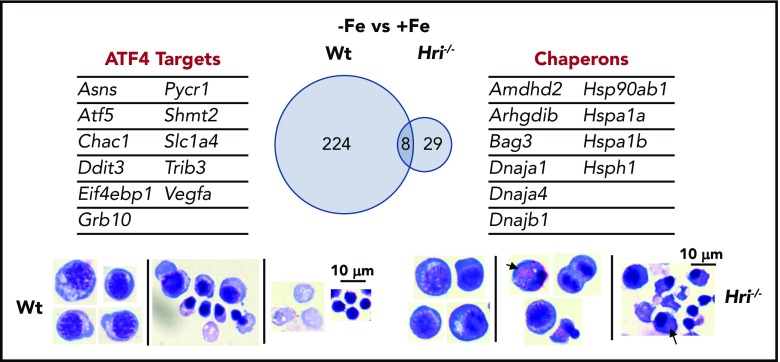
Figure 5.
HRI-ISR prevents cytoplasmic unfolded protein response and enables terminal erythropoiesis. Differentially expressed mRNAs between +Fe and −Fe conditions of Wt and Hri−/− BasoEs are shown. Only 37 mRNAs are differentially expressed in Hri−/− BasoEs during ID, in contrast to 232 mRNAs that are differentially expressed in Wt BasoEs. Importantly, ATF4 target genes are most highly activated, specifically in Wt BasoEs, during ID to mitigate oxidative stress and to enable terminal erythropoiesis. Thus, HRI is necessary for transcriptome adaptation to ID. The majority of upregulated mRNAs are cytoplasmic chaperons in Hri−/− BasoEs during ID as a response to denatured unfolded globin synthesized in excess of heme. However, increased chaperone expression alone in the absence of HRI is not sufficient to overcome the proteotoxicity. Hri−/− FL erythroid progenitors suffer severe proteotoxicity of aggregated protein inclusions during terminal erythroid differentiation from 30 hours to 42 hours when Hb synthesis is intensified. Lower panels, C57/BL6 stain. Adapted from Zhang et al with permission.46