Abstract
Purpose
The use of susceptibility weighted imaging in high field magnetic resonance imaging scanners can detect the nigrosome-1 area located in the caudo-lateral region of the pars compacta in the substantia nigra. This structure comprises a significant amount of dopaminergic neurons and degenerates in the early stages of Parkinson’s disease. Essential tremor is a neurological condition that in some cases could be confused with the early stages of Parkinson’s disease with a possible error in clinical diagnosis. Our purpose is to evaluate the accuracy of nigrosome-1 detection by high resolution magnetic resonance imaging to discriminate Parkinson’s disease from essential tremor.
Methods
A case–control study compared patients with a clinical diagnosis of Parkinson’s disease and essential tremor. Magnetic resonance imaging studies were performed using a 3T magnetic resonance imaging scanner. The susceptibility weighted imaging sequence was obtained in the axial plane with an isotropic voxel of 0.75 mm. Two independent neuroradiologists evaluated the images without access to clinical patient data.
Results
Sixteen patients were included in each group (Parkinson’s disease and essential tremor). Average age: Parkinson’s disease group: 71.3 (SD 6.3) and essential tremor group: 68.3 (SD 12.3). For the first evaluator, the nigrosome-1 area was absent in 15 patients with Parkinson’s disease and in two with essential tremor and for the second evaluator was absent in 15 patients with Parkinson’s disease and four with essential tremor. The sensitivity/specificity for the diagnosis of Parkinson’s disease was 93.75%/87.5% for the first evaluator and 93.75%/75% for the second evaluator.
Conclusion
The detection of the nigrosome-1 area is a useful tool in the differential diagnosis between Parkinson’s disease and essential tremor, with high sensitivity and specificity.
Keywords: Parkinson’s disease, essential tremor, nigrosome-1
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative cause of parkinsonism. It is characterised by motor and non-motor clinical manifestations. Its diagnosis may pose a challenge, particularly in the early stages and is based on motor abnormalities, according to clinical criteria coined in the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB).1 However, these diagnostic criteria may have a significant error rate when applied by general doctors in the community.2,3 A study showed that 26% of 502 patients diagnosed with PD had no parkinsonism and, in this group, 29% fulfilled the diagnostic criteria for essential tremor (ET).4
ET is an entity characterised by action tremor (postural and/or kinetic), with unclear physiopathology. Recent evidence suggests that a specific cerebello-thalamo-cortical loop is abnormal5 in patients with ET. This disorder usually involves the upper limbs during voluntary movements, but may sometimes involve other segments of the body, especially the head and vocal cords. It is associated with family history in approximately 50% of cases.6 Diagnosis (as in the case of PD) is also based on clinical findings, patient history and neurological examination, according to the criteria of the Consensus for Essential Tremor of the Movement Disorders Society (MDS).7 It is typically considered a benign disease but, in some cases, may develop a significant degree of disability.
Both entities are common disorders in elderly patients. The prevalence has been calculated at 5% for ET8 and 1.8% for PD9 in patients over 65 years of age.
Traditionally the role of conventional magnetic resonance imaging (MRI) in the diagnosis of PD has been focused on excluding other causes of parkinsonism. However, recent advances in MRI technology, including high field 3T, have led to improving spatial resolution, and the use of special techniques (diffusion tensor or functional MRI)10,11 have allowed describing structural and functional changes in the brain of PD patients that could be useful in the early diagnosis.
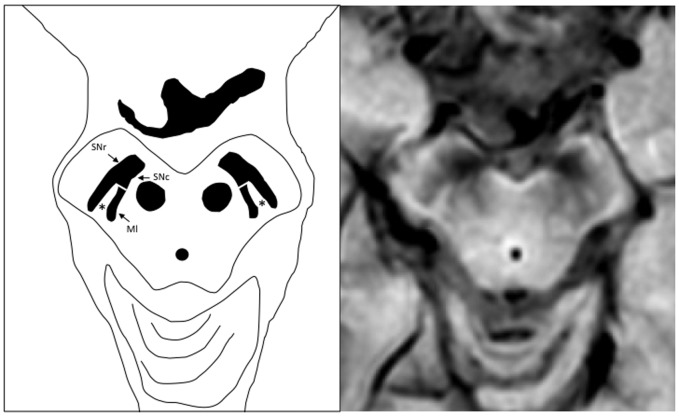
One of the recent findings relates to the ability to discriminate the subregions of the substantia nigra (SN) in 7T MRI, in particular, the region known as nigrosome-1, which is located in the caudo-lateral substantia nigra compacta (SNc)12 that degenerates early in the course of the disease (Figure 1). This region is liable to be detected with susceptibility weighted imaging (SWI).
Figure 1.
Schematic representation of the substantia nigra in the susceptibility weighted imaging sequence. SNr: substantia nigra pars reticulata; SNc: substantia nigra pars compacta; Ml: medial lemniscus. The nigrosome-1 (*) area is observed in the caudo-lateral region of SNc (swallow tail sign).
The SN is anatomically and functionally divided into two parts: the SNc which is inferior (caudal) and posterior in the midbrain, and the substantia nigra reticulate (SNr) which is rostral (superior) and anterior.
There is some confusion between the radiological and pathological terminology, regarding the anatomy. Radiologists prefer the terms anterior, posterior, superior and inferior. However, every anatomical/histological description uses the terms ventral, dorsal, rostral and caudal.
The brain structure most affected in Parkinson’s disease is the SNc, and within this structure, degeneration of dopaminergic neurons is not uniform. Using immunostaining, Damier and colleagues13,14 were able to identify in the SNc areas with intense staining which were designated as nigral matrix, and five dopaminergic neuron clusters with poor staining designated as nigrosomes. They used immunostaining for a molecule called calbindin D28k, which is a major calcium binding protein, found in nigrostriatal afferent fibres.
Of all dopamine-containing neurons in the SNc, 60% were located in the matrix and 40% in nigrosomes.
The largest of these nigrosomes was designated as nigrosome-1 and is located in the caudal and lateral SN, and its population of neurons is the most affected in PD.
On SWI nigrosome-1 is detected as an ovoid hyperintense area flanked laterally and medially by a low signal corresponding to the SNc and the medial lemniscus, respectively. This low signal determines the so-called ‘swallow tail sign’15 (Figure 1). In PD and other parkinsonian entities, the nigrosome-1 high signal gradually disappears due to neuronal loss, as demonstrated by Damier et al.14 with possible involvement of iron deposition, which has been reported by several authors.16,17
This finding has been replicated in 3T MRI using the SWI sequence, wherein the loss of nigrosome-1 in patients with PD has a sensitivity of 79–100%, 90–100% specificity and diagnostic accuracy of 86–91%, compared with patients without PD.15,18 The loss of nigrosome-1 has also been reported in atypical parkinsonism such as progressive supranuclear palsy and multiple system atrophy.18–20 However, the utility of this sign to differentiate patients with PD from other extrapyramidal syndromes such as ET has not been evaluated, which is relevant for treatment decisions.
This research was aimed at identifying the association between the absence of nigrosome-1 and clinically diagnosed PD as compared to patients with ET. It evaluates the usefulness of this observation to discriminate patients with PD from patients with ET. We also aimed to estimate the sensitivity and specificity of the absence of nigrosome-1 in the diagnosis of PD compared to patients with ET.
Materials and methods
A case–control study was designed to compare PD patients (cases) to ET patients (controls).
Population
Patients with at least 3 years of follow-up by the neurology department were included in the PD group and patients with 5 years of follow-up were included in the ET group. The diagnosis of PD and ET was made according to the current clinical criteria for each one. Consecutive patients who consulted in out-clinic of abnormal movements were included, according to inclusion criteria: patients with a clinical diagnosis of PD according to the UKPDSBB criteria and diagnosis of ET according to the criteria of the MDS with 3 years of follow-up by a movement disorder specialist.
Age, sex, years of evolution, PD and ET phenotype, Hoehn and Yahr stage MDS-Unified Parkinson’s Disease rating scale (UPDRS) part III scale (for PD severity), Fahn–Tolosa–Marin scale (for ET severity), and side of symptom onset were collected.
Imaging acquisition
The presence of the nigrosome-1 area was evaluated in all participants with a three-dimensional (3D) high resolution SWI, shot fast field 3D multi-echo. TR/TE 16.55/23.29 flip angle 10°, number of slices: 120, voxel size: 0.75 × 0.75 × 0.75 mm3, acquisition time 2 : 40 minutes. SWI was obtained in the axial plane parallel to the anterior commissure-posterior commissure plane in 3T Ingenia (Philips, The Netherlands).
Imaging evaluation
The images were assessed by two neuroradiologists blinded to the clinical diagnosis to determine the presence of nigrosome-1, following an axial plane parallel to the anterior commissure-posterior commissure line, according to the method described by Schwarz et al.15 The finding was evaluated independently for each side and was classified into three categories: normal, probably normal and absent. They were reclassified as normal nigrosome-1 when the evaluation was normal or probably normal on both sides, and abnormal when nigrosome-1 was absent in at least one of the two sides (Figure 2).
Figure 2.
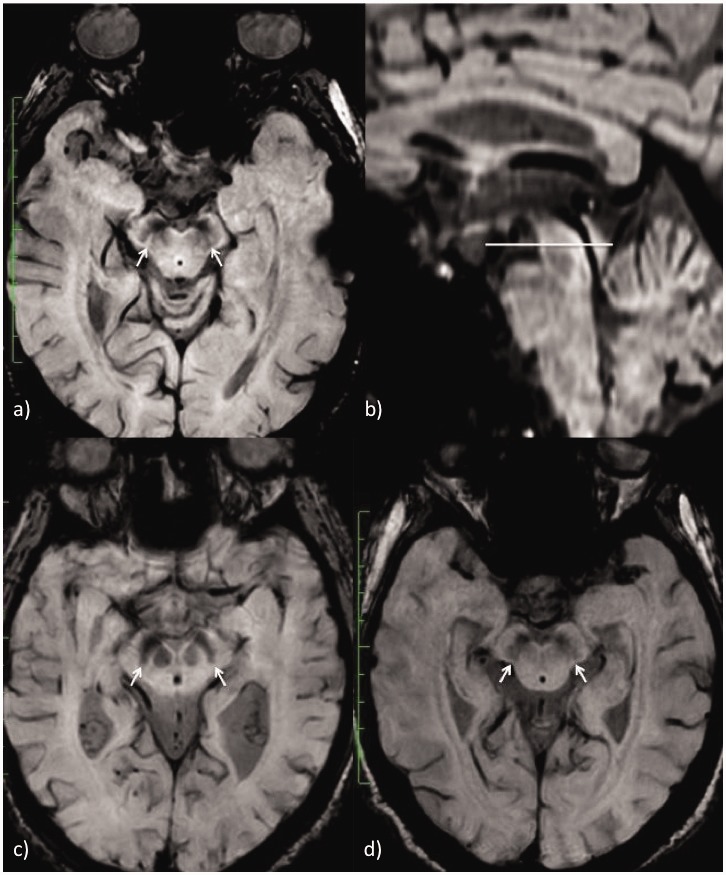
Different categories of nigrosome-1 in the axial plane through the substantia nigra: (a) nigrosome-1 visible on both sides in a patient diagnosed with ET, (c) nigrosome-1 absent on both sides in a PD patient, (d) nigrosome-1 probably present on both sides in an ET patient. The axial plane is oriented following the anterior commissure-posterior commissure line (b). PD: Parkinson’s disease; ET: essential tremor.
Statistical analysis
Continuous variables were evaluated by the Mann–Whitney U test and categorical variables were evaluated using the chi-square test. The kappa coefficient for interobserver reliability assessment was estimated. The sensitivity, specificity, positive predictive value and negative predictive value for the absence of nigrosome-1 were calculated for each evaluator. The level of significance was set at P ≤ 0.05. The odds ratio as a measure of association with confidence intervals (CIs) of 95% was used. The inclusion of patients and data collection were performed following the recommendations of the Declaration of Helsinki. The ethics committee of research protocols of our institution approved the protocol. All patients signed informed consent for inclusion in the study.
Results
Sixteen patients were included in each group (PD and ET). The demographic and clinical characteristics of both groups are described in Table 1.
Table 1.
Demographic and clinical characteristics.
| PD (N = 16) | ET (N = 16) | P value | |
|---|---|---|---|
| Gender (male %) | 50% | 81.2% | 0.13 |
| Age (mean, SD) | 71.3 ± 6.3 | 68.3 ± 12.3 | 0.29 |
| Years of evolution (mean, SD) | 7.1 ± 3.4 | 21.1 ± 15.2 | 0.001 |
| Clinical phenotype (%) | Rigid akinetic 6.25% Tremor-dominant 37.5% Mixed phenotype 56.3% | Classic presentation 100% + Head tremor 31.2% + Voice tremor 6.2% + Perioral tremor 6.2% | – |
| Clinical scale (mean, SD) | Hoehn and Yahr 1.94 ± 0.54 MDS-UPDRS III 22.1 ± 9.7 | Fahn–Tolosa–Marin scale 16.25 ± 10.9 | – |
| Treatment | L-DOPA 93.7% Dopamine agonist 56.25% Monoamine oxidase inhibitors 31.25% | Propranolol 31.3% Propanolol + benzodiazepine 12.5% | – |
PD: Parkinson’s disease; ET: essential tremor; L-DOPA: levodopa.
Two neuroradiologists evaluated the MRI of the patients without access to their clinical information. Evaluator 1 (MSPA) had 5 years of experience in neuroimaging and evaluator 2 (JAF) had 30 years of experience in neuroimaging.
The first evaluator described the nigrosome-1 area as absent in 17 patients, 15 of which were diagnosed as having PD and two as having ET. For the second evaluator, nigrosome-1 area was absent in 19 patients: 15 with PD and four with ET. For the first evaluator, sensitivity for the diagnosis of PD was 93% (95% CI 68–99%), specificity of 88% (95% CI 63–98%), positive predictive value of 88% (95% CI 65–96%), negative predictive value of 94% (95% CI 69–99%) and diagnostic accuracy of 91% (95% CI 75–98%). Sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy for the second evaluator were: 93% (95% CI 64–99%), 75% (95% CI 54–94%), 88.2% (95% CI 55–88%), 92% (95% CI 69–99%) and 84% (95% CI 67–95%), respectively.
Only one patient with PD was classified into the normal nigrosome-1 group by both raters (Table 2).
Table 2.
Interpretation of nigrosome-1 by two evaluators.
| Evaluator 1 |
Evaluator 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| PD (N of patients) |
ET (N of patients) |
PD (N of patients) |
ET (N of patients) |
|||||
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Normal | 2 | 1 | 10 | 9 | 1 | 1 | 3 | 8 |
| Probably normal | 3 | 2 | 5 | 5 | 2 | 1 | 9 | 4 |
| Absent | 11 | 13 | 1 | 2 | 13 | 14 | 4 | 4 |
PD: Parkinson’s disease; ET: essential tremor.
According to the clinical phenotype of PD the only patient with the rigid akinetic phenotype had normal nigrosome-1 on both sides. Five patients with tremor-dominant phenotype and six patients with mixed phenotype had a bilateral absence of nigrosome-1. The remaining four patients had unilateral loss of nigrosome-1, three of them with mixed phenotype and one with tremor-dominant phenotype.
The kappa coefficient for the right side was 0.71 (P < 0.00001), for the left side was 0.80 (P < 0.00001) and for the overall assessment of the absence of nigrosome-1 was 0.87 (P < 0.00001).
With the information collected by evaluator 1, we found one patient (6.25%) with symptoms of the right side that did not present alterations in the nigrosome. Eleven patients (68.75%) had a bilateral alteration of the nigrosome, of which five had an onset of symptoms of the right side and six of the left side. The remaining four patients (25%) had an onset of symptoms on the side contralateral to the affected nigrosome (two on the right side and two on the left side).
Discussion
In this study, we compared PD patients with ET patients due to the possibility of overlapping clinical findings between the two entities, as reported previously.
The results obtained showed high sensitivity and specificity of the technique for discrimination between the two groups. These findings relate to other publications comparing PD patients with healthy controls such as Schwarz et al.,15 who found a sensitivity of 100% and a specificity of 91–93%, and Cosottini et al.,21 who found a sensitivity of 71–86% and a specificity of 92–100%, both using a 3T MRI scanner. Cosottini et al.21 evaluated the same group of patients with a 7T MRI scanner with improved sensitivity (86–93%) and specificity of 100%. Comparisons of diagnostic accuracy should be carefully considered because each study used protocols with different slice thicknesses and included patients with varying degrees of severity. In the prospective study of Schwarz et al.,15 patients had a mean MDS-UPDRS part III (scale of clinical severity) of 32.5, and in the study of Cosottini et al.21 it was 18.3. In contrast, our sample of PD patients had a mean of 22.1. As the loss of nigrosome-1 is a progressive neurodegenerative process, this loss is likely to be more conspicuous in patients with a longer evolution and more severe disability.
In a prospective study by Meijer et al.,22 patients with newly diagnosed PD and atypical parkinsonism were included, with mean clinical monitoring of 21.6 and 28.4 months, respectively. These authors found a marginal utility for differential diagnosis by detecting nigrosome-1. One possible explanation given by the authors suggested that the MRI protocol included a SWI sequence with a thickness of 3 mm, resulting in slices too thick for proper assessment of the nigrosome-1 area, whose measurements are in the submillimetre range.22
We found four patients (evaluator 2) with a clinical diagnosis of ET presenting with an absence of the nigrosome-1 area and only one patient with normal nigrosome-1 in the PD group (both evaluators). The absence of the nigrosome-1 area in patients with ET could be related to the spatial resolution of the SWI sequence employed in this study (0.75 × 0.75 × 0.75 mm), which is slightly higher than that of other publications.15,21,23
Another hypothesis is the relation not yet fully established between ET and the subsequent development of PD. There are a few authors who have studied this relationship.24–28 In a review by Thenganatt et al.,29 the relationship between PD and ET is described, which includes clinical superposition, especially with benign tremulous parkinsonism. This clinical phenotype presents with moderate to severe rest, postural and action tremor, and partial response to levodopa therapy.30 We included five patients with the tremor-dominant phenotype, but none of them fulfilled the criteria for benign tremulous parkinsonism. Benito-Leon et al.31 found that a history of ET increased by four the risk of developing PD. However, the low number of cases (six) who had this progression made it difficult to draw firm conclusions.31
On the other hand, there are functional neuroimaging studies that have shown a dopaminergic deficiency in the striatum in patients with ET versus healthy controls.32 Transcranial ultrasound reported hyperechogenicity in the substantia nigra in patients with ET, which could be a risk marker for PD.33
In a histopathological examination, Louis et al. described the existence of Lewy bodies in the locus coeruleus of patients with ET,34 as described in PD histopathology.
Recently published evidence shows that patients with late-onset ET (>46 years) have a less frequent family history of tremor and lower clinical response to alcohol, suggesting that they could belong to a different clinical entity.35 In our sample, three of the four ET patients with an absence of nigrosome-1 had a late onset of the disease.
We found a good inter-observer variability for detecting each side of nigrosome-1 and an excellent one for the overall assessment of the absence of the nigrosome-1 area. Inter-observer variability may affect the diagnostic accuracy and thus the usefulness of the method for the diagnosis of PD. In our experience, the SWI sequence has a relatively low signal-to-noise ratio and is especially prone to susceptibility or motion artifacts. These facts reduce the quality of the images in patients with pneumatisation of petroclival structures or patients with head tremor. The aforementioned characteristics regarding the SWI sequence could partially explain the differences in the assessment of the evaluators.
The limitations of this study may include the absence of a method of confirmatory diagnosis for patients with PD, such as metabolic studies of the dopaminergic pathway (DAT-SCAN or positron emission tomography with 18F-fluoro-levodopa), which may be useful for more accurate diagnostic classification, according to other researchers’ reports.23 We have tried to overcome this difficulty including patients with more than 3 years of PD, narrowing the possibility of error in the clinical diagnosis.
Prospective studies are required in patients with PD and other movement disorders, including atypical parkinsonism, for an appropriate assessment of the usefulness of nigrosome-1 detection in this clinical setting for the diagnosis of PD.
Conclusion
We compared the accuracy of nigrosome-1 detection using SWI to discriminate clinically diagnosed PD patients and ET patients in a case–control study. We found high sensitivity and high specificity for the detection of the nigrosome-1 area and adequate inter-observer variability.
Nigrosome-1 imaging could be useful in the detection of nigral changes in PD patients that hopefully may aid in this challenging diagnostic process.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol, Neurosurg, Psychiatry 1992; 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain: a journal of neurology 2002; 125(Pt 4): 861–870. [DOI] [PubMed] [Google Scholar]
- 3.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol, Neurosurg, Psychiatry 2002; 73: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing 1999; 28: 99–102. [DOI] [PubMed] [Google Scholar]
- 5.Helmich RC, Toni I, Deuschl G, et al. The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep 2013; 13: 378. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED. Clinical practice. Essential tremor. N Engl J Med 2001; 345: 887–891. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorders Society on tremor. Movement Disord 1998; 13: 2–23. [DOI] [PubMed] [Google Scholar]
- 8.Deuschl G, Petersen I, Lorenz D, et al. Tremor in the elderly: essential and aging-related tremor. Movement Disord: official journal of the Movement Disorder Society 2015; 30: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology 1995; 45: 2143–2146. [DOI] [PubMed] [Google Scholar]
- 10.Pan P, Zhang Y, Liu Y, et al. Abnormalities of regional brain function in Parkinson’s disease: a meta-analysis of resting state functional magnetic resonance imaging studies. Sci Rep. Epub ahead of print 1 December 2017. DOI: 10.1038/srep40469. [DOI] [PMC free article] [PubMed]
- 11.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009; 72: 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blazejewska AI, Schwarz ST, Pitiot A, et al. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7T MRI. Neurology 2013; 81: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D (28 K) immunohistochemistry. Brain: a journal of neurology 1999; 122(Pt 8): 1421–1436. [DOI] [PubMed] [Google Scholar]
- 14.Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain: a journal of neurology 1999; 122(Pt 8): 1437–1448. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz ST, Afzal M, Morgan PS, et al. The ‘swallow tail’ appearance of the healthy nigrosome – a new accurate test of Parkinson’s disease: a case–control and retrospective cross-sectional MRI study at 3T. PloS One 2014; 9: e93814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch EC, Brandel JP, Galle P, et al. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: an X-ray microanalysis. J Neurochem 1991; 56: 446–451. [DOI] [PubMed] [Google Scholar]
- 17.Riederer P, Dirr A, Goetz M, et al. Distribution of iron in different brain regions and subcellular compartments in Parkinson’s disease. Ann Neurol 1992; 32(Suppl.): S101–S104. [DOI] [PubMed] [Google Scholar]
- 18.Reiter E, Mueller C, Pinter B, et al. Dorsolateral nigral hyperintensity on 3.0 T susceptibility-weighted imaging in neurodegenerative parkinsonism. Movement Disord 2015; 30: 1068–1076. [DOI] [PubMed] [Google Scholar]
- 19.Bae YJ, Kim JM, Kim E, et al. Loss of nigral hyperintensity on 3 tesla MRI of parkinsonism: comparison with 123I–FP–CIT SPECT. Movement Disord 2016; 31: 684–692. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama A, Sato N, Kimura Y, et al. MR findings in the substantia nigra on phase difference enhanced imaging in neurodegenerative parkinsonism. Parkinsonism Relat Disord 2018; 48: 10–16. [DOI] [PubMed] [Google Scholar]
- 21.Cosottini M, Frosini D, Pesaresi I, et al. Comparison of 3T and 7T susceptibility-weighted angiography of the substantia nigra in diagnosing Parkinson disease. AJNR Am J Neuroradiol 2015; 36: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer FJ, Steens SC, van Rumund A, et al. Nigrosome-1 on susceptibility weighted imaging to differentiate Parkinson’s disease from atypical parkinsonism: an in vivo and ex vivo pilot study. Polish J Radiol 2016; 81: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh Y, Sung YH, Lee J, et al. Nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. AJNR Am J Neuroradiol 2015; 36: 2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zorzon M, Capus L, Pellegrino A, et al. Familial and environmental risk factors in Parkinson’s disease: a case–control study in north-east Italy. Acta Neurol Scand 2002; 105: 77–82. [DOI] [PubMed] [Google Scholar]
- 25.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord 2007; 13: 67–76. [DOI] [PubMed] [Google Scholar]
- 26.Romero JP, Benito-Leon J and Bermejo-Pareja F. The NEDICES study: recent advances in the understanding of the epidemiology of essential tremor. Tremor Other Hyperkinetic Movements (New York, NY) 2012; 2: tre-02-70-346-2. [DOI] [PMC free article] [PubMed]
- 27.Lang AE, Kierans C, Blair RD. Family history of tremor in Parkinson’s disease compared with those of controls and patients with idiopathic dystonia. Adv Neurol 1987; 45: 313–316. [PubMed] [Google Scholar]
- 28.Koller WC, Busenbark K, Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Essential Tremor Study Group. Ann Neurol 1994; 35: 717–723. [DOI] [PubMed] [Google Scholar]
- 29.Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord 2016; 22(Suppl. 1): S162–S165. [DOI] [PubMed] [Google Scholar]
- 30.Josephs KA, Matsumoto JY, Ahlskog JE. Benign tremulous parkinsonism. Arch Neurol 2006; 63: 354–357. [DOI] [PubMed] [Google Scholar]
- 31.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: a population based study. J Neurol, Neurosurgery, Psychiatry 2009; 80: 423–425. [DOI] [PubMed] [Google Scholar]
- 32.Isaias IU, Canesi M, Benti R, et al. Striatal dopamine transporter abnormalities in patients with essential tremor. Nucl Med Commun 2008; 29: 349–353. [DOI] [PubMed] [Google Scholar]
- 33.Sprenger FS, Wurster I, Seppi K, et al. Substantia nigra hyperechogenicity and Parkinson’s disease risk in patients with essential tremor. Movement Disord: official journal of the Movement Disorder Society 2016; 31: 579–583. [DOI] [PubMed] [Google Scholar]
- 34.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain: a journal of neurology 2007; 130(Pt 12): 3297–3307. [DOI] [PubMed] [Google Scholar]
- 35.Hopfner F, Ahlf A, Lorenz D, et al. Early- and late-onset essential tremor patients represent clinically distinct subgroups. Movement Disord: official journal of the Movement Disorder Society 2016; 31: 1560–1566. [DOI] [PubMed] [Google Scholar]