Abstract
Sarcocystis spp. are causative agents of bovine eosinophilic myositis and/or myocarditis, which are chronic subclinical myopathies that are occasionally responsible for condemnation at slaughterhouses. Sarcocystis cruzi is a protozoan parasite of worldwide distribution transmitted by canids, most commonly associated with subclinical infection in cattle. Although S. cruzi infections can rarely lead to fatal systemic disease, fatal cardiac cases with confirmation of the etiologic diagnosis have not been reported, to our knowledge. We describe herein an unusual case of S. cruzi–induced fatal bovine eosinophilic myocarditis. A 22-mo-old, Holstein–Hereford heifer, in a group of 110 cattle on pasture, manifested growth retardation and died in February 2017. Autopsy revealed myriad yellow-green 1–3-mm coalescing foci, surrounded by fibrosis, affecting ~75% of the ventricular myocardium. Pulmonary edema, ascites, and hydrothorax were consistent with chronic congestive heart failure. Histology revealed severe eosinophilic, granulomatous, necrotizing myocarditis, with multinucleate giant cells, fibrosis, and mineralization. Numerous thin-walled protozoan cysts resembling Sarcocystis spp. were present in the necrotic foci and within the sarcoplasm of adjacent cardiomyocytes. PCR and sequencing of the 18S rRNA gene revealed 99.9–100% homology with S. cruzi. Sarcocystosis can be a rare cause of fatal myocarditis in cattle.
Keywords: cattle, eosinophilic myocarditis, fatality, Sarcocystis cruzi, sarcocystosis
Sarcocystis spp. are Apicomplexa protozoa of worldwide distribution that infect a wide range of vertebrates,4 typically without significant clinical disease. Species differ in life cycle, host specificity, morphology, and pathogenicity. Sarcocystis spp. have a life cycle with 2 obligate hosts: an intermediate host in which asexual reproduction occurs with tissue cyst formation, and a carnivore–omnivore definitive host, in which enteric sexual reproduction takes place with the release of sporocysts in feces.4
The main Sarcocystis spp. that parasitize cattle are S. cruzi (syn. S. bovicanis), S. hirsuta, and S. hominis, whose principal definitive hosts are dogs, cats, and humans, respectively. Sarcocystis rommeli (formerly misnamed S. sinensis) has also been described infecting cattle; however, its definitive host remains unknown.5 The species S. bovifelis was proposed instead of S. rommeli based on cyst morphology and the possible role of felids as definitive hosts.4,7 Morphologically, S. cruzi has thin-walled cysts (<1 μm); cysts of S. hirsuta, S. rommeli, and S. hominis have thicker walls (2–7 μm).
Generally, canids are definitive hosts for the Sarcocystis species that are the most virulent for intermediate hosts. The severity of clinical signs and lesions depends mainly on the number of ingested sporocysts and the immunologic status of the host.4 Eosinophilic myositis and myocarditis associated with Sarcocystis spp. infections in cattle are usually incidental postmortem findings but can be the reason for condemnation at slaughterhouses. Moreover, histologic examination of cardiac and skeletal muscles in healthy ruminants from many countries reveals a high frequency of sarcocysts, up to 100%, without associated inflammation.13,15,18 Despite high prevalence, these parasites rarely cause clinical disease,4,6 thus sarcocystosis has been considered to be a subclinical myopathy (i.e. presence of muscle cysts with or without associated inflammation, but without detectable clinical signs).19 Herein we describe an unusual case of severe eosinophilic myocarditis with fatal outcome induced by S. cruzi infection in a heifer.
Our case occurred in February 2017, at a mixed dairy–beef farm in Colonia, Uruguay. The herd was composed of 110, 8–12-mo-old crossbred heifers and steers, and one 22-mo-old heifer. Cattle grazed on pastures in a 75-hectare area, with water supply from natural watercourses. The affected animal was a 22-mo-old Holstein–Hereford heifer, with a history of growth retardation, whose clinical condition suddenly declined, and died spontaneously shortly thereafter.
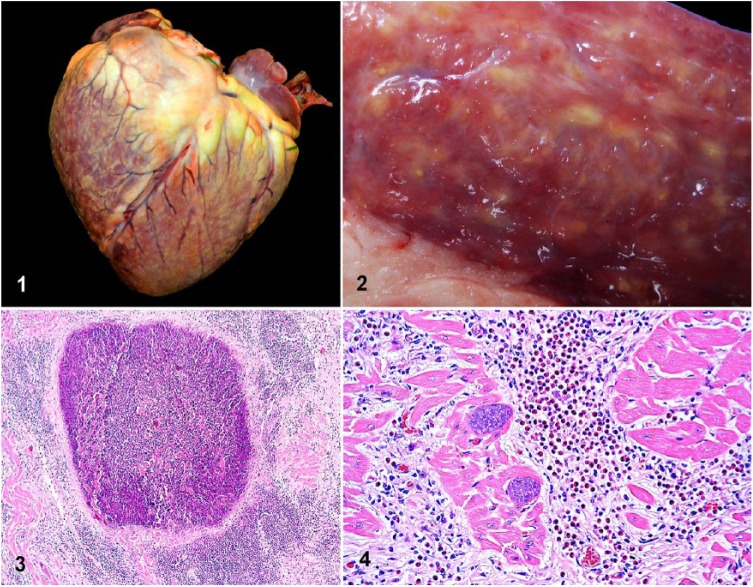
Autopsy revealed myriad, irregular, yellow-green, 1–3-mm coalescing foci with variably distinct borders, and white striations disseminated throughout the myocardium (Figs. 1, 2). Lesions predominantly effaced the ventricular free walls and interventricular septum, and to a lesser extent the atrial myocardium, overall involving ~75% of the heart. In addition, there was moderate diffuse enhancement of the lobular pattern in the liver and diffuse pulmonary congestion and edema, with marked expansion of the interlobular septa, stable froth within the bronchial and tracheal lumens, mild ascites, and hydrothorax. These lesions were compatible with chronic congestive heart failure. No other gross lesions were identified.
Figures 1–4.
Eosinophilic granulomatous myocarditis in a heifer. Figure 1. Myriad 1–3-mm, irregular, yellow-green coalescing foci disseminated throughout the myocardium are visible from the epicardial surface. Figure 2. Transmural section of the left ventricular free wall with granulomas and white streaks throughout the myocardium. Figure 3. A myocardial granuloma composed of a well-demarcated central area of necrosis surrounded by dense interstitial mixed inflammatory cell infiltrates and fibrosis. H&E. Figure 4. Abundant eosinophils, fewer lymphocytes, and macrophages infiltrate the myocardial interstitium, which is also expanded by fibrosis. Adjacent cardiomyocytes contain intact intrasarcoplasmic, thin-walled, basophilic Sarcocystis cruzi cysts. H&E.
Samples of heart, lung, brain, kidney, spleen, lymph node, skeletal muscles, tongue, liver, urinary bladder, and intestine were immersion-fixed in neutral-buffered formalin for 48 h, embedded in paraffin, sectioned at 4–5 µm, and stained with hematoxylin and eosin (H&E) for histologic and micromorphometric evaluation under an optical microscope (Axio-Scope.A1, Carl-Zeiss, Darmstadt, Germany) coupled with a digital camera (Axiocam-512, Carl-Zeiss) and ZEN software (Carl-Zeiss). Fresh heart was collected at autopsy and stored at −20°C.
Histology revealed severe, chronic, disseminated eosinophilic, granulomatous necrotizing myocarditis, with myocardial fibrosis, multinucleate giant cells, and mineralization. The granulomas had a well-demarcated necrotic center surrounded by dense interstitial mixed inflammatory cell infiltrates and fibrosis. The inflammatory infiltrates were characterized by abundant eosinophils, fewer lymphocytes, epithelioid macrophages, and occasional multinucleate giant cells that expanded the myocardial interstitium. Numerous preserved intra- and extra-lesional, ovoid, protozoan cysts containing uniformly sized elongated zoites were observed within the sarcoplasm of cardiomyocytes in, or adjacent to, areas of severe interstitial inflammation. Degenerate sarcocysts were occasionally present within the center of the necrotic foci and surrounded by a dense infiltrate of eosinophils, macrophages, and lymphocytes (Figs. 3, 4, Supplementary Figs. 1–4). Micromorphometric evaluation of 100 preserved cysts revealed that, on average, the major and minor diameters were 46 μm (20–205 μm) and 34 μm (15–73 μm). The cyst wall thickness was ≤1 μm in all examined cysts, regardless of their diameter. In the lungs, there was diffuse congestion of the alveolar capillaries, with edema and fibrin in the interstitium and alveolar spaces, and microhemorrhages and/or erythrocyte diapedesis into the alveolar spaces, which occasionally contained uni- or bi-nucleate alveolar macrophages. Diffuse periacinar hepatocellular degeneration and necrosis were found in the liver. The pulmonary and hepatic lesions were attributed to chronic heart failure and terminal ischemia, respectively. Additionally, there was focal mild eosinophilic glossitis with very infrequent extralesional intrasarcoplasmic preserved sarcocysts. No significant findings were noted in other tissues; no sarcocysts were detected in skeletal muscle sections other than tongue.
DNA extracted from 2 frozen samples of heart (QIAamp cador pathogen mini kit; Qiagen, Hilden, Germany) was processed by real-time PCR with specific probes for S. cruzi, S. hirsuta, S. hominis, and S. sinensis/S. rommeli.14 A positive result was obtained only with the S. cruzi–specific probe. Additionally, a fragment of the Sarcocystis spp. 18S rRNA gene was amplified by PCR in both DNA samples. Amplification products were purified and sequenced.17 Sequences were aligned and analyzed (R9 software; Geneious, Auckland, NZ). Consensus sequences were compared with sequences available in GenBank by BLASTn analysis. Sequences of 844 and 843 bp were obtained and registered in GenBank (MK275239–MK275240), and both shared 100–99.9% identity with other sequences of S. cruzi (KT901167, KC209738, AB682780, JX679467, among others). Sarcocystis levinei (KU247922), a species identified in buffalo, was the next-highest match at 99.4% homology. Conventional PCRs for Toxoplasma gondii and Neospora caninum were negative.
The extensive and severe cardiac lesions, along with the pulmonary and hepatic lesions, were highly suggestive of chronic heart failure as the cause of death in this heifer. The numerous cysts compatible with Sarcocystis spp. within the heart, and the degenerate cysts within the necrotic centers of the myocardial granulomas, in conjunction with the molecular identification of S. cruzi and the absence of detection of any other causative agent, strongly argues that S. cruzi was the cause of the myocarditis.
Other conditions characterized by eosinophilic granulomatous myocarditis, such as helminthic and fungal infections, were ruled out by the absence of intralesional helminthic and fungal elements on H&E and Gomori methenamine silver stains. Syndromes associated with multisystemic eosinophilic granulomas described in cattle exposed to some toxicants, including plants such as Vicia villosa (hairy vetch),16 were ruled out by the pathology examination, which revealed that severe inflammation was restricted to the heart, and by the lack of history of exposure to these toxicants.
S. cruzi infection in cattle is usually subclinical, although acute and chronic clinical disease have been described in natural and experimental cases.4 Acute sarcocystosis, initially called “Dalmeny disease,” was first reported in Canada in 1961.2 The clinicopathologic presentation is variable and depends on the dose of sporocysts ingested. Although experimental administration of ≤1,000 sporocysts causes no clinical signs, doses of ≥5 million sporocysts are invariably fatal. Cattle fed 200,000 sporocysts develop clinical disease, some die of acute sarcocystosis, whereas others survive the acute stage and show growth retardation (chronic sarcocystosis).4 Acute sarcocystosis, starting at ~4 wk postinfection, is characterized clinically by persistent fever, anorexia, weight loss, hair loss, anemia, weakness, muscle twitching, prostration, drooling, neurologic signs, and death; pregnant cows may abort, and lactating cows have reduced milk production.4 The histopathologic hallmark of acute sarcocystosis is the presence of numerous S. cruzi schizonts in vascular endothelial cells of many organs, along with vasculitis, necrosis, and/or inflammatory infiltrates in muscles, brain, placenta, and other tissues.1,4,12 Thus, acute sarcocystosis results from systemic vascular infection with early or immature S. cruzi asexual stages.
Unlike the acute presentation, chronic sarcocystosis is characterized clinically by growth retardation, and histopathologically by the presence of mature S. cruzi cysts containing bradyzoites within the sarcoplasm of striated myocytes that develop ~8 wk postinfection, without immature stages detectable in the vascular endothelium.4 Most cases of chronic sarcocystosis are not associated with significant lesions in infected muscles, other than the typical intrasarcoplasmic cysts.13,15 However, a small proportion of cattle chronically infected with S. cruzi develop eosinophilic myositis or myocarditis with bradyzoite-containing cysts.9
Even in cattle that develop considerable lesions, eosinophilic myositis or myocarditis are not apparent clinically. In most cases, the lesions are incidental postmortem findings at slaughterhouses.4,9 Typically, affected muscles have characteristic green discoloration detectable grossly and are trimmed from the carcass at meat inspection. Although fatal cases of eosinophilic myocarditis in cattle have been reported, to our knowledge, fatal cases attributable to chronic eosinophilic myocarditis with definitive identification of S. cruzi have not been documented.
To date, there is enough evidence, including experimental reproduction of the lesions, to causally associate bovine eosinophilic, granulomatous myositis or myocarditis with various Sarcocystis species, including the zoonotic S. hominis.3,4,18–20 However, the pathogenic mechanisms leading to lesion development are not understood completely. Hypersensitivity reactions are thought to play a role in cattle with S. cruzi–associated eosinophilic myocarditis detected at slaughter based on increased S. cruzi–specific IgE serum levels in affected animals, in contrast to cattle infected with S. cruzi but without lesions of eosinophilic myocarditis.9 Additionally, lesions have been reproduced experimentally by intramuscular injection of adjuvanted antigen of Sarcocystis obtained from cases of bovine eosinophilic myocarditis at slaughter,18 which also suggests that the lesion represents an immunologic reaction against Sarcocystis spp. antigen.
Cattle with eosinophilic myositis or myocarditis may be genetically predisposed to produce high levels of Sarcocystis-specific IgE in response to bradyzoite antigen,9 as occurs in individuals with a genetic predisposition to type I hypersensitivity reactions to foreign antigens. This genetic predisposition hypothesis could explain the usual low morbidity of eosinophilic myositis or myocarditis, despite the high Sarcocystis spp. prevalence. The manifestation of clinical sarcocystosis with granulomatous and eosinophilic myositis induced by Sarcocystis fayeri in 2 genetically related horses by the same stallion also suggests a possible genetic basis for lesion and disease development.10 Additional theories for potential pathogenesis include infection by more pathogenic strains or genotypes of Sarcocystis spp., infective dose, breed, and the immune status of the intermediate hosts,4 or increased sarcolemmal fragility.
Although massive Sarcocystis spp. infections can lead to acute fatal disease, chronic fatal cases are seldom diagnosed. A fatal case of eosinophilic myocarditis that resulted in sudden death was reported in an 18-mo-old Hereford heifer in Iowa11; however, no association with Sarcocystis infection was made. A presumptive case of chronic Sarcocystis spp.–induced eosinophilic myocarditis with fatal outcome was reported in a 2-y-old Hereford heifer in Uruguay.6 The etiologic diagnosis was based on morphologic evaluation of the protozoan, although the species involved was not confirmed. Interestingly, these cases, including our case, occurred sporadically in Hereford or Hereford-cross heifers 1.5–2 y old, which indirectly favors the hypothesis of a possible genetic or breed predisposition for eosinophilic myocarditis or myositis.
Although the source of infection was not determined in our case, domestic dogs and wild foxes are common in the area. The observation of cysts in myocytes, without schizonts recognized in blood vessels, suggested that the infection had taken place at least 8 wk before death.4 The nature of the lesions, particularly the mature interstitial myocardial fibrosis, was also consistent with a protracted course. The lesions involving the heart and the morphology of the thin-walled cysts strongly suggested S. cruzi as the species involved.4,20 This agrees with other studies in which sarcocysts were analyzed in meat samples from Uruguay, with S. cruzi the only species found.8,15 S. cruzi has been demonstrated to be the species with the highest prevalence (>90%) in several countries, including Argentina,13,14 noting that the myocardium is the most frequently affected muscle. Meanwhile, the prevalence of S. hirsuta and S. hominis is variable in different parts of the world, and these species locate predominantly in the esophagus and other muscles, rather than the myocardium.13 Molecular studies identifying Sarcocystis spp. infecting Uruguayan cattle are limited.15
Because several Sarcocystis spp. can be associated with eosinophilic myositis, including a zoonotic species, the need has been raised for species confirmation through molecular methods and/or transmission electron microscopy.19 In our case, S. cruzi was the only species identified by PCR and sequencing. S. cruzi should be considered an unusual cause of sporadic fatal myocarditis in cattle.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638719856651 for Fatal Sarcocystis cruzi–induced eosinophilic myocarditis in a heifer in Uruguay by Virginia Aráoz, Caroline da Silva Silveira, Gastón Moré, Georgget Banchero, Franklin Riet-Correa and Federico Giannitti in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Yisell Perdomo from INIA for technical assistance and Dr. Bradd Barr for critically reviewing this manuscript.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded by grant PL-015 N-15156 from INIA.
Supplementary material: Supplementary material for this article is available online.
ORCID iDs: Gastón Moré  https://orcid.org/0000-0002-0475-717X
https://orcid.org/0000-0002-0475-717X
Federico Giannitti  https://orcid.org/0000-0001-8799-6848
https://orcid.org/0000-0001-8799-6848
References
- 1. Carrigan MJ. An outbreak of sarcocystosis in dairy cattle. Aust Vet J 1986;63:22–24. [DOI] [PubMed] [Google Scholar]
- 2. Corner AH, et al. Dalmeny disease. An infection of cattle presumed to be caused by an unidentified protozoon. Can Vet J 1963;4:252–264. [PMC free article] [PubMed] [Google Scholar]
- 3. Dubey JP. Clinical sarcocystosis in calves fed Sarcocystis hirsuta sporocysts from cats. Vet Pathol 1983;20:90–98. [DOI] [PubMed] [Google Scholar]
- 4. Dubey JP, et al. Sarcocystosis of Animals and Humans. 2nd ed. Boca Raton, FL: CRC Press, 2015. [Google Scholar]
- 5. Dubey JP, et al. Sarcocystis rommeli, n. sp. (Apicomplexa: Sarcocystidae) from cattle (Bos taurus) and its differentiation from Sarcocystis hominis. J Eukaryot Microbiol 2016;63:62–68. [DOI] [PubMed] [Google Scholar]
- 6. Dutra F. Miocarditis sarcocística en vaquillona [Sarcocystic myocarditis in a heifer]. Treinta y Tres, Uruguay: División de Laboratorios Veterinarios, Ministerio de Ganadería Agricultura y Pesca, 2014 Archivo Veterinario del Este 22–23 Spanish. [Google Scholar]
- 7. Gjerde B. The resurrection of a species: Sarcocystis bovifelis Heydorn et al., 1975 is distinct from the current Sarcocystis hirsuta in cattle and morphologically indistinguishable from Sarcocystis sinensis in water buffaloes. Parasitol Res 2016;115:1–21. [DOI] [PubMed] [Google Scholar]
- 8. Gjerde B. Molecular characterization of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res 2016;115:1473–1492. [DOI] [PubMed] [Google Scholar]
- 9. Granstrom DE, et al. Type-I hypersensitivity as a component of eosinophilic myositis (muscular sarcocystosis) in cattle. Am J Vet Res 1989;50:571–574. [PubMed] [Google Scholar]
- 10. Herd HR, et al. Sarcocystis fayeri-induced granulomatous and eosinophilic myositis in 2 related horses. Vet Pathol 2015;52:1191–1194. [DOI] [PubMed] [Google Scholar]
- 11. Jaspers R. Bovine eosinophilic myocarditis. Iowa State Univ Vet 1962. –3;25(2):article 7. [Google Scholar]
- 12. Landsverk T. An outbreak of sarcocystosis in a cattle herd. Acta Vet Scand 1979;20:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moré G, et al. Prevalence of Sarcocystis spp. in Argentinean cattle. Vet Parasitol 2011;177:162–165. [DOI] [PubMed] [Google Scholar]
- 14. Moré G, et al. Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet Parasitol 2013;197:85–94. [DOI] [PubMed] [Google Scholar]
- 15. Pritt B, et al. Detection of Sarcocystis parasites in retail beef: A regional survey combining histological and genetic detection methods. J Food Protec 2008;71:2144–2147. [DOI] [PubMed] [Google Scholar]
- 16. Robinson WF, Robinson NA. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 3 St. Louis, MO: Elsevier, 2016:36. [Google Scholar]
- 17. Scioscia NP, et al. Pampas fox (Lycalopex gymnocercus) new intermediate host of Sarcocystis svanai (Apicomplexa: Sarcocystidae). Parasitol Int 2017;66:214–218. [DOI] [PubMed] [Google Scholar]
- 18. Vangeel L, et al. Intramuscular inoculation of cattle with Sarcocystis antigen results in focal eosinophilic myositis. Vet Parasitol 2012;183:224–230. [DOI] [PubMed] [Google Scholar]
- 19. Vangeel L, et al. Different Sarcocystis spp. are present in bovine eosinophilic myositis. Vet Parasitol 2013;197:543–548. [DOI] [PubMed] [Google Scholar]
- 20. Wouda W, et al. Eosinophilic myositis due to Sarcocystis hominis in a beef cow. J Comp Pathol 2006;135:249–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638719856651 for Fatal Sarcocystis cruzi–induced eosinophilic myocarditis in a heifer in Uruguay by Virginia Aráoz, Caroline da Silva Silveira, Gastón Moré, Georgget Banchero, Franklin Riet-Correa and Federico Giannitti in Journal of Veterinary Diagnostic Investigation