Abstract
Background:
Repository corticotropin injection (RCI) has regulatory approval for many indications, including symptomatic sarcoidosis. This large case series of patients with advanced symptomatic sarcoidosis treated with RCI describes patient characteristics, RCI utilization patterns, concomitant therapies, and physicians’ assessments of treatment response.
Methods:
Patients ⩾18 years with symptomatic sarcoidosis, treated with RCI in the previous 36 months, who had completed a course of RCI or received RCI for ⩾6 months at the time of data collection were included.
Results:
The study included 302 patients (mean age, 51 years; 52%, women) with a mean 4.8 years since initial diagnosis of sarcoidosis. Most patients (76%) had extrapulmonary involvement, primarily in the skin (28%), joints (25%), heart (22%), and eyes (22%); 34% had multiple (⩾2) organ involvement. The mean duration of RCI treatment was 32.5 weeks, with 61.6% of patients continuing RCI therapy for ⩾6 months. The RCI utilization pattern indicated an individualized approach to therapy, with a higher starting dose associated with a shorter duration of therapy compared with a lower starting dose. The percentage of patients who used corticosteroids decreased from 61.3% during the 3 months before initiation of RCI to 12.9% 3 months after RCI therapy; the mean daily dose of corticosteroid decreased from 18.2 mg to 9.9 mg. The proportion of patients given <10 mg/day of prednisone increased from 21% before RCI use to 47% 3 months after RCI use. According to physicians’ assessments of change in patients’ health status after RCI therapy, overall status improved in 95% of patients, overall symptoms in 73%, lung function in 38%, and inflammation in 33%.
Conclusions:
The findings suggest that RCI is a viable treatment option for patients with advanced symptomatic sarcoidosis and provide insights on patient characteristics and practice patterns to help clinicians determine appropriate use.
The reviews of this paper are available via the supplemental material section.
Keywords: Acthar Gel (natural ACTH), drug therapy, health care utilization, repository corticotropin injection, RCI, sarcoidosis
Introduction
Sarcoidosis, an inflammatory systemic granulomatous disease with unknown etiology, affects the lungs in 90% of patients and impacts mostly young and middle-aged adults.1–4 The disease can affect many extrapulmonary organs, including the eyes, joints, skin, liver, spleen, lymph nodes, salivary glands, heart, and nervous system.1 Many patients (30–50%) are asymptomatic and are diagnosed upon detection of abnormalities during chest radiographs performed for routine screening.4 Approximately 33% of patients with sarcoidosis present with constitutional symptoms such as fatigue, fever (usually low-grade), malaise, night sweats, and weight loss; 30–50% have pulmonary symptoms such as dyspnea, dry cough, and chest pains.1 Whereas most patients with sarcoidosis experience spontaneous remission, 10–30% have a chronic progressive disease that can cause severe organ impairment.4,5
The estimated overall incidence and prevalence of sarcoidosis in the United States (U.S.) are 8 and 60 cases per 100,000 persons, respectively.6 Each year in the U.S., over 25,000 patients are diagnosed with sarcoidosis, and 185,000 seek medical care for the disease.6 Rates of hospitalization for sarcoidosis increased by approximately 26% from 2005 to 2014 in the U.S.; during the same period, inhospital mortality associated with sarcoidosis decreased by approximately 26%.7 Studies have reported mortality ranging from <1% to 8% for patients with sarcoidosis, with the variability attributed to factors such as care setting, disease severity, geographic location, age, ethnicity, and sex.8 Pulmonary fibrosis with respiratory failure, cardiac complications, and neurologic involvement are the most common causes of death from sarcoidosis.5
Prednisone and repository corticotropin injection (RCI; Acthar® Gel; Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA) are the only drugs that have the U.S. Food and Drug Administration (FDA)-approved indications for sarcoidosis.9 Corticosteroids are the mainstay of systemic therapy for patients with symptomatic sarcoidosis.2,3 However, evidence links long-term and high-dose corticosteroid use with increased risk of adverse events (AEs) and higher health care utilization costs in various patient populations.10–12 Other treatment options for sarcoidosis include off-label use of antimetabolites, immunosuppressants, tumor necrosis factor inhibitors, and antimalarial agents.2,13 Limited evidence supports the use of these agents in patients with sarcoidosis.
RCI, a naturally sourced complex mixture of prolonged-release adrenocorticotropic hormone (ACTH) analogs and other pituitary peptides, acts through multiple mechanisms of action that include anti-inflammatory and immunomodulatory pathways.14–16 RCI is one of the therapies used for patients with corticosteroid-refractory disease or intolerance for corticosteroids.3,13 Two small studies reported results with RCI therapy in patients with sarcoidosis. A retrospective chart review found that, of 29 patients who received RCI treatment for ⩾3 months, 11 (38%) had objective improvements in ⩾1 organ, 16 (55%) remained stable, and two (7%) relapsed after 6 months.17 A small prospective comparison of two doses of RCI in 18 patients with advanced pulmonary sarcoidosis reported that, regardless of RCI dose, patients experienced significant reductions in prednisone dose and improvements in the diffusion capacity of the lung for carbon monoxide measurements.18 More real-world data are needed to provide guidance on the optimal applications of RCI therapy for patients with sarcoidosis.
We performed this retrospective medical record study with a large case series of patients with symptomatic sarcoidosis treated with RCI. The objectives were to describe patient demographic and clinical characteristics, utilization patterns of RCI and concomitant therapies, and physicians’ assessments of RCI effects on patients’ health status.
Methods
Study design
To obtain a representative sample of patients, we merged a national database of RCI prescribers with the American Medical Association Physician Masterfile listing. We contacted a small randomized sample from the merged listing, via telephone and email, to invite pulmonologists, rheumatologists, primary care physicians, dermatologists, cardiologists, ophthalmologists, gastroenterologists, and neurologists. Physicians from different specialties were invited because sarcoidosis may impact organs throughout the body. Physicians who had treated at least one patient with symptomatic sarcoidosis with RCI during the previous 36 months were eligible for the study.
Data were collected on the last six consecutive patients seen by participating physicians who met the study’s eligibility criteria. To ensure that high-volume sites did not dominate the sample, each eligible physician could contribute data on a maximum of six patients.
Patients
Eligible patients were adults (age ⩾18 years) with a diagnosis of sarcoidosis who had undergone treatment with RCI in the previous 36 months and who had a completely accessible medical record. Another key inclusion criterion was the completion of an individualized course of RCI (based on the disease severity and initial response of the patient) or having received RCI for ⩾6 months at the time of data collection. This criterion served to accommodate the chronic nature of sarcoidosis, which requires ongoing therapy, while allowing for a fair assessment of RCI after an adequate duration of therapy if the course of therapy had not concluded before data collection. Reasons for exclusion from the study were the absence of symptoms.
Data collection and analyses
Physicians or their designated staff persons extracted data from the medical records of qualified patients through a secure online system, with technical support available to clarify questionnaire items and minimize data errors. The data collection system incorporated real-time and post-survey data checks. The data collection process comprised a 5-min physician screener form (to ensure eligibility and agreement to participate) and a patient data collection form that took approximately 15 min per patient to complete. Data were anonymized, deidentified, and reported in the aggregate to protect patient and physician confidentiality. Collected data included demographic and clinical characteristics (e.g. comorbidities, concomitant medications, sarcoidosis symptoms, organ involvement), RCI treatment, and other medications for symptomatic sarcoidosis.
The planned sample size of ⩾300 patients was not based on formal statistical hypothesis testing because of the descriptive nature of the study. We tabulated data using IBM® SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA) and employed descriptive statistics to report findings. Descriptive statistics included mean ± standard deviation for continuous data and frequency count and proportion for categorical variables.
This study was based on pre-existing information that did not directly involve studies with human subjects. We used existing deidentified data and did not collect personal health information. An Institutional Review Board (Solutions Institutional Review Board, Little Rock, AR, USA) reviewed the study and verified it as exempt because of the lack of identifiers, use of existing data, and no direct contact with patients [45CFR46.101(b)(4): Existing Data & Specimens, No Identifiers].
Results
From September 2017 to November 2017, we screened 171 physicians, and 98 eligible physicians (57%) provided data on 302 patients with symptomatic sarcoidosis. The participating physicians specialized in pulmonology (n = 23, 23%), rheumatology (n = 18, 18%), primary care (n = 17, 17%), dermatology (n = 11, 11%), cardiology (n = 11, 11%), ophthalmology (n = 10, 10%), gastroenterology (n = 6, 6%), and neurology (n = 2, 2%).
Patient demographic and clinical characteristics
Patients had a mean age of 51 years, and 52% were women (Table 1). The most common comorbidities were hypertension (40%), hyperlipidemia (28%), and diabetes (18%); 24% of patients had no comorbid conditions. Most patients (n = 193, 64%) had chest imaging and biopsy confirmation of stage 3 or stage 4 sarcoidosis, and many (n = 90, 30%) had been hospitalized for sarcoidosis during the previous 12 months. Some patients (n = 81; 27%) had concurrent use of all three of the major medication classes described: corticosteroids (⩾5 mg/day prednisone equivalent for ⩾60 days), nonbiological oral medications (e.g. hydroxychloroquine, methotrexate, azathioprine, leflunomide, mycophenolate, other nonsteroidal oral agents), and biologics.
Table 1.
Demographic and clinical characteristics of patients with advanced symptomatic sarcoidosis.
| Characteristic | Patients with advanced symptomatic sarcoidosis (N = 302) |
|---|---|
| Mean age, years (SD) | 51 (12) |
| Sex, n (%) | |
| Women | 157 (52) |
| Men | 145 (48) |
| Age group, year, n (%) | |
| 18–24 | 1 (0.3) |
| 25–34 | 29 (9.6) |
| 35–44 | 61 (20.2) |
| 45–54 | 90 (29.8) |
| 55–64 | 78 (25.8) |
| ⩾65 | 43 (14.2) |
| Race/ethnicity, n (%)a | |
| African American | 168 (55.6) |
| American Indian/Alaska native | 10 (3.3) |
| Asian | 19 (6.3) |
| Caucasian/non-Hispanic | 78 (25.8) |
| Hispanic/Latino | 26 (8.6) |
| Don’t know | 7 (2.3) |
| Geographic regions, n (%) | |
| Northeast | 90 (29.8) |
| Midwest | 70 (23.2) |
| South | 83 (27.5) |
| West | 59 (19.5) |
| Time since diagnosis in years, mean (SD) | 4.8 (6.6) |
| Time period diagnosis in years, n (%) | |
| <1 | 53 (17.5) |
| 1–2 | 68 (22.5) |
| 2–3 | 52 (17.2) |
| 3–5 | 40 (13.2) |
| 5–10 | 51 (16.9) |
| ⩾10 | 38 (12.6) |
| Comorbid conditions, n (%)a | |
| Hypertension | 122 (40.4) |
| Hyperlipidemia | 86 (28.5) |
| Diabetes | 56 (18.5) |
| Heart conditions | 41 (13.6) |
| Respiratory conditions | 38 (12.6) |
| Gastrointestinal conditions | 33 (10.9) |
| Mood disorder | 31 (10.3) |
| Chronic joint disease/rheumatoid arthritis | 23 (7.6) |
| None | 73 (24.2) |
SD, standard deviation.
Sum >100% because respondents were allowed to check more than one category.
All 302 patients (100%) had evidence of pulmonary involvement, 42% of patients had involvement in one extrapulmonary organ, and 34% had involvement in multiple (⩾2) extrapulmonary organs (Figure 1a). As shown in Figure 1b, the most common sites of extrapulmonary involvement were skin (28%), joints (25%), heart (22%), and eyes (22%).
Figure 1.
Number of extrapulmonary organs involved (a) and sites of extrapulmonary organ involvement (b) in patients with symptomatic sarcoidosis treated with RCI (N = 302).
RCI, repository corticotropin injection.
Shortness of breath (43%), fatigue (42%), bone and joint pain (28%), wheezing/coughing (25%), and abnormal heartbeat (23%) were the most common signs and symptoms documented when patients started their most recent course of RCI therapy (Table 2). Presented symptoms tended to be mild or moderate in severity.
Table 2.
Presence and severity of signs and symptoms before initiation of RCI.
| Sign or symptoma | Presenting sign or symptom (N = 302) n (%) | Mild n |
Moderate n |
Severe n |
|---|---|---|---|---|
| Shortness of breath | 131 (43) | 42 | 69 | 20 |
| Fatigue | 126 (42) | 40 | 59 | 27 |
| Bone and joint pain | 85 (28) | 23 | 48 | 14 |
| Wheezing/coughing | 75 (25) | 24 | 41 | 10 |
| Abnormal heartbeats | 68 (23) | 29 | 35 | 4 |
| Depressed mood | 64 (21) | 30 | 25 | 9 |
| Chest pain | 63 (21) | 29 | 29 | 5 |
| Skin rash | 57 (19) | 22 | 26 | 9 |
| Anaemia | 52 (17) | 27 | 21 | 4 |
| Night sweats | 51 (17) | 21 | 24 | 6 |
| Weight loss | 51 (17) | 30 | 18 | 3 |
| Eye symptoms | 50 (17) | 16 | 26 | 8 |
RCI, repository corticotropin injection.
Physicians’ responses to the question, ‘Which symptoms did the patient have when initiating the most recent course of RCI? Indicate the severity.’
RCI treatment patterns
Of 302 patients included in the study, 242 patients (80%) had used RCI for the first time, and only 60 patients (20%) had previously used RCI. At the time of data collection, 142 patients (47%) had completed a course of RCI therapy, and 160 patients (53%) were continuing RCI therapy. The mean duration of RCI treatment was 32.5 ± 35.6 weeks. Most patients (n = 186, 62%) had continued RCI therapy for ⩾6 months, including 141 patients with an RCI treatment duration of 6 to <12 months and 45 patients with ⩾12 months of treatment. Among the 116 patients (38%) who had used RCI for <6 months, 93 patients had continued therapy for ⩽3 months, and 23 patients had received RCI treatment for 3 to < 6 months.
RCI dosing varied because patients had individualized dosing and dose adjustments. There were 49 different initial dosing regimens for RCI. Most patients (n = 122, 40%) initiated RCI at 41 U/week to 80 U/week, with a mean treatment duration of 31.4 weeks. The 113 patients (38%) who initiated RCI at >80 U/week had a mean treatment duration of 28.6 weeks, and the 67 patients (22%) who initiated RCI at ⩽40 U/week had a mean treatment duration of 41.2 weeks. These treatment patterns suggest that physicians had adopted one of these RCI treatment approaches: a higher dose with a shorter duration or a lower dose with a longer duration.
Physicians tended to maintain patients on the initial dose of RCI; 79% of patients did not have any dose adjustments, 11% had a dose reduction, 3% had a dose increase, and 7% had multiple dose adjustments.
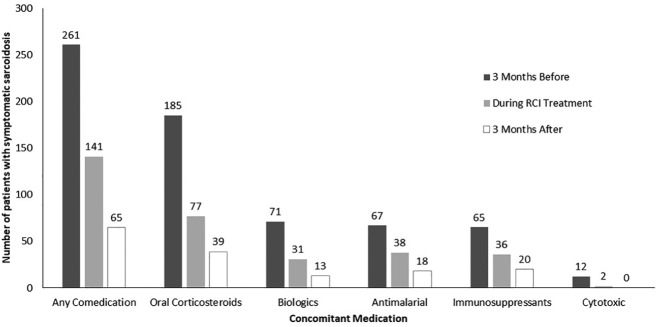
Concomitant medications
Figure 2 shows medication use for the treatment of symptomatic sarcoidosis before, during, and after RCI therapy. During the 3 months before initiating RCI therapy, 261 patients (86%) had received other medication for symptomatic sarcoidosis, including oral corticosteroids (n = 185, 61%), biologics (n = 71, 24%), and antimalarial agents (n = 67, 22%). During RCI treatment, 141 patients (47%) used concomitant medications, primarily oral corticosteroids (n = 77, 25%), immunosuppressants (n = 36, 12%), and antimalarial agents (n = 38, 13%). At 3 months after RCI treatment, 65 patients (22%) used concomitant medications, primarily oral corticosteroids (n = 39, 13%), immunosuppressants (n = 20, 7%), and antimalarial agents (n = 18, 6%). These patterns suggest that reported concomitant medication use decreased during and 3 months after RCI treatment.
Figure 2.
Concomitant medication use in patients with symptomatic sarcoidosis 3 months before, during, and 3 months after treatment with RCI (N = 302).
RCI, repository corticotropin injection.
Among 258 patients (85%) with known previous corticosteroid exposure at any time before initiation of RCI therapy, 177 patients (69%) had received high-dose prednisone (⩾10 mg/day) for ⩾6 months. Information on prednisone dose was available for 179 of 185 (97%) patients treated with corticosteroids within 3 months before initiation of RCI treatment. These patients had a mean prednisone dose of 18.2 mg/day, and 37 patients (21%) had daily doses <10 mg. Dose information was available for 38 of 39 patients (97%) treated with corticosteroids within 3 months after RCI use. These patients had a mean prednisone dose of 9.9 mg/day, and 18 patients (47%) had daily doses <10 mg.
Physicians’ assessments of improvement
According to physicians’ assessments of change in patients’ health status following RCI treatment (Table 3), most patients (95%) had improved, and 164 patients (54%) had ⩾2 types of improvements in sarcoidosis symptoms. The most commonly reported types of sarcoidosis symptom improvements were overall symptoms (73%), lung function (38%), inflammation (33%), reduction or discontinuation of corticosteroid use (32%), and improved patient quality of life (32%).
Table 3.
Physicians’ assessments of changes in patients’ health following treatment with RCI.
| Category | n (%) (N = 302) |
|---|---|
| Patient’s current statusa | |
| Improved | 288 (95) |
| Not improved | 14 (5) |
| Type of treatment responseb | |
| Overall symptoms | 219 (73) |
| Lung function | 116 (38) |
| Inflammation | 100 (33) |
| Corticosteroid use (reduction or discontinuation) | 98 (32) |
| Quality of life | 96 (32) |
| Fatigue | 88 (29) |
| Other organ functions | 39 (13) |
| Pulmonary fibrosis | 34 (11) |
| Size of granulomas (inflamed lumps) | 29 (10) |
| Reducing immunosuppressant use | 22 (7) |
| Number of granulomas (inflamed lumps) | 20 (7) |
RCI, repository corticotropin injection.
Physicians’ responses to the question, ‘What is the patient’s status as of the end of RCI therapy or the 6 months point in therapy for ongoing treatment patients?’
Physicians’ responses to the question, ‘Please select the outcomes below that have improved as a result of RCI treatment.’ Since respondents could select all options that applied, the sum exceeds 100%.
Discussion
This retrospective analysis of RCI treatment patterns and outcomes included 302 patients with advanced symptomatic sarcoidosis, with a mean of 4.8 years since initial diagnosis. Most patients had signs of advanced disease, such as chest imaging and biopsy confirmation of stage 3 or 4 sarcoidosis (64%), hospitalization for sarcoidosis during the previous 12 months (30%), or concurrent use of the three major medication classes (i.e. high-dose corticosteroids, nonbiologic oral medications, biologics; 27%). Further, 76% of patients had extrapulmonary involvement, most commonly in the skin (28%), joints (25%), heart (22%), and eyes (22%). The mean duration of RCI treatment was 32.5 weeks (7.5 months), and 61.6% of patients continued RCI therapy for ⩾6 months. Our analysis of dosing and duration of RCI use indicated an individualized approach to therapy. Patients who received a higher starting dose tended to have a shorter duration of therapy than patients who received a lower starting dose. We also observed reduced use of other medications after initiation of RCI therapy. The percentage of patients who used corticosteroids decreased from 61.3% during the 3 months before initiation of RCI therapy to 12.9% after the start of RCI therapy. During the same period, the mean daily dose of corticosteroid decreased from 18.2 mg to 9.9 mg. The proportion of patients given <10 mg/day of prednisone increased from 21% before RCI use to 47% 3 months after RCI use; 91 patients (50.8%) discontinued use of corticosteroids. According to physicians’ assessments of changes in patients’ health status at the end of RCI therapy, overall treatment response was observed in 95% of patients, symptoms improved in 73%, lung function improved in 38%, and inflammation improved in 33%.
Our study captured primary data from one of the largest sarcoidosis case series of patients. The two previous studies of RCI therapy in patients with sarcoidosis had small sample sizes and focused on the effectiveness and safety of RCI rather than the variability in the clinical use of the medication.17,18 Conducting a real-world study of patients using RCI is difficult because it is used in a small subset of patients with symptomatic sarcoidosis. The current study details RCI utilization patterns and quantifies the reduced need for background medication. Our findings add to the nascent literature on the effectiveness of RCI treatment for patients with advanced symptomatic sarcoidosis. These data are essential for generating new hypotheses to be tested in future studies.
Findings from our study are consistent with published studies such as A Case Control Etiologic Study of Sarcoidosis (ACCESS), which included 736 patients,19 with the lung being the primary organ involved and skin being the second most common organ involved. However, our patient cohort had higher rates of extrapulmonary involvement for many organs including skin (28% versus 16%), eyes (22% versus 12%), and heart (22% versus 2%), compared with ACCESS respectively.19 These findings suggest that, in our study, patients with extrapulmonary organ involvement were more likely to be treated with RCI. Further, given the higher rates of extrapulmonary involvement for our patient cohort and physician selection criteria, data were collected from a broad range of physician specialty types. On the contrary, previous studies have collected data primarily from pulmonary physicians. Our study reported improvements in health status and pulmonary function as well as reductions in prednisone dosing in patients with symptomatic sarcoidosis after initiation of RCI therapy. In a study by Baughman and colleagues,17 of 29 patients who completed 3 months of RCI therapy, 38% had objective improvements in at least one involved organ and disease in the target organs improved in 10 of 14 patients with extrapulmonary involvement. However, the treatment response rates reported in our study are about 2.5 times higher than in the study by Baughman and colleagues.17 This might be due to bias resulting from physician assessment. Baughman and colleagues reported reductions in prednisone dosages and improvement in lung function as measured by the diffusion capacity of the lung for carbon monoxide in patients who completed 24 weeks of RCI therapy.18
Our study provides real-world evidence to support an individualized dosage strategy for incorporating RCI in the treatment of patients with advanced symptomatic sarcoidosis. Analysis of RCI dosing and duration revealed a trend linking higher starting doses with shorter treatment duration and lower starting doses with longer treatment. This individualized approach seemed to have worked well for the 95% of patients who had an ‘improved’ health status according to the physicians’ assessments.
Another important treatment objective for patients with sarcoidosis is the reduction of background medications, especially oral corticosteroids, which are the first-line treatment for symptomatic sarcoidosis, although their long-term use can cause substantial toxicities. We observed reductions in corticosteroid use following RCI therapy in our study cohort. Effective and safe treatments that improve patient status may offer a viable alternative to reduce the burden of high-dose, long-term use of corticosteroids. Future research is needed to determine whether this is a useful therapeutic goal for clinical practice.
Limitations
The study is limited by its reliance on data retrospectively collected via a survey of respondents who had access to patient medical records, which might have had errors and omissions. We tried to minimize the impact of potential missing data by focusing on patients’ clinical aspects and physicians’ assessments, the types of information that are usually readily available in medical records or best known to the respondents who submitted data for our study. The study did not quantify patients’ outcomes such as diagnostic measurements and safety endpoints. Further, because of its inherent retrospective design, there may be a risk of bias, resulting in overestimation of the effectiveness of RCI based on physicians’ assessments. Because this was an exploratory and hypothesis-generating study, we did not make any comparisons between RCI and a control group or other treatments, which may also result in bias. It is important to note the following: RCI is indicated for symptomatic sarcoidosis;15 RCI is included in treatment guidelines from the Foundation for Sarcoidosis Research;11 and a phase IV, double-blind randomized placebo-controlled clinical trial is ongoing to examine the safety and efficacy of Acthar Gel in patients with pulmonary sarcoidosis (ClinicalTrials.gov Identifier: NCT03320070).
Another limitation is the use of the physicians’ assessments of improvement in a patient’s health status as a key descriptive endpoint to evaluate RCI therapy. This subjective endpoint relied on each individual physician’s interpretation of each patient’s medical record and the physician’s standards for assessing improvement. We did not collect data on test results or other objective measures that physicians might have used to make their determinations. The retrospective and noncomparative study design did not allow us to discern whether patients were responding to known concomitant treatments or to therapies not known to the physician or not recorded in the medical records. The use of physicians’ assessment may have underestimated the use of biologics in these patients; however, this cannot be fully ascertained from the current study.
Our study did not capture adverse reactions in this cohort of patients with symptomatic sarcoidosis undergoing treatment with RCI and various concomitant medications, including corticosteroids. The previously mentioned retrospective chart review by Baughman and colleagues reported that, within 3 months of initiating RCI, five patients discontinued treatment because of peripheral edema or agitation and six patients with the same AEs continued treatment after dose reduction.17 One patient had skin hyperpigmentation that did not require RCI dosage modification, and none of the patients required changes in ongoing treatment for pre-existing diabetes or hypertension. Baughman and colleagues reported that, among 18 patients enrolled in the 24-week study of RCI treatment, 1 stopped treatment within 2 weeks because of toxicity, and 7 had RCI doses halved.18 AEs reported by patients included jitteriness (n = 6), headache (n = 3), edema (n = 2), and nausea (n = 1). According to the prescribing information, the most common AEs associated with RCI treatment are fluid retention, changes in glucose tolerance, elevation in blood pressure, behavioral and mood change, increased appetite, and weight gain. Our study did not capture drivers of the decision to treat patients with RCI as well as detailed information on prior therapies (standard steroid-sparing agents such as methotrexate, leflunomide, azathioprine, mycophenolate, adalimumab, and infliximab).
The findings from our study may be generalizable to a broader population of patients with symptomatic sarcoidosis treated with RCI in the U.S. because we used probability sampling to obtain a nationally representative sample of patients. To achieve this goal, we used two national databases to obtain a random sample of 98 physicians who managed study-eligible patients. We then obtained a random sample of 302 patients under the care of these physicians.
Conclusion
We found that most patients treated with RCI responded to the treatment, especially with regard to symptoms, lung function, and inflammation. Further, patients with advanced symptomatic sarcoidosis received individualized RCI treatment that was associated with reduced use of other sarcoidosis medications, including corticosteroids. Our findings provide insights on patient characteristics and practice patterns to help clinicians determine appropriate use of RCI.
Supplemental Material
Supplemental material, Author_response_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank Winnie Nelson for assisting in the design and implementation of the study. Dr Nelson was previously employed by Mallinckrodt Pharmaceuticals.
Monica Nicosia, Global Outcomes Group, provided medical writing assistance and Esther Tazartes, Global Outcomes Group, provided editorial assistance; these services were funded by Mallinckrodt Pharmaceuticals (Bedminster, NJ, USA).
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: The study was funded by Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA.
Conflict of interest statement: All authors had full access to all of the data in this study. All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given approval for this version to be published. JK and GJW are employees of Mallinckrodt Pharmaceuticals and declare that they have no other conflicts of interest. YQ is a former employee of Mallinckrodt Pharmaceuticals and declares that he does not have any other conflict of interest. IC, JRG, KH, and SC were research consultants for the study and declare that they have no conflicts of interest.
ORCID iD: Ishveen Chopra  https://orcid.org/0000-0002-9921-1726
https://orcid.org/0000-0002-9921-1726
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Ishveen Chopra, Manticore Consultancy, Bethesda, MD, USA.
Yimin Qin, Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA.
John Kranyak, Mallinckrodt Pharmaceuticals, Bedminster, NJ, USA.
Jack R. Gallagher, Clarity Pharma Research LLC, Spartanburg, SC, USA
Kylee Heap, Clarity Pharma Research LLC, Spartanburg, SC, USA.
Susan Carroll, Clarity Pharma Research LLC, Spartanburg, SC, USA.
George J. Wan, Mallinckrodt Pharmaceuticals, 1425 U.S. Route 206, Bedminster, NJ 07921, USA.
References
- 1. Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160: 736–755. [DOI] [PubMed] [Google Scholar]
- 2. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014; 383: 1155–1167. [DOI] [PubMed] [Google Scholar]
- 3. Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and management of sarcoidosis. Am Fam Physician 2016; 93: 840–848. [PubMed] [Google Scholar]
- 4. Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician 2004; 70: 312–322. [PubMed] [Google Scholar]
- 5. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165. [DOI] [PubMed] [Google Scholar]
- 6. Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 7. Patel N, Kalra R, Doshi R, et al. Hospitalization rates, prevalence of cardiovascular manifestations, and outcomes associated with sarcoidosis in the United States. J Am Heart Assoc 2018; 7: e007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med 2014; 20: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FSR. Sarcoidosis treatment guidelines, https://www.stopsarcoidosis.org/wp-content/uploads/FSR-Physicians-Protocol1.pdf (2017, accessed 23 June 2019).
- 10. Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin 2018; 34: 1519–1527. [DOI] [PubMed] [Google Scholar]
- 11. Rice JB, White AG, Johnson M, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ 2018; 21: 846–852. [DOI] [PubMed] [Google Scholar]
- 12. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med 2015; 3: 813–822. [DOI] [PubMed] [Google Scholar]
- 14. Ross AP, Ben-Zacharia A, Harris C, et al. Multiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormone. Front Neurol 2013; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acthar Gel (repository corticotropin injection) [prescribing information]. Bedminster, NJ: Mallinckrodt ARD LLC, 2019. [Google Scholar]
- 16. Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol 2015; 27: 216–226. [DOI] [PubMed] [Google Scholar]
- 17. Baughman RP, Barney JB, O’Hare L, et al. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med 2016; 110: 66–72. [DOI] [PubMed] [Google Scholar]
- 18. Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung 2017; 195: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164: 1885–1889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records by Ishveen Chopra, Yimin Qin, John Kranyak, Jack R. Gallagher, Kylee Heap, Susan Carroll and George J. Wan in Therapeutic Advances in Respiratory Disease