Short abstract
Background
We have previously reported that histamine-induced pruritus was attenuated in toll-like receptor 4 (TLR4) knockout mice due to decreased transient receptor potential V1 (TRPV1) sensitivity. Our results implied that TLR4 potentiated TRPV1 activation in sensory neurons; however, the molecular mechanism has yet to be elucidated. In this study, we investigated the molecular mechanisms of TLR4-mediated TRPV1 potentiation using TLR4-deficient sensory neurons and a heterologous expression system.
Methods
Primary sensory neurons were obtained from wild-type or TLR4 knockout mice, and HEK293T cells expressing TRPV1 and TLR4 were prepared by transient transfection. TRPV1 activity was analyzed by calcium imaging, fluorophotometry, and patch-clamp recording. Subcellular protein distribution was tested by immunocytochemistry and cell surface biotinylation assay. Protein interaction was assessed by western blot and immunoprecipitation assay.
Results
Direct association between TRPV1 and TLR4 was detected in HEK293T cells upon heterologous TRPV1 and TLR4 expression. In an immunoprecipitation assay using TLR4-deletion mutants and soluble toll/interleukin-1 receptor (TIR) protein, the cytoplasmic TIR domain of TLR4 was required for TLR4-TRPV1 association and TRPV1 potentiation. In TLR4-deficient sensory neurons, the activation-induced desensitization of TRPV1 increased, accompanied by enhanced TRPV1 clearance from the cell membrane upon activation compared to wild-type neurons. In addition, heterologous TLR4 expression inhibited activation-induced TRPV1 endocytosis and lysosomal degradation in HEK293T cells.
Conclusion
Our data show that direct association between TRPV1 and TLR4 through the TIR domain enhances TRPV1 activity by blocking activation-induced TRPV1 desensitization.
Keywords: TRPV1, TLR4, desensitization, pain, pruritus
Introduction
Transient receptor potential V1 (TRPV1) is a nonselective cation channel of the TRP family that was originally identified as a vanilloid receptor.1 The function of this channel has been extensively studied in the sensory nervous system: it is expressed in a subset of sensory neurons and activated by various noxious stimuli such as heat, protons, and various endogenous inflammation-associated lipids.1,2 Opening of the TRPV1 channel leads to an increase in intracellular calcium, resulting in activation of nociceptive neurons and subsequent sensation of pain.1 TRPV1 also plays an important role in pruritus, especially in histamine-induced itch sensation.3 It is reported that activation of the histamine receptor H1R1 leads to intracellular calcium increase and inward current generation, subsequently eliciting itch sensation via TRPV1.3
Since TRPV1 activation in sensory neurons is critical for pain and itch sensation, the sensitivity of TRPV1 is controlled by many regulatory molecules involved in pain and pruritus. For instance, the sensitivity of TRPV1 to noxious heat is increased by inflammatory mediators such as prostaglandins and bradykinin, thereby increasing pain sensation in the inflammatory conditions.4 On the other hand, TRPV1 can be desensitized upon prolonged activation or repeated exposures to agonists.5 Although the exact mechanisms for sensitization/desensitization of TRPV1 are far from being completely understood, it has been shown that TRPV1 activity is regulated by phosphorylation.4,6,7 In addition, activation-induced internalization and degradation can take part in desensitization upon prolonged stimulation.8 Crosstalk between various receptors on sensory neurons and their intracellular signaling pathways has been reported to regulate TRPV1 activity. For example, the prostaglandin E2 receptor induces cyclic adenosine monophosphate increase and protein kinase A (PKA) activation. This, in turn, phosphorylates TRPV1 on Ser116, blocking TRPV1 desensitization.9 In addition, activation of the nerve growth factor receptor sensitizes TRPV1 by rapidly increasing membrane trafficking of TRPV1.10
Recently, it was reported that activation of toll-like receptor 4 (TLR4), an innate immune receptor that recognizes pathogen- or tissue damage-associated molecular patterns, by lipopolysaccharide (LPS) or paclitaxel sensitized TRPV1 on the sensory neurons.11,12 We also reported that TLR4 expression enhances histamine-induced pruritus by potentiating TRPV1 activity.13 In our previous study, the TRPV1-mediated intracellular calcium signal and inward current are compromised in TLR4-deficient sensory neurons. In contrast, heterologous TLR4 expression potentiated capsaicin-induced TRPV1 activation,13 of which the underlying mechanism is still unknown. In this study, we investigated the molecular mechanisms underlying TLR4-mediated TRPV1 activity regulation. Here, we present data that TLR4 enhances TRPV1 activity by direct association via its toll/interleukin-1 receptor (TIR) domain. The TLR4-TRPV1 interaction then inhibits activation-induced desensitization of TRPV1 by blocking TRPV1 internalization and subsequent degradation.
Material and methods
Mice
Eight-week-old C57BL/6 mice were purchased from Daehan Biolink (Eumseong, Korea). TLR4 knockout (KO) mice in a C57BL/6 background were generously provided by Dr. S. Akira (Osaka University, Japan). Mice were housed at 23 ± 2°C with a 12-h light–dark cycle and fed food and water ad libitum. All surgical and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Seoul National University.
Measurement of licking and biting behavior
Capsaicin was prepared as a 1.6 µg solution in ethanol and was administrated into the right hind paw by intraplantar injection using a 31 G insulin syringe. Mice were observed for 5 min after capsaicin application, and total duration of hind paw licking or biting was measured.
Primary dorsal root ganglia neuron culture
Dorsal root ganglia (DRGs) collected from eight-week-old mice were incubated in Hank’s Balanced Salt Solution (HBSS) (Welgene, Daegu, Korea) containing 0.33 mg/ml papain (Worthington, Lakewood, NJ, USA) and 0.65 mg/ml L-cysteine (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at 37°C and then in HBSS containing 4 mg/ml collagenase (Roche, Mannheim, Germany) and 5 mg/ml dispase (Invitrogen, Carlsbad, CA, USA) for 10 min at 37°C. Samples were washed with Dulbecco’s Modified Eagle’s medium (DMEM)/F12 in 10% (v/v) fetal bovine serum (FBS) (Invitrogen), 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Welgene). The cell suspension was filtered through a 70-µm cell strainer and cultured in a poly-D-lysine (PDL; Sigma-Aldrich)-coated culture dish or glass coverslips.
Cell culture and transfection
HEK293T cells were maintained in DMEM containing 10% FBS, 100 U/ml ampicillin, 100 µg/ml streptomycin, and 2 mM L-glutamine in a humidified incubator containing 5% CO2. HEK293T cells were transfected using Effectene transfection reagent (Qiagen, Venlo, the Netherlands) according to the manufacturer’s instructions.
Immunofluorescence
For immunocytochemistry, transfected HEK293T cells were seeded onto a PDL-coated cover glass and fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 15 min. After rinsing in 0.1 M PBS, cells were blocked with 0.1 M PBS containing 5% normal goat serum, 5% FBS, 2% bovine serum albumin, and 0.1% Triton X-100 for 1 h at room temperature (RT). Cells were incubated overnight at 4°C with rabbit anti-HA (1:1000; Cell Signaling, Danvers, MA, USA) antibody. Cells were incubated for 1 h at RT with Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Groove, PA, USA) and mounted with VectaShield medium (Vector Labs, Burlingame, CA, USA).
Calcium assay
Calcium response in DRG neurons was measured by single-cell calcium imaging using Fura 2-acetoxymethyl ester (Fura-2 AM) (Invitrogen). Cells were plated on PDL-coated cover glasses and incubated overnight. Cells were then incubated for 50 min at RT with 2 µM Fura-2 AM in HBSS containing 25 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.5) and washed with HBSS-HEPES twice before assays. A baseline reading was collected for 60 s before the addition of capsaicin. To test cell viability, 100 mM KCl was added after treatment. Intracellular calcium level was measured by digital video microfluorometry with an intensified charge-coupled device camera (CasCade, Roper Scientific, Trenton, NJ, USA) coupled to a microscope and analyzed with MetaFluor software (Universal Imaging Corp., Downingtown, PA, USA). Fura-2 AM excitation wavelengths were selected by a Lambda DG-4 monochromator wavelength changer (Shutter Instrument, Novato, CA, USA).
To determine intracellular calcium level in the HEK293T cell population, cells were detached from plates 24 h after transfection and stained with 2 µM Fura-2 AM in Locke’s solution (154 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 2.2 mM CaCl2, 5.0 mM HEPES, 10 mM glucose, pH 7.4) for 50 min at 37°C. Cells were then washed twice with Locker’s solution and suspended at 1 × 106 cells/ml for assays. Intracellular calcium level was monitored with dual excitation at 340 nm and 380 nm and emission at 500 nm by a spectrofluorophotometer (Shimadzu RF-5301-PC, Shimadzu, Kyoto, Japan). The ratio of emission after 340 nm and 380 nm excitation (340 nm/380 nm) was used as the index of intracellular calcium concentration ([Ca++]i). The net change in [Ca++]i upon drug treatment (Δ ratio (340 nm/380 nm)) was calculated by subtracting basal [Ca++]i from the peak [Ca++]i achieved after exposure to the drug.
Western blot assay
For western blots, protein samples were separated using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk in TBST (20 mM Tris, pH 7.4, 0.1% Tween 20, 150 mM NaCl), membranes were incubated with anti-HA-Tag (Cell Signaling, Danvers, MA, USA), anti-TRPV1 (Santa Cruz, Dallas, TX, USA), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling), anti-β-actin (Sigma-Aldrich), or anti-α-tubulin (Sigma-Aldrich) antibodies. Proteins were detected with horseradish peroxidase-conjugated secondary antibodies using the West Save Gold western blot detection kit (Ab Frontier, Seoul, Korea). Signals were visualized by MicroChemi (DNR Bio-imaging Systems, Jerusalem, Israel).
Whole-cell recording
HEK293T cells overexpressing TLR4 and/or TRPV1 were seeded in 12 mm2 glass dishes two days after DNA transfection. Whole-cell recordings were performed the following day. Whole-cell patch-clamp recordings to measure currents were performed at RT using the HEKA EPC10 amplifier (HEKA Elektronik GmbH, Lambrecht/Pfalz, Germany). Pipettes were fabricated from borosilicate glass (Sutter Instrument, CA, USA) and were pulled with a Flaming/Brown micropipette Puller (Model P-97, Sutter Instrument). When filled with solution, the resistance of the pipettes ranged from 4 to 6 MΩ. The recording chamber was continuously perfused (2 ml/min). Resistance was compensated for (>80%), and leak subtraction was performed. Pulse v8.30 software (HEKA) was used for experiments and analysis. The internal pipette solution was composed of (in mM): 125 KCl, 5 NaCl, 2.5 CaCl2, 2 MgCl2, 5 EGTA, 10 D-glucose, and 5 HEPES adjusted to pH 7.3 with NaOH, 295∼300 mOsm. Extracellular solution contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 D-glucose, adjusted to pH 7.3 with NaOH, 300∼310 mOsm. Capsaicin 1uM in this experiment was dissolved in extracellular solution. Currents were normally evoked from a holding potential of –60 mV.
Cell-surface biotinylation assay
Surface biotinylation was performed on acutely dissociated DRG neurons following established protocols. Cells were biotinylated with 500 µg/ml EZlink sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Waltham, MA, USA) in PBS+/+ (PBS containing Ca2+ and Mg2+) solution at 4°C for 30 min. Unreacted biotin was quenched using PBS+/+ solution containing 0.1 M glycine for 15 min. Then, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris, 137 mM NaCl, 2.7 mM KCl, 1% Triton X-100, 0.1% SDS, pH 7.4, and a proteinase inhibitor mixture). A 10% volume of the lysate was saved for determination of total protein, and the remainder was incubated with NeutrAvidin plus Ultralink beads (Thermo Fisher Scientific) overnight at 4°C. After three washes with RIPA buffer, bound proteins were eluted with 5× SDS loading buffer by boiling for 5 min and were analyzed by western blot with an anti-TRPV1 antibody.
Fluorescence-activated cell sorting analysis
HEK293T cells expressing TRPV1-green fluorescent protein (GFP) were harvested after 0.25% of trypsin-ethylenediaminetetraacetic acid treatment for 3 min in 37°C and fixed with 2% PFA. FACSverse flow cytometer (BD Biosciences) was used to measure the GFP+ population. Among the total cells, GFP+ cells were gated to calculate TRPV1-expressing cell population. Data were acquired and analyzed with BD FACSuiteTM software (BD Biosciences).
Statistical analysis
All data are presented as mean value with standard error of the mean (SEM). Differences between groups were determined by one-way analysis of variance with Bonferroni, Tukey’s multiple comparison test, or Student’s t-test. Differences were considered significant when p was less than 0.05.
Results
TLR4 directly interacts with TRPV1
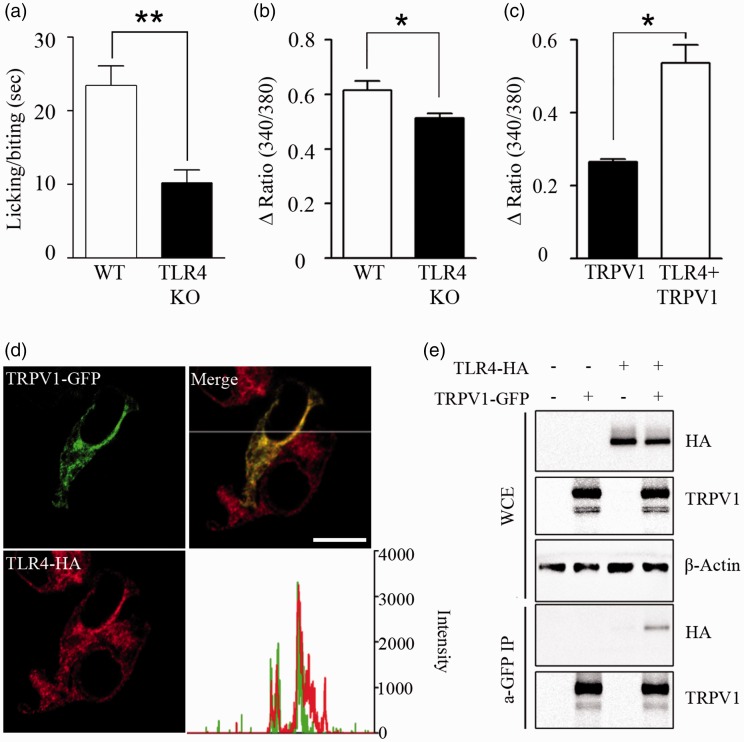
We have previously reported that TLR4 enhances histamine-induced pruritus by potentiating TRPV1 activity.13 To confirm that TLR4 affects TRPV1 activity in vivo, we injected capsaicin in the hind paw of wild-type (WT) and TLR4 KO mice and then measured the paw licking or biting time for 5 min. In the TLR4 KO mice, the licking or biting time after capsaicin injection was less than half of that of WT mice (Figure 1(a)). As previously reported, capsaicin-induced intracellular calcium increase is reduced in the sensory neurons from TLR4 KO mice (Figure 1(b)). In addition, heterologous TLR4 expression enhanced capsaicin-induced intracellular calcium signal in HEK293T cells (Figure 1(c)). To investigate the mechanisms of TRPV1 activity regulation by TLR4 expression, we first tested the subcellular localization of TLR4 and TRPV1 in HEK293T cells by quantifying subcellular immunofluorescence intensity. Under confocal microscope, the TLR4 and TRPV1 signals closely matched, which suggested a possibility of colocalization of these two proteins within the cells (Figure 1(d)). To test whether these two membrane receptors directly interact, immunoprecipitation was performed with lysate from TLR4- and TRPV1-expressing HEK293T cells. In these cells, immunoprecipitation of TRPV1 also pulled down TLR4 protein (Figure 1(e)), supporting a direct association between TLR4 and TRPV1 on the cell membrane.
Figure 1.
Direct interaction between TLR4 and TRPV1. (a) To confirm the effect of TLR4 expression on TRPV1-activated mice behavior, WT and TLR4 KO mice were administered capsaicin (1.6 µg) by intraplantar injection on the right hind paw. Licking/biting time of the right hind paw was measured for 5 min after capsaicin injection (n = 7, **p < 0.01). (b) DRG neurons from WT and TLR4 KO mice were cultured and treated with capsaicin (1 µM), and intracellular calcium was monitored by calcium imaging assays (n = 5, *p < 0.05). (c) HEK293T cells transiently overexpressed with TRPV1-GFP or TRPV1-GFP plus TLR4-HA (n = 6) were loaded with Fura-2 AM. Cells were treated with capsaicin (10 µM), and intracellular calcium level was measured by spectrofluorophotometer population assay. Mean with SEM are shown (n = 6, *p < 0.05). (d) TRPV1 and TLR4 were transiently overexpressed in HEK293T cells, and sub-cellular fluorescence intensities were analyzed under a confocal microscope. The fluorescence intensities of TRPV1 and TLR4 along the x-axis are shown in a graph (right). The subcellular localization of TRPV1 merged with that of TLR4. Scale bar, 20 µm. (e) HEK293T cells were transfected with TLR4-HA, TRPV1-GFP, or TLR4-HA plus TRPV1-GFP expression vectors. Total cell extracts were immunoprecipitated with anti-GFP antibody, and then TLR4 expression was measured using anti-HA antibody (lower two panels). In addition, expression levels of TLR4, TRPV1, and β-actin in the WCE were measured (upper three panels). Representative gel pictures are shown (n = 3).
WT: wild-type; TLR4: toll-like receptor 4; KO: knockout; TRPV1: transient receptor potential V1; GFP: green fluorescent protein; WCE: whole-cell extracts.
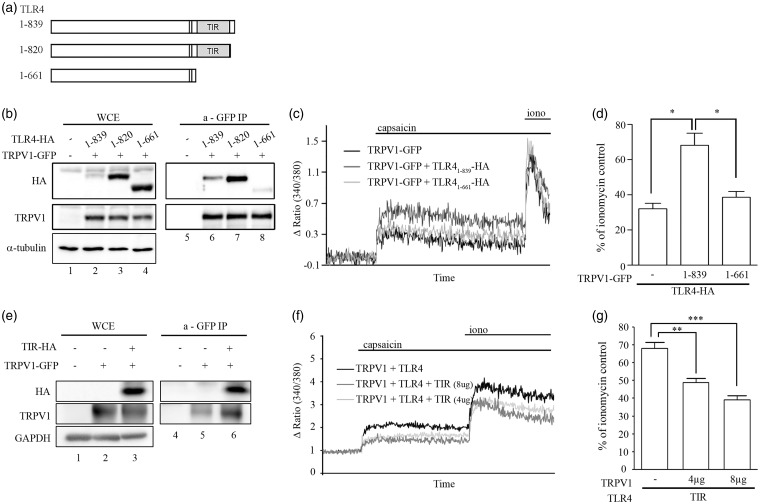
TLR4 associates with TRPV1 through the TIR domain
Next, we determined the TRPV1 binding site on the TLR4 protein. It is well-known that TLR family members interact with intracellular signaling proteins via a cytoplasmic protein–protein interaction domain called TIR.14 To test if this TIR domain is also involved in the TLR4 interaction with TRPV1, we created two types of TLR4 C-terminal truncation mutants with (TLR41–820) or without (TLR41–661) the TIR domain (Figure 2(a)). After overexpression of these TLR4-deletion mutants in HEK293T cells, along with full-length TLR4 (TLR41–839) and TRPV1, association between TLR4 and TRPV1 was measured by a co-immunoprecipitation assay. Compared to full-length TLR4, mutant TLR4 without the TIR domain (TLR41–661) showed significantly reduced physical interaction with TRPV1. However, protein interaction between TRPV1 and TLR41–820 was comparable to that between TRPV1 and full-length TLR4 (Figure 2(b)). These data show that TLR4 interacts with TRPV1 through the TIR domain. Interestingly, we observed higher expression levels of TLR4 mutants compared to full-length TLR4, even though the same amount of plasmid was used for transfection. This implies the presence of a putative negative expression regulator at the C-terminal sequence (820–839). To test if TLR4-TRPV1 interaction potentiates TRPV1 activity, capsaicin-induced intracellular calcium signals were compared between TRPV1/TLR4 and TRPV1/TLR41–661 co-expressing cells. The percentage of capsaicin-induced intracellular calcium level compared to that of ionomycin-induced calcium level increased up to 68% in the TRPV1/TLR4-overexpressing HEK293T cells (Figure 2(c) and (d)). However, it was reduced by 39% when cells were co-expressed with TLR41–661, which is comparable to the level of HEK293T cells expressing TRPV1 alone (Figure 2(c) and (d)). To further confirm the TIR-dependent TLR4-TRPV1 interaction, we overexpressed the TIR domain (672–819 amino acid region of TLR4) along with TRPV1 in the HEK293T cells. In the immunoprecipitation assay, soluble TIR protein alone was pulled down with TRPV1 (Figure 2(e)), indicating the TIR domain is sufficient for interaction with TRPV1. In addition, soluble TIR overexpression inhibited the capsaicin-induced intracellular calcium signal of TRPV1/TLR4-overexpressing HEK293T cells at a dose-dependent manner (Figure 2(f) and (g)). Taken together, these data indicate that TLR4 interaction with TRPV1 via its TIR domain enhances capsaicin-induced TRPV1 activation.
Figure 2.
TLR4 interacts with TRPV1 through the TIR domain. (a) Schematic diagram of the domain structure of TLR4 and the deletion mutants used in the study. (b) Lysates of HEK293T cells expressing TRPV1-GFP or TRPV1-GFP plus TLR4-HA (TLR41–839, TLR41–820, or TLR41–661) were immunoprecipitated with anti-GFP antibody. The amount of each type of TLR4 before (lanes 1–4) and after (lanes 5–8) GFP pull-down was measured with western blot using anti-HA and anti-α-tubulin (a marker for cytosolic proteins) antibodies. (c), (d) HEK293T cells transiently overexpressed with TRPV1-GFP or TRPV1-GFP plus TLR4-HA (TLR41–839 or TLR41–661) were loaded with Fura-2 AM. Cells were treated with capsaicin (10 µM) followed by ionomycin (0.3 µg/ml), and intracellular calcium level was measured by spectrofluorophotometer population assay. Representative traces are shown (c), and the percentage of the capsaicin-induced intracellular calcium level compared to the ionomycin-activated calcium level is shown in a graph ((d), n = 3, *p < 0.05). (e) Lysates of HEK293T cells expressing TRPV1-GFP plus TIR domain (TIR-HA) were immunoprecipitated with anti-GFP antibody. The amount of TIR before (lanes 1–3) and after (lanes 4–6) GFP pull-down was measured with western blot using anti-HA and anti-GAPDH (a marker for cytosolic proteins) antibodies. (f), (g) HEK293T cells transiently overexpressing TRPV1-GFP plus TLR4-HA or TRPV1-GFP plus TLR4-HA plus TIR domain (4 µg or 8 µg) were loaded with Fura-2 AM and treated with capsaicin (10 µM) followed by ionomycin (0.3 µg/ml). Intracellular calcium was monitored by population assay. Representative traces are shown (f), and the percentage of the capsaicin-induced intracellular calcium level compared to the ionomycin-activated calcium level is shown in a graph. The data represent mean with SEM ((g), n = 5, **p < 0.01, ***p < 0.001).
TLR4: toll-like receptor 4; TRPV1: transient receptor potential V1; GFP: green fluorescent protein; WCE: whole-cell extracts; TIR: toll/interleukin-1 receptor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
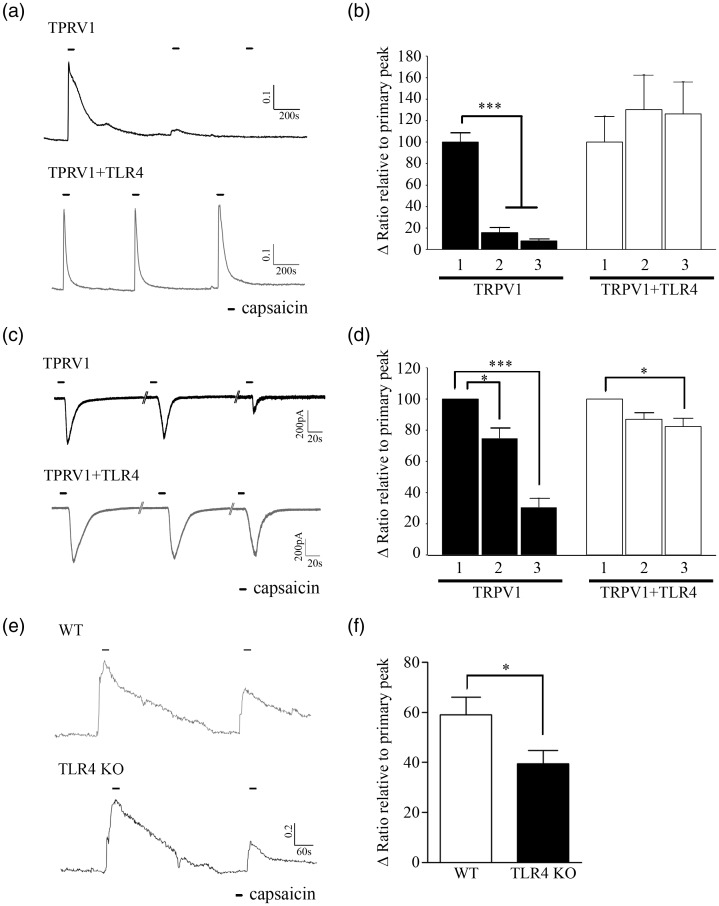
TLR4 deficiency enhances activation-induced TRPV1 desensitization
TRPV1 activation induced by capsaicin stimuli is usually followed by nociceptor desensitization.15 We tested if TLR4-TRPV1 interaction regulates TRPV1 activity by affecting its activation-induced desensitization. To investigate this, we measured the TRPV1 activity by measuring intracellular calcium increase upon repeated capsaicin treatment in TRPV1-expressing (TRPV1+) and TRPV1/TLR4-expressing (TRPV1+/TLR4+) HEK293T cells. In TRPV1+ cells, the intracellular calcium increase caused by the second capsaicin stimuli was reduced more than 80% compared to that of first stimuli, whereas it was comparable to the first peak in TRPV1+/TLR4+ cells (Figure 3(a) and (b)). Likewise, in electrophysiological studies, repeated capsaicin treatment reduced the capsaicin-induced inward current in TRPV1+ HEK293T cells by 69%, whereas it was reduced only by 18% in TRPV1+/TLR4+ HEK293T cells (Figure 3(c) and (d)). We also compared the capsaicin-activated TRPV1 desensitization in WT and TLR4 KO sensory neurons. Compared to the primary capsaicin stimulus, the intracellular calcium increase upon the second capsaicin treatment was significantly ameliorated in both WT and TLR4 KO sensory neurons. When we calculated the ratio of the second calcium increase relative to the first, it was reduced by 59% in TLR4 KO sensory neurons, whereas it was reduced by only 39% in WT sensory neurons (Figure 3(c)). Taken together, these data indicate that TLR4 deficiency enhances the activation-induced desensitization of TRPV1.
Figure 3.
TLR4 inhibits capsaicin-induced TRPV1 desensitization. (a), (b) HEK 293T cell transiently overexpressing TRPV1-GFP or TRPV1-GFP plus TLR4-HA were loaded with Fura2-AM. Cells were treated with capsaicin (1 µM) three times, and intracellular calcium was monitored by calcium imaging assays. A representative calcium trace is shown. Scale bar, 0.1 ratio/200 s (a). The level of calcium increase from the second and third capsaicin treatments was normalized to that of the level of first treatment and presented in a graph ((b), n = 11 and 15, ***p < 0.001). ((c), // = 2min), (d) Whole-cell patch-clamp recording was performed using HEK293T cells transiently overexpressing TRPV1-GFP or TRPV1-GFP plus TLR4-HA. Representative traces of capsaicin-induced inward currents are shown. Scale bar, 200 pA/20 s (c). The amplitude of second and third capsaicin-induced inward current was normalized to that of the first capsaicin-induced inward current and shown in a graph ((d), n = 5, *p < 0.05, ***p < 0.001). (e), (f) TRPV1 activity was assessed by calcium imaging in WT or TLR4 KO sensory neurons. Following acquisition of baseline and capsaicin (1 µM) treatment for 2 min, cells were rinsed with normal HEPES buffer prior to second capsaicin treatment. A representative calcium trace is shown. Scale bar, 0.2 ratio/60 s (e). The level of calcium increase from the second capsaicin treatment was normalized to that of the first treatment and presented in a graph. The data represent mean with SEM ((f), n = 3 with 138 and 105 cells measured, respectively, *p < 0.05).
TRPV1: transient receptor potential V1; TLR4: toll-like receptor 4; WT: wild-type; KO: knockout.
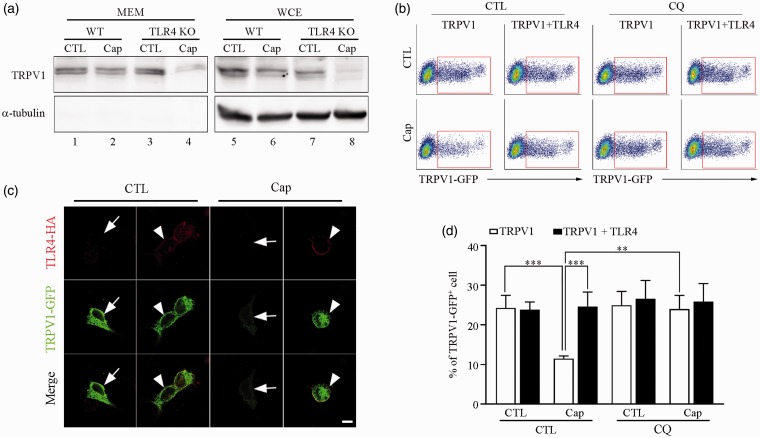
TLR4 inhibits activation-induced TRPV1 endocytosis and lysosomal degradation
TRPV1 endocytosis was considered as one of the mechanisms of TRPV1 desensitization upon its activation. Therefore, we tested whether the enhanced capsaicin-induced desensitization in the absence of TLR4 was due to increased endocytosis of TRPV1 from the plasma membrane. To examine this, TRPV1 expression levels in WT and TLR4 KO sensory neurons were compared by western blot analysis. In TLR4 KO sensory neurons, TRPV1 expression in both the membrane fraction and whole-cell extracts significantly decreased upon capsaicin treatment (Figure 4(a)). This decrease was much greater than that of WT neurons, suggesting that TRPV1 is endocytosed and degraded from the membrane fraction more efficiently in the absence of TLR4 (Figure 4(a), compare lanes 2 and 4). To confirm this, we compared the TRPV1 expression pattern of the HEK293T cells transiently expressing TRPV1 with or without TLR4 after capsaicin treatment. Without capsaicin treatment, similar levels of TRPV1 expression were detected on the cell membrane and in the cytosol of both TLR4+ (Figure 4(b), arrowhead) and TLR4-negative (TLR4–; arrow) HEK293T cells. However, after capsaicin treatment, TRPV1 expression was significantly reduced in the TLR4– HEK293T cells compared to the TLR4+ cells (Figure 4(b)). These data were confirmed by fluorescence-activated cell sorting (FACS) analysis. Capsaicin treatment reduced the percentage of TRPV1+ HEK293T cells in the TRPV1-transfected HEK293T cell population by more than 50%, while the percentage of TRPV1+ cells in the TRPV1 plus TLR4-transfected cell population was comparable between vehicle and capsaicin-treated samples (Figure 4(c) and (d)). To test whether lysosomal TRPV1 degradation is involved in the decrease of TRPV1+ cells after capsaicin treatment, we inhibited lysosomal protein degradation using chloroquine. With chloroquine pretreatment, the percentage of the TRPV1+ cell population in TRPV1-transfected cells was not substantially altered after capsaicin activation (Figure 4(c) and (d)). Taken together, these data indicate that TLR4 inhibits activation-induced TRPV1 endocytosis and subsequent lysosomal degradation, which might be responsible for the enhanced TRPV1 desensitization observed in TLR4-deficient neurons.
Figure 4.
TLR4 inhibits activation-induced TRPV1 internalization and degradation. (a) Biotinylated membrane proteins were prepared from primary cultured WT or TLR4 KO sensory neurons with or without capsaicin stimulation (1 µM) for 15 min. Biotinylated proteins (MEM, lanes 1–4) and whole-cell extracts (lanes 5–8) were used for western blot assay to detect TRPV1 expressed on cell membranes and α-tubulin. (b) HEK293T cells transiently overexpressing TRPV1-GFP and TLR4-HA were incubated with capsaicin (1 µM) for 15 min. Cells were stained with anti-HA antibody to detect TLR4-expressing cells. TRPV1 expression patterns on the cells were detected under confocal microscopy. Representative pictures are shown (arrows: TLR4–/TRPV1+ cells; arrowheads: TLR4+/TRPV1+ cells). Scale bar, 10 µm. (c), (d) HEK293T cells were transfected with TRPV1-GFP or TLR4-HA plus TRPV1-GFP expression vectors. In an experiment, cells were pretreated with 200 µg/ml CQ for 30 min and then exposed to 1 µM of capsaicin. Cells were fixed with 2% of PFA, and FACS analysis was performed using GFP signal to measure TRPV1-expressing cell population. Representative FACS plots (c) and quantified FACS data (d) are shown. The data represent mean with SEM (n = 5, **p < 0.01, *** p < 0.005).
WT: wild-type; TLR4: toll-like receptor 4; KO: knockout; TRPV1: transient receptor potential V1; CQ: chloroquine; GFP: green fluorescent protein. CTL: Control; MEM: Membrane.
Discussion
In this study, we present data for the first time showing that TLR4 potentiates TRPV1 activity by direct protein–protein interactions. TLR4 is a member of the TLR family that recognizes LPS of Gram-negative bacterial infections. Upon activation, TLR4 triggers a series of intracellular signaling pathways, activating NF-κB and MAP kinase and eventually resulting in inflammatory gene expression in the innate immune cells. In addition, TLR4 activation can induce intracellular calcium increase, although the mechanisms have not been clearly elucidated. Recent studies indicate that TLR4 can crosstalk with membrane channel molecules of the TRP family and thereby affect intracellular calcium signaling. In endothelial cells, TLR4 stimulation leads to activation of TRPC6 and thereby induces calcium influx.16 In macrophages, TLR4-induced proinflammatory cytokine expression is dependent on TRPV2-dependent calcium influx.17 However, in these studies, TLR4-induced second messenger molecules such as diacylglycerol were proposed to activate the TRP channel indirectly.16 In this regard, our data showing TRP channel activity regulation by direct protein interaction between TLR4 and TRPV1 are quite distinct and provide a novel regulatory mechanism of TRPV1 by TLR4.
Of interest, our study using the TIR deletion mutant of TLR4 or soluble TIR expression supports the premise that TLR4-TRPV1 interaction is mediated by the TIR domain of TLR4. Furthermore, attenuation of TRPV1 activity by soluble TIR overexpression demonstrates that TIR domain-dependent TLR4-TRPV1 interaction is responsible for the TRPV1 activity increase by TLR4. Considering that TRPV1 does not have a TIR domain, our data suggest that the TIR domain of TLR4 can interact with protein motifs other than TIR. However, our data do not exclude the possibility that certain TIR-containing adaptor molecules mediate TLR4-TRPV1 association, and this should be investigated in future studies. In this study, we did not provide evidence of endogenous TLR4-TRPV1 protein interaction in sensory neurons, which we suspect is partly due to inefficiency of the antibody used for immunoprecipitating native proteins. Still, our data using TLR4 KO sensory neurons support a functional interaction between endogenous TLR4 and TRPV1 proteins.
As an underlying mechanism for TLR4-mediated TRPV1 activity potentiation, we revealed that TLR4 blocks desensitization of TRPV1 by inhibiting activation-induced TRPV1 endocytosis and subsequent lysosomal degradation. TRPV1 sensitivity is regulated at multiple levels. TRPV1 excitability can be increased by phosphorylation, decreasing the threshold to agonist stimuli.9,18 In addition, TRPV1 is sensitized by increased membrane trafficking of this channel upon stimuli.6,10 In contrast, TRPV1 is desensitized upon agonist activation by internalization and subsequent lysosomal degradation in a calcium-dependent manner.8 Recent studies showed that the level of TRPV1 sensitization/desensitization is regulated by protein–protein interactions with other cytosolic proteins such as AKAP79/150 and β-arrestin. Upon binding to TRPV1, AKAP79/150 recruits kinases such as PKA and PKC that phosphorylate TRPV1, thereby enhancing its sensitization or blocking its desensitization.19 Meanwhile, binding of β-arrestin to TRPV1 competes against TRPV1 phosphorylation by kinases and desensitizes the TRPV1 channel.20 Considering the critical role of AKAP79/150 and β-arrestin on TRPV1 sensitization/desensitization, it can be speculated TLR4 interaction with TRPV1 might affect the binding affinity of TRPV1 to AKAP75/150 or β–arrestin and thereby regulate TRPV1 desensitization. This possibility needs to be tested in future studies.
In this study, we focused on the role of TLR4 on TRPV1 sensitization/desensitization in sensory neurons, which we propose as one of the underlying mechanisms of the reduced pruritic responses observed in TLR4 KO mice.21 Of note, TRPV1 is expressed not only in sensory neurons but also in other cell types. For instance, recent studies discovered that TRPV1 is expressed in immune cells, including helper T cells, macrophages, and dendritic cells.22 It is well-known that intracellular calcium serves as an important signaling molecule in activation of the above immune cells.23 Considering that all these immune cells also express TLR4,24 it is reasonable to speculate that TLR4-TRPV1 interaction in these immune cells can also play a role in TRPV1-induced calcium signaling and subsequent immune cell activation. Therefore, the TRPV1 sensitization mechanism by TLR4 discovered in this study might play important roles in physiological/pathological immune responses beyond pruritus.
In conclusion, our data show that TLR4-TRPV1 association via the TLR domain can potentiate TRPV1 activity. Additionally, TLR4 inhibits activation-induced TRPV1 internalization and subsequent degradation, which might be responsible for TLR4-mediated TRPV1 potentiation.
Authors’ Contributions
All authors read and approved the final manuscript. Hyunjung Min, Woo-Hyun Cho, and Hyunkyoung Lee performed experiments, analyzed data, and wrote the manuscript. Boomin Choi performed western blot; Se-Young Choi and Yoon-Jung Kim performed calcium assay using a spectrofluorophotometer and analyzed data. Yeonhee Joo performed TLR4 mutagenesis and subcloning experiments. Han K Lee and Sung J Jung designed and performed patch-clamp recording and analyzed data; Soojin Lee and Sung J Lee designed and monitored this work and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea, which is funded by the Ministry of Education, Science, and Technology (NRF-2016M3C7A1905074, NRF-2016R1A2B4006288).
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 2.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999; 400: 452–457. [DOI] [PubMed] [Google Scholar]
- 3.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 2007; 27: 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 2002; 88: 544–548. [DOI] [PubMed] [Google Scholar]
- 5.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother 1967; 31: 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 7.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem 2004; 279: 25665–25672. [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Salvador L, Andres-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem 2012; 287: 19462–19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 2003; 278: 50080–50090. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005; 24: 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci 2015; 35: 13487–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. . LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 13.Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, Lee SJ. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain 2014; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in toll-like receptor signalling. Nat Rev Immunol 2007; 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 15.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem 2001; 276: 11021–11030. [DOI] [PubMed] [Google Scholar]
- 16.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med 2012; 209: 1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashiro K, Sasano T, Tojo K, Namekata I, Kurokawa J, Sawada N, Suganami T, Kamei Y, Tanaka H, Tajima N, Utsunomiya K, Ogawa Y, Furukawa T. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem Biophys Res Commun 2010; 398: 284–289. [DOI] [PubMed] [Google Scholar]
- 18.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 2003; 100: 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28: 4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Por ED, Gomez R, Akopian AN, Jeske NA. Phosphorylation regulates TRPV1 association with beta-arrestin-2. Biochem J 2013; 451: 101–109. [DOI] [PubMed] [Google Scholar]
- 21.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 2006; 52: 691–703. [DOI] [PubMed] [Google Scholar]
- 22.Bertin S, Aoki-Nonaka Y, de Jong PR, Nohara LL, Xu H, Stanwood SR, Srikanth S, Lee J, To K, Abramson L, Yu T, Han T, Touma R, Li X, González-Navajas JM, Herdman S, Corr M, Fu G, Dong H, Gwack Y, Franco A, Jefferies WA, Raz E. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells. Nat Immunol 2014; 15: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh-Hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol 2008; 20: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol 2007; 179: 41–44. [DOI] [PubMed] [Google Scholar]