Abstract
Bovine tuberculosis (bTB), caused by Mycobacterium bovis, is endemic in Kruger National Park, South Africa, home to the largest population of white rhinoceros (Ceratotherium simum) in the world. In 2016, the first cases of naturally occurring bTB were reported in white rhinoceros; however, there is a lack of understanding of infection and disease process in this species. Prevention and control of transmission depends on the availability of accurate tools to detect M. bovis infection. Interferon gamma (IFN-γ) assays are a reliable detection method for TB in other animal species, and studies have indicated that these tests can be used in white rhinoceros. We sought to screen and optimize a commercial IFN-γ enzyme-linked immunosorbent assay (ELISA) to detect endogenous white rhinoceros IFN-γ in mitogen-stimulated whole blood as a basis for developing a test for M. bovis infection. Optimizations included identifying ELISA antibodies and determining the effect of sample matrix, ELISA plate incubation temperature, ELISA linearity, assay reproducibility, and the assay’s limit of quantification. The optimized assay employed an equine IFN-γ antibody pair that was used to create a commercial ELISA kit. This ELISA had a linear response to recombinant equine and endogenous rhinoceros IFN-γ (range: 7.8–125 pg/mL). When incubated at 37°C, the ELISA was highly reproducible, with an optimal recovery and a low limit of quantification, indicating that the Mabtech equine IFN-γ ELISAPRO kit is a robust assay for measuring white rhinoceros IFN-γ.
Keywords: Ceratotherium simum; ELISA, equine; gamma interferon; white rhinoceros
Introduction
The white rhinoceros (Ceratotherium simum) is classified as “Near Threatened” by the International Union for Conservation of Nature and is under high poaching pressure in a number of African countries.4 In South Africa, the majority of white rhinoceros are found in Kruger National Park (KNP), a bovine tuberculosis (bTB) endemic area rife with poaching. Even though Mycobacterium bovis infection has been reported in a wide range of wildlife, it was only in 2016 that the first cases were found in wild rhinoceros.9,10 In order to understand M. bovis infection and disease processes, it is crucial to recognize the role of the host immunologic response. An effective immune response against M. bovis is dependent on T helper type 1 (Th1) cell-mediated immunity.3 Interferon gamma (IFN-γ) is a key cytokine in this response and has been shown to be an important biomarker used in the diagnosis of mycobacterial infections in domestic cattle, wildlife, and humans.3,6 However, immune responses are not well characterized in most wildlife species such as rhinoceros. Therefore, understanding the comparative immunobiology of M. bovis infection requires the development of assays to detect and measure immune responses.8
The white rhinoceros IFN-γ gene has been cloned and expressed, with the recombinant protein used for the production of rhinoceros IFN-γ–specific antibodies.11 The inferred IFN-γ amino acid sequence was shown to have 90% homology to that of equids.11 Using rhinoceros-specific and commercial bovine IFN-γ antibodies in ELISAs, a previous study12 demonstrated that antigen-specific IFN-γ production is a promising immunologic technique for the detection of M. bovis infection in white rhinoceros. Notably, the bovine-specific IFN-γ antibody (Ab) pair used12 was cross-reactive with equine IFN-γ, and could detect endogenous white rhinoceros IFN-γ. Those findings suggest that commercial reagents may be utilized for developing immunoassays in wildlife.12 Therefore, our aim was to screen and optimize a commercial IFN-γ ELISA to detect and measure endogenous white rhinoceros IFN-γ in mitogen-stimulated whole blood. The optimized assay could then be further evaluated as a potential test for M. bovis infection.
Materials and methods
Animals
Blood samples were collected opportunistically from immobilized white rhinoceros in KNP, South Africa, during routine management procedures or for other approved activities according to the standard operating procedures for the capture, transportation, and maintenance in holding facilities of wildlife (South African National Parks). Ethical approval for this project was granted by the Stellenbosch University Animal Care and Use Committee (SU-0966), and a section 20 research permit was issued by the Department of Agriculture, Forestry and Fisheries (DAFF; 12/11/1/7/2).
Whole blood stimulation
Rhinoceros whole blood was collected in sealed lithium heparin vacutainers (BD Biosciences, Franklin Lakes, NJ) and, for each animal, 1-mL aliquots were transferred to 2 empty serum vacutainer tubes with gas-permeable caps. Pokeweed mitogen (PWM; MilliporeSigma, St. Louis, MO) in phosphate-buffered saline, pH 7.4 (PBS; Thermo Fisher Scientific, Waltham, MA) was added to one tube at a final assay concentration of 10 µg/mL, and 10 µL of sterile PBS to the other tube. The tubes were designated as PWM and Nil, respectively, and incubated for 24 h at 37°C in 5% CO2. Thereafter, blood was transferred to 2-mL microcentrifuge tubes, and plasma was harvested following centrifugation at 2,000 × g for 5 min. Plasma samples derived from mitogen-stimulated and unstimulated whole blood were screened using bovine antibodies as described previously,12 and 5 samples with high IFN-γ concentrations (compared to nil concentrations for each animal) were selected and pooled to create a reference sample with sufficient volume for repeated ELISAs. Plasma samples were then stored at −80°C until analyzed.
Screening of anti–IFN-γ antibodies
Commercial ELISA Ab pairs were selected as potential candidates for the detection of rhinoceros IFN-γ (Table 1). Capture antibodies were diluted to 2 µg/mL in 1× PBS (Thermo Fisher Scientific). A 96-well microtiter plate (Greiner Bio-one, Heidelberg, Germany) was coated by adding 100 μL/well of diluted capture Ab and incubating the plate overnight at 4°C. The plate was washed 4 times (300 µL/well) with wash buffer solution (PBS with 0.05% Tween 20; MilliporeSigma). Thereafter, 200 μL blocking buffer (BB; wash solution with 0.1% bovine serum albumin; Roche, Basel, Switzerland), was added to each well and the plate incubated at room temperature (RT; 19°C on the day of analysis) for 1 h. After washing the plate 4 times, the pooled PWM plasma was diluted 1:2 in BB and 100 µL added to each well in duplicate. The plate was covered, incubated at RT for 2 h, and then washed 4 times. Detection antibodies were diluted to 1 µg/mL in BB, 100 µL added to each well, and incubated at RT for 1 h. Following incubation, the plate was washed 4 times, and 100 μL/well of streptavidin–horseradish peroxidase (HRP; R&D Systems, Minneapolis, MN) diluted 1:200 in BB was added and incubated at RT for 1 h. The plate was washed as above, and 100 μL of colorimetric tetramethylbenzidine (TMB) enzyme substrate (BD Biosciences) was added to each well and incubated at RT in the dark for 20 min. The reaction was stopped by adding 100 μL of 2 M H2SO4 solution to each well. The optical density (OD) of each test and control wells was measured at 450 nm and 630 nm as reference wavelength (VersaMax ELISA microplate reader with SoftMax Pro software; Molecular Devices, San Jose, CA). The ELISA results were calculated as the OD value measured at 630 nm subtracted from that measured at 450 nm; negative assay controls were used to ensure that the test well signal was specific to the PWM plasma sample and normalized OD values. Those ELISAs with a detectable PWM signal (based on mean OD of test wells) were selected for further analysis.
Table 1.
Commercial interferon gamma (IFN-γ) enzyme-linked immunosorbent assay (ELISA) kits and development kits screened for the detection of endogenous rhinoceros IFN-γ.
| Analyte | Antibody type | Plasma dilution | Manufacturer | Product information |
|---|---|---|---|---|
| Development ELISA | ||||
| Bovine IFN-γ | MT17.1,a MT30b | 1:2 | Mabtech, Nacka Strand, Sweden | 3119-1H-20 |
| Bovine IFN- γ | bIFN-γ-1,a PAN-biotinb | 1:2 | Mabtech | 3115-1H-20 |
| Canine IFN-γ | MT13,a MT166-biotinb | 1:2 | Mabtech | 3113-1H-6 |
| Equine IFN-γ | MT166,a MT13-biotinb | 1:2 | Mabtech | 3117-1H-6 (batch 11) |
| Ferret IFN-γ | MTF14,a MTF19-biotinb | 1:2 | Mabtech | 3112-1H-6 |
| Porcine IFN-γ | pIFN-γ-1,a p2CIIb | 1:2 | Mabtech | 3130-1H-20 |
| Precoated ELISA kit | ||||
| Equine IFN-γ | NA | 1:2 | MilliporeSigma, St. Louis, MO | RAB0583-1KT |
| Equine IFN-γ | NA | 1:2 | RayBiotech, Peachtree Corners, GA | MBS109347 |
| Rhinoceros IFN-γ | NA | U | MyBioSource, San Diego, CA | ELE-IFNg |
Superscripts a and b indicate capture and detection antibody, respectively. NA = not applicable; U = undiluted.
Selection of an IFN-γ ELISA
The pooled PWM rhinoceros plasma was diluted 1:8 in BB and assayed in duplicate using the bovine IFN-γ Ab pair (kit 3115; Mabtech, Nacka Strand, Sweden) and equine IFN-γ Ab pair (kit 3117) as described above. Furthermore, these samples were assayed using 3 IFN-γ ELISA kits that included precoated plates and supplied reagents according to their manufacturer’s instructions (Table 1). The ELISA displaying the greatest mean OD result for duplicate test wells was selected for further validation.
Validation of IFN-γ ELISA
A customized precoated equine IFN-γ ELISAPRO kit (Mabtech, catalog: 3117-1HP-10) utilizing the selected equine anti–IFN-γ Ab pair was used according to the manufacturer’s instructions, except for the incubation temperature, as described below. A 10-µL aliquot of recombinant equine IFN-γ (rIFN-γ) standard solution (500,000 pg/mL) was diluted in 5 mL of sample diluent buffer (provided in the kit) to create a working solution of 1,000 pg/mL. This was serially diluted 1:2 (2-fold dilutions) to produce a dilution series of 1,000–7.8 pg/mL. ELISA results were measured and calculated as above; the relationship between OD and IFN-γ concentration was described using linear regression analysis using the standard curve to determine rhinoceros IFN-γ concentrations, as described previously (Cox KL, et al. Immunoassay methods. In: Sittampalam GS, et al., eds. Assay Guidance Manual [internet]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92434/; GraphPad v.5; GraphPad Software, San Diego, CA).
To characterize assay performance at various incubation temperatures, the rIFN-γ dilution series described above was assayed in duplicate, with all incubations performed at RT, 30°C, or 37°C, except for the TMB substrate step, which was performed in the dark at RT. Results for each ELISA were analyzed by regression analysis as described above. Hereafter, all ELISA steps, except the TMB step, were performed at 37°C.
To determine the recovery of rIFN-γ in a rhinoceros plasma matrix, Nil plasma was utilized from 3 randomly selected rhinoceros. The concentrations of IFN-γ in the rhinoceros Nil samples were measured using the equine IFN-γ ELISAPRO kit. For each animal, rIFN-γ was spiked into a reference sample consisting of 100% sample diluent, and 2 test samples, consisting of 50% plasma (1:2 dilution in sample diluent) and 25% plasma (1:4 dilution in sample diluent), respectively. Concentrations of rIFN-γ in each sample were calculated with reference to a standard curve as described above. The recovery (%) of spiked rIFN-γ in the test samples was calculated as: ([rIFN-γ] in test sample ÷ [rIFN-γ] in reference sample) × 100%.
Assay linearity and parallelism were evaluated for IFN-γ concentrations of 7.8–125 pg/mL. Pooled PWM plasma was diluted in sample diluent to obtain a plasma sample with ~125 pg/mL endogenous IFN-γ, as measured in the equine IFN-γ ELISA. This sample was serially diluted 1:2 in sample diluent to form a 6-point dilution series, using a 1,000-pg/mL solution of rIFN-γ. Duplicate samples were assayed, plasma IFN-γ concentrations were calculated as described above, and results for both dilution series were analyzed by regression analysis. The ELISA linearity was characterized as the correlation coefficient (R2) value of the rIFN-γ regression. To determine the parallelism of the ELISA, regression slopes for the rIFN-γ and endogenous IFN-γ were compared using an F test (GraphPad v.5).
Assay repeatability and reproducibility were determined using plasma from 3 rhinoceros. For each animal, PWM samples were diluted 1:5 in pooled Nil plasma and assayed in triplicate on the same ELISA plate, as above. This was repeated daily for 3 d. Intra-assay precision (within-run repeatability) was calculated as the coefficient of variation (CV) of the results for the 3 replicates on day 1. Inter-assay precision (between-run reproducibility) was calculated as the CV of the results of the 3 daily assays.
In order to determine the limit of detection (LOD) and limit of quantification (LOQ) of the ELISA, 24 replicates of sample diluent were analyzed on the same plate as a dilution series of rIFN-γ consisting of 7.8, 3.9, 2.0, and 1.0 pg/mL. For the 24 replicates, the mean OD value and standard deviation (SD) were calculated. The LOD (OD) was calculated as the mean + 3 SD, and the LOQ (OD) was calculated as the mean + 10 SD.1 The values of these parameters as a concentration of rIFN-γ were then extrapolated from the standard curve by regression analysis.
The final modified equine ELISAPRO protocol was performed as follows: the precoated plate was washed 5 times with 1× wash solution provided in the kit. Thereafter, serially diluted (2-fold dilutions in sample diluent) equine IFN-γ recombinant standard and rhinoceros plasma samples were added to duplicate wells (100 µL/well), and the plate incubated at 37°C for 2 h. After incubation and 5 washes, equine detection Ab, prepared at a 1:500 dilution in sample diluent to a final concentration of 1 µg/mL, was added at 100 µL/well, and the plate incubated at 37°C for 1 h. After 5 washes, 100 μL/well of streptavidin–HRP (1:1,000 dilution) was added and incubated at 37°C for 1 h. After 5 washes, 100 μL of TMB enzyme substrate was added to each well and incubated in the dark at RT for 15 min. The reaction was stopped by addition of 100 μL of stop solution (provided in kit). Results were determined as described above.
Results
Of the 7 ELISA Ab pairs that we screened, the Mabtech bovine pair (kit 3115) and equine pair (kit 3117) resulted in a detectable signal when assaying PWM-stimulated blood of white rhinoceros. Moreover, compared with the 3 precoated ELISAs, the in-house equine IFN-γ Ab pair had the greatest signal for PWM plasma, and this was selected for further evaluation as a precoated ELISA (Fig. 1).
Figure 1.

The comparative sensitivity of selected ELISAs for rhinoceros interferon gamma (IFN-γ). Whole blood from 5 rhinoceros was incubated overnight with pokeweed mitogen (PWM; 10 μg/mL). Hereafter, pooled PWM rhinoceros plasma was diluted 1:8 and assayed using selected IFN-γ ELISAs. The Mabtech equine ELISA displayed the greatest mean optical density (OD) for this sample.
The Mabtech precoated equine ELISAPRO kit displayed a linear response for rIFN-γ concentrations of 7.8–125 pg/mL (R2 > 0.99), and this was consistent at incubation temperatures of RT, 30°C, and 37°C (Fig. 2). Thereafter, to facilitate reproducibility between laboratories, because most laboratories have incubators set at 37°C and because high ambient temperatures may be present in laboratories where rhinoceros occur, the ELISA was performed at 37°C. The individual IFN-γ concentrations of Nil plasma samples were <8 pg/mL (data not shown), and mean recovery of rIFN-γ was 93% in a 50% plasma sample and 88% in a 25% plasma sample (Table 2). Subsequently, rhinoceros plasma was assayed at a 1:2 dilution in kit sample diluent. The linear response of the ELISA for rIFN-γ was not significantly different from that of endogenous rhinoceros IFN-γ (R2 > 0.99; F = 0.12; p > 0.5; Fig. 3).
Figure 2.
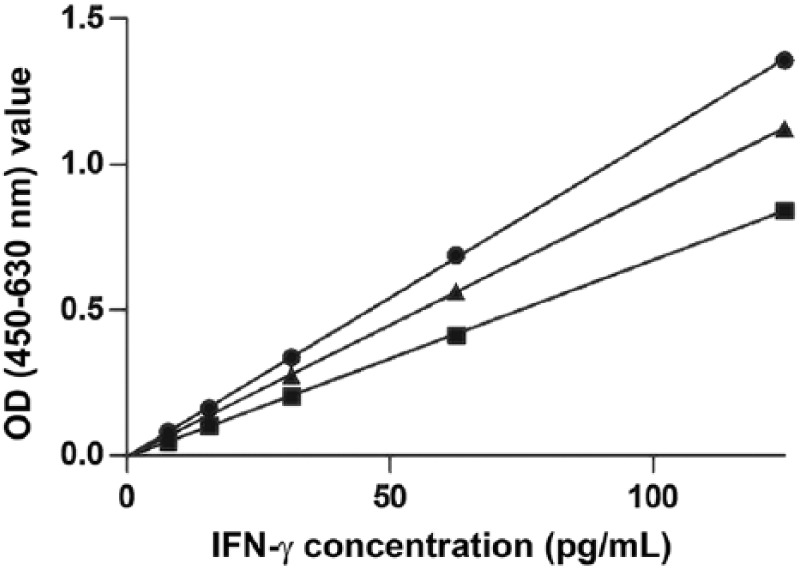
Regression analysis of a dilution series of recombinant equine interferon gamma (IFN-γ) of 7.8–125 pg/mL, and measured using the Mabtech equine IFN-γ ELISAPRO kit, at room temperature (■), 30°C (•), and 37°C (▲). At all temperatures, the assay displayed a linear response (R2 > 0.99). OD = optical density.
Table 2.
Recovery of recombinant equine interferon gamma (IFN-γ) in 3 rhinoceros plasma matrices using the Mabtech equine IFN-γ ELISAPRO kit assay.
| Sample recovery |
|||
|---|---|---|---|
| 50% plasma | 25% plasma | SD | |
| Animal 1 | 81 | 77 | 2.7 |
| Animal 2 | 96 | 94 | 0.8 |
| Animal 3 | 102 | 93 | 5.9 |
| Mean recovery % | 93 | 88 | 3.5 |
SD = standard deviation.
Figure 3.
Regression analysis of dilution series of recombinant equine interferon gamma (IFN-γ) A. and rhinoceros IFN-γ in plasma B. of 7.8–125 pg/mL, and measured using the Mabtech equine IFN-γ ELISAPRO kit. Both samples displayed linear responses (R2 > 0.99) with no significant difference between lines (F = 0.12; p > 0.5). OD = optical density.
Using 3 different rhinoceros samples, intra-assay precision was 0.4–2.8% and inter-assay precision was 3.4–6.4% (Table 3). The LOD and LOQ were calculated as 1.5 and 5.4 pg/mL, respectively. However, because these extrapolated values were regarded as imprecise, the nearest empirical values (i.e., 2 pg/mL and 7.8 pg/mL),were accepted as the LOD and LOQ, respectively.
Table 3.
Intra-assay and inter-assay precisions of pokeweed mitogen-stimulated whole blood from 3 rhinoceros diluted in pooled Nil plasma assayed in triplicate for 3 d using the Mabtech equine IFN-γ ELISAPRO kit assay.
| Intra-assay precision |
Inter-assay precision |
|||||
|---|---|---|---|---|---|---|
| Animal | Mean (pg/mL) | SD | CV | Mean (pg/mL) | SD | CV |
| 1 | 111.0 | 1.7 | 1.6 | 104.0 | 6.2 | 5.9 |
| 2 | 105.0 | 2.6 | 2.8 | 102.0 | 3.5 | 3.4 |
| 3 | 112.0 | 0.6 | 0.4 | 104.0 | 6.7 | 6.4 |
CV = coefficient of variation; SD = standard deviation.
Discussion
We selected the Mabtech equine IFN-γ ELISAPRO kit as the optimal system for the measurement of white rhinoceros endogenous IFN-γ. When incubation steps were performed at 37°C, the ELISA displayed good recovery of IFN-γ in a rhinoceros plasma matrix, a linear response to both recombinant equine IFN-γ and endogenous rhinoceros IFN-γ, and high reproducibility.
Notably, the equine IFN-γ ELISAPRO kit results displayed greater sensitivity than the bovine in-house ELISA used previously.12 The identification of an equine ELISA as the optimal assay for measuring white rhinoceros IFN-γ was anticipated given the phylogenetic relationship between rhinoceros and equine species14 and the high homology of the IFN-γ sequences.11 Moreover, the antibodies used in this ELISA are known to cross-react with IFN-γ of other species (https://www.mabtech.com/knowledge-center/tutorials-and-guidelines/veterinary-reagents), and the use of ELISAs for IFN-γ detection in African wildlife has been reported previously.5,6
The ELISA incubation steps were performed at 37°C in contrast to the manufacturer’s instructions. Rhinoceros typically are found in areas that are distant from environmentally controlled laboratories, and temperature fluctuation could result in variation in ELISA results. Therefore, 37°C was selected as the incubation temperature that can be achieved practically in most laboratories regardless of ambient temperatures. This temperature is utilized in the commercial cattle-type IFN-γ release assay.2 A possible drawback of this protocol is that the LOQ of the ELISAPRO kit was calculated as 7.8 pg/mL, which is greater than that reported by the manufacturer. Nonetheless, the ELISA displayed a linear response across a wide range of temperatures (19–37°C). These characteristics of the ELISAPRO kit highlight the utility of the assay for measuring rhinoceros IFN-γ under highly standardized conditions as applicable. The ELISAPRO kit showed excellent performance in measuring endogenous rhinoceros IFN-γ in plasma samples. Rhinoceros plasma showed minimal interference with the ELISA, and recovery of IFN-γ in this matrix was within the acceptable range of 80–120% (Guideline ICH Harmonised Tripartite. Validation of analytical procedures: text and methodology Q2(R1). Proc Intern Conf Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2005 Nov; Geneva, Switzerland. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf).
Moreover, parallelism was excellent, and ELISA responses were indistinguishable for recombinant equine and endogenous rhinoceros IFN-γ. This is in contrast to other African wildlife species such as warthogs, in which detection of IFN-γ in stimulated blood samples has proven difficult.13 In addition, intra-and inter-assay precision showed CVs of <10% and 15%, respectively, which is considered an acceptable range,7 indicating the high reproducibility of the equine IFN-γ ELISAPRO kit. Therefore, this kit is well suited for measuring rhinoceros IFN-γ in clinical samples. However, further research is underway to investigate the use of this ELISA for the detection of M. bovis antigen-specific cytokine secretion from stimulated white rhinoceros whole blood samples.
Acknowledgments
We thank Leana Rossouw, Guy Hausler, Tebogo Manamela, and the Veterinary Wildlife Services capture team from Kruger National Park for assistance during sample collection from the rhinoceros and preparation in the laboratory. We thank our collaborators from Mabtech, Drs. Eva Gelius and Jens Gertow, for supplying us with antibodies and customizing the ELISA with the selected antibody pairs.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded by the NRF South African Research Initiative (SARChI, grant 86949), the American Association of Zoological Medicine Wild Animal Health Fund (1-2017), and the South African Medical Research Council. We declare that the content of this publication is the sole responsibility of the authors and that the funding agencies were not involved in any of the research design, implementation, analyses, or writing. Therefore, the publication does not necessarily represent the views of the funders.
ORCID iDs: Sven DC. Parsons  https://orcid.org/0000-0002-9033-9686
https://orcid.org/0000-0002-9033-9686
Michele A. Miller  https://orcid.org/0000-0002-5883-6076
https://orcid.org/0000-0002-5883-6076
References
- 1. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantification. Clin Biochem Rev 2008;29:S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 2. Bernitz N, et al. Detection of Mycobacterium bovis infection in African buffaloes (Syncerus caffer) using QuantiFERON®-TB gold (QFT) tubes and the Qiagen cattletype® IFN-gamma ELISA. Vet Immunol Immunopathol 2018;196:48–52. [DOI] [PubMed] [Google Scholar]
- 3. de la Rua-Domenech R, et al. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 2006;81:190–210. [DOI] [PubMed] [Google Scholar]
- 4. Emslie R. Ceratotherium simum. The IUCN Red List of Threatened Species 2012. Cambridge, UK: International Union for Conservation of Nature and Natural Resources, 2012. [Google Scholar]
- 5. Goosen WJ, et al. The evaluation of candidate biomarkers of cell-mediated immunity for the diagnosis of Mycobacterium bovis infection in African buffaloes (Syncerus caffer). Vet Immunol Immunopathol 2014;162:198–202. [DOI] [PubMed] [Google Scholar]
- 6. Gormley E, et al. Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam®) assay. Vet Microbiol 2006;112:171–179. [DOI] [PubMed] [Google Scholar]
- 7. Li HW, et al. Comparative evaluation of three new commercial immunoassays for anti-mullerian hormone measurement. Hum Reprod 2016;31:2796–2802. [DOI] [PubMed] [Google Scholar]
- 8. Maas M, et al. Facts and dilemmas in diagnosis of tuberculosis in wildlife. Com Immunol Microbiol Infect Dis 2013;36:269–285. [DOI] [PubMed] [Google Scholar]
- 9. Miller MA, et al. Mycobacterium bovis in a free-ranging black rhinoceros, Kruger National Park, South Africa, 2016. Emerg Infect Dis 2017;23:557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller MA, et al. Conservation of white rhinoceros threatened by bovine tuberculosis, South Africa, 2016–2017. Emerg Infect Dis 2018;24:2373–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morar D, et al. Cloning, sequencing and expression of white rhinoceros (Ceratotherium simum) interferon-gamma (IFN-γ) and the production of rhinoceros IFN-γ specific antibodies. Vet Immunol Immunopathol 2007;115:146–54. [DOI] [PubMed] [Google Scholar]
- 12. Parsons SDC, et al. The kinetics of the humoral and interferon-gamma immune responses to experimental Mycobacterium bovis infection in the white rhinoceros (Ceratotherium simum). Front Immunol 2017;8:1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roos EO, et al. IP-10: a potential biomarker for detection of Mycobacterium bovis infection in warthogs (Phacochoerus africanus). Vet Immunol Immunopathol 2018;201:43–48. [DOI] [PubMed] [Google Scholar]
- 14. Steiner CC, Ryder OA. Molecular phylogeny and evolution of the perissodactyla. Zool J Linn Soc 2011; 163:1289–1303. [Google Scholar]



