Abstract
Malignant Leydig cell tumor (MLCT) is a rare testicular tumor in dogs. We report herein 2 dogs with MLCT and cutaneous metastasis. Grossly, marked enlargement and distortion of the involved testes were noted; on cut surface, the parenchyma was completely replaced by neoplastic tissue. In addition, these tumors had extensive necrosis and hemorrhage. Case 1 had a rapidly growing cutaneous mass in the left angle of the mouth; the lesion was well-circumscribed and had an indistinct lobular pattern. Case 2 had multiple cutaneous masses in the dorsal neck region, the thoracic back region, and the right hindlimb. Microscopically, the tumor lobules were composed of oval-to-polyhedral cells with eosinophilic cytoplasm and resembled testicular tumors. By immunohistochemistry, the neoplastic cells in both the testicular and cutaneous tumors were positive for inhibin-alpha and melan A. The mitotic counts of the primary tumors from cases 1 and 2 were 21 and 11 per 10 high-power fields, respectively. Based on these findings, the cases were diagnosed as MLCT with cutaneous metastasis. Ki-67 expression in the neoplastic cells of the 2 cases was higher than in benign Leydig cell tumors. Our findings may be helpful for the diagnosis of canine MLCT.
Keywords: canine, Ki-67 labeling index, malignant Leydig cell tumor
Testicular tumors are among the most common neoplasms in aged male dogs,2,7 and the incidence of such tumors in dogs is higher than in any other mammalian species.2 Testicular tumors may be divided into 2 categories: sex cord-stromal tumors and germ cell tumors.2,7 Sex cord-stromal tumors are derived from either Leydig cells or Sertoli cells. Leydig cell tumors (LCTs) are the most common type of testicular tumor in dogs, rats, and mice,5 and are usually regarded as benign tumors in dogs.2,7 Although some authors have described malignant LCTs (MLCTs) in dogs,3,18 the diagnostic criteria for malignancy are unclear.
In human medicine, Ki-67 expression is helpful for the distinction of benign and malignant tumors.1,13,15,16 It has been reported that the Ki-67 labeling index (LI) is associated with poor prognosis in various human cancers.11 The consistent relationship between the Ki-67 LI and increased number of mitotic figures also has been reported in human cancers.10 In veterinary medicine, the Ki-67 LI has been reported to be significantly associated with tumor metastasis and overall survival, and to be useful for the distinction of malignant and benign lesions in canine mammary tumors.14 We describe herein the histopathologic features of 2 canine cases of MLCT with distant cutaneous metastasis. In addition, we compared the mitotic count (MC) and Ki-67 LI of the neoplastic cells in our cases with those of benign LCTs (BLCTs).
Case 1 was a 5.5 kg, 14-y-old Shetland Sheepdog with a history of unilateral cryptorchidism. The intra-abdominal testis was removed 13 y ago; information on the pathology of the undescended testis was unavailable. The remaining testis, which had descended into the scrotum, was resected because of rapid swelling. At 10 mo after surgery, the referring veterinarian noted a rapidly growing cutaneous mass in the left angle of the mouth, and this mass was resected. At that time, the dog was under treatment for acute pancreatitis.
Case 2 was a 14.7 kg, 13-y-old mixed-breed dog with enlargement of the right testis. At the time of bilateral orchiectomy, a cutaneous mass was detected in the dorsal neck region and was resected. Two months after orchiectomy, 3 additional cutaneous nodules were observed. Two of the nodules were located in the thoracic back region; the third nodule was located in the right hindlimb.
Both dogs died suddenly ~6 wk after the last surgery. During preoperative examination, the condition of both dogs was generally good. There was no evidence of visceral metastasis by ultrasonography and chest radiography. No lymphadenopathy was noted on palpation. Unfortunately, autopsies were not performed.
On gross examination, the affected testes were markedly enlarged and distorted in both cases (Table 1). On cut surface, normal testicular parenchyma was replaced by a yellow or tan-to-brown mass (Fig. 1A). The testicular masses had a multinodular appearance and contained areas of hemorrhage and necrosis. In case 2, the tumor involved the visceral lamina and invaded the cavum vaginale; an adhesion was observed between the tumor and the parietal lamina. Macroscopically, the cutaneous lesions of each case had similar features. Each lesion was a well-circumscribed, yellow-to-tan nodule. The nodule in case 1 had an indistinct lobulated appearance on cut surface.
Table 1.
Signalment and proliferative indices of cases of malignant and benign canine Leydig cell tumors.
| Case | Age (y) | Maximum dimension of testicular tumor size (cm) | Proliferative
indices |
Condition of the contralateral testis | |
|---|---|---|---|---|---|
| MC | Ki-67 LI | ||||
| 1 | 14 | 6.0 | 21 | 17.9 | Not present |
| 2 | 13 | 8.0 | 11 | 20.5 | Atrophic |
| BCLT 1 | 15 | 1.5 | 0 | 1.9 | Sertoli cell tumor |
| BCLT 2 | 10.5 | 4 | 1 | 3.2 | Atrophic |
| BCLT 3 | 9 | 1.3 | 0 | 2.2 | No remarkable change |
| BCLT 4 | 14 | 1.1 | 4 | 2.5 | Atrophic |
| BCLT 5 | 12 | 1.4 | 2 | 9.2 | Atrophic |
| BCLT 6 | 11.5 | 0.2 | 0 | 1.6 | Hypoplastic with cryptorchidism |
| BCLT 7 | 13 | 0.8 | 0 | 5.3 | Leydig cell tumor with multilocular cystic lesion |
| BCLT 8 | 14 | 1.3 | 3 | 6.4 | Atrophic |
| BCLT 9 | 12 | 1.5 | 3 | 7.9 | Not available |
| BCLT 10 | 14.5 | 2.2 | 0 | 6.3 | Atrophic |
BLCT = benign Leydig cell tumor; LI = labeling index; MC = the number of mitotic figures in 10 consecutive fields with a total area of 2.37 mm2. Ki-67 LI values were determined by semi-automated analysis of 5 images (200×) with Ki-67 immunostaining using the Fiji/ImageJ ImmunoRatio plugin.19
Figure 1.
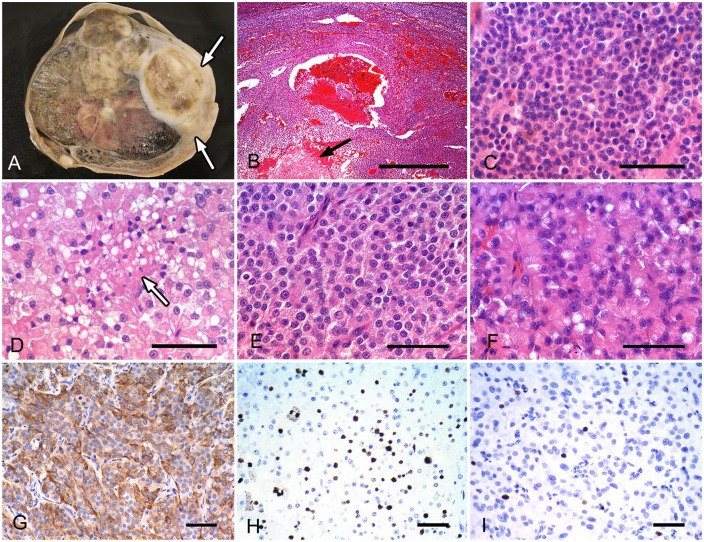
Macroscopic and histologic features of the malignant canine Leydig cell testicular tumor (A–D). A. The testicular parenchyma has been replaced by testicular tumor tissue and has multifocal necrosis. Adhesion between the tunica vaginalis and the tumor mass is also present (arrows). B. Tumor lobules are separated by a thin fibrovascular stroma; intralobular necrosis (arrow) is present. H&E. Bar = 500 μm. C. The tumor has mild-to-moderate atypia and a high mitotic count. H&E. Bar = 50 μm. D. Neoplastic cells had more abundant cytoplasm; focal necrosis was present (arrow). H&E. Bar = 50 μm. E–G. Histologic features of the metastatic cutaneous malignant Leydig cell tumors. E, F. Round-to-polygonal neoplastic cells, with eosinophilic cytoplasm, resemble Leydig cells. H&E. Bars = 50 μm. G. Neoplastic cells are positive for inhibin-alpha. Bar = 50 μm. H, I. Ki-67 immunostaining of the malignant (H) and benign Leydig cell tumor (I). Bars = 50 μm.
Histologically, most of the findings in the testicular tumors were similar in both cases. The neoplasm was composed of multiple lobules separated by a fibrovascular connective tissue stroma (Fig. 1B). The neoplastic cells were oval-to-polyhedral and had variable amounts of granular or vacuolated eosinophilic cytoplasm with distinct cell borders (Figs. 1C, 1D). Nuclei were round-to-oval with finely to coarsely stippled chromatin, and contained 1–3 prominent nucleoli in most neoplastic cells. The neoplastic cells had mild-to-moderate anisocytosis and anisokaryosis (Figs. 1C, 1D). In case 1, there were variations in cell density and nuclear pleomorphism of neoplastic cells. Areas of increased cell density with a high nuclear-to-cytoplasmic ratio and moderate nuclear pleomorphism also had increased numbers of mitoses (Fig. 1C). The neoplasm was composed of a mixture of areas of more and less pleomorphism. These findings were not observed in case 2. In both cases, neoplastic cells that were located in extensive areas of necrosis and hemorrhage had invaded the stromal connective tissue. In case 2, neoplastic cells had also infiltrated the tunica vaginalis through the tunica albuginea. Vascular invasion by neoplastic cells was observed in both cases, and intravascular neoplastic cells within the pampiniform plexus were seen in case 2. The MC is defined as the number of mitotic figures of the neoplastic cells in 10 consecutive fields, using the 40× objective lens and the 10× ocular lens (FN 22 mm), with a total area of 2.37 mm2. The MC was 21 and 11 in cases 1 and 2, respectively. Microscopically, the cutaneous tumors in both cases were composed of oval-to-polyhedral cells that resembled those seen in testicular tumors (Figs. 1E, 1F). The neoplastic cells were arranged in trabeculae and nests separated by fibrovascular septa with extensive invasion into the surrounding dermis, subcutis, and/or deep skeletal muscle. In case 1, the cutaneous neoplasm resembled the testicular neoplasm in the areas of increased cell density with a high nuclear-to-cytoplasmic ratio (Figs. 1C, 1E). Canine LCTs have been shown to be strongly positive for both inhibin-alpha and melan A.12 Neoplastic cells in both of our testicular and cutaneous tumors were positive for inhibin-alpha and melan A (Table 2; Fig. 1G). Based on these findings, we diagnosed the 2 cases presented herein as MLCT with cutaneous metastasis.
Table 2.
Antibodies used for immunohistochemical investigation of canine Leydig cell tumors.
| Antibody against: | Source | Type/clone | Dilution | Pretreatment |
|---|---|---|---|---|
| Inhibin-alpha | Bio-Rad AbD Serotec, Kidlington, UK | mAb/R1 | 1:50 | Citrate buffer, pH 6.0, HIER, 10 min |
| Melan A | Leica Biosystems, Newcastle, UK | mAb/A103 | 1:50 | EDTA buffer, pH 9.0, HIER, 10 min |
| Ki-67 | Nichirei Biosciences, Tokyo, Japan | mAb/SP6 | Ready-to-use | EDTA buffer, pH 9.0, HIER, 20 min |
EDTA = ethylenediamine tetra-acetic acid; HIER = heat-induced epitope retrieval; mAb = monoclonal antibody.
Increased mitotic activity and increased Ki-67 expression have been recorded in human MLCTs.17 We compared the MC and Ki-67 LI of the neoplastic cells in our cases with the 10 BLCT cases randomly selected from our laboratory records (Table 1). Macroscopically, all cases of BLCTs had a well-circumscribed mass with an average maximum dimension of 1.5 cm (range: 0.2–4 cm; Table 1). Microscopically, neoplastic cells showed little-to-mild cytologic atypia with low MC (x– MC = 1.3, range: 0–4; Table 1). Also, necrosis of neoplastic tissue and invasion into adjacent parenchyma were not observed in all cases. We also performed immunohistochemistry using monoclonal antibody to Ki-67 (Table 2). Germ cells within the seminiferous tubules were used as positive controls for Ki-67 immunostaining.12 For assessment of the Ki-67 LI, 5 regions with higher Ki-67 expression were captured from tumor sections in each case at high magnification (20× objective). Automatic color segmentation was performed on 5 images from each case (Fiji/ImageJ ImmunoRatio plugin; imagej.nih.gov).19 The average Ki-67 LI of the 2 MLCT cases and the 10 BLCTs was 19.2 and 5.0, respectively (Table 1; Figs. 1H, 1I). This result suggested that Ki-67 staining might be useful in this determination when metastases or invasion is not clearly evident.
In humans, 10% of LCTs are classified as malignant.17 The pathologic features of human MLCT have been reported to include large size (>5 cm), necrosis, cytologic atypia, increased mitotic activity, increased Ki-67 expression, and vascular invasion.17 MLCTs also develop in rats and mice, and the diagnostic criteria for this tumor type in these animals resemble the criteria in humans, except that mitotic figures are infrequent in the tumor tissues of these animals.5 In canine cases of MLCT, enlargement and distortion of the testis and replacement of the testicular tissue by tumor with marked necrosis have been described previously.3 In our study, the involved testes exhibited marked enlargement and distortion, replacement of the parenchyma by tumor tissue, and extensive necrosis with hemorrhage. Canine LCTs have been reported to be generally small and to produce little, or only subtle, distortion of the affected testis.2 The affected testes in the 10 cases of BLCT that we reviewed were smaller than the 2 MLCT cases presented herein. In addition, tumor necrosis was not observed in the cases of canine BLCT that we reviewed.
Ki-67 expression was significantly higher in adenocarcinomas than in adenomas of the adrenal, pituitary, and parathyroid glands,1,15,16 and Ki-67 LI is a prognostic indicator of neuroendocrine tumors.9 The Ki-67 index is indicative of the clinical behavior of LCT in humans.6 In addition, the Ki-67 indices of BLCTs have been reported as zero, and a MLCT had an index of 20%.6 We showed that the MC and the Ki-67 LI were higher in our 2 MLCT cases than in our 10 BLCT cases. These results suggest that marked tumor necrosis reflected high proliferative activity in these tumors. Therefore, increased MC and Ki-67 expression are also considered features indicative of a malignant phenotype in canine LCTs, which is identical to what is observed in human LCTs. These findings may help in the diagnosis of canine MLCT.
A further large-scale study is necessary to evaluate the diagnostic criteria for canine MLCTs. The number of mitotic figures was reported to be low in MLCT tissues in a dog.18 A plausible reason for these differences may be the heterogeneity of proliferative activity within tumor tissues. In case 1, heterogeneity of cell density and nuclear pleomorphism was observed in the testicular neoplasm. In the areas of increased cell density, there was moderate nuclear pleomorphism, and Ki-67–labeled cells were clearly increased. In addition, the cutaneous neoplasm in case 1 resembled the testicular neoplasm in the areas of increased cell density.
Cutaneous metastasis of malignant neoplasms is uncommon in both animals and humans. The most common sources of cutaneous metastases in humans are breast, skin, lung, uterine, kidney, and gastrointestinal cancers.4 Two cases of canine MLCT with metastasis were reported previously, and one showed multiple skin metastases; the other showed metastasis to the left hindlimb musculature.3,18 In our study, both of the cases exhibited cutaneous metastatic lesions, which were recognized in case 2 at the time of initial diagnosis. It has been reported that ~20% of human patients with MLCT had metastatic lesions at the time of initial diagnosis and that the common sites of metastasis included the regional lymph nodes, liver, lung, and bone.8 Previous studies in dogs3,18 showed that metastasis of MLCT to the lung and brain was suspected according to clinical signs and radiologic examination. Both of our cases died within 2 mo of the last surgery; however, autopsies were not performed. No clinical evidence of tumor metastasis was seen in the regional lymph nodes or visceral organs. In the present and previously reported cases of canine MLCT, 3 of 4 cases had cutaneous metastasis. Therefore, canine MLCT may be predisposed to cutaneous metastasis, although the reason for this is unknown.
Acknowledgments
We thank Enago (www.enago.jp) for the English language review.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Abbona GC, et al. Proliferative activity in parathyroid tumors as detected by Ki-67 immunostaining. Human Pathol 1995;26:135–138. [DOI] [PubMed] [Google Scholar]
- 2. Agnew DW, et al. Tumors of the genital systems. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:689–722. [Google Scholar]
- 3. Canadas A, et al. Multiple cutaneous metastasis of a malignant Leydig cell tumour in a dog. J Comp Pathol 2016;155:181–184. [DOI] [PubMed] [Google Scholar]
- 4. Cox NH, et al. Systemic disease and the skin. In: Burns T, et al. eds. Rook’s Textbook of Dermatology. 8th ed. Vol. 3 Oxford, UK: Wiley-Blackwell, 2010:62–117. [Google Scholar]
- 5. Creasy D, et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol 2012;40(Suppl 6):S40–S121. [DOI] [PubMed] [Google Scholar]
- 6. Hekimgil M, et al. Leydig cell tumor of the testis: comparison of histopathological and immunohistochemical features of three azoospermic cases and one malignant case. Pathol Int 2001;51:792–796. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy PC, et al. Histological Classification of Tumors of the Genital System of Domestic Animals. 2nd series. (Vol. 4, WHO International Classification of Tumors of Domestic Animals). Washington, DC: Armed Forces Institute of Pathology, 1999. [Google Scholar]
- 8. Kim I, et al. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 1985;9:177–192. [DOI] [PubMed] [Google Scholar]
- 9. Kimura N, et al. Ki-67 is an indicator of progression of neuroendocrine tumors. Endocr Pathol 1994;5:223–228. [DOI] [PubMed] [Google Scholar]
- 10. Lehr HA, et al. Mitotic figure counts are significantly overestimated in resection specimens of invasive breast carcinomas. Mod Pathol 2013;26:336–342. [DOI] [PubMed] [Google Scholar]
- 11. Li LT, et al. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 2015;11:1566–1572. [DOI] [PubMed] [Google Scholar]
- 12. Owston MA, Ramos-Vara JA. Histologic and immunohistochemical characterization of a testicular mixed germ cell sex cord-stromal tumor and a Leydig cell tumor in a dog. Vet Pathol 2007;44:936–943. [DOI] [PubMed] [Google Scholar]
- 13. Pekmezci M, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol 2015;28:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peña LL, et al. Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: relationship to clinical and pathologic variables. J Vet Diagn Invest 1998;10:237–246. [DOI] [PubMed] [Google Scholar]
- 15. Prevedello DM, et al. Relevance of high Ki-67 in pituitary adenomas: case report and review of the literature. Neurosurg Focus 2005;19:E11. [DOI] [PubMed] [Google Scholar]
- 16. Schmitt A, et al. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology 2006;49:298–307. [DOI] [PubMed] [Google Scholar]
- 17. Sesterhenn IA, et al. Sex cord/gonadal stromal tumors. In: Eble JN, et al., eds. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press, 2004:250–258. [Google Scholar]
- 18. Togni A, et al. Metastasized Leydig cell tumor in a dog. Schweiz Arch Tierheilkd 2015;157:111–115. [DOI] [PubMed] [Google Scholar]
- 19. Tuominen VJ, et al. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res 2010;12:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]