 25.68, P < .01; respectively). Correlation between preoperative CK20 mRNA levels and patient survival are shown in Figure 3.
Figure 3.
Correlation between preoperative CK20 mRNA expression and patient survival. A, The overall survival rate of patients based on preoperative CK20-negative and CK20-positive expression in peripheral blood (P < .01). B, The disease-free survival rate of patients based on preoperative CK20-negative and CK20-positive expression in peripheral blood (P < .01). CK20 indicates cytokeratin 20; mRNA, messenger RNA.
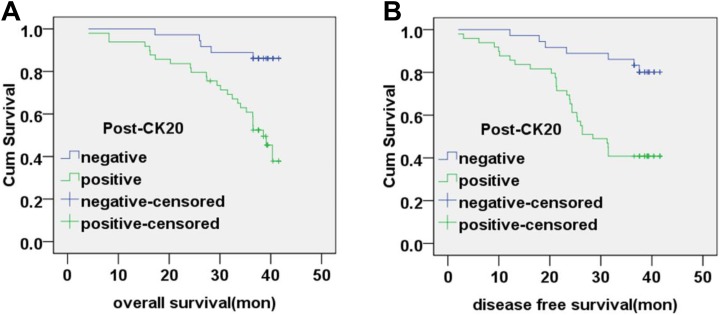
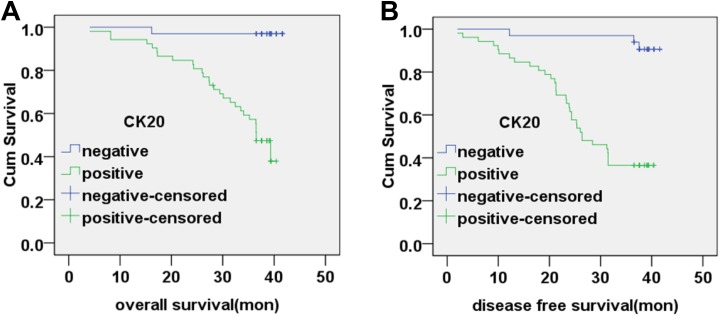
The OS time was 39.52 ± 0.93 months (95% CI, 37.69-41.35) for postoperative CK20-negative patients and 33.73 ± 1.50 months (95% CI: 30.77-36.69) for postoperative CK20-positive patients. The DFS time was 38.41 ± 1.26 months (95% CI: 35.94-40.87) for postoperative CK20-negative patients and 29.01 ± 1.76 months (95% CI, 25.57-32.46) for postoperative CK20-positive patients. The OS and DFS time for postoperative CK20-positive patients was significantly poorer than that of CK20-negative patients (χ2 = 13.613, P < .01; χ2 = 11.065, P < .01; respectively). Correlation between postoperative CK20 mRNA levels and patient survival is shown in Figure 4.
Figure 4.
Correlation between postoperative CK20 mRNA expression and patient survival. A, The overall survival rate of patients based on postoperative CK20-negative and CK20-positive expression in peripheral blood (P < .01). B, The disease-free survival rate of patients based on postoperative CK20-negative and CK20-positive expression in peripheral blood (P < .01). CK20 indicates cytokeratin 20; mRNA, messenger RNA.
Detection of CK20 Expression in Lymph Nodes by IHC and qRT-PCR
A total of 1568 lymph nodes were removed from 85 patients with GC, with a median of 18.5 (range: 7-90) lymph nodes per patient. By H&E staining, 414 lymph nodes and 42 patients were positive for metastasis, with a median of 4.8 (range: 1-89) lymph nodes per patient. Lymph nodes positive for metastasis by H&E staining were all positive via pan-CK IHC. For specimens detected by CK20-IHC, 44 (10.6%) lymph nodes positive for metastasis by H&E staining showed negative CK expression. All lymph nodes that were negative for metastasis by H&E staining were further evaluated for CK staining using pan-CK IHC. Among these, an additional 28 (2.43%) lymph nodes representing 16 patients showed positive pan-CK expression by IHC. When evaluated by qRT-PCR, an additional 137 (11.87%) lymph nodes representing 34 patients showed positive CK20 mRNA expression. All IHC-positive lymph nodes were confirmed as positive by qRT-PCR. The sensitivity of qRT-PCR was significantly higher than H&E staining and IHC in GC lymph nodes (χ2 = 31.87, P < .01), and there was a statistically significant difference between CK20 detection by RT-PCR compared with pan-CK detection by IHC in lymph nodes negative for metastasis by H&E staining (χ2 = 9.18, P = .002).
Based on the expression of CK20 mRNA, we identified 137 lymph nodes and 34 patients with micrometastases. This resulted in a change in clinicopathological staging in 22 cases. Correlation between the postoperative CK20 mRNA expression in lymph nodes and clinicopathological factors was closely related to the degree of differentiation (P = 3.19 × 10−4), invasive depth (P = 4.188 × 10−5), microvessel invasion (P = 1.673 × 10−4), the tumor diameter (P = .019), Borrmann type (P = .049), tumor-related antigens including CEA (P = .034), CA724 (P = .008), and CA199 (P = .037), as well as preoperative (P = .024) and postoperative CK20 expression in peripheral blood (P = .004). However, no association was observed between postoperative CK20 mRNA expression and demographic characteristics, tumor location, or the status of lymphatic metastasis (P > .05). The correlation between CK20 mRNA levels in lymph nodes and clinicopathological characteristics is shown in Table 4.
Table 4.
Correlations Between CK20 mRNA Expression in Lymph Nodes and Clinicopathological Features of 85 Patients With Gastric Cancer.
| Clinicopathological Feature | Negative, n (%) | Positive, n (%) | χ2 Test | P Value |
|---|---|---|---|---|
| 51 Cases (60.0%) | 34 Cases (40.0%) | |||
| Gender | 1.561 | .212 | ||
| Male | 38 (74.5) | 21 (61.7) | ||
| Female | 13 (25.5) | 13 (38.2) | ||
| Age (years) | 0.554 | .457 | ||
| ≥60 | 41 (80.4) | 25 (73.5) | ||
| <60 | 10 (19.6) | 9 (26.5) | ||
| Tumor location | 0.151 | .985 | ||
| Upper | 19 (37.2) | 13 (38.2) | ||
| Middle | 14 (27.5) | 9 (26.5) | ||
| Lower | 14 (27.5) | 10 (29.4) | ||
| Diffuse/multiple lesions | 4 (7.8) | 2 (5.9) | ||
| Tumor diameter (cm) | 7.976 | .019 | ||
| <3 | 20 (39.2) | 5 (14.7) | ||
| 3-5 | 14 (27.5) | 8 (23.5) | ||
| >5 | 17 (33.3) | 21 (61.8) | ||
| Histological grade | 12.952 | 3.19 × 10−4 | ||
| Well/moderately differentiated | 29 (56.9) | 6 (17.6) | ||
| Poorly differentiated/undifferentiated | 22 (43.1) | 28 (82.4) | ||
| Borrmann type | 7.787 | .049 | ||
| I | 5 (9.8) | 0 (0.0) | ||
| II | 21 (41.2) | 8 (23.5) | ||
| III | 10 (19.6) | 11 (32.4) | ||
| IV | 15 (29.4) | 15 (44.1) | ||
| Lymph node status (H&E staining) | 4.194 | .241 | ||
| N0 | 31 (60.8) | 12 (35.3) | ||
| N1 | 7 (13.7) | 3 (8.8) | ||
| N2 | 5 (9.8) | 7 (20.6) | ||
| N3 | 8 (15.7) | 12 (35.3) | ||
| Invasive depth | 16.784 | 4.188 × 10−5 | ||
| T1-T2 | 32 (62.7) | 6 (17.6) | ||
| T3-T4 | 19 (37.3) | 28 (82.4) | ||
| CEA | 4.508 | .034 | ||
| Normal | 42 (82.4) | 21 (61.8) | ||
| High | 9 (17.6) | 13 (38.2) | ||
| CA724 | 7.054 | .008 | ||
| Normal | 42 (82.4) | 19 (55.9) | ||
| High | 9 (17.6) | 15 (44.1) | ||
| CA199 | 4.359 | .037 | ||
| Normal | 42 (82.4) | 23 (67.6) | ||
| High | 9 (17.6) | 11 (32.4) | ||
| Tumor emboli in the microvessels | 14.167 | 1.673 × 10−4 | ||
| Negative | 30 (58.8) | 6 (17.6) | ||
| Positive | 21 (41.2) | 28 (82.4) | ||
| Pre-CK20 in peripheral blood | 5.006 | .024 | ||
| Negative | 35 (68.6) | 15 (44.1) | ||
| Positive | 16 (31.4) | 19 (55.8) | ||
| Post-CK20 in peripheral blood | 8.224 | .004 | ||
| Negative | 28 (54.9) | 8 (23.5) | ||
| Positive | 23 (45.1) | 26 (76.5) |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CK20, cytokeratin 20; H&E, hematoxylin and eosin; mRNA, messenger RNA.
Correlation Between CK20 mRNA in Lymph Nodes and Patient Survival
The OS time was 38.76 ± 1.39 months (95% CI: 36.03-41.49) for CK20 mRNA-negative lymph node patients and 34.59 ± 1.32 months (95% CI: 31.99-37.16) for CK20 mRNA-positive lymph node patients. The OS time for CK20 mRNA-positive lymph node patients was significantly poorer than that of CK20 mRNA-negative lymph node patients (χ2 = 22.435, P < .01).
The DFS time was 38.41 ± 1.26 months (95% CI, 38.64-42.13) for CK20 mRNA-negative lymph node patients and 29.01 ± 1.76 months (95% CI, 25.57-32.46) for CK20 mRNA-positive lymph node patients. The DFS time for CK20 mRNA-positive lymph node patients was significantly poorer than that of negative patients (χ2 = 24.042, P < .01). Correlation between the CK20 mRNA status in lymph nodes and patient survival is shown in Figure 5.
Figure 5.
Correlation between CK20 mRNA expression in lymph nodes and patient survival. A, The overall survival rate of patients based on CK20-positive expression and CK20-negative expression in lymph nodes (P < .01). B, The disease-free survival rate of patients based on CK20-positive expression and CK20-negative expression in lymph nodes (P < .01). CK20 indicates cytokeratin 20; mRNA, messenger RNA.
Factors Associated With Survival of Patients With GC
On univariate analysis, tumor diameter, histological grade, Borrmann type, invasive depth, CEA, CA724, CA199, tumor emboli in the microvessels, preoperative CK20 in peripheral blood, postoperative CK20 in peripheral blood, and lymph node metastasis status by H&E staining, pan-CK expression by IHC, and CK20 mRNA expression by qPCR were significantly associated with survival (Table 5). On Cox regression analysis, histological grade, preoperative CK20 in peripheral blood, postoperative CK20 in peripheral blood, and lymph node status by H&E staining, pan-CK expression by IHC, and CK20 mRNA expression by qPCR were independently associated with survival of patients with GC (Table 6).
Table 5.
Univariate Analysis Showing Variables Associated With Survival of Patients With Gastric Cancer.
| Clinicopathological Feature | Died, n (%) | Survived, n (%) | χ2 Test | P Value |
|---|---|---|---|---|
| 29 Cases (34.1%) | 56 Cases (65.9%) | |||
| Gender | 0.004 | .95 | ||
| Male | 20 (69.0) | 39 (69.6) | ||
| Female | 9 (31.0) | 17 (30.4) | ||
| Age (years) | 3.73 | .06 | ||
| ≥60 | 19 (65.5) | 47 (83.9) | ||
| <60 | 10 (34.5) | 9 (16.1) | ||
| Tumor location | 3.72 | .29 | ||
| Upper | 10 (34.5) | 22 (39.3) | ||
| Middle | 6 (20.7) | 17 (30.3) | ||
| Lower | 9 (31.0) | 15 (26.8) | ||
| Diffuse/multiple lesions | 4 (13.8) | 2 (3.6) | ||
| Tumor diameter (cm) | 13.69 | .001 | ||
| <3 | 4 (13.8) | 21 (37.5) | ||
| 3-5 | 4 (13.8) | 18 (32.1) | ||
| >5 | 21 (72.4) | 17 (30.4) | ||
| Histological grade | 21.35 | 3.81 × 10−6 | ||
| Well/moderately differentiated | 2 (6.9) | 33 (58.9) | ||
| Poorly differentiated/undifferentiated | 27 (93.1) | 23 (41.1) | ||
| Borrmann type | 10.34 | .016 | ||
| I | 0 (0.0) | 5 (8.9) | ||
| II | 5 (17.3) | 24 (42.9) | ||
| III | 9 (31.0) | 12 (21.4) | ||
| IV | 15 (51.7) | 15 (26.8) | ||
| Invasive depth | 7.53 | .006 | ||
| T1-T2 | 7 (24.1) | 31 (55.4) | ||
| T3-T4 | 22 (75.9) | 25 (44.6) | ||
| CEA | 8.23 | .004 | ||
| Normal | 16 (55.2) | 47 (83.9) | ||
| High | 13 (44.8) | 9 (16.1) | ||
| CA724 | 5.98 | .014 | ||
| Normal | 16 (55.2) | 45 (80.4) | ||
| High | 13 (44.8) | 11 (19.6) | ||
| CA199 | 7.79 | .005 | ||
| Normal | 17 (58.6) | 48 (85.7) | ||
| High | 12 (41.4) | 8 (14.3) | ||
| Tumor emboli in the microvessels | 11.36 | .001 | ||
| Negative | 5 (17.2) | 31 (55.4) | ||
| Positive | 24 (82.8) | 25 (44.6) | ||
| Pre-CK20 in peripheral blood | 17.73 | 2.54 × 10−5 | ||
| Negative | 8 (27.6) | 42 (75.0) | ||
| Positive | 21 (72.4) | 14 (25.0) | ||
| Post-CK20 in peripheral blood | 1.36 | .001 | ||
| Negative | 5 (17.2) | 31 (55.4) | ||
| Positive | 24 (82.8) | 25 (44.6) | ||
| Lymph node status (H&E staining) | 19.58 | 9.63 × 10−6 | ||
| Negative | 5 (17.2) | 38 (67.9) | ||
| Positive | 24 (82.8) | 18 (32.1) | ||
| Lymph node status (IHC pan-CK) | 28.49 | 9.37 × 10−8 | ||
| Negative | 2 (6.9) | 38 (67.9) | ||
| Positive | 27 (93.1) | 18 (32.1) | ||
| Lymph node status (PCR CK20) | 18.89 | 1.38 × 10−5 | ||
| Negative | 2 (6.9) | 31 (55.4) | ||
| Positive | 27 (93.1) | 25 (44.6) |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CK20, cytokeratin 20; H&E, hematoxylin and eosin; IHC, immunocytochemistry; PCR, polymerase chain reaction.
Table 6.
Cox Regression Analysis Showing Variables Independently Associated With Survival of Patients With Gastric Cancer.
| B | SE | Wald | Significance | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Histological grade | −1.87 | 0.61 | 9.29 | 0.002 | 0.156 | 0.05 | 0.51 |
| Preoperative CK20 in peripheral blood | 1.62 | 0.43 | 14.00 | <0.001 | 5.04 | 2.16 | 11.77 |
| Postoperative CK20 in peripheral blood | 1.14 | 0.52 | 4.80 | 0.03 | 3.13 | 1.13 | 8.71 |
| Lymph node status (H&E staining) | −1.52 | 0.64 | 5.58 | 0.02 | 0.22 | 0.06 | 0.77 |
| Lymph node status (IHC pan-CK) | 4.25 | 0.95 | 19.83 | <0.001 | 70.01 | 10.79 | 454.25 |
| Lymph node status (PCR CK-20) | 1.87 | 0.76 | 6.10 | 0.01 | 6.48 | 1.47 | 28.57 |
Abbreviations: CA, carbohydrate antigen; CEA, carcino-embryonic antigen; CI, confidence interval; CK20, cytokeratin 20; H&E, hematoxylin and eosin; IHC, immunocytochemistry; PCR, polymerase chain reaction; SE, standard error.
Discussion
Despite efforts regarding prevention, early diagnosis, and improved therapeutic strategies, mortality rates for patients with GC with resectable tumors remain high. Invasion and distant metastasis are the leading factors influencing the clinical outcome of patients with GC.30 Several studies have also shown that peripheral blood and lymph node micrometastasis prior to surgery might be key indicators for GC recurrence and distant metastasis.17,31,32 Therefore, identifying markers to determine the potential of metastasis and disease progression in patients with GC will help us tailor postoperative adjuvant therapies and improve clinical outcomes. However, currently, there are no markers available that can predict peripheral blood or lymph node metastasis or evaluate the prognosis of patients with GC.
Previous studies have shown that CK18 and CK19 mRNA could be detected in peripheral blood using nested RT-PCR in healthy participants, but CK20 mRNA could not be detected.33 Our results indicated that CK20 mRNA is expressed in different GC cell lines and that detection of CK20 using RT-PCR is a relatively sensitive and specific method to distinguish gastric cells from PBMNs. We also found that none of the patients with benign neoplasm showed positive CK20 expression in the peripheral blood before or after surgery. The CK20 was only expressed in the peripheral blood from patients with GC. Because CK20 is specifically found in gastrointestinal epithelium,34,35 our results indicate that epithelial cells or epithelial carcinoma cells exist in the peripheral blood circulation.
In our study, we detected 35 (41.2%) of 85 patients with primary GC with positive CK20 expression by qRT-PCR prior to surgical resection. In contrast, we detected 49 (57.6%) of 85 patients with positive CK20 expression by qRT-PCR on the first day after the operation. Postoperatively, there was no association between positive CK20 mRNA detection and histological grade (P > .05). Our findings indicate that micrometastasis in the peripheral blood is associated with surgery and that the operative procedure may be a factor in promoting micrometastasis. We also found that OS and DFS for patients with positive CK20 expression were significantly poorer than that of patients with negative CK20 expression (P < .01). This suggests that CK20 expression in the peripheral blood is indicative of micrometastasis and poor prognosis in patients with GC.
Currently, micrometastases in lymph nodes are defined as tumor cell clusters of between 0.2 and 2.0 mm in size in the greatest dimension. According to the seventh TNM classification by the International Union Against Cancer,36 lymph node micrometastasis should be reflected in the staging of the disease. The IHC is a widely accepted technique for detecting lymph node micrometastasis in GC using epithelial markers such as CK.37 Using IHC, Cai et al reported the incidence of lymph node micrometastasis was 25% in T1b GC,38 while Kim et al reported a 10% incidence of lymph node micrometastasis in pT1N0 GC.24 In our study, we found that 44 (10.6%) lymph nodes positive for micrometastasis based on H&E staining were negative for CK20 expression based on IHC, indicating that use of an anti-CK20 antibody to detect lymph node micrometastases may result in false negatives. However, all lymph nodes positive for micrometastasis based on H&E staining were also positive for pan-CK expression based on IHC, and the incidence of lymph node micrometastasis based on pan-CK IHC was 2.43% (28/1154) among the lymph nodes that were called as negative by H&E staining.
When micrometastasis is detected by RT-PCR, CK is usually employed as a target marker.39,40 The RT-PCR was previously shown to increase the detection rate of micrometastasis to a level higher than that achieved by IHC.40 According to the report by Arigami et al, RT-PCR identified lymph node micrometastasis in 31.3% of patients, whereas IHC detected lymph node micrometastasis in 11.3% of patients.41 Similarly, we found the sensitivity of qRT-PCR was significantly higher than IHC (P < .01).
According to the seventh edition of the AJCC staging system,28 if the number of macrometastatic nodes is less than 15, detection of only one micrometastasis could change the N stage. From our study in which the expression of CK20 mRNA was detected in lymph nodes, we identified 137 lymph nodes in 34 patients with micrometastases. Our findings changed the clinicopathological staging in 22 cases, and we found that micrometastasis in lymph nodes is significantly related to indicators of poor prognosis.
Conclusions
This study showed that CK20 mRNA expression in the lymph nodes is indicative of lymph node micrometastasis. Until now, the clinical impact of lymph node micrometastasis on GC prognosis was controversial. Our results indicate that expression of CK20 mRNA in lymph nodes was significantly associated with reduced OS and DFS. Therefore, detecting CK20 mRNA expression in the peripheral blood and lymph nodes might help to diagnose micrometastasis in the circulation and thus identify patients with GC with poor prognosis. Indeed, micrometastasis is thought to be a key etiology of recurrence and distant metastasis after resection of primary gastric tumors. We suggest the status of micrometastases in the peripheral blood in patients with early or advanced GC should be investigated at an early stage, so their treatment plan can be adjusted accordingly. In addition, the detection of micrometastases in the lymph nodes can allow more accurate clinicopathological staging of GC to guide treatment and improve prognosis.
Abbreviations
- cDNA
complementary DNA
- CA
carbohydrate antigen
- CEA
carcino-embryonic antigen
- CI
confidence interval
- CK20
cytokeratin 20
- DFS
disease-free survival
- GC
gastric cancer
- H&E
hematoxylin and eosin
- IHC
immunocytochemistry
- mRNA
messenger RNA
- OR
odds ratio
- OS
overall survival
- PBMN
peripheral blood mononuclear cell
- qRT-PCR
quantitative real-time polymerase chain reaction
- ROC
receiver operating characteristic
- TNM
tumor–node–metastasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by China Postdoctoral Science Foundation (grant number 2018M632400), Scienceand Technology Support Program (Social Development) Project of Taizhou City (grant number TS201824), and the National Natural Science Foundation of China (grant numbers 81172279 and 81572343).
ORCID iD: Xiaolan You  https://orcid.org/0000-0002-5982-0316
https://orcid.org/0000-0002-5982-0316
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(5):531–546. [DOI] [PubMed] [Google Scholar]
- 4. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40(5):584–591. [DOI] [PubMed] [Google Scholar]
- 5. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14(2):87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maehara Y, Oshiro T, Endo K, et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119(4):397–402. [DOI] [PubMed] [Google Scholar]
- 8. Siewert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg. 1996;83(8):1144–1147. [DOI] [PubMed] [Google Scholar]
- 9. Ishida K, Katsuyama T, Sugiyama A, Kawasaki S. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer. 1997;79(6):1069–1076. [DOI] [PubMed] [Google Scholar]
- 10. Yin T, Ji XL, Shen MS. Relationship between lymph node sinuses with blood and lymphatic metastasis of gastric cancer. World J Gastroenterol. 2003;9(1):40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou YN, Xu CP, Han B, et al. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8(6):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Culp LA, Lin W, Kleinman NR, O’Connor KL, Lechner R. Earliest steps in primary tumor formation and micrometastasis resolved with histochemical markers of gene-tagged tumor cells. J Histochem Cytochem. 1998;46(5):557–568. [DOI] [PubMed] [Google Scholar]
- 13. Funke I, Schraut W. Meta-analyses of studies on bone marrow micrometastases: an independent prognostic impact remains to be substantiated. J Clin Oncol. 1998;16(2):557–566. [DOI] [PubMed] [Google Scholar]
- 14. Timar J, Csuka O, Orosz Z, Jeney A, Kopper L. Molecular pathology of tumor metastasis. Pathol Oncol Res. 2002;8(3):204–219. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi N, Ito I, Yanagisawa A, et al. Genetic diagnosis of lymph node metastasis in colorectal cancer. Lancet. 1995;345(8960):1257–1259. [DOI] [PubMed] [Google Scholar]
- 16. Shi J, Qu Y, Li X, et al. Increased expression of EHF via gene amplification contributes to the activation of HER family signaling and associates with poor survival in gastric cancer. Cell Death Dis. 2016;7(10):e2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiao YF, Chen CG, Yue J, Ma MQ, Ma Z, Yu ZT. Prognostic significance of preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of patients with gastric cardia cancer. World J Gastroenterol. 2017;23(8):1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng X, Fan L, Zhou P, et al. Detection of circulating tumor cells and circulating tumor microemboli in gastric cancer. Transl Oncol. 2017;10(3):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang C, Hu X, Liu XY, et al. Effect of tumor-associated macrophages on gastric cancer stem cell in omental milky spots and lymph node micrometastasis. IntJ Clin Exp Pathol. 2015;8(11):13795–13805. [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng B, Ni CH, Chen H, et al. New evidence guiding extent of lymphadenectomy for esophagogastric junction tumor: application of Ber-Ep4 joint with CD44v6 staining on the detection of lower mediastinal lymph node micrometastasis and survival analysis. Medicine (Baltimore). 2017;96(14):e6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St. Clair CM, Eriksson AGZ, Ducie JA, et al. Low-volume lymph node metastasis discovered during sentinel lymph node mapping for endometrial carcinoma. Ann Surg Oncoly. 2016;23(5):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeuck TL, Wittekind C. Gastric carcinoma: stage migration by immunohistochemically detected lymph node micrometastases. Gastric Cancer. 2015;18(1):100–108. [DOI] [PubMed] [Google Scholar]
- 23. Ishii K, Kinami S, Funaki K, et al. Detection of sentinel and non-sentinel lymph node micrometastases by complete serial sectioning and immunohistochemical analysis for gastric cancer. J Exp Clin Cancer Res. 2008;27(5):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35(4):409–414. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Yu JC, Kang WM, Wang WZ, Liu YQ, Gu P. The predictive effect of cadherin-17 on lymph node micrometastasis in pN0 gastric cancer. Ann Surg Oncol. 2012;19(5):1529–1534. [DOI] [PubMed] [Google Scholar]
- 26. Kim JH, Park JM, Jung CW, et al. The significances of lymph node micrometastasis and its correlation with E-cadherin expression in pT1-T3N0 gastric adenocarcinoma. J Surg Oncol. 2008;97(2):125–130. [DOI] [PubMed] [Google Scholar]
- 27. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113–123. [DOI] [PubMed] [Google Scholar]
- 28. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 29. You X, Wang Y, Wu J, et al. Prognostic significance of galectin-1 and vasculogenic mimicry in patients with gastric cancer. Onco Targets Ther. 2018. May;11(5):3237–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang Z, Sheng H, Zheng X, et al. Upregulation of circulating cytokeratin 20, urokinase plasminogen activator and C-reactive protein is associated with poor prognosis in gastric cancer. Mol Clin Oncol. 2015;3(6):1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yong JZ, Chun DZ, Dong QD. Impact of lymph node micrometastasis on gastric carcinoma prognosis: a meta-analysis. World J Gastroenterol. 2015;21(5):1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou J, Ma X, Bi F, Liu M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget. 2017;8(15):25713–25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Denis MG, Lipart C, Lebhorgne J, et al. Detection of disseminated tumor cells in peripheral blood of colorectal cancer patients. Int J Cancer. 1997,74(5):540–544. [DOI] [PubMed] [Google Scholar]
- 34. Burchill SA, Selby PJ. Molecular detection of low-level disease in patients with cancer. J Pathol. 2000;190(1):6–14. [DOI] [PubMed] [Google Scholar]
- 35. Natsugoe S, Nakashima S, Matsumoto M, et al. Paraaortic lymph node micrometastasis and tumor cell microinvolvement in advanced gastric carcinoma. Gastric Cancer. 1999;2(3):179–185. [DOI] [PubMed] [Google Scholar]
- 36. Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7−/20+ phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ru Y, Zhang L, Chen Q, et al. Detection and clinical significance of lymph node micrometastasis in gastric cardia adenocarcinoma. J Int Med Res. 2012;40(1):293–299. [DOI] [PubMed] [Google Scholar]
- 38. Cai J, Ikeguchi M, Maeta M, Kaibara N. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127(1):32–39. [DOI] [PubMed] [Google Scholar]
- 39. Wu ZY, Li JH, Zhan WH, He YL, Wan J. Effect of lymph node micrometastases on prognosis of gastric carcinoma. World J Gastroenterol .2007;13(30):4122–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsumoto M, Natsugoe S, Ishigami S, et al. CK20 indicates cytokeratin 20; mRNA, messenger RNA. Surgery. 2002;131(6):630–635. [DOI] [PubMed] [Google Scholar]
- 41. Arigami T, Natsugoe S, Uenosono Y, et al. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer. 2005;93(6):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]