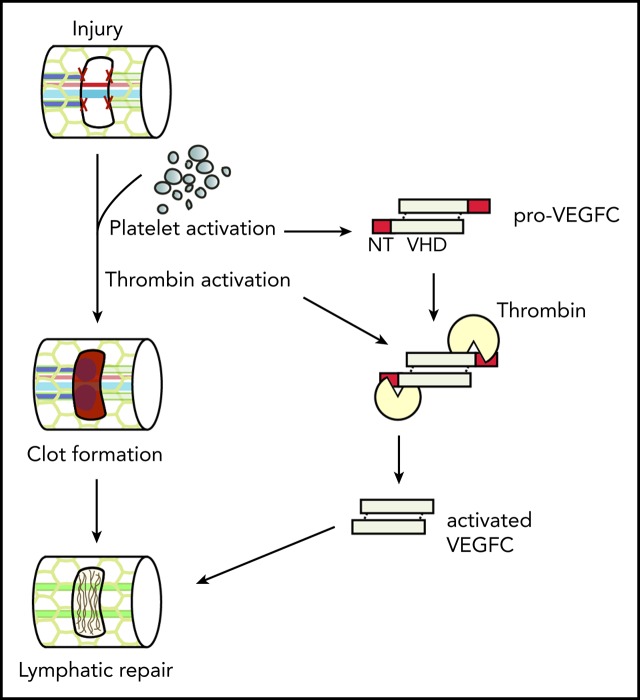
Key Points
Platelet activation supports lymphatic vessel growth during wound healing through release of the lymphangiogenic factor VEGFC.
Thrombin and plasmin support lymphatic vessel growth through proteolytic activation of the lymphangiogenic factors VEGFC and VEGFD.
Abstract
Hemostasis associated with tissue injury is followed by wound healing, a complex process by which damaged cellular material is removed and tissue repaired. Angiogenic responses are a central aspect of wound healing, including the growth of new lymphatic vessels by which immune cells, protein, and fluid are transported out of the wound area. The concept that hemostatic responses might be linked to wound healing responses is an old one, but demonstrating such a link in vivo and defining specific molecular mechanisms by which the 2 processes are connected has been difficult. In the present study, we demonstrate that the lymphangiogenic factors vascular endothelial growth factor C (VEGFC) and VEGFD are cleaved by thrombin and plasmin, serine proteases generated during hemostasis and wound healing. Using a new tail-wounding assay to test the relationship between clot formation and lymphangiogenesis in mice, we find that platelets accelerate lymphatic growth after injury in vivo. Genetic studies reveal that platelet enhancement of lymphatic growth after wounding is dependent on the release of VEGFC, but not VEGFD, a finding consistent with high expression of VEGFC in both platelets and avian thrombocytes. Analysis of lymphangiogenesis after full-thickness skin excision, a wound model that is not associated with significant clot formation, also revealed an essential role for VEGFC, but not VEGFD. These studies define a concrete molecular and cellular link between hemostasis and lymphangiogenesis during wound healing and reveal that VEGFC, the dominant lymphangiogenic factor during embryonic development, continues to play a dominant role in lymphatic growth in mature animals.
Visual Abstract
Introduction
The hemostatic response to tissue injury has been long recognized to prepare for biological events beyond the immediate cessation of bleeding, such as supporting immune responses and wound healing.1-3 Fibrin network formation and platelet retraction function to limit wound area,4 whereas platelet α- and dense-granule releasate contributes numerous molecular mediators of immune cell recruitment and tissue repair.5 Platelet α-granules are rich with growth factors known to stimulate angiogenesis and cell growth in the injury site, including insulin-like growth factor 1, platelet-derived growth factor, transforming growth factor-β, and vascular endothelial growth factor A (VEGFA; reviewed in Blair and Flaumenhaft6). Platelet α-granules have also been shown to release VEGFC,7 the dominant mediator of lymphangiogenesis during development,8 and VEGFC transcripts have been detected in platelets.9 However, a functional role for platelets in supporting lymphatic vessel growth associated with subsequent wound healing has not been defined.
Lymphatic vessels participate in essential homeostatic functions, including management of interstitial fluid, adaptive immune responses, and the transport of absorbed lipids from the gut,10,11 as well as in pathologic responses such as wound healing and tumor metastasis.12,13 Lymphatic vascular growth has been extensively studied in the context of embryonic development, using mammalian, avian, and fish models.14,15 These studies have identified a number of molecular pathways essential for the specification and growth of lymphatic vessels in the developing embryo. It is commonly thought that the molecular and genetic circuitry by which lymphatic vessels develop will also be used in the mature animal, but this hypothesis remains largely untested.
Genetic studies in developing mice and fish have identified VEGFC as an essential lymphangiogenic factor that acts primarily through the receptor tyrosine kinase VEGFR3 to drive the growth of lymphatic vessels.8,16-18 In contrast to VEGFA, VEGFC is secreted as a propeptide that requires proteolytic cleavage of its N- and C-termini to generate a mature growth factor capable of activating VEGFR3.19,20 Recent studies have revealed that the secreted protein CCBE1 and the metalloprotease ADAMTS3 are required specifically for VEGFC proteolysis.20,21 These findings are consistent with the observations that developing mice lacking CCBE1 or ADAMTS3, and fish lacking CCBE1, fail to form lymphatic vessels,22-25 a phenotype identical to that of animals lacking VEGFC. In vitro studies have also identified plasmin, a serine protease involved in clot degradation, as capable of cleaving and activating VEGFC.26 Plasmin has also been demonstrated to cleave VEGFD, a second lymphangiogenic factor that requires proteolysis to activate VEGFR3 but is not an ADAMTS3 substrate.26 Thus, the hemostatic system may drive postinjury lymphangiogenesis by delivering VEGFC to the site via platelet degranulation and/or generating the enzymes required to activate the lymphangiogenic factors VEGFC and VEGFD. A previous study using nongenetic and nonmolecular methods supports the role for PRP in promoting lymphatic repair27; however, the molecular mechanisms by which PRP may promote lymphangiogenesis in the context of wound healing remains unclear.
To test whether hemostatic responses can stimulate lymphangiogenesis and define the roles of VEGFC and VEGFD in lymphangiogenesis associated with wound healing, we used an established skin wounding model and a novel tail lymphatic injury model in which lymphatic vessel repair occurs in the setting of clot formation. Using this tail lymphatic injury model, we found that addition of platelet-rich plasma (PRP) to the injury site significantly accelerates lymphatic vessel repair compared with addition of platelet-poor plasma (PPP). Furthermore, we found that genetic loss of VEGFC, but not VEGFD, leads to impaired lymphatic vessel repair compared with mice with wild-type levels of VEGFC. Consistent with a central role for platelets in this process, addition of wild-type, but not VEGFC-deficient, PRP to the injury site of VEGFC conditional knockout (cKO) animals rescues the defect in lymphatic vessel repair after tail injury. In the skin wounding model in which there is very little bleeding, and hence few platelets in the injury site, we also found that VEGFC was the major driver of lymphangiogenesis after wounding, whereas VEGFD had no discernable role. These studies establish VEGFC as a molecular link between platelet activation during hemostasis and lymphatic growth during wound healing.
Methods
VEGFC and VEGFD processing and western blot
Full-length FLAG-tagged VEGFC or VEGFD (VEGFC-VHD-FLAG or VEGFD-VHD-FLAG) constructs were cloned into the pcDNA 3.1+ plasmid, as described previously.20 Primers used for VEGFD are included in the supplemental Data, available on the Blood Web site. Wild-type HEK293T cells were seeded at a density of 3 × 105 cells per 10 cm2, and transfected with the indicated plasmids 16 hours after seeding, using Lipofectamine 2000 (Invitrogen) or FuGENE Reagent 6 (Promega). Transfected cells were left in Opti-MEM I (Thermo Fisher), and conditioned media were collected 72 hours after transfection. The conditioned media were then incubated with either conditioned medium from ADAMTS3-transfected WT HEK293T cells or plasmin (HTI HCPM-0140) or thrombin (HTI HCT-0020), at the indicated concentration (Figure 1) at 37°C. After the indicated incubation time, the samples were collected and analyzed for processing by western blotting. Western blotting was performed using anti-FLAG (Sigma-Aldrich A8592-1MG) and developed using ECL reagent (Thermo Fisher Scientific). For thrombin inhibitor experiments, conditioned medium containing FLAG-tagged VEGFC was prepared as before, and preincubated with either vehicle control or the indicated concentration of Dabigatran (Selleckchem S2196) at 37°C for 30 minutes. Thrombin (HTI HCT-0020) was then added, and after incubating at 37°C for 18 hours, samples were collected and analyzed for VEGFC processing using near-infrared western blot detection, using anti-FLAG M2 (Sigma-Aldrich F1804), and detected using the Odyssey Fc Imager (LI-COR).
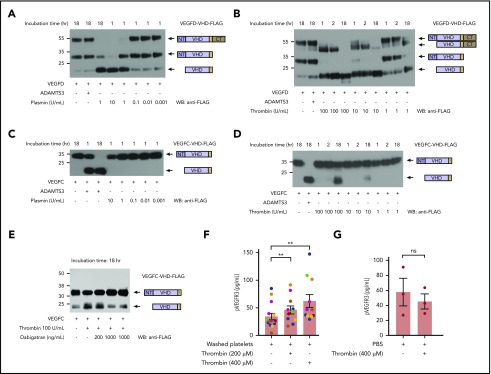
Figure 1.
The lymphangiogenic factors VEGFC and VEGFD are proteolytically activated by thrombin and plasmin. (A-D) Proteolytic release of VEGFC VHD and VEGFD VHD by plasmin, thrombin, and ADAMTS3 proteases. Conditioned supernatant containing VEGFD VHD-FLAG or VEGFC VHD-FLAG was incubated with the indicated concentrations of thrombin and plasmin for 1, 2, or 18 hours, and VHD-FLAG detected by western blotting with anti-FLAG antibodies. The protein domains within the detected bands are shown schematically on the right. Note that VEGFD is efficiently cleaved by plasmin and, to a lesser extent, thrombin, but not by ADAMTS3, whereas VEGFC is efficiently cleaved by ADAMTS3 and thrombin, but not by plasmin. (E) Thrombin cleavage of VEGFC is inhibited by addition of thrombin-specific inhibitor dabigatran. (F) Thrombin-activated washed platelets drive VEGFR3 phosphorylation in cultured LECs. Washed human platelets were exposed to the indicated concentrations of thrombin, added to cultured LECs and phospho-VEGFR3 measured by ELISA. Each color represents results obtained using platelets from a single donor. (G) LEC exposure to thrombin alone does not increase phospho-VEGFR3. Error bars indicate SEM. **P < .01; ns, not significant.
Isolation and activation of human platelets
Venous blood was obtained from healthy adult human volunteers (3 men and 4 women), and diluted 1:10 into a 3.8% sodium citrate solution. Washed platelets were then prepared by 2 sequential centrifugation steps, as follows. Whole blood was first spun at 500g for 8 minutes, the plasma layer transferred to a fresh tube, and spun at 1100g for 9 minutes. The supernatant was removed, and the pelleted platelets were resuspended in phosphate-buffered saline (hereafter referred to as “washed platelets”). The washed platelets were transferred into 1.5-mL Eppendorf tubes and either left untreated or activated for 20 phosphate-buffered saline with the indicated amount of thrombin. All steps were performed at room temperature. Human studies were approved by the University of Pennsylvania Institutional Review Board.
Preparation of PRP and PPP, and treatment of injury site with PRP or PPP
Blood was collected from the inferior vena cava of donor mice between 6 and 12 weeks of age from the same colony. Blood was drawn into a syringe containing 3.8% sodium citrate. PRP was prepared by 2 centrifugation steps as follows. Whole blood was first spun at 300g × 5 minutes to pellet red blood cells. The plasma layer, including the buffy coat, was then collected, transferred into a fresh Eppendorf tube, and centrifuged again at 1000g × 5 minutes to pellet platelets. The top half of the supernatant was removed and transferred into a fresh Eppendorf tube, and used as PPP if required for the experiment. The pelleted platelets were resuspended in the remaining supernatant and used as PRP. Citrated PRP and PPP were allowed to sit at room temperature (30 minutes-3 hours) while the tail lymphatic injury surgery was performed on the recipient mouse. After resection of the collecting lymphatic vessels, the injury site was cleaned using a clean Kimwipe to remove any blood or Evans blue dye (EBD). The citrated PRP or PPP was then activated with 200 mM calcium chloride (1:10), and ∼5 μL of the re-calcified PRP or PPP added to the cleaned injury site. PRP/PPP was allowed to clot before removing the mouse from anesthesia.
LEC stimulation and phospho-VEGFR3 enzyme-linked immunosorbent assay (ELISA)
Neonatal lymphatic endothelial cells (LECs; Lonza CC2505) were seeded at a density of 0.5 × 106 cells per well in a 6-well plate 16 to 24 hours before the experiment. On the day of the experiment, LECs were serum-starved in 1 mL of 0.1% bovine serum albumin in nonsupplemented EBM2 media (Lonza) for 6 hours at 37°C. Serum-starved LECs were treated with the untreated or activated washed platelets for 15 minutes at 37°C. The media were then aspirated and the cells washed 3 times with cold phosphate-buffered saline before being lysed with 150 μL phospho-VEGFR3 lysis buffer (1% NP-40 Alternative, 20 mM Tris at pH 8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM activated sodium orthovanadate, and protease inhibitors; 1 tablet of cOmplete, Mini, EDTA-free Protease inhibitor Cocktail, Roche). Samples were collected, and the ELISA was performed according to manufacturer’s instructions in the Human Phospho-VEGFR3 ELISA Kit (R&D Systems DYC2724).
Mice
A conditional allele of the Vegfc gene was generated by introducing loxP sites flanking exon 3 using gene targeting in ES cells. Details of targeting are described in supplemental Methods and shown in supplemental Figure 1. Correctly targeted homozygous animals (Vegfcfl/+) were crossed with FLPe mice to remove the FRT-flanked neomycin resistance cassette. Rosa-CreERT2 mice have been previously described,28,29 and Vegfd−/− mice have been previously reported.30 For global conditional deletion of Vegfc, mice were given 200 μL of 25 mg/mL tamoxifen (Sigma) dissolved in corn oil by gavage daily for 5 days, and then placed on tamoxifen chow on the fifth day through the duration of the experiment. Both Vegfcfl/fl and Vegfcfl/fl; Rosa-CreERT2 animals were given tamoxifen. Wild-type Swiss Webster mice (Charles River) were used for characterization of the tail injury model. All other experimental and control animals were maintained on a mixed genetic background. Animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Tail lymphatic injury model
Twelve- to 17-week-old mice were anesthetized with isoflurane, and hair was removed from the tail using a razor blade or depilatory cream. EBD was injected near of the tip of the tail to label the lymphatic vessels, and a small, 1-mm-wide excisional wound on the left lateral side of the tail, 3 cm from the base of the tail, was then made using a clean razor blade and small surgical scissors, taking care not to damage the underlying major artery and vein (Figure 2). The injury site was cleared of blood continuously until the cessation of bleeding to provide a clear open wound site. The 1-mm segment of exposed collecting lymphatics running next to the tail vein was then carefully resected. After resection, either the injury site was gently massaged to initiate bleeding from the surrounding tissue into the injury site, or the injury site was filled with approximately 5 μL ex vivo blood products recalcified PRP or recalcified PPP. The blood was then allowed to clot in the injury site before removing the animals from anesthesia. The wounds were left without dressing, as any dressing that can be reached by the mice is quickly chewed away by the mice. Of note, the wound site 3 cm from the base of the tail was chosen because it cannot be reached or disturbed by the animal.
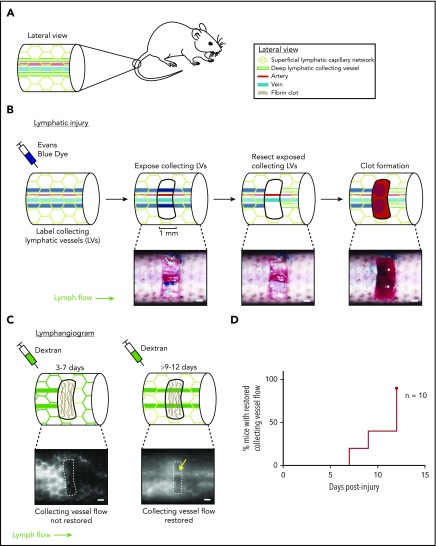
Figure 2.
Characterization of a mouse hemostatic tail lymphatic injury model. (A) Anatomy of the blood and lymphatic vessels in the mouse tail. (B) Schematic of the hemostatic tail lymphatic injury model (top). Images of the injury model at each step are shown in the bottom panels. Collecting lymphatic vessels in the tail are first labeled by EBD injection. A 1-mm section of skin is then removed without damaging the underlying vessels, and the exposed section of lymphatic collecting vessel is resected. Blood from disrupted blood capillaries fills the injury site and forms a blood clot. (C) Lymphangiogenesis is assessed postinjury by lymphangiography with fluorescent dextran. Before restoration of collecting lymphatic vessel function (3-7 days), fluorescent dextran is taken up and drained by surface lymphatic capillaries. After restoration of collecting lymphatic vessel function (9-12 days), fluorescent dextran drainage across the injury site via the collecting lymphatic vessels is observed (yellow arrow). (D) Healing curve showing percentage of animals with restored flow in collecting lymphatic vessels over time in wild-type animals (n = 10). LV = lymphatic vessel. Scale bars = 500 µm.
Lymphangiography
To assess healing of the tail lymphatic vessels, 10 kDa fluorescent dextran (Invitrogen D22910, D22912) was injected subcutaneously into the tail, distal to the injury site on days 7, 9, and 12 for wild-type animals, and days 5, 7, 9, and 12 for wild-type animals treated with PRP or PPP. For Vegfc and Vegfd animals, the lymphangiogram was performed on days 7, 9, 12, 15, 18, and 21. Dextran flow across the injury site was assessed using an Olympus MVX10 microscope and Olympus DP74 camera with the cellSens software. Images were taken in either the red or green channel, depending on the fluorophore linked to the dextran, and converted to grayscale using the RGB to luminance function on Fiji (Fiji Is Just ImageJ). Collecting lymphatic vessel flow was considered not restored if fluorescent signal was not detected extending across the entire 1-mm injury site (supplemental Video 1). Collecting lymphatic vessel flow was scored as restored if a fluorescent pulse was visualized going across the injury site in a vessel-like structure and at the right anatomical location (supplemental Video 2).
Full-thickness skin injury model
Twelve- to 16-week-old mice were used for the skin wounding experiments. In brief, mice were anesthetized with isoflurane, and hair was shaved off the dorsum of the animals. The surgical site was then cleaned with alcohol and povidone iodine to create a clean site for wounding. A 15 × 15-mm square was measured and marked out on the dorsal skin using a ruler, and a full-thickness skin excisional wound was made by lifting the skin away from the underlying fascia and removing the area of skin outlined using surgical scissors. After the surgery, the mouse was returned to a clean cage and allowed to recover. The wound was left uncovered and without a dressing. The dorsal placement of the wound prevents the mouse from reaching the wound with its mouth.
Histology and immunohistochemistry
Mice were sacrificed by CO2 asphyxiation, and dorsal skin containing either healthy or wounded skin was removed from the mice and fixed in 2% paraformaldehyde overnight at 4°C. Tissues were then dehydrated to 100% EtOH, paraffin embedded, and sectioned, as described previously.28 Sections were subjected to hematoxylin and eosin and/or immunohistochemical staining, using antibodies against Prox1 (Abcam ab76696) and Lyve1 (R&D AF2125), as previously described.31 Images were acquired with an Olympus BX53 microscope and Olympus DP80 camera, using ×10, ×20, and ×40 objectives, saved using the cellSens software, and processed in ImageJ.
Quantification of lymphangiogenesis
Paraffin-embedded wound tissues were cross-sectioned from the anterior to posterior edges of the wound at a thickness of 6 µm. For quantification, sections from the center, anterior, and posterior edges of the wound were used, and random 20× fields (approximately 3-4 per section) from each section were taken on the microscope, and the number of LECs (Lyve1+Prox1+ double positive cells) in each 20× image was counted. The number of LECs in each field was summed and averaged over the total number of random fields taken. A minimum of 10 random 20× fields were counted per animal.
Statistical analysis
P values in the ELISA were calculated using the Wilcoxon matched-pairs signed rank test. P values in the tail lymphatic injury assay were calculated using the Gehan-Breslow-Wilcoxon method. P values for quantification of lymphangiogenesis after skin wounding was calculated using an unpaired, 2-tailed Student’s t-test.
Results
Coagulation and anticoagulation proteases activate lymphangiogenic factors
Recent studies have demonstrated that the lymphangiogenic factors VEGFC and VEGFD are secreted as inactive propeptides that require proteolytic cleavage to their mature form (the VEGF homology domain [VHD]), to activate VEGFR3.19-21 Biochemical and genetic evidence demonstrate that VEGFC is cleaved by the metalloprotease ADAMTS3 and requires the presence of CCBE1, a nonenzymatic cofactor.20,21,32 In contrast, VEGFD is not cleaved by ADAMTS3, but is instead cleaved at a site consistent with serine protease activity.20 These results are consistent with a previous report that plasmin can cleave VEGFD as well as VEGFC,26 and that plasmin-cleaved VEGFD and VEGFC can bind VEGFR3 and drive receptor dimerization.26 To further explore a potential role for coagulation and anticoagulation proteases in lymphangiogenesis, we expressed full-length VEGFC and VEGFD proteins in which the VHD domain was FLAG-tagged,20 incubated them with either plasmin or thrombin, and used western blotting to assay cleavage. Plasmin effectively cleaved VEGFD (Figure 1A), but we could not detect any cleavage of VEGFC (Figure 1C). The highest levels of plasmin did lead to minor depletion of the full-length protein without appearance of the VHD band, an observation that could indicate cleavage of the FLAG-tag from the protein. Thrombin efficiently cleaved VEGFD, and at higher concentrations was also able to cleave VEGFC (Figure 1B,D). We further confirmed the specificity of thrombin-dependent cleavage by adding the specific thrombin inhibitor dabigatran, which inhibited the cleavage of VEGFC in a dose-dependent manner (Figure 1E). These results and prior studies suggest that serine proteases generated during and after hemostasis may stimulate lymphangiogenesis by proteolytically activating VEGFD and/or VEGFC.
Activated platelets stimulate VEGFR3 signaling in cultured LECs
VEGFC transcripts are detected in platelets using quantitative polymerase chain reaction, and VEGFC protein is released by activated platelets.7 VEGFC transcripts have also been detected in human platelets by RNAseq,9 and we have found that VEGFC expression is upregulated 276-fold in avian thrombocytes vs lymphocytes (Schmaier et al33 and data not shown). These findings suggested that platelets may serve as an important and evolutionarily conserved source of VEGFC to link lymphangiogenesis to wound healing. To determine whether platelets are able to stimulate lymphangiogenesis by releasing VEGFC, we exposed cultured human LECs to thrombin-activated platelets and analyzed VEGFR3 phosphorylation. Thrombin-activated platelets stimulated VEGFR3 phosphorylation in the cultured LECs (Figure 1F), but exposure of LECs to thrombin alone did not drive VEGFR3-phosphorylation (Figure 1G). These ex vivo studies suggest that platelet activation may stimulate lymphatic growth through release and delivery of VEGFC during the wound healing process.
Development of a murine hemostatic lymphatic injury model
Lymphangiogenesis has been extensively studied in the developing embryo, where trauma is absent and hemostasis is minimal. Postnatal models of lymphangiogenesis have been optimized for direct visualization of lymphatic vessel growth (eg, in the cornea or ear skin), conditions for which bleeding and hemostasis are negative factors and have therefore been excluded. To further test a link between hemostasis and lymphangiogenesis, we developed a tail lymphatic injury model in mice in which the regrowth and restored function of a resected section of collecting lymphatic vessel occurs in the setting of thrombus formation and is followed over time, using tail lymphangiography. This model is based on a well-characterized tail skin denudation model in which the superficial and deep lymphatic networks are removed from a large section of the mouse tail.34,35 We modified this protocol to create a smaller injury site that both does not fully disrupt lymphatic flow through the tail and creates a hemostatic injury/clot site to which blood products such as PRP may be added in trans to test their effect on lymphatic growth. We believe these components of the model represent physiological injuries in which lymphatic vessels are disrupted and need to be repaired, rather than re-growing new vessels, which is seen in the denudation model. The mouse tail contains a superficial lymphatic capillary network that feeds into a deeper set of collecting lymphatic vessels, which are located lateral to the tail artery and vein (Figure 2A). In the hemostatic tail lymphatic injury model, the collecting lymphatic vessels are first labeled by subcutaneous injection of EBD near the tip of the tail (Figure 2B, left). After EBD injection, a 1-mm section of skin is removed from the lateral side of the tail 3 cm from the base of the tail without damaging the underlying blood and lymphatic vessels. The exposed collecting lymphatic vessels are then carefully resected without damaging the adjacent tail artery and vein, and the wound site allowed to fill passively with blood from surrounding interrupted blood capillaries, or actively with ex vivo-derived PRP or PPP, and a clot is allowed to form (Figure 2B). Lymphangiogenic repair is subsequently assessed functionally, using injection of 10 kDa fluorescent dextran into the tail distal to the injury site (tail lymphangiography). Uptake of the dextran into superficial and collecting lymphatic vessels can be visualized under a fluorescent microscope, and lymphangiogenic repair scored by whether or not the fluorescent dextran drains across the collecting lymphatic vessels at the injury site (Figure 2C; supplemental Videos 1 and 2). Using this hemostatic tail lymphatic injury model, we found that after injury and formation of an endogenous blood clot, lymphatic flow was restored within 9 to 12 days after wounding in most animals (Figure 2D).
Platelets promote lymphangiogenesis after wounding
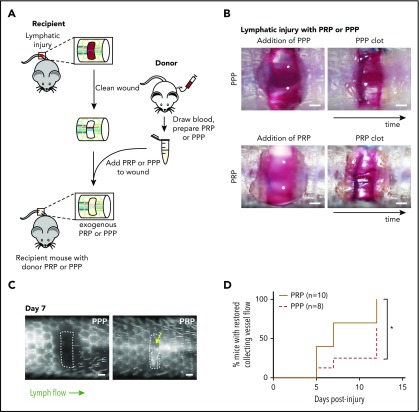
To determine whether platelets contribute significantly to lymphangiogenesis after wounding, the murine hemostatic lymphatic injury model was used as described here, except that PRP or PPP was added to the wound site immediately after resection of the collecting lymphatic vessels (Figure 3A-B). Addition of PRP to the injury site significantly accelerated lymphangiogenic repair, with restoration of lymphatic flow across the injury site observed in approximately half the time required after addition of PPP (Figure 3C-D). This finding provides the proof of principle that platelets can contribute significantly to lymphangiogenesis after wounding in vivo.
Figure 3.
Platelets promote lymphangiogenesis after wounding. (A) Schematic of PRP or PPP treatment to tail injury. After lymphatic injury in the recipient mouse, the injury site is cleaned and PRP or PPP prepared from blood from a donor mouse added. (B) Images showing application of PRP or PPP to the tail injury site. (C) Representative tail lymphangiography across the injury site on day 7 after tail lymphatic injury in wild-type mice to which PRP or PPP was added to the injury site. Note that flow across collecting lymphatic vessels is not restored in the PPP-treated animal, but is restored in the PRP-treated animal (yellow arrow). (D) Healing curves for restoration of lymphatic flow after addition of PRP or PPP to the injury site. Scale bars = 500 µm. *P < .05.
VEGFC is required for lymphangiogenesis after wounding
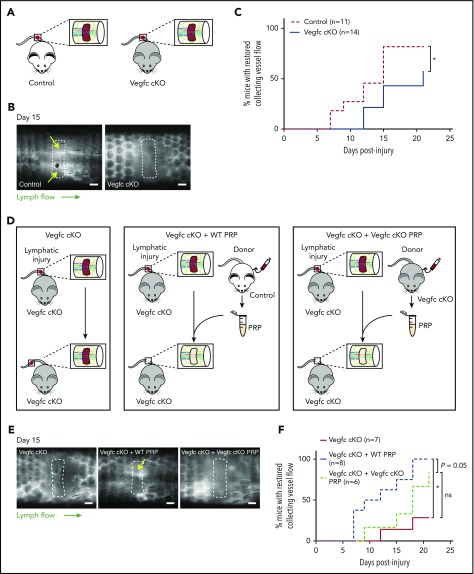
Prior studies demonstrating an indispensable role of VEGFC in developmental lymphangiogenesis, and our finding that VEGFC may be proteolytically activated by the central coagulation enzyme thrombin (Figure 1), suggested that VEGFC may play an important role in lymphangiogenesis during wound healing. To test a requirement for VEGFC during lymphangiogenesis after wounding, we generated Vegfc-floxed animals (Vegfcfl) in which Vegfc gene expression can be conditionally deleted using specific Cre-drivers (supplemental Figure 1). To test the requirement for Vegfc during lymphangiogenesis in the murine hemostatic lymphatic injury model, we used Vegfcfl/fl; Rosa-creERT2 animals in which Vegfc is globally deleted on tamoxifen induction (hereafter called “Vegfc cKO” animals; Figure 4A). Restoration of lymphatic flow after lymphatic injury was significantly impaired in Vegfc cKO animals compared with Vegfcfl/fl control littermates (Figure 4B-C). These findings reveal that VEGFC plays a central role in lymphangiogenesis in mature animals after wounding, as well as in embryos during lymphatic vascular development.
Figure 4.
Platelet-derived VEGFC promotes lymphangiogenesis after wounding. (A) Schematic of the hemostatic tail lymphatic injury model comparing control (Vegfcfl/fl) and Vegfc cKO (Vegfcfl/fl; Rosa-creERT2) mice. (B) Representative images of tail lymphangiograms across the injury site on day 15 after tail injury in control and Vegfc cKO animals. Yellow arrows indicate restored collecting vessel flow. (C) Healing curves for restoration of lymphatic flow in control (Vegfcfl/fl) and Vegfc cKO (Vegfcfl/fl; Rosa-creERT2) mice. (D) Schematic of the hemostatic tail lymphatic injury model to assess lymphangiogenic repair in Vegfc cKO mice, Vegfc cKO mice with addition of wild-type PRP and Vegfc cKO mice with the addition of Vegfc KO PRP. (E) Representative images of tail lymphangiograms across the injury site on day 15 after tail injury in Vegfc cKO mice, Vegfc cKO mice with addition of WT PRP and Vegfc cKO mice with addition of Vegfc KO PRP. Yellow arrow indicates restored collecting vessel flow. (F) Healing curves for Vegfc cKO mouse tail lymphatic collecting vessels in Vegfc cKO mice, and Vegfc cKO mice with addition of WT or Vegfc cKO PRP to the injury site. Scale bars = 500 µm. *P < .017 (using the Bonferroni method to correct for multiple comparisons).
Platelet-derived VEGFC promotes lymphangiogenesis after wounding
The studies described here suggested that platelet-derived VEGFC might play an important role in linking hemostasis and lymphangiogenesis after wounding. To test this hypothesis, we took advantage of the ability to use PRP in trans to support lymphangiogenesis in the murine hemostatic lymphatic injury model. After tail injury in Vegfc cKO animals, the injury site was allowed to fill with endogenous whole blood (“Vegfc cKO”) or filled with PRP harvested from either wild-type (“Vegfc cKO + WT PRP”) or Vegfc cKO animals (“Vegfc cKO + Vegfc cKO PRP”; Figure 4D). Addition of WT PRP to the injury site rescued the defect in lymphangiogenesis in Vegfc cKO animals, whereas the addition of Vegfc cKO PRP did not significantly improve lymphangiogenesis in the Vegfc cKO animals (Figure 4E-F). Although the power of these studies is limited both statistically (because of the variability of the response) and perhaps biologically (eg, because of roles of other lymphangiogenic factors such as VEGFA), these findings suggest that platelet-derived VEGFC is likely to participate in lymphatic repair after injury.
VEGFD is not required for lymphangiogenesis after wounding
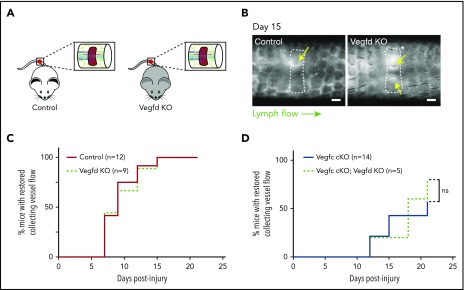
Although previous studies have failed to define an essential role for VEGFD during developmental lymphatic growth, our biochemical studies (Bui et al20; Fig. 1) and those of others26 indicate that VEGFD may be cleaved and activated by the coagulation protease thrombin and the thrombolytic protease plasmin. In addition, the finding that genetic deletion of VEGFC did not lead to a complete loss of lymphangiogenesis after wounding suggested that VEGFD may also participate in this process. To test this hypothesis, we performed the murine hemostatic lymphatic injury model in Vegfd KO animals (Vegfd-/0 males and Vegfd−/− females; Figure 5A). In contrast to mice lacking VEGFC, Vegfd KO mice demonstrated restored lymphatic flow after wounding at a rate that was indistinguishable from that of control animals (Vegfd+/0 males and Vegfd+/− females; Figure 5B-C), indicating that VEGFD is not required for lymphangiogenesis after wounding using this model. Finally, to test whether VEGFC may compensate for the loss of VEGFD, we tested lymphangiogenesis after wounding in animals that lacked both VEGFC and VEGFD (Vegfc cKO;Vegfd KO animals). Vegfc cKO;Vegfd KO animals exhibited a rate of restoration of lymphatic flow that was indistinguishable from that of Vegfc cKO animals (Figure 5D), consistent with a lack of redundancy between VEGFC and VEGFD in this response. These studies demonstrate that, unlike VEGFC, VEGFD does not participate significantly in lymphangiogenesis after wounding using the hemostatic tail lymphatic injury model.
Figure 5.
VEGFD is not required for lymphangiogenesis in the mouse hemostatic tail injury model. (A) Schematic of tail injury in control (Vegfd+/0 and Vegfd+/−) and Vegfd KO (Vegfd−/0 and Vegfd−/−) mice. (B) Representative images of tail lymphangiograms across the injury site on day 15 after tail injury in control and Vegfd KO animals. Yellow arrows indicate restored lymph flow across the injury site. (C) Healing curves for restoration of lymphatic flow in control and Vegfd KO animals. (D) Healing curves for restoration of lymphatic flow in Vegfc cKO and Vegfc cKO;Vegfd KO animals. Note that VEGFD is not required even in the absence of VEGFC. Scale bars = 500 µm. ns, not significant.
VEGFC but not VEGFD is required for lymphangiogenesis in skin injury model
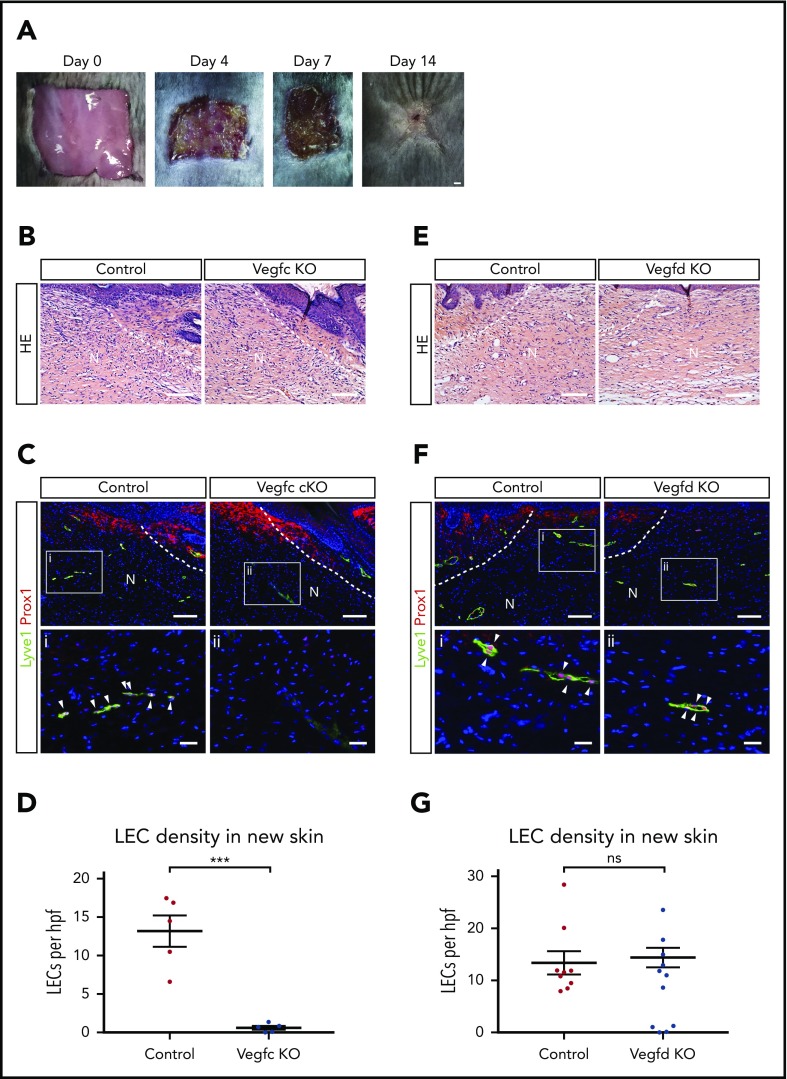
The studies described here support an important role for platelets and VEGFC in driving lymphatic growth and repair after injury, but whether and to what extent these results predict lymphangiogenic responses after wounding that is associated with less extensive bleeding and hemostasis is not clear. To address this question, we next tested the roles of VEGFC and VEGFD for lymphangiogenesis, using an established dorsal skin excision model that has been well characterized.36 In this model, skin excision is associated with minimal bleeding and minimal platelet recruitment (supplemental Figure 2), but generation of fibrin and the VEGFC/VEGFD-activating proteases thrombin and plasmin is present37 (Figure 6A). As reported by previous studies, after full-thickness excision, there is wound contraction and re-epithelialization that is accompanied by regrowth of lymphatic vessels but not hair follicles during a 14-day period (Hong et al38 and Ito et al39; supplemental Figure 3). After full-thickness skin excision, Vegfc cKO animals exhibited growth of new skin that was indistinguishable from that of control animals when assessed using hematoxylin and eosin staining of tissue sections 14 days after injury (Figure 6B). However, we observed a complete failure of lymphatic vessel growth in the new skin of Vegfc cKO animals, assessed by quantitative analysis of LECs that are marked by co-expression of the nuclear transcription factor PROX1 and the cell membrane glycoprotein LYVE1 (Figure 6C-D), whereas angiogenesis within the new skin was not affected (supplemental Figure 4). In contrast, loss of VEGFD did not perturb skin regeneration or lymphangiogenesis after full-thickness wounding (Figure 6E-G). Thus VEGFC, the major player in developmental lymphangiogenesis, is also the major player in postnatal lymphangiogenesis after wounding.
Figure 6.
VEGFC but not VEGFD is required for lymphangiogenesis after full-thickness dorsal skin excision. (A) Images showing growth of new skin within 14 days after full-thickness skin wounding. The area of new skin is identified by the lack of hair growth on day 14. (B) Hematoxylin and eosin (HE) staining reveals no obvious morphological differences within new skin after wounding of control or Vegfc cKO animals. (C-D) VEGFC is required for lymphangiogenesis after full thickness skin wounding. (C) Immunostaining of new skin 14 days after wounding reveals the presence of numerous Lyve1+Prox1+ LECs (white arrowheads) in control mice, but none in Vegfc cKO mice. (D) Quantitation of LEC density within new skin of control and Vegfc cKO animals. n = 5 for both groups. (E) HE staining reveals no obvious morphological differences within new skin after wounding of control or Vegfd KO animals. (F-G) VEGFD is not required for lymphangiogenesis after full-thickness skin wounding. (F) Immunostaining of new skin 14 days after wounding reveals the presence of numerous Lyve1+Prox1+ LECs (white arrowheads) in control and Vegfd KO mice. (G) Quantitation of LEC density within new skin of control and Vegfd KO animals. n = 9,7 (control, Vegfd KO). N, new skin; hpf, high-power field. **P < .01. Scale bars: A = 1 mm; B,C (top), E,F (top) = 100 µm; i, ii = 25 µm. Error bars indicate SEM.
Discussion
Platelets and coagulation proteases play a central role in hemostasis, a response to wounding that prevents lethal blood loss in vertebrates in which blood circulates in a closed vascular system. Whether and to what extent platelets and coagulation proteases also contribute to later aspects of wound healing such as vessel regrowth is less understood. A link between hemostatic responses and lymphangiogenesis has been suggested by studies demonstrating VEGFC expression in platelets and proteolytic activation of the primary lymphangiogenic factors VEGFC and VEGFD by plasmin,26 an abundant fibrinolytic enzyme generated at wound sites. In the present study, we have used new mouse genetic lines and both a new and an established wounding model to examine the relationship between hemostatic responses and lymphangiogenesis after wounding. Our results demonstrate that platelets may play a significant role in stimulating lymphangiogenesis after wounding, and identify VEGFC as the primary factor associated with lymphatic vessel growth after wounding.
Hemostasis stimulated by wounding is rapidly followed by a large number of biological responses that facilitate wound healing, including the growth of new blood and lymphatic vessels to nourish and drain the recovering tissue.40 Platelet activation and degranulation is a central event in hemostatic responses, and platelet granules are known to contain a large number of factors such as platelet-derived growth factors and VEGFs that are capable of stimulating angiogenesis.6 Thus, it has been postulated that platelet activation may be a potent and important regulator of the angiogenic responses associated with wound healing.41-43 Most of the data supporting this putative role for platelets, however, are derived from in vitro studies in which releasate from activated platelets stimulates the growth of cultured endothelial cells.43 In contrast, in vivo evidence for platelet participation in angiogenic responses has been lacking. We find that PRP accelerates lymphangiogenesis after wounding, that PRP harvested from VEGFC-deficient animals is unable to drive lymphangiogenesis to the extent of wild-type PRP, and that the loss of lymphangiogenesis observed after tail wounding in VEGFC-deficient animals can be rescued by wild-type PRP. These studies are consistent with prior nongenetic, nonmolecular studies supporting a role for platelets in lymphatic repair,27 and support a model in which platelet activation during hemostasis provides an early stimulus for the angiogenic responses that will follow. They also suggest that there are likely to be other physiologic and pathologic conditions in which activated platelets may regulate lymphatic vessel growth and repair.
These studies uncover a new aspect of the complex relationship between platelets and lymphatic vessels. Platelets do not leave the blood circulation without being activated, and are not normally present in the lymphatic vasculature. However, we and others have recently demonstrated that lymphatic endothelial cells are potent activators of platelets (mediated by LEC Podoplanin activation of the platelet CLEC-2 receptor), and that lymphatic endothelial activation of platelets is required to prevent blood from entering the lymphatic system at sites of lymphovenous anastomosis (a unique intravascular form of hemostasis termed lymphovenous hemostasis).44-46 However, a complete lack of platelets, either because of loss of the transcription factor Meis1 required for megakaryocyte formation47 or ablation of megakaryocytes using diphtheria toxin,44 does not compromise lymphatic vessel growth during development. Thus, it appears that platelet hemostatic function is required to safeguard lymphatic function at sites of lymphovenous junction and that activation of platelets (and release of platelet-derived VEGFC) plays an important lymphangiogenic role during wound healing, but not during embryonic vascular development.
A significant challenge for investigating the relationship between hemostasis and lymphangiogenesis has been the lack of an in vivo model with which to test it. In the present study, we have developed a novel mouse tail wounding model that permits such investigation. Notable advantages of this murine hemostatic lymphatic injury model are that it directly tests lymphatic function after wounding and is not limited to inferring changes in function from changes in lymphatic vessel anatomy or density, the ability to add PRP or PPP in trans permits rapid testing of platelets or plasma proteins from genetically modified animals, and the focal nature of the injury is similar to that associated with common surgical procedures known to result in clinical lymphatic dysfunction such as brachial edema after axillary lymph node resection. The ability of PRP to stimulate repair of collecting lymphatic vessels in the mouse tail suggests that PRP application may also stimulate repair of collecting vessels after lymph node removal in patients. Future studies will be needed to test the idea that platelets may be used as a natural VEGFC delivery mechanism to stimulate therapeutic lymphatic vessel growth and repair. Preclinical studies in mice have shown that VEGFC delivery can stimulate lymphangiogenesis after wounding and improve wound healing and edema resolution in skin wounds,48,49 providing further support for exploring methods of delivering VEGFC to sites of lymph node removal or injury sites.
A final significant finding of this study is that using 2 distinct wounding models, we observed a dominant role for VEGFC in postnatal lymphatic vessel growth, with no detectable role for VEGFD. Gain-of-function studies have demonstrated that VEGFC and VEGFD can stimulate VEGFR3 signaling to a similar extent, and are capable of inducing similar lymphangiogenic responses in vivo,50,51 but loss-of-function studies have primarily demonstrated a requirement for VEGFC in mammalian lymphatic vessel growth.8,18,52 The discovery that both VEGFC and VEGFD require proteolytic activation to become active ligands capable of driving VEGFR3 signaling,20,21,53 and that VEGFD is specifically activated by serine proteases such as plasmin,26 suggested that the role of VEGFD might be more related to lymphatic growth after wounding in postnatal animals than programmed lymphatic growth in developing embryos.20 After testing this hypothesis using 2 distinct postnatal wound healing models, we find no evidence for such a specialized postnatal role for VEGFD. Prior studies of mice with postnatal deletion of VEGFC ± VEGFD have revealed a degree of redundant function for the 2 factors in maintaining lacteal growth in the intestine,54 but we detected no such redundancy in wound healing responses. Failure to detect a VEGFD contribution in vivo under conditions known to generate serine proteases that specifically activate VEGFD in vitro suggests that the failure to define a role for this lymphangiogenic factor in mammals is not likely to merely reflect the lack of an appropriate biological setting. Instead, VEGFD is likely to play a subtle role in lymphatic growth or function that remains to be defined.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank members of the M.L.K. laboratory for their valuable insights over the course of these studies. The authors also would like to thank Arben Nace and Ying Zheng for their help with the skin wounding model.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL121650 and HL120846 (to M.L.K.), and by the Agency for Science, Technology and Research (Singapore) (L.L.).
Footnotes
We will share publication-related data through e-mails to the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.L., H.B., O.F., J.Y., L.L., D.E., W.M., M.C., G.O., J.D.W., and M.L.K. designed and performed experiments; L.L., J.D.W., and M.L.K. analyzed results; L.L., J.D.W., and M.L.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Welsh, Translational Research Center, Room 11-123, 3400 Civic Center Blvd, Building 421, Philadelphia, PA 19104-5159; e-mail: jwels@pennmedicine.upenn.edu; Mark L. Kahn, Translational Research Center, Room 11-123, 3400 Civic Center Blvd, Building 421, Philadelphia, PA 19104-5159; e-mail: markkahn@pennmedicine.upenn.edu.
REFERENCES
- 1.Iannacone M. Platelet-mediated modulation of adaptive immunity. Semin Immunol. 2016;28(6):555-560. [DOI] [PubMed] [Google Scholar]
- 2.Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci (Landmark Ed). 2018;23:726-751. [DOI] [PubMed] [Google Scholar]
- 3.Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 2019;179:56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97(12):3691-3698. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Davis RP, Jenne CN. Platelets as modulators of inflammation. Semin Thromb Hemost. 2018;44(2):91-101. [DOI] [PubMed] [Google Scholar]
- 6.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80(1):171-175. [PubMed] [Google Scholar]
- 8.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74-80. [DOI] [PubMed] [Google Scholar]
- 9.Bray PF, McKenzie SE, Edelstein LC, et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 2013;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;35(1):31-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cifarelli V, Eichmann A. The intestinal lymphatic system: functions and metabolic implications. Cell Mol Gastroenterol Hepatol. 2019;7(3):503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Kataru RP, Koh GY. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012;33(7):350-356. [DOI] [PubMed] [Google Scholar]
- 13.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371-1380. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest. 2014;124(3):888-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koltowska K, Betterman KL, Harvey NL, Hogan BM. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development. 2013;140(9):1857-1870. [DOI] [PubMed] [Google Scholar]
- 16.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(2):290-298. [PMC free article] [PubMed] [Google Scholar]
- 17.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276(5317):1423-1425. [DOI] [PubMed] [Google Scholar]
- 18.Ober EA, Olofsson B, Mäkinen T, et al. Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep. 2004;5(1):78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joukov V, Sorsa T, Kumar V, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16(13):3898-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bui HM, Enis D, Robciuc MR, et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest. 2016;126(6):2167-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeltsch M, Jha SK, Tvorogov D, et al. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation. 2014;129(19):1962-1971. [DOI] [PubMed] [Google Scholar]
- 22.Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41(12):1272-1274. [DOI] [PubMed] [Google Scholar]
- 23.Hogan BM, Bos FL, Bussmann J, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41(4):396-398. [DOI] [PubMed] [Google Scholar]
- 24.Janssen L, Dupont L, Bekhouche M, et al. ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis. 2016;19(1):53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos FL, Caunt M, Peterson-Maduro J, et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res. 2011;109(5):486-491. [DOI] [PubMed] [Google Scholar]
- 26.McColl BK, Baldwin ME, Roufail S, et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D [published correction appears in J Exp Med. 2003;198(7):following 1126]. J Exp Med. 2003;198(6):863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann M, Wettstein R, Senaldi C, et al. Impact of platelet rich plasma and adipose stem cells on lymphangiogenesis in a murine tail lymphedema model. Microvasc Res. 2015;102:78-85. [DOI] [PubMed] [Google Scholar]
- 28.Zou Z, Enis DR, Bui H, et al. The secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesis. Blood. 2013;121(16):3228-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura A, Kirsch DG, McLaughlin ME, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661-665. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin ME, Halford MM, Roufail S, et al. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25(6):2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakus Z, Gleghorn JP, Enis DR, et al. Lymphatic function is required prenatally for lung inflation at birth. J Exp Med. 2014;211(5):815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha SK, Rauniyar K, Karpanen T, et al. Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci Rep. 2017;7(1):4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmaier AA, Stalker TJ, Runge JJ, et al. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood. 2011;118(13):3661-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72(3):161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabibiazar R, Cheung L, Han J, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3(7):e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011;2011:969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romer J, Bugge TH, Pyke C, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2(3):287-292. [DOI] [PubMed] [Google Scholar]
- 38.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18(10):1111-1113. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316-320. [DOI] [PubMed] [Google Scholar]
- 40.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314-321. [DOI] [PubMed] [Google Scholar]
- 41.Italiano JE Jr., Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7 Suppl 1173-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klement GL, Yip TT, Cassiola F, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battinelli EM, Markens BA, Italiano JE Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hess PR, Rawnsley DR, Jakus Z, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2013;124(1):273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson SP, Lowe K, Finney BA. Platelets in lymph vessel development and integrity. Adv Anat Embryol Cell Biol. 2014;214:93-105. [DOI] [PubMed] [Google Scholar]
- 46.Janardhan HP, Trivedi CM. Establishment and maintenance of blood-lymph separation. Cell Mol Life Sci. 2019;76(10):1865-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carramolino L, Fuentes J, Garcia-Andres C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106(7):1197-1201. [DOI] [PubMed] [Google Scholar]
- 48.Saaristo A, Tammela T, Farkkilā A, et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169(3):1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saaristo A, Tammela T, Timonen J, et al. Vascular endothelial growth factor-C gene therapy restores lymphatic flow across incision wounds. FASEB J. 2004;18(14):1707-1709. [DOI] [PubMed] [Google Scholar]
- 50.Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20(6):1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rissanen TT, Markkanen JE, Gruchala M, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92(10):1098-1106. [DOI] [PubMed] [Google Scholar]
- 52.Küchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16(12):1244-1248. [DOI] [PubMed] [Google Scholar]
- 53.Stacker SA, Stenvers K, Caesar C, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274(45):32127-32136. [DOI] [PubMed] [Google Scholar]
- 54.Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7(11):1418-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.