Abstract
Background:
Sepsis and septic shock are common in noninvasive ventilation (NIV) patients. However, studies on the association between sepsis and NIV failure are lacking.
Methods:
A prospective multi-center observational study was performed in 16 Chinese intensive care units (ICUs). Patients who used NIV due to hypoxemic respiratory failure were enrolled. Sepsis and septic shock were diagnosed according to the guideline of sepsis-3.
Results:
A total of 519 patients were enrolled. Sepsis developed in 365 patients (70%) and septic shock developed in 79 patients (15%). However, 75 patients (14%) had no sepsis. NIV failure was 23%, 38%, and 61% in patients, with no sepsis, sepsis, and septic shock, respectively. Multivariate analysis found that sepsis [odds ratio (OR) = 1.95, 95% confidence interval (CI): 1.06–3.61] and septic shock (OR = 2.47, 95% CI: 1.12–5.45) were independently associated with NIV failure. In sepsis and septic shock population, the NIV failure was 13%, 31%, 37%, 53%, and 67% in patients with sequential organ failure assessment (SOFA) scores of ⩽2, 3–4, 5–6, 7–8, and ⩾9, respectively. Patients with nonpulmonary induced sepsis had similar NIV failure rate compared with those with pulmonary induced sepsis, but had higher proportion of septic shock (37% versus 10%, p ⩽ 0.01) and lower ICU mortality (10% versus 22%, p ⩽ 0.01).
Conclusions:
Sepsis was associated with NIV failure in patients with hypoxemic respiratory failure, and the association was stronger in septic shock patients. NIV failure increased with the increase of organ dysfunction caused by sepsis.
The reviews of this paper are available via the supplemental material section.
Keywords: noninvasive ventilation, organ dysfunction, sepsis
Introduction
Noninvasive ventilation (NIV) has been used increasingly in recent years.1 It decreases the work of breathing in patients with hypoxemic respiratory failure, and avoids the need for endotracheal intubation in responders.2,3 However, the NIV failure rate is high (31–54%) in patients with hypoxemic respiratory failure.4–7 Patients who experience NIV failure are more likely to die in hospital.8–10 A possible reason is delayed intubation-associated complications.11,12 Therefore, identifying patients who respond badly to NIV has potential value to reduce delayed intubation.
Sepsis syndrome is a common reason for morbidity and mortality in intensive care units (ICUs). The incidence of severe sepsis and septic shock is 11.8–12.6 patients per 100 ICU admissions.13,14 If all patients with sepsis syndrome were included in the calculation, the incidence would be higher. Previous studies have reported that sepsis was associated with NIV failure.4,15 However, they failed to describe the level of severity, efficacy and outcomes in septic patients who received NIV.
The aim of this study was to identify the association between sepsis and NIV failure in patients with hypoxemic respiratory failure. In addition, we further explored the outcomes between patients whose sepsis was induced by pulmonary and nonpulmonary infection.
Methods
From September 2017 to July 2018, we performed a prospective multi-center observational study in 16 Chinese ICUs. Patients admitted to ICU for NIV due to hypoxemic respiratory failure were candidates. We enrolled patients as follows: respiratory rate more than 25 breaths/min, or clinical presentation of respiratory distress at rest (such as active contraction of the accessory inspiratory muscles or paradoxical abdominal motion), PaO2 of <60 mmHg at room air or PaO2/ FiO2 <300 with supplemental oxygen. However, we excluded patients as follows: pneumothorax, age less than 18 years, PaCO2 more than 50 mmHg, receipt of intubation for invasive mechanical ventilation preceding 48 h of NIV, and reaching the criteria of intubation but refuse to intubate before NIV. In addition, patients with cardiogenic pulmonary edema were also excluded. The Ethics Committee and Institutional Review Board (the First Affiliated Hospital of Chongqing Medical University) approved the study protocol (No. 2016150). Written informed consent was obtained from patients or their family members.
NIV was managed by attending physicians, respiratory therapists, and nurses. The attending physicians managed the whole use of NIV, including initiation and termination of NIV. The respiratory therapists and nurses assisted with managing the NIV, including interface selection, parameter setting, humidification management, and variable recording. All participating centers have a training protocol on how to use NIV, and all participants received strictly training before use of NIV. Selection of the oronasal mask was based on the patient’s facial type. The straps of the mask were kept as tight as possible while remaining comfortable for the patient. If the oronasal mask was not tolerated, a nasal mask, full-face mask, or other interface can be selected. In patients with labored breathing, the bilevel positive pressure ventilation mode was used. In patients with cardiogenic pulmonary edema or pulmonary atelectasis, continuous positive airway pressure (CPAP) can be selected. The parameters were recommended to be gradually increased based on the patient tolerance. Positive-end expiratory pressure was maintained at 6–10 cm H2O. Inspiratory pressure was initially set at 8 cm H2O, and then increased in increments of 2 cm H2O to achieve the best control of dyspnea and tolerance of the patient. When the respiratory failure was reversed, liberation from NIV was considered. The reversal of respiratory failure was referenced as follows: the PaO2/FiO2 >300, respiratory rate <25 cycles/min, and no clinical symptoms indicating respiratory distress. The parameters were gradually decreased and the ventilator used intermittently until the patient was totally liberated from the ventilator. However, if respiratory failure progressively deteriorated and reached the criteria of intubation, intubation for invasive mechanical ventilation was recommended.
The criteria for intubation were as follows. Major criteria: respiratory or cardiac arrest, loss of consciousness (such as a sudden change from being awake to unconscious), development of conditions necessitating intubation to protect the airway (coma or seizure disorders) or to manage copious tracheal secretions, heart rate <50 beats/min with loss of alertness, hemodynamic instability (mean arterial blood pressure <65 mmHg) without response to fluids and vasoactive agents. Minor criteria: failure to maintain a PaO2/FiO2 more than 150, respiratory rate more than 35 breaths/min, acidosis with pH less than 7.35, inability to correct dyspnea, lacking improvement signs of respiratory muscle fatigue. Intubation was recommended if the patient reached one major criterion or more than two minor criteria. However, this was based on the attending physicians’ discretion. NIV failure was defined as a requirement for intubation.
Sepsis and septic shock were diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).16 Immunosuppression was considered if one of the following was present: solid tumor with white blood cell counts (WBC) <1 × 109/l, hematological malignancy (such as myeloma, lymphoma, leukemia, etc.), administration of steroids at a dose greater than 0.3 mg/kg per day of prednisolone for more than 1 month, an immunosuppresive drug taken for more than 1 month, HIV infection, and solid organ transplant.17–19 Patients with chronic obstructive pulmonary disease, chronic bronchitis, sequelae of pulmonary tuberculosis, bronchiectasis, obesity-hypoventilation syndrome or chest-wall deformity were diagnosed as chronic respiratory disease.20 Patients with chronic heart failure, coronary artery disease, hypertensive or valvular heart diseases, and dilated myocardial disease were diagnosed as chronic heart disease.20
Statistical analysis
Normally distributed continuous variables were reported as mean value and standard deviation. Abnormally distributed continuous variables were reported as median value and interquartile range. The difference in two groups was analyzed by unpaired Student’s t test or Mann–Whitney U test when appropriate; the difference in three groups was analyzed by one-way ANOVA or Kruskal-Wallis H test when appropriate. Categorical variables were reported as number and percentage, and difference between groups were analyzed by Chi-squared or Fisher’s exact test. Variables with a p value less than 0.2 in the univariate analysis and other clinical meaningful variables were entered in a stepwise multivariate logistic regression analysis to identify independent risk factors associated with NIV failure. A p value less than 0.05 was considered statistically significant.
Results
From September 2017 to July 2018, a total of 519 patients were enrolled. Sepsis developed in 365 patients (70%) and septic shock developed in 79 patients (15%). However, 75 patients (14%) had no sepsis or septic shock. The baseline data are summarized in Table 1. The majority of sepsis syndrome was resulted from pulmonary source (78% in sepsis and 42% in septic shock, respectively). Univariate analysis revealed that sepsis and septic shock were highly associated with NIV failure [odds ratio (OR) = 2.10, 95% confidence interval (CI): 1.17–3.75 and OR = 5.28, 95% CI: 2.61–10.69, respectively] (Table 2). Multivariate analysis also showed that sepsis and septic shock were independently associated with NIV failure (OR = 1.95, 95% CI: 1.06–3.61 and OR = 2.47, 95% CI: 1.12–5.45, respectively).
Table 1.
Baseline data.
| No sepsis (n = 75) | Sepsis (n = 365) | Septic shock (n = 79) | p a | |
|---|---|---|---|---|
| Age, years | 56 ± 19 | 62 ± 18 | 60 ± 19 | 0.03 |
| Male (%) | 47 (63%) | 246 (67%) | 54 (68%) | 0.70 |
| APACHE II score | 13 ± 6 | 15 ± 5 | 18 ± 5 | <0.01 |
| SOFA score | 4.7 ± 2.2 | 4.9 ± 2.2 | 8.6 ± 3.2 | <0.01 |
| Presence of immunosuppression | 5 (7%) | 49 (13%) | 8 (10%) | 0.22 |
| Site of infection | ||||
| Respiratory | 2 (2%) | 286 (78%) | 33 (42%) | |
| Abdominal | – | 41 (11%) | 23 (29%) | |
| Urinary | – | 17 (5%) | 16 (20%) | |
| Others | – | 21 (6%) | 7 (9%) | |
| Comorbidity | ||||
| Hypertension | 26 (35%) | 145 (40%) | 23 (29%) | 0.18 |
| Diabetes mellitus | 12 (16%) | 86 (24%) | 17 (22%) | 0.35 |
| Solid tumor | 18 (24%) | 47 (13%) | 13 (17%) | 0.05 |
| Chronic heart disease | 12 (16%) | 71 (20%) | 16 (20%) | 0.75 |
| Chronic respiratory disease | 13 (17%) | 56 (15%) | 8 (10%) | 0.40 |
| Reasons for NIV | ||||
| Pneumonia | 2 (3%) | 242 (66%) | 34 (43%) | <0.01 |
| Pancreatitis | 18 (24%) | 26 (7%) | 4 (5%) | <0.01 |
| Postoperative respiratory failure | 9 (12%) | 35 (10%) | 10 (13%) | 0.64 |
| Trauma | 8 (11%) | 14 (4%) | 2 (3%) | 0.02 |
| Pulmonary embolism | 6 (8%) | 6 (2%) | 0 (0%) | <0.01 |
| Pulmonary cancer | 5 (7%) | 2 (0.5%) | 1 (1%) | <0.01 |
| Asthma | 4 (5%) | 0 (0%) | 0 (0%) | <0.01 |
| Poison | 2 (3%) | 1 (0.3%) | 0 (0%) | 0.03 |
| Ketoacidosis | 0 (0%) | 2 (0.5%) | 1 (1%) | 0.58 |
| Cholangitis/cholecystitis | 0 (0%) | 9 (3%) | 6 (8%) | 0.01 |
| Interstitial lung disease | 5 (7%) | 2 (0.5%) | 0 (0%) | <0.01 |
| Others | 16 (21%) | 26 (7%) | 21 (27%) | <0.01 |
| Laboratory tests | ||||
| White blood cell counts, ×109/l | 12 ± 6 | 12 ± 8 | 14 ± 9 | 0.22 |
| Platelet counts, ×109/l | 181 ± 106 | 196 ± 114 | 171 ± 126 | 0.16 |
| Hemoglobin, mg/dl | 10.9 ± 2.9 | 11.0 ± 2.8 | 10.1 ± 3.0 | 0.07 |
| Albumin, g/l | 31 ± 6 | 30 ± 6 | 29 ± 7 | 0.21 |
| Potassium, mmol/l | 4.0 ± 0.7 | 4.0 ± 0.7 | 4.2 ± 0.9 | 0.11 |
| Sodium, mmol/l | 138 ± 7 | 138 ± 6 | 139 ± 9 | 0.10 |
| Chlorine, mmol/l | 104 ± 7 | 103 ± 7 | 105 ± 8 | 0.09 |
| Creatinine, μmol/l | 65 (52–88) | 77 (57–128) | 122 (74–264) | <0.01 |
| Total bilirubin, μmol/l | 17 (10–29) | 15 (10–24) | 18 (11–37) | 0.10 |
| Variables collected at beginning of NIV | ||||
| GCS | 14.6 ± 1.6 | 14.6 ± 1.2 | 14.4 ± 1.1 | 0.49 |
| Heart rate, beats/min | 113 ± 21 | 114 ± 23 | 125 ± 21 | <0.01 |
| Respiratory rate, breaths/min | 31 ± 8 | 32 ± 7 | 33 ± 8 | 0.44 |
| Mean arterial pressure, mmHg | 99 ± 18 | 96 ± 16 | 80 ± 16 | <0.01 |
| pH | 7.42 ± 0.09 | 7.43 ± 0.08 | 7.39 ± 0.11 | <0.01 |
| PaCO2, mmHg | 36 ± 7 | 34 ± 7 | 32 ± 9 | <0.01 |
| PaO2/FiO2, mmHg | 172 ± 71 | 146 ± 50 | 165 ± 66 | <0.01 |
APACHE II, acute physiology and chronic health evaluation II; GCS, Glasgow coma scale; NIV, noninvasive ventilation; SOFA, sequential organ failure assessment.
Comparison between three groups.
Table 2.
Univariate and multivariate analysis for NIV failure.
| Univariate analysis OR (95% CI) | p | Multivariate analysis OR (95% CI) | p | |
|---|---|---|---|---|
| Age, years | 1.008 (0.998–1.018) | 0.14 | 1.014 (1.002–1.025) | 0.02 |
| Infection condition | ||||
| Sepsis (versus nonsepsis) | 2.10 (1.17–3.75) | <0.01 | 1.95 (1.06–3.61) | 0.03 |
| Septic shock (versus nonsepsis) | 5.28 (2.61–10.69) | <0.01 | 2.47 (1.12–5.45) | 0.03 |
| Solid tumor | 1.23 (0.76–2.00) | 0.40 | – | – |
| Chronic heart disease | 1.06 (0.68–1.65) | 0.80 | – | – |
| Chronic respiratory disease | 0.92 (0.56–1.52) | 0.75 | – | – |
| Presence of immunosuppression | 1.77 (1.04–3.01) | 0.04 | – | – |
| APACHE II score | 1.09 (1.06–1.13) | <0.01 | – | – |
| SOFA score | 1.24 (1.16–1.34) | <0.01 | 1.22 (1.12–1.32) | <0.01 |
| Platelet counts | 0.999 (0.997–1.000) | 0.07 | – | – |
| Albumin, g/l | 0.96 (0.93–0.99) | <0.01 | 0.96 (0.93–1.00) | 0.03 |
| Variables collected at beginning of NIV | ||||
| Respiratory rate, breaths/min | 1.07 (1.04–1.10) | <0.01 | 1.07 (1.04–1.10) | <0.01 |
| Heart rate, beats/min | 1.01 (1.00–1.02) | 0.04 | – | – |
| Mean arterial pressure, mmHg | 0.988 (0.978–0.999) | 0.03 | – | – |
| GCS | 0.92 (0.80–1.05) | 0.22 | – | – |
| pH | 0.23 (0.03–1.61) | 0.14 | – | – |
| PaCO2, mmHg | 0.97 (0.94–0.99) | <0.01 | – | – |
| PaO2/FiO2, mmHg | 0.997 (0.994–1.000) | 0.06 | – | – |
APACHE II, acute physiology and chronic health evaluation II; CI, confidence internal; GCS, Glasgow coma scale; NIV, noninvasive ventilation; OR, odds ratio; SOFA, sequential organ failure assessment.
NIV failure increased from 23% to 38% from no-sepsis to sepsis patients, and it increased further to 61% in septic shock patients (Table 3). ICU and hospital mortality also increased from no-sepsis to septic shock patients. Sequential organ failure assessment (SOFA) score was independently associated with NIV failure (OR = 1.22, 95% CI: 1.12–1.32). Among the patients with sepsis and septic shock, NIV failure increased with increase in SOFA scores (Figure 1).
Table 3.
Outcomes.
| No sepsis (n = 75) | Sepsis (n = 365) | Septic shock (n = 79) | p a | |
|---|---|---|---|---|
| NIV failure | 17 (23%) | 139 (38%) | 48 (61%) | <0.01 |
| ICU stay | 6.4 (4.0–9.0) | 7.2 (4.2–13.2) | 6.8 (3.4–12.8) | 0.09 |
| Hospital stay | 15.8 (8.9–21.8) | 16.0 (8.1–25.5) | 16.0 (8.3–29.0) | 0.84 |
| ICU mortality | 7 (9%) | 64 (18%) | 19 (24%) | 0.05 |
| Hospital mortality | 9 (12%) | 79 (22%) | 21 (27%) | 0.07 |
ICU, intensive care unit; NIV, noninvasive ventilation.
Comparison between three groups.
Figure 1.
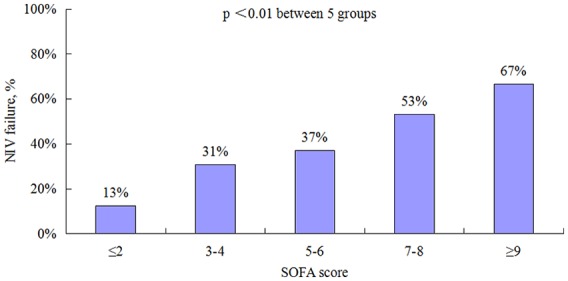
NIV failure rate in patients with sepsis and septic shock on different SOFA scores.
NIV, noninvasive ventilation; SOFA, sequential organ failure assessment.
Table 4 shows the characteristics of the patients whose infection came from pulmonary and nonpulmonary sources. Septic shock patients were more common in nonpulmonary infection (37% versus 10%, p < 0.01). They also had higher SOFA scores, higher creatinine, higher total bilirubin, and higher heart rate. The NIV failure rate was similar between pulmonary and nonpulmonary infection (43% versus 40%, p = 0.59). However, patients with nonpulmonary infection had lower ICU and hospital mortality than those with pulmonary infection (10% versus 22%, p < 0.01, and 14% versus 26%, p < 0.01, respectively).
Table 4.
Comparisons between sepsis and septic shock patients with different infection origins.
| Pulmonary origin (n = 319) | Nonpulmonary origin (n = 125) | p | |
|---|---|---|---|
| Age, years | 63 ± 18 | 60 ± 17 | 0.10 |
| Male (%) | 223 (70%) | 77 (62%) | 0.11 |
| APACHE II score | 16 ± 5 | 16 ± 4 | 0.55 |
| SOFA score | 5.1 ± 2.5 | 6.6 ± 3.2 | <0.01 |
| Presence of immunosuppression | 53 (17%) | 4 (3%) | <0.01 |
| Presence of sepsis | 286 (90%) | 79 (63%) | <0.01 |
| Presence of septic shock | 33 (10%) | 46 (37%) | <0.01 |
| Comorbidity | |||
| Hypertension | 123 (39%) | 45 (36%) | 0.66 |
| Diabetes mellitus | 65 (20%) | 38 (30%) | 0.03 |
| Solid tumor | 38 (12%) | 22 (18%) | 0.12 |
| Chronic heart disease | 64 (20%) | 23 (18%) | 0.79 |
| Chronic respiratory disease | 57 (18%) | 7 (6%) | <0.01 |
| Laboratory tests | |||
| White blood cell counts, ×109/l | 12 ± 8 | 15 ± 10 | <0.01 |
| Platelet counts, ×109/l | 193 ± 109 | 187 ± 135 | 0.61 |
| Hemoglobin, mg/dl | 11.0 ± 2.8 | 10.4 ± 3.0 | 0.06 |
| Albumin, g/l | 30 ± 6 | 29 ± 6 | 0.10 |
| Potassium, mmol/l | 4.0 ± 0.7 | 4.1 ± 0.8 | 0.36 |
| Sodium, mmol/l | 137 ± 7 | 140 ± 7 | <0.01 |
| Chlorine, mmol/l | 102 ± 7 | 105 ± 7 | <0.01 |
| Creatinine, μmol/l | 77 (57–127) | 110 (70–191) | <0.01 |
| Total bilirubin, μmol/l | 15 (10–23) | 19 (12–40) | <0.01 |
| Variables collected at beginning of NIV | |||
| GCS | 14.6 ± 1.4 | 14.6 ± 0.8 | 0.86 |
| Heart rate, beats/min | 113 ± 23 | 123 ± 21 | <0.01 |
| Respiratory rate, breaths/min | 32 ± 7 | 32 ± 7 | 0.69 |
| Mean arterial pressure, mmHg | 94 ± 17 | 90 ± 17 | <0.01 |
| pH | 7.43 ± 0.08 | 7.40 ± 0.10 | <0.01 |
| PaCO2, mmHg | 34 ± 7 | 32 ± 8 | 0.04 |
| PaO2/FiO2, mmHg | 141 ± 49 | 171 ± 60 | <0.01 |
| NIV failure | 137 (43%) | 50 (40%) | 0.59 |
| ICU stay | 7.1 (4.2–13.1) | 7.1 (3.9–13.7) | 0.89 |
| Hospital stay | 15.0 (7.8–24.7) | 18.8 (9.8–36.3) | <0.01 |
| ICU mortality | 71 (22%) | 12 (10%) | <0.01 |
| Hospital mortality | 83 (26%) | 17 (14%) | <0.01 |
APACHE II, acute physiology and chronic health evaluation II; GCS, Glasgow coma scale; NIV, noninvasive ventilation; SOFA, sequential organ failure assessment.
Discussion
Current study explored the association between NIV failure and the severity of sepsis. We found that sepsis was independently associated with NIV failure in patients with hypoxemic respiratory failure, and the association was stronger in septic shock patients. The organ dysfunction caused by sepsis were positively correlated with NIV failure.
The use of NIV in hypoxemic respiratory failure is controversial.21 Delayed intubation due to improper use of NIV increases hospital mortality.12,22 Our study found that patients with sepsis were more likely to experience NIV failure, and much more likely if they had septic shock. So, NIV should be used cautiously in such patients. Furthermore, Serpa and colleagues have suggested that NIV should be used in selected cases of mild respiratory failure with preserved or relatively stable hemodynamic status, and that frequent reassessments of therapeutic effect are required in order to prevent delay in intubation.23
The overall NIV failure rate in our study was 39%. This rate was at the median value reported previously.4–7 However, in patients with no-sepsis, the NIV failure rate was 23%, much lower than the median value. In this group, oxygenation was highest and APACHE II and SOFA scores were lowest. So finding the lowest NIV failure rate in this group is not surprising. On the contrary, the NIV failure rate in patients with septic shock was 61%, the highest among the three groups in our study. Patients in this group had higher APACHE II scores, higher SOFA scores, and higher heart rate. Previous studies have demonstrated that patients with high APACHE II score, SOFA score, and heart rate were more likely to experience NIV failure.6,8,24 Therefore, these reasons explain the high NIV failure rate in patients with septic shock.
Sepsis is common in ICUs. A recent multi-center observational study reported an incidence of 30.2 per 100 ICU beds in Brazilian ICUs.25 Previous studies have reported that sepsis is associated with NIV failure.4,15 Although these studies provided important knowledge in this field, they failed to stratify the severity of sepsis. In the current study, we stratified sepsis and reported associations between NIV failure and sepsis severity. As use of NIV in patients with hypoxemic respiratory failure is controversial, our data provide some references for use of NIV in this population.
Sepsis results mostly from pulmonary infection.13,26 Our study confirmed this. However, unlike previous studies, we found the interesting result that patients with sepsis from pulmonary infection had a similar NIV failure rate but higher mortality than those whose sepsis came from nonpulmonary infection, although nonpulmonary infection patients had a higher proportion of septic shock, higher SOFA score, and higher heart rate. Previous studies have reported that pulmonary-infection-induced sepsis resulted in higher mortality than nonpulmonary infection.27–29 And pulmonary-infection-induced sepsis became an independent risk factor for 28-day in-hospital mortality.29 The reasons were unclear, but may possibly have been due to ARDS. In our study, the PaO2/FiO2 in the pulmonary infection group was much lower than in the nonpulmonary infection group, meaning that pulmonary infection leads to greater severity of lung injury. This may explain the higher mortality in the pulmonary infection group.
This study has several limitations. We excluded patients with hypercapnic respiratory failure because these patients receive much benefit from NIV.30 This may bias the incidence of sepsis and septic shock in the NIV population. In addition, this is a multi-center observational study performed in 16 Chinese ICUs. We applied recommended criteria for intubation. However, the decision to intubate was at the attending physicians’ discretion. This may bias NIV failure rate. On the efficacy of NIV, we found only that NIV failure was associated with sepsis and septic shock. Whether patients with sepsis syndrome benefit from NIV requires to be confirmed by randomized control trials.
In conclusion, sepsis was associated with NIV failure in patients with hypoxemic respiratory failure, and the association was stronger in septic shock patients. NIV failure increased with the increase of organ dysfunction caused by sepsis. Patients with sepsis induced by pulmonary infection were more likely to die in hospital.
Supplemental Material
Supplemental material, Author_response_1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.2 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.2 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Acknowledgments
Authors Jun Duan, Lijuan Chen, and Guopeng Liang contributed equally.
Footnotes
Author contributions: Jun Duan initiated this study, drafted the manuscript, and took responsibility for the integrity of the work as a whole. Jun Duan, Lijuan Chen, Guopeng Liang, and Weiwei Shu joined in study design. Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai, and Lintong Zhou joined in patient recruitment and data collection. All authors joined in manuscript revision and approved the final version.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported by Chongqing Health Commission Projects (2017MSXM019).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jun Duan  https://orcid.org/0000-0003-1685-0117
https://orcid.org/0000-0003-1685-0117
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Jun Duan, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Youyi Road 1, Yuzhong District, Chongqing 400016, P.R. China.
Lijuan Chen, Department of Respiratory and Critical Care Medicine, Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital, Chengdu, Sichuan, P.R. China.
Guopeng Liang, Department of Critical Care Medicine, West China hospital of Sichuan University, Chengdu, Sichuan, P.R. China.
Weiwei Shu, Department of Critical Care Medicine, Yongchuan Hospital of Chongqing Medical University, Yongchuan, Chongqing, P.R. China.
Liucun Li, Department of Respiratory and Critical Care Medicine, the Second Xiangya Hospital, Central South University, Changsha, Hunan, P.R. China.
Ke Wang, Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Shengyu Wang, Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Xi’an Medical University, Xi’an, P.R. China.
Xiaoyi Liu, Department of Critical Care Medicine, the Central Hospital of Dazhou, Dazhou, Shichuan, P.R. China.
Chunfeng He, Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Army Medical University, Chongqing, P.R. China.
Dehua He, Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, P.R. China.
Qimin Chen, Department of Critical Care Medicine, the Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, P.R. China.
Bilin Wei, Department of Critical Care Medicine, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, P.R. China.
Baixu Chen, Department of Critical Care Medicine, West China hospital of Sichuan University, Chengdu, Sichuan, P.R. China.
Yuzhen Shu, Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Yao Tian, Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Xi’an Medical University, Xi’an, P.R. China.
Liping Fan, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Xiaoli Han, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Rui Zhang, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Xiangmei Yang, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Yan Peng, Department of Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Dong Wan, Department of Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Xiaoying Chen, Department of Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Lin Ye, Department of Cardiothoracic Surgery, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Shijing Tian, Department of Surgical Intensive Care Unit, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Qiong Huang, Department of Coronary Care Unit, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Lei Jiang, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Linfu Bai, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
Lintong Zhou, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, P.R. China.
References
- 1. Demoule A, Chevret S, Carlucci A, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med 2016; 42: 82–92. [DOI] [PubMed] [Google Scholar]
- 2. L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172: 1112–1118. [DOI] [PubMed] [Google Scholar]
- 3. Ferrer M, Esquinas A, Leon M, et al. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med 2003; 168: 1438–1444. [DOI] [PubMed] [Google Scholar]
- 4. Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 2001; 27: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 5. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35: 18–25. [DOI] [PubMed] [Google Scholar]
- 6. Duan J, Han X, Bai L, et al. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med 2017; 43: 192–199. [DOI] [PubMed] [Google Scholar]
- 7. Thille AW, Contou D, Fragnoli C, et al. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care 2013; 17: R269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez A, Ferri C, Martin-Loeches I, et al. Risk factors for noninvasive ventilation failure in critically ill subjects with confirmed influenza infection. Respir Care 2017; 62: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 9. Neuschwander A, Lemiale V, Darmon M, et al. Noninvasive ventilation during acute respiratory distress syndrome in patients with cancer: trends in use and outcome. J Crit Care 2017; 38: 295–299. [DOI] [PubMed] [Google Scholar]
- 10. Correa TD, Sanches PR, de Morais LC, et al. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: a prospective, observational, cohort study. BMC Pulm Med 2015; 15: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mosier JM, Sakles JC, Whitmore SP, et al. Failed noninvasive positive-pressure ventilation is associated with an increased risk of intubation-related complications. Ann Intensive Care 2015; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi T, Toba S, Sekiguchi Y, et al. Protocol-based noninvasive positive pressure ventilation for acute respiratory failure. J Anesth 2011; 25: 42–49. [DOI] [PubMed] [Google Scholar]
- 13. Finfer S, Bellomo R, Lipman J, et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 2004; 30: 589–596. [DOI] [PubMed] [Google Scholar]
- 14. SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 2016; 42: 1980–1989. [DOI] [PubMed] [Google Scholar]
- 15. Meeder AM, Tjan DH, van Zanten AR. Noninvasive and invasive positive pressure ventilation for acute respiratory failure in critically ill patients: a comparative cohort study. J Thorac Dis 2016; 8: 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016; 4: 646–652. [DOI] [PubMed] [Google Scholar]
- 18. Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care 2015; 19: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemiale V, Mokart D, Resche-Rigon M, et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA 2015; 314: 1711–1719. [DOI] [PubMed] [Google Scholar]
- 20. Ferrer M, Sellares J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 21. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: pii: 1602426. [DOI] [PubMed] [Google Scholar]
- 22. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350: 2452–2460. [DOI] [PubMed] [Google Scholar]
- 23. Serpa Neto A, Schultz MJ, Festic E. Ventilatory support of patients with sepsis or septic shock in resource-limited settings. Intensive Care Med 2016; 42: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Rajhi A, Murad A, Li PZ, et al. Outcomes and predictors of failure of non-invasive ventilation in patients with community acquired pneumonia in the ED. Am J Emerg Med 2018; 36: 347–351. [DOI] [PubMed] [Google Scholar]
- 25. Machado FR, Cavalcanti AB, Bozza FA, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 2017; 17: 1180–1189. [DOI] [PubMed] [Google Scholar]
- 26. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med 2007; 35: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 27. He XL, Liao XL, Xie ZC, et al. Pulmonary infection is an independent risk factor for long-term mortality and quality of life for sepsis patients. Biomed Res Int 2016; 2016: 4213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujishima S, Gando S, Daizoh S, et al. Infection site is predictive of outcome in acute lung injury associated with severe sepsis and septic shock. Respirology 2016; 21: 898–904. [DOI] [PubMed] [Google Scholar]
- 29. Kim WY, Lee YJ, Yeon Lim S, et al. Clinical characteristics and prognosis of pneumonia and sepsis: multicenter study. Minerva Anestesiol 2013; 79: 1356–1365. [PubMed] [Google Scholar]
- 30. Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 7: CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock by Jun Duan, Lijuan Chen, Guopeng Liang, Weiwei Shu, Liucun Li, Ke Wang, Shengyu Wang, Xiaoyi Liu, Chunfeng He, Dehua He, Qimin Chen, Bilin Wei, Baixu Chen, Yuzhen Shu, Yao Tian, Liping Fan, Xiaoli Han, Rui Zhang, Xiangmei Yang, Yan Peng, Dong Wan, Xiaoying Chen, Lin Ye, Shijing Tian, Qiong Huang, Lei Jiang, Linfu Bai and Lintong Zhou in Therapeutic Advances in Respiratory Disease


