Abstract
Cases of cranial superficial epigastric vein (CSEV) phlebitis with subsequent septicemia were observed in dairy farms in Minas Gerais, Brazil. Autopsy on 4 affected cows, from 2 farms, revealed CSEV thrombophlebitis with perivascular abscesses, pulmonary abscesses, valvular endocarditis, arthritis, thromboembolic nephritis, and renal infarcts. Microscopic examination revealed fibrosing and lymphoplasmacytic phlebitis with occasional endothelial loss, subendothelial areas of necrosis, and abundant fibrin deposition. Trueperella pyogenes, Escherichia coli, and Staphylococcus aureus were isolated from lesions of 3 different cows. Thrombophlebitis of the CSEV is a rare condition in dairy cows; however, it has become more frequent in dairy farms in southeastern Brazil after repeated venipuncture of this vein, likely with contaminated needles used for administration of oxytocin.
Keywords: diseases of dairy cattle, phlebitis, phlebothrombosis, venipuncture
Venous thrombosis, thrombophlebitis, and septic thrombophlebitis are frequently associated with traumatic or repeated venipuncture in cattle.8 Common predisposing factors that increase the risk of venous injury include the type of needle or catheter, inappropriate preparation of the site, improper restraint of the animal, inexperience of the operator, and inoculation of irritant substances.8,10 The jugular vein is the most commonly damaged blood vessel following venipuncture in dairy cattle, followed by the cranial superficial epigastric vein (CSEV) and the tail or caudal vein.8 CSEV venipuncture is indicated only in emergency cases or when both jugular veins are inaccessible.8 The CSEV drains 90% of the blood from the mammary gland, and thus lesions occluding or reducing its diameter will negatively influence milk production.5
Oxytocin is a neuropeptide hormone that stimulates uterine contractions and milk letdown.1 CSEV venipuncture has become a common procedure in Brazilian dairy farms after the popularization of oxytocin use to increase milk production.1 Exogenous oxytocin is applied intravenously twice a day before milking, with more than 500 applications required in a typical Brazilian 280-d lactation season (Santos MV. Ocitocina injetável durante a ordenha—solução ou complicação? [Oxytocin injection during milking—solution or complication?]. Inforleite 2013;40–42. Portuguese).
CSEV thrombophlebitis and septicemia has been reported rarely worldwide, and has not been described in Brazilian dairy cattle, to our knowledge.8 However, the increase in administration of oxytocin via the CSEV in dairy farms is reflected in our diagnostic caseload, with multiple affected cows referred to the Veterinary Hospital and Autopsy Service. Herein we describe 4 cases originating from 2 outbreaks of septicemia in dairy cattle associated with CSEV thrombophlebitis caused by repetitive venipuncture for the administration of oxytocin.
In June 2017, three 3–4-y-old Girolando cows (cases 1–3) were referred to the Veterinary Hospital at the Federal University of Minas Gerais (VH-UFMG; Brazil) with a history of chronic weight loss, anorexia, and anemia. Given their overall poor condition, all 3 cows were euthanized via pentobarbital overdose. Thirty other lactating cows from the same farm had died with similar clinical signs in a 2-mo period. All affected cows had received doses of 0.1 mL of oxytocin (10 IU/mL) that was administered via the CSEV twice a day. In September 2017, a 4-y-old Girolando cow (case 4) from a second farm was referred to the VH-UFMG with a history of chronic weight loss, arched back, and apathy for 3 wk. The cow was also euthanized, given her poor body condition. This cow had also received twice daily doses of 0.1 mL of oxytocin via the CSEV. No other similar case was reported in this farm.
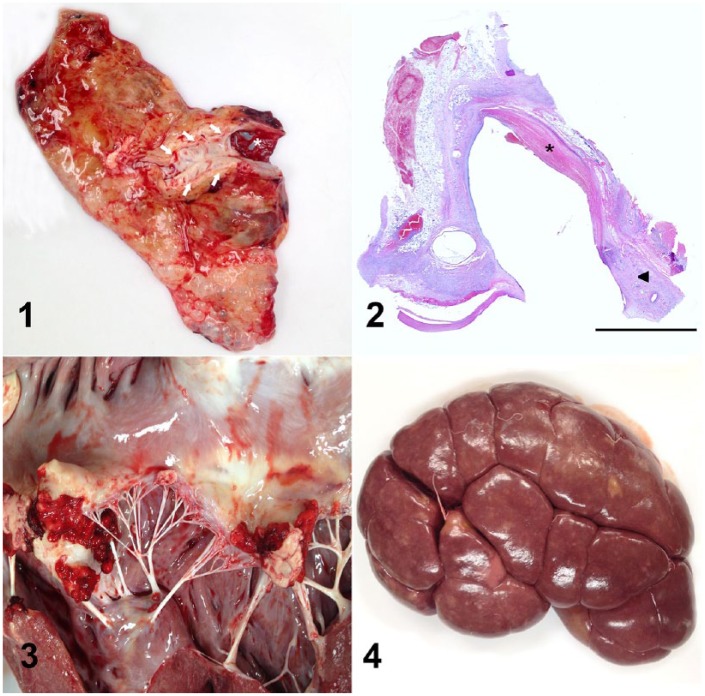
An autopsy was performed on the 4 affected cows, and revealed extensive swelling of the ventral abdominal and mammary areas corresponding to the anatomic distribution of the CSEV tributaries; this was mainly the result of edema and fibrosis. In case 1, a 4-cm diameter abscess expanded the ventral abdominal subcutaneous tissue adjacent to the epigastric vein. The CSEV wall in all 4 cases was thickened, irregular, and firm, as a result of fibrosis, and had friable dark red areas of necrosis occasionally admixed with purulent exudate. Locally extensive areas of the vascular lining were roughened and covered with 2–3-mm thick layers of yellow-to-red strands of fibrin (Fig. 1). The mammary and inguinal lymph nodes of all cases were enlarged and had pinpoint white foci throughout the cortex. Throughout the lungs of cases 1 and 2, there were 0.5–2-cm diameter abscesses with fibrous adhesions to the parietal pleura. The carpal joints of cases 1 and 2, the distal interphalangeal joints of case 1, and the left coxofemoral joint of case 2 were moderately swollen. The articular surfaces of these joints were irregular and covered with small-to-moderate amounts of purulent exudate and fibrin strands. The free margins of the aortic and mitral valves of cases 1 and 4, respectively, were effaced by vegetative, yellow-to-red, friable aggregates of fibrin and necrotic debris (Fig. 3). The renal cortices in cases 1 and 4 contained multiple, white-to-red, wedge-shaped infarcts and yellow, 0.1–0.3-mm diameter nodules (embolic nephritis; Fig. 4). Sections of multiple organs were collected, fixed in buffered 10% formalin, processed routinely for histology, and stained with hematoxylin and eosin.
Figures 1–4.
Cranial superficial epigastric vein (CSEV) phlebitis and septicemia in dairy cows. Figure 1. The intimal surface of the CSEV (arrows) of case 4 is roughened and covered by thin layers of fibrin (asterisk). The adjacent areas were markedly swollen and firm, as a result of edema and fibrosis. Figure 2. The wall of the CSEV of case 4 was markedly thickened by fibrosis and moderate numbers of lymphocytes, plasma cells, and macrophages, mainly in the tunica adventitia (arrowhead). There were extensive areas of endothelial loss, covered by strands of fibrin, and expansion of the subendothelial spaces with fibrin, neutrophils, and cell debris (asterisk). H&E. Bar = 5 mm. Figure 3. Vegetative endocarditis of the mitral valve of case 4. Figure 4. The surface of the renal cortex of case 4 contained irregular pale tan areas (infarcts) and yellow, 0.1–0.3-mm diameter nodules of embolic nephritis.
Histologically, the wall of the CSEV of all cows was markedly thickened by fibrosis and moderate numbers of lymphocytes, plasma cells, and macrophages, mainly in the tunica adventitia. There were extensive areas of endothelial loss, covered by strands of fibrin, and expansion of the intima by neutrophils, fibrin, and cell debris (Fig. 2). Pulmonary abscesses were characterized by nodular aggregates of degenerate neutrophils and necrotic debris surrounded by thick layers of fibrous connective tissue. The free margins of the aortic valve of case 1 and mitral valve of case 4 were covered with fibrin and blood admixed with moderate numbers of neutrophils, eosinophilic cellular debris, and bacterial colonies. The renal infarcts in cases 1 and 4 were characterized by extensive areas of coagulative necrosis bordered by a thick layer of neutrophils and hemorrhage; suppurative embolic nephritis was present, as well. No bacterial colonies were observed in the examined histologic sections of the kidney of cases 1 and 4, even though these lesions likely originated from bacterial emboli from the CSEV and the valvular endocarditis. There was prominent lymphoid hyperplasia of the mammary and inguinal lymph nodes of all cases.
Fresh samples of the vascular lesions and adjacent soft tissues from cases 1, 2, and 4, as well as from lung and endocardium from cases 2 and 4, respectively, were submitted for aerobic bacterial culture. Samples were incubated at 37°C for 48 h on blood agar and MacConkey agar. Pure colonies were submitted to primary tests: Gram, catalase, and oxidase. Bacterial species were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Escherichia coli was isolated from the vascular tissue of case 1, Trueperella pyogenes was isolated from the vascular tissue and lung of case 2, and Staphylococcus aureus was isolated from the vascular tissue and endocardium of case 4.
Reports of thrombophlebitis consist mainly of natural or experimental cases of jugular thrombophlebitis in horses and cattle after long-term catheterization.6,7,9,10 CSEV thrombophlebitis is rare in dairy cattle,8 but has become more common in our routine diagnostic caseload because of repeated CSEV venipuncture for administration of oxytocin. Thrombophlebitis and cellulitis have been reported secondarily to CSEV lesions, with occasional septicemia and secondary valvular endocarditis and pneumonia.2,3 The occurrence of CESV thrombophlebitis following repeated venipuncture and the importance of this lesion as the primary site of bacterial infection that can spread systemically via the bloodstream and cause lesions elsewhere is demonstrated in our report.
The CSEV is an effective site for intravenous (IV) administration of pharmaceuticals given its large size and relatively easy accessibility compared to the jugular or coccygeal veins.4 In Brazil, oxytocin is injected via CSEV in the pit parlor just before milking.1 The use of oxytocin is recommended in mixed-breed cows such as Girolando (Bos taurus × Bos indicus), which are common in southeastern and northeastern Brazil. For some of these mixed-breed animals, milk letdown relies on the presence of the calf close to the cow; smaller amounts of oxytocin are released by the cow in the absence of the calf.1 The use of oxytocin is allowed by the Brazilian agricultural regulatory agencies given that there have been no reported adverse effects from human consumption of milk from treated cows. However, repeated application of this hormone is considered to be a risk factor for the dissemination of Trypanosoma vivax and bovine leukemia virus (Santos MV, 2013).
In our cases, pulmonary abscesses, valvular endocarditis, embolic nephritis, and purulent arthritis occurred secondarily to the dissemination of bacterial emboli from the infected CSEV. Lesions in the heart and lungs have been described as the main consequences of CSEV thrombophlebitis.2 Renal infarcts and emboli in our cases were present only in cows with endocarditis, suggesting they were secondary to emboli from the valvular lesions.
The culture of different bacterial species from the tissues in our cases suggests that contaminated needles are more likely the cause of these lesions than repeated venipuncture. The bacteria cultured from affected tissues from these 2 outbreaks are common pyogenic organisms that are typically involved in cases of thrombophlebitis in cows.7,8 Although the needles utilized for the procedure in our cases were not cultured, thrombophlebitis and septicemia in cattle have been reported as a result of the use of contaminated needles or secondary to hematomas formed resulting from extensive vascular damage after the procedure.6,7,10 Further investigation is required to confirm bacterial contamination of needles or, possibly, oxytocin vials if future cases occur.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by funds from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig; process 2070.01.0003082/2018-25)
References
- 1. Araujo WAG, et al. Ocitocina exógena e a presença do bezerro sobre a produção e qualidade do leite de vacas mestiças [Exogenous oxytocin and calf presence on performance and milk quality of crossbred cow]. Braz J Vet Res Anim Sci 2012;49:465–470. Portuguese. [Google Scholar]
- 2. Braun U. Entzündung grosser Venen [Inflammation of the large vessels]. In: Dirksen G, et al., eds. Innere Medizin und Chirurgie des Rindes [Internal Medicine and Surgery of Cattle]. 4th ed. Berlin: Parey Buchverlag, 2002:191–193. German. [Google Scholar]
- 3. Braun U, et al. Ultrasonographic findings in three cows with cellulitis. Vet Rec 2005;157:26–28. [DOI] [PubMed] [Google Scholar]
- 4. Braun U, Hoegger R. B-mode and colour Doppler ultrasonography of the milk vein in 29 healthy Swiss Braunvieh cows. Vet Rec 2008;163:47–49. [DOI] [PubMed] [Google Scholar]
- 5. Gracner G, et al. Correlation between the milk vein internal diameter surface and milk yield in Simmental cows. Turk J Vet Anim Sci 2005;39:741–744. [Google Scholar]
- 6. Muller CDVS, et al. Influence of different types of catheters on the development of diseases of the jugular vein in 45 horses. J Equine Vet Sci 2016;46:89–97. [Google Scholar]
- 7. Pardon B, et al. Nosocomial intravascular catheter infections with extended spectrum Beta-lactamase-producing Escherichia coli in calves after strain introduction from a commercial herd. Transbound Emerg Dis 2017;64:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peek S, McGuirk SM. Cardiovascular diseases. In: Divers TJ, Peek S, eds. Rebhun’s Diseases of Dairy Cattle. 2nd ed. St. Louis, MO: Saunders Elsevier, 2007:43:78. [Google Scholar]
- 9. Pusterla N, Braun U. Ultrasonographic evaluation of the jugular vein of cows with catheter-related thrombophlebitis. Vet Rec 1995;137:431–434. [DOI] [PubMed] [Google Scholar]
- 10. Rouleau G, et al. Factors influencing the development of jugular thrombophlebitis in cattle and comparison of 2 types of catheter. Can Vet J 2013;44:399–404. [PMC free article] [PubMed] [Google Scholar]