Abstract
Zinc oxide (ZnO) is commonly fed to pigs at pharmacologic concentrations (2,000–3,000 ppm) for the first 3 wk post-weaning to increase growth and reduce enteric bacterial disease. The safety of this high-dose treatment is assumed based upon lower bioavailability of ZnO compared to other common forms of Zn in feed; however, limited data are available regarding the specific serum and tissue concentrations of Zn expected in animals experiencing overload following feeding of excessive ZnO. Fifty-five 3-wk-old pigs were divided into 5 groups receiving various concentrations of ZnO (0–6,000 ppm) for 3 wk. Pigs receiving 6,000 ppm ZnO had higher mean pancreatic Zn concentrations (p < 0.001) compared to other treatments, and higher pancreatic Zn concentrations were associated with pancreatic acinar cell apoptosis (p < 0.0001). Hepatic Zn concentrations were highest for pigs receiving 6,000 ppm ZnO (mean ± SEM; 729 ± 264 ppm) and significantly higher than all other groups (p < 0.0001), with controls having concentrations <60 ppm. Similarly, serum Zn was highest in pigs receiving 6,000 ppm ZnO (4.81 ± 2.31 ppm) and significantly higher than all groups (controls, <1 ppm). Additionally, as pigs became overloaded with Zn, there were significant reductions in serum Cu and both serum and hepatic Se. Hepatic and serum Zn concentrations >500 ppm and >2 ppm, respectively, are indicative of Zn overload, and dietary trace mineral analysis is warranted if expected inclusion rates are ≤3,000 ppm ZnO.
Keywords: growth promotion, swine, zinc oxide, zinc toxicity
Introduction
Zinc is an essential trace mineral that is required for protein production and growth in livestock and is an important component of enzymes involved in cellular replication, including DNA polymerase.2,20 Adequate zinc is required for optimal innate immune function, and deficiency can lead to increased susceptibility to bacterial infections and alterations in tight junctions.2,8 Pharmacologic concentrations of zinc oxide (ZnO; 2,000–3,000 ppm) are commonly fed to newly weaned pigs in many parts of the world for various durations to control diarrhea and improve growth.4,12 However, the mechanism of action for observed growth effects is not clearly understood,19 and there is significant discord in the literature concerning the actual effects of supplementation, with multiple reports showing increases in average daily gain (ADG),4,10,12,15 whereas others show no effect.3,18,27 Differences in study design and animal numbers as well as pig genetics, intestinal microbial populations, and other environmental factors may contribute to this discord.
Common protocols employed in the United States include feeding 3,000 ppm ZnO for 1 wk followed by 2,000 ppm for 2 additional weeks in the nursery (Smith JW II, et al. The effects of dietary mineral regimen on starter pig growth performance and blood and immune parameters. Proc Kansas State University Swine Day. Report of Progress 746; Nov 1995; Manhattan, KS. Available from: http://hdl.handle.net/2097/3135). The safety of this short-term, high-dose therapeutic treatment is assumed based upon the lower bioavailability of ZnO relative to other forms of Zn commonly included in feed10,27 and the reversibility of tissue accumulation after ZnO withdrawal.14 However, if diet manufacturing errors occur and supra-pharmacologic concentrations of ZnO are fed inadvertently, Zn toxicity may occur.
Zn homeostasis is regulated at multiple locations in the gastrointestinal tract, and there is an inverse relationship between metallothionein (MT) synthesis and Zn absorption.1 Approximately 20–30% of cytosolic Zn is bound to MT, which thereby restricts transfer from the intestinal lumen to the portal circulation.1 High dietary Zn increases MT production in enterocytes, which then limits systemic Zn absorption through intracellular retention until enterocytes are sloughed.20 Intestinal MT binds Cu preferentially to Zn, and may sequester Cu, leading to potential Cu deficiency when pharmacologic concentrations of Zn are fed, if dietary Cu concentrations are inadequate.13 The pancreas is involved in Zn excretion, and excretion increases in periods of Zn excess.6 Fecal excretion is the primary method of Zn elimination in supplemented pigs,22 and excretion is increased after tissue loading occurs, which is typically during the first 9–11 d.18,26 Like many heavy metals, Zn will accumulate in the soil and may potentially cause serious environmental pollution, through groundwater and surface water contamination, particularly in areas of intensive pig farming where pigs are fed and excreting high concentrations of Zn.7
Reported clinical signs of Zn toxicity in swine include reduced rate of gain, reduced feed efficiency, depression, and lameness24,25; however, limited data are available regarding the specific serum and tissue levels of Zn expected in animals experiencing overload following excessive ZnO administration that might occur during a feed manufacturing error. Accordingly, our objectives were to characterize the effects of supra-pharmacologic dietary ZnO concentrations on early-weaned nursery pig performance parameters, as well as serum, liver, and pancreatic concentrations of Zn. Furthermore, we aimed to assess alterations in concentrations of other specific trace minerals (Cu, Fe, and Se) and any pathologic changes detectable by histopathology. In aggregate, these data will serve as reference values for veterinary diagnostic purposes where Zn overload is a concern.
Material and methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of Iowa State University (IACUC 1-18-8696-S). Fifty-five 3-wk-old pigs (mean ± SEM; 4.50 ± 0.21 kg BW; F25 sows × 6.0 sires; Genetiporc, PIC, Hendersonville, TN) were randomly allocated across 5 treatments (n = 11) and housed individually in pens in 1 of 2 rooms. All pigs had previously received 200 mg of iron (gleptoferron; Ceva Biomune, Lenexa, KS) by IM injection when 3 d old. Each room had pens of identical size (122 × 42 × 61 cm [48 × 16.5 × 24 in]) and had an identical layout. The rooms were maintained at a 12/12 h light/dark cycle, and were temperature-controlled within the thermoneutral zone of the pigs for the duration of the experiment. Individual pens were equipped with a nipple waterer and feeder.
Experimental diets
Pigs were fed a mash diet in 2 phases: phase 1 consisted of 0–7 d post-weaned; phase 2 consisted of 8–21 d post-weaned. All diets were formulated to meet or exceed National Research Council21 estimates of requirements of weaned pigs and did not contain antimicrobials (Supplementary Table 1). The phase 1 basal diet was formulated and mixed to contain 0, 3,000, or 6,000 ppm Zn using ZnO. The phase 2 basal diet was formulated and mixed to contain 0, 2,000, 4,000, or 6,000 ppm Zn using ZnO. Using these diets, 5 treatment groups were constructed to mimic industry scenarios: group 1 = negative control (NC; phase 1 and phase 2 containing 0 ppm ZnO); group 2 = 3,000 ppm ZnO in phase 1 then 0 ppm ZnO in phase 2 (ZnO3-NC); group 3 = 3,000 ppm ZnO in phase 1 then 2,000 ppm ZnO in phase 2 (ZnO3-2); group 4 = 6,000 ppm ZnO in phase 1 then 4,000 ppm ZnO in phase 2 (ZnO6-4); or group 5 = 6,000 ppm ZnO in phase 1 and 6,000 ppm ZnO in phase 2 (ZnO6).
Animal observations and performance assessments
Nursery pig performance and clinical observations were recorded daily for the 21-d study duration to determine visual signs of Zn overload including a rough hair coat and loss of physical condition. Individual body weight was measured at d 0, 7, 14, and 21 by weighing each pig in a calibrated scale cart (Mosdal Scale Systems, Lanesboro, MN) for determination of ADG (kg/d). Feeders were weighed daily on a Mosdal scale cart to determine the average daily feed intake (ADFI, kg/d) by feed disappearance. Fresh feed was added and recorded as needed, approximately every 2–3 d. Feed efficiency was determined by the ratio of daily gain to daily feed intake (G:F). All performance parameters were collected and assessed by the same person.
Autopsy and tissue sampling
At d 21, all pigs were euthanized via captive bolt followed by exsanguination and immediately subjected to autopsy evaluation. The gastric mucosa was inspected for evidence of gastritis, ulceration, or thickening of the pars esophagea. Samples of liver and pancreas were collected for trace mineral analysis, and sections of liver, pancreas, stomach, duodenum, jejunum, ileum, and colon were placed in 10% neutral-buffered formalin for routine processing, sectioning, and hematoxylin and eosin staining. Sections of duodenum were collected 15 cm after the pylorus; jejunum from the approximate midpoint of the intestine; and ileum from 15 cm proximal to the cecum. Sections of colon were collected from the apex of the spiral colon.
Trace mineral analyses and hematology
Serum and whole blood (EDTA) samples (5-mL vacutainer tubes; BD, Franklin Lakes, NJ) were collected from all pigs via jugular venipuncture on days 0, 7, 14, and 21. For serum collection, blood samples were allowed to clot at room temperature, centrifuged (2,000 × g for 10 min at 4°C), and the serum was removed and stored at −80°C until submission to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) for routine trace mineral analysis. The EDTA blood samples were submitted to the ISU-VDL within 2 h of collection for complete blood count by the laboratory’s standard operating procedures (SOPs). Day 21 autopsy samples of liver and pancreas were also submitted for routine trace mineral analysis at the ISU-VDL. For feed and tissue trace mineral analysis (Table 1), samples were analyzed for Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Na, P, K, Se, and Zn using inductively coupled plasma–mass spectrometry (ICP-MS; Analytik Jena, Woburn, MA) in CRI mode with hydrogen as the skimmer gas. Serum samples were analyzed for Ca, Cu, Fe, Mg, Mn, Mo, P, K, Se, and Zn. Standards for elemental analyses were obtained from Inorganic Ventures (Christiansburg, VA); digestion vessels, trace mineral grade nitric acid, and hydrochloric acid were obtained from Fisher Scientific (Pittsburgh, PA). Fresh tissue and feed samples were processed and analyzed following the established SOPs of the laboratory, on a wet weight basis. Briefly, 1.0-g samples were weighed into 50-mL digestion tubes to which 10 mL of 70% nitric acid was added, and samples were placed in a microwave digester. After digestion, all samples were filtered twice (Whatman #40 filter paper; GE Healthcare Life Sciences, Buckinghamshire, UK) and diluted to 25 mL using 18 MΩ water. A final 1:10 dilution using 1% nitric acid was prepared and analyzed by ICP-MS. For serum, 0.25 mL of sample was diluted 1:20 using 1% nitric acid and analyzed by ICP-MS. For quality control, Bi, Sc, In, Li, Y, and Tb were used as internal standards for the ICP-MS. Instrument precision is assessed by the laboratory using reference samples, internal controls, and ongoing statistical process control charting. Trace mineral results are reported as ppm on a wet-weight basis after digestion.
Table 1.
Trace mineral analysis (ppm) of diets (as-fed basis).
| Treatment group | |||||
|---|---|---|---|---|---|
| 1 (NC) | 2 (ZnO3-NC) | 3 (ZnO3-2) | 4 (ZnO6-4) | 5 (ZnO6) | |
| Phase 1 diets (d–7) | |||||
| Cadmium | 0.053 | 0.064 | 0.064 | 0.099 | 0.099 |
| Calcium | 7,600 | 8,070 | 8,070 | 6,730 | 6,730 |
| Chromium | 1 | 3.06 | 3.06 | 4.08 | 4.08 |
| Cobalt | 0.137 | 0.176 | 0.176 | 0.165 | 0.165 |
| Copper | 23 | 23 | 23 | 20 | 20 |
| Iron | 141 | 462 | 462 | 503 | 503 |
| Magnesium | 1,040 | 1,080 | 1,080 | 1,140 | 1,140 |
| Manganese | 58.3 | 98.5 | 98.5 | 86.3 | 86.3 |
| Molybdenum | 0.65 | 0.89 | 0.89 | 0.99 | 0.99 |
| Phosphorus | 4,840 | 5,840 | 5,840 | 5,860 | 5,860 |
| Potassium | 7,250 | 7,490 | 7,490 | 7,890 | 7,890 |
| Selenium | 0.51 | 0.59 | 0.59 | 0.55 | 0.55 |
| Sodium | 2,320 | 2,340 | 2,340 | 2,360 | 2,360 |
| Zinc | 159 | 3,820 | 3,820 | 6,140 | 6,140 |
| Phase 2 diets (d 8–21) | |||||
| Cadmium | 0.097 | 0.097 | 0.11 | 0.097 | 0.117 |
| Calcium | 10,500 | 10,500 | 10,110 | 6,200 | 6,320 |
| Chromium | 2.33 | 2.33 | 2.78 | 3.31 | 4.19 |
| Cobalt | 0.217 | 0.217 | 0.22 | 0.17 | 0.191 |
| Copper | 22 | 22 | 26 | 18 | 18 |
| Iron | 415 | 415 | 223 | 420 | 508 |
| Magnesium | 1,330 | 1,330 | 1,340 | 1,220 | 1,250 |
| Manganese | 84.1 | 84.1 | 73.7 | 61.7 | 72.1 |
| Molybdenum | 1.12 | 1.12 | 1.11 | 0.94 | 1 |
| Phosphorus | 7,287 | 7,290 | 6,840 | 6,730 | 7,040 |
| Potassium | 8,490 | 8,490 | 8,560 | 7,580 | 7,820 |
| Selenium | 0.71 | 0.71 | 0.66 | 0.54 | 0.53 |
| Sodium | 2,540 | 2,540 | 2,430 | 1,980 | 2,280 |
| Zinc | 246 | 246 | 2,580 | 3,260 | 5,700 |
NC = negative control.
Histopathology
Sections of liver, stomach, pancreas, and colon were examined by a veterinary pathologist (ER Burrough) blinded to treatment group and scored semi-quantitatively. For liver, 0 = normal, 1 = mild hepatocellular vacuolation affecting <50% of hepatocytes, 2 = moderate vacuolation affecting 50–75% of hepatocytes, and 3 = severe vacuolation affecting >75% of hepatocytes often with periportal inflammation. For stomach, 0 = normal, 1 = mild multifocal lymphocytic infiltration, 2 = moderate multifocal lymphocytic infiltration, and 3 = severe lymphocytic infiltration. For pancreas, 0 = normal with rare acinar cell apoptosis, 1 = mild multifocal cytoplasmic vacuolation with rare-to-scattered acinar cell apoptosis, 2 = moderate multifocal cytoplasmic vacuolation with scattered acinar cell apoptosis, and 3 = severe multifocal cytoplasmic vacuolation and frequent acinar cell apoptosis. For colon, 0 = normal, 1 = mild lymphocytic infiltration of the lamina propria, 2 = moderate lymphocytic infiltration of the lamina propria, and 3 = severe lymphocytic infiltration of the lamina propria and neutrophilic exudation into crypt lumens.
In sections of small intestine, villus length (μm) and crypt depth (μm) in the duodenum, jejunum, and ileum were assessed by counting 5 well-oriented villus-crypt units per section and computing the mean for each segment from each animal.
Statistical analyses
All analyses were performed using a commercial statistical software package (JMP Pro 11; SAS Institute, Cary, NC). Distribution of data was assessed using a normal quantile plot. Trace mineral values, intestinal villus measurements, and performance parameters (ADG, ADFI, and G:F) were compared using a one-way ANOVA with Tukey–Kramer adjustment for normally distributed data and with a nonparametric Steel–Dwass test if normality was not met. For changes in serum trace minerals and hematologic parameters over time, a Wilcoxon signed rank test was performed. Semi-quantitative histology scores were compared using the Fisher exact test, and p ≤ 0.05 was considered statistically significant.
Results
Growth performance, feed efficiency, and autopsy findings
No statistically significant growth performance differences were detected between groups (Table 2). At autopsy, a few pigs had evidence of serous atrophy of cardiac fat that was either mild (n = 7; 3 in group 2, 2 in group 4, and 2 in group 5) or severe (n = 2; 1 in each of groups 4 and 5); however, there was no statistical association with dietary treatment or presence of feed in the stomach. Similarly, multifocal congestion of the gastric mucosa and variable hyperkeratosis of the pars esophagea was observed in a few pigs (n = 10; 3 in group 2, 2 in group 3, 1 in group 4, and 4 in group 5) but this was not associated with dietary treatment.
Table 2.
Nursery pig growth performance parameters (d 0–21).
| Unit | Treatment group | ||||||
|---|---|---|---|---|---|---|---|
| 1 (NC) | 2 (ZnO3-NC) | 3 (ZnO3-2) | 4 (ZnO6-4) | 5 (ZnO6) | |||
| ADG | kg/d | 0.152 ± 0.022 | 0.175 ± 0.016 | 0.210 ± 0.025 | 0.203 ± 0.023 | 0.165 ± 0.025 | p > 0.39 |
| ADFI | kg/d | 0.222 ± 0.017 | 0.227 ± 0.021 | 0.259 ± 0.021 | 0.255 ± 0.020 | 0.250 ± 0.024 | p > 0.72 |
| G:F | 0.670 ± 0.064 | 0.801 ± 0.080 | 0.792 ± 0.046 | 0.772 ± 0.036 | 0.641 ± 0.055 | p > 0.30 | |
ADFI = average daily feed intake; ADG = average daily gain; G:F = ratio of gain to feed consumed; NC = negative control. Values represent mean ± SEM.
Trace minerals and hematology
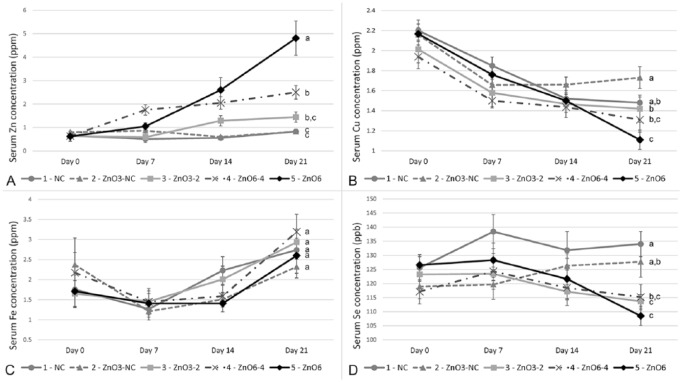
At d 0, all groups had mean serum Zn levels within the marginal range of 0.40–0.80 ppm based on reference concentrations for pigs (Fig. 1).24 By the end of phase 1 (d 7), significant differences in mean serum Zn were observed compared to controls for pigs in groups receiving 6,000 ppm ZnO (groups 4 and 5; p = 0.002 and 0.032, respectively), with both groups having mean concentrations >1.0 ppm and within or exceeding what is reported as the adequate range of 0.70–1.50 ppm.24 On d 14, significant differences in mean serum Zn were again observed compared to controls (groups 3–5; p = 0.045, 0.001, and 0.005, respectively), with pigs in groups receiving 6,000 ppm and 4,000 ppm ZnO (groups 5 and 4, respectively) in phase 2 (d 7–21) having mean concentration >2.0 ppm and within what is reported as a toxic range of 1.40–3.30 ppm.24 At d 14, pigs receiving 2,000 ppm ZnO (group 3) had mean concentration within the adequate range, whereas the 2 groups (1 and 2) with no supplemental ZnO in the phase 2 diet had mean serum Zn concentration within the marginal range.
Figure 1.
Serum trace mineral concentrations over time. Graphs reflect mean serum concentrations (± SEM) of zinc (A), copper (B), iron (C), and selenium (D) by dietary treatment at days 0, 7, 14, and 21. At 21 d, concentrations not connected by the same letter are significantly different (p ≤ 0.05). Treatment groups: 1 (negative control [NC]; phase 1 and phase 2 containing 0 ppm ZnO); 2 (ZnO3-NC; 3,000 ppm ZnO in phase 1, then 0 ppm ZnO in phase 2); 3 (ZnO3-2; 3,000 ppm ZnO in phase 1, then 2,000 ppm ZnO in phase 2); 4 (ZnO6-4; 6,000 ppm ZnO in phase 1, then 4,000 ppm ZnO in phase 2); 5 (ZnO6; 6,000 ppm ZnO in phase 1, then 6,000 ppm ZnO in phase 2).
At the end of the study (d 21), mean serum Zn concentration was significantly higher in pigs receiving 6,000 ppm ZnO for 3 wk (group 5; mean ± SEM; 4.81 ± 2.31 ppm) compared to all other groups (p < 0.001). The mean increase in serum Zn from d 0 to d 21 was also significantly greater for the ZnO 6,000 ppm group than all other groups (p < 0.001). At d 21, mean serum Zn concentration for the 2 groups receiving ZnO at 4,000 or 6,000 ppm (groups 4 and 5, respectively) were in the toxic range, whereas the mean serum Zn concentration for the other 3 groups were all within the adequate range (0.70–1.50 ppm).
Serum Cu concentrations were significantly lower at d 21 for the 2 groups receiving the highest levels of ZnO (groups 4 and 5) compared with the groups receiving no ZnO (groups 1 and 2) in the phase 2 diet (p < 0.03, all analyses). The mean decrease in serum Cu from d 0 to d 21 was also significantly greater for group 5 than all other groups (p < 0.01). Serum Se concentrations were also significantly lower at d 21 for the 3 groups (groups 3–5) receiving the highest concentrations of ZnO compared with the groups receiving no ZnO in the phase 2 diet (p < 0.04, all analyses). The mean decrease in serum Se from d 0 to d 21 was also significantly greater for the ZnO 6,000 ppm group than all other groups (p < 0.001). No significant differences in serum Fe concentrations were detected over the course of the study.
Only pigs receiving ZnO at 6,000 ppm for one or more weeks (groups 4 and 5) had hepatic Zn concentrations >500 ppm or serum concentrations >2.0 ppm at autopsy (Table 3). Pigs receiving 6,000 ppm ZnO in either phase (groups 4 and 5) had higher mean pancreatic Zn concentrations relative to all other treatments (p ≤ 0.008, all analyses; Table 3). A significant difference in hepatic Se was also detected between group 1 and groups 3 and 4 (p < 0.001, all analyses), with the highest mean concentrations in the non-supplemented NC pigs (group 1; mean ± SEM; 0.538 ± 0.020 ppm) and the lowest mean concentrations in the group 3 pigs receiving the typical industry treatment of 3,000 ppm followed by 2,000 ppm ZnO (0.411 ± 0.020 ppm). No differences in mean hepatic Cu and Fe concentrations were detected among any of the 5 treatment groups (p > 0.12, all analyses).
Table 3.
Zinc concentrations at the end of phase 2 diet (d 21) by treatment.
| Treatment group | |||||
|---|---|---|---|---|---|
| 1 (NC) | 2 (ZnO3-NC) | 3 (ZnO3-2) | 4 (ZnO6-4) | 5 (ZnO6) | |
| Liver | |||||
| Min. | 29 | 36 | 104 | 194 | 312 |
| Mean | 39.2 ± 10.7a | 60.7 ± 26.7a | 347 ± 117b | 520 ± 245b,c | 729 ± 264c |
| Max. | 59 | 115 | 490 | 1117 | 1120 |
| Pancreas | |||||
| Min. | 17.9 | 19.7 | 65.8 | 117.5 | 161.8 |
| Mean | 21.4 ± 3.3a | 32.1 ± 10.8a | 136 ± 53a | 472 ± 320b | 670 ± 375b |
| Max. | 25.6 | 55.8 | 216.6 | 1144 | 1344 |
| Serum | |||||
| Min. | 0.7 | 0.5 | 0.9 | 0.8 | 1.4 |
| Mean | 0.83 ± 0.13a | 0.83 ± 0.32a | 1.44 ± 0.36a,b | 2.39 ± 0.94b | 4.81 ± 2.31c |
| Max. | 1.1 | 1.7 | 2.0 | 4.0 | 8.7 |
NC = negative control. Mean values represent mean ± SEM. Within row means with differing superscripts (a,b,c) indicate a significant difference (p ≤ 0.05).
Statistically significant reductions in both hematocrit and hemoglobin values were detected over the course of the study (p < 0.001 and p = 0.006, respectively; Table 4); however, these reductions were not specific for a particular group.
Table 4.
Hematology summaries (d 0–21) by treatment.
| Treatment group | ||||||
|---|---|---|---|---|---|---|
| 1 (NC) | 2 (ZnO3-NC) | 3 (ZnO3-2) | 4 (ZnO6-4) | 5 (ZnO6) | ||
| HCT d 0 | 0.401 ± 0.008 | 0.395 ± 0.012 | 0.400 ± 0.006 | 0.432 ± 0.011 | 0.368 ± 0.049 | |
| HCT d 21 | 0.369 ± 0.016 | 0.369 ± 0.008 | 0.381 ± 0.011 | 0.371 ± 0.008 | 0.354 ± 0.021 | |
| Mean ∆ HCT* | −0.021 | −0.031 | −0.021 | −0.057 | −0.021 | p < 0.001 |
| Hb d 0 | 117 ± 3 | 114 ± 3 | 117 ± 3 | 124 ± 3 | 109 ± 14 | |
| Hb d 21 | 111 ± 4 | 111 ± 2 | 114 ± 3 | 110 ± 2 | 104 ± 7 | |
| Mean ∆ Hb* | −3.0 | −3.6 | −3.6 | −10.5 | −6.8 | p = 0.006 |
Hb = hemoglobin (g/L); HCT = hematocrit (L/L); NC = negative control. Values represent mean ± SEM.
Values represent matched pairs analysis (Wilcoxon signed rank test). A significant decrease was observed for both HCT and Hb over 21 d for all groups; however, significant differences between treatments were not observed.
Histopathology (pancreas and intestinal morphology assessments)
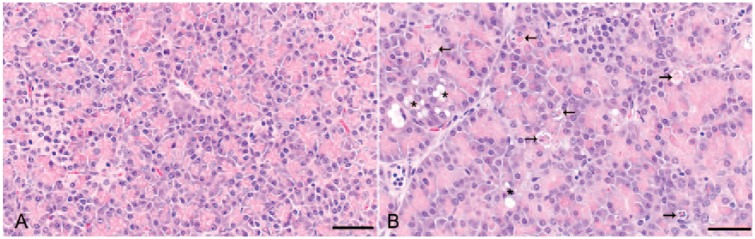
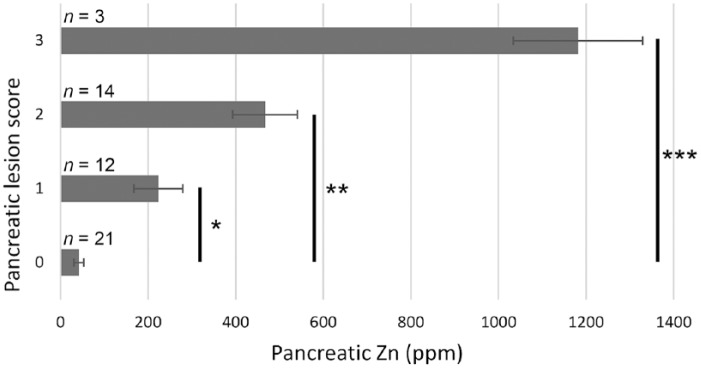
Pancreatic lesions scoring 2 or 3 were only observed in sections from pigs still receiving ZnO in the phase 2 diets (groups 3–5; Fig. 2). Additionally, acinar cells in samples scoring 2 or 3 often were enlarged with more abundant cytoplasm than control tissues. A significant difference in pancreatic acinar cell apoptosis scores by treatment group was observed (p < 0.0001), with pigs receiving the 2 highest ZnO treatment concentrations (groups 4 and 5) having the greatest frequency of lesions. Pancreatic lesion scores were also positively correlated with pancreatic tissue Zn concentrations (R2 = 0.7333), and mean pancreatic Zn concentrations for all samples at each semiquantitative lesion score were also significantly different (Fig. 3; p < 0.05, all analyses).
Figure 2.
A. Normal pancreas from an 8-wk-old pig receiving no dietary ZnO. B. Pancreas from an 8-wk-old pig receiving 6,000 ppm ZnO for 21 d. Note multifocal cytoplasmic vacuolation (asterisks), often more abundant acinar cell cytoplasm, and frequent acinar cell apoptosis (arrows). H&E. Bars = 50 µm.
Figure 3.
Mean pancreatic Zn concentration by pancreatic lesion score at 21 d. * = p < 0.05; ** = p < 0.01; *** = p < 0.0001.
Colitis lesions with scores of 2 or 3 were only observed in sections from pigs without ZnO in the phase 2 diets (groups 1 and 2), and no colitis lesions were observed in sections from pigs in treatment group 5. The observed difference in colitis lesion rates between groups was statistically significant (p = 0.028). Mild-to-moderate hepatocyte vacuolation and lymphocytic gastritis were observed; however, there were no significant associations with dietary treatment group (p > 0.3, all analyses).
Mean villus length was only statistically significant in the jejunum, with group 5 pigs receiving 6,000 ppm ZnO having significantly longer villi (p < 0.03, all comparisons; Table 5). Crypt depths did not differ significantly in any intestinal sections. As a result, group 5 pigs had the highest V:C ratio in the jejunum segments compared to all other groups (p = 0.007; Table 5).
Table 5.
Comparison of intestinal villus height and crypt depth by treatment after 21 d.
| Treatment group | ||||||
|---|---|---|---|---|---|---|
| 1 (NC) | 2 (ZnO3-NC) | 3 (ZnO3-2) | 4 (ZnO6-4) | 5 (ZnO6) | ||
| Duodenum | ||||||
| Villus height | 742 ± 27 | 762 ± 45 | 811 ± 40 | 892 ± 42 | 824 ± 35 | p = 0.056 |
| Crypt depth | 205 ± 10 | 197 ± 8 | 218 ± 14 | 219 ± 10 | 215 ± 9 | p = 0.519 |
| V:C ratio | 3.66 ± 0.14 | 3.92 ± 0.27 | 3.77 ± 0.16 | 4.11 ± 0.15 | 3.90 ± 0.25 | p = 0.597 |
| Jejunum | ||||||
| Villus height | 639 ± 29a | 595 ± 28a | 678 ± 27a | 675 ± 28a | 804 ± 29b | p < 0.001 |
| Crypt depth | 163 ± 7 | 152 ± 5 | 169 ± 9 | 172 ± 5 | 169 ± 7 | p = 0.229 |
| V:C ratio | 3.94 ± 0.12a | 3.96 ± 0.24a | 4.09 ± 0.22a,b | 3.91 ± 0.07a | 4.81 ± 0.25b | p = 0.007 |
| Ileum | ||||||
| Villus height | 500 ± 26 | 462 ± 18 | 495 ± 25 | 493 ± 25 | 511 ± 25 | p = 0.672 |
| Crypt depth | 146 ± 6 | 138 ± 6 | 135 ± 6 | 134 ± 5 | 136 ± 6 | p = 0.562 |
| V:C ratio | 3.43 ± 0.11 | 3.35 ± 0.11 | 3.67 ± 0.10 | 3.72 ± 0.16 | 3.77 ± 0.16 | p = 0.134 |
NC = negative control; V:C ratio = the ratio of villus height (μm) to crypt depth (μm). Values represent mean ± SEM. Means with differing superscripts (a,b) indicate a significant difference (p ≤ 0.05).
Discussion
In the United States, pharmacologic Zn concentrations are achieved using ZnO that is often included in phase 1 nursery diets at 3,000 ppm for 1 wk followed by a reduction to 2,000 ppm in phase 2 diets for 2 additional weeks. Accordingly, we used this feeding regimen but did not observe statistically significant differences in ADG, ADFI, and G:F compared to controls.
One potential mechanism for the reported growth-promoting effects of pharmacologic concentrations of ZnO is rapid restoration of circulating Zn concentrations immediately following weaning transition of pigs. Weaning piglets at 21 d of age creates Zn deficiency, as detected by plasma concentrations, compared to unweaned pigs in the same litter, and this can be restored with supplemental ZnO post-weaning.5 All pigs in our study had mean serum Zn concentrations in the marginal range at d 0; the mean serum Zn concentration was rapidly elevated and restored to the adequate range by d 7 with 6,000 ppm ZnO, and by d 14 with 3,000 ppm ZnO followed by 2,000 ppm.
Another potential mechanism for the growth-promoting effects of ZnO is a reduction in enteric pathogens and improved gut health given that zinc sulfate has been shown to inhibit growth of many potential enteric bacterial pathogens in vitro.28 Feeding ZnO at 2,500 ppm has been shown to reduce post-weaning diarrhea,22 and this level of dietary supplementation has also been associated with improved stability of the intestinal microflora and overall diversity of coliforms during the first 2 wk post-weaning compared to controls.16 In our study, colitis was more often observed in pigs with no supplemental ZnO in the phase 2 diet, and there was no colitis observed in pigs receiving the highest levels of ZnO, suggesting a positive impact of supplementation at the mucosal level in the colon.
Increases in intestinal villus length and reductions in crypt depth have been demonstrated in pigs fed pharmacologic doses of Zn, and it has been suggested that this may lead to improved growth as a result of increased nutrient absorptive surface area in the small intestine.18 However, this effect of pharmacologic Zn has not been consistently observed across nursery pig studies, with others demonstrating no effects on intestinal morphology.11,19 Similarly, we only observed longer villi in the jejunum of pigs fed the highest concentration of Zn; this effect was not detected in other intestinal segments and was not linear across treatment concentration.
Compared with other commonly available forms of Zn, such as ZnSO4, ZnO is less bioavailable to pigs.29 Based on whole body retention, the bioavailability of ZnO is reportedly as low as 20%.23 In theory, this lower bioavailability reduces the likelihood of Zn toxicity and allows more Zn to remain in the intestinal lumen where it can exert antimicrobial effects. However, feed-grade sources of ZnO vary widely in color, texture, Zn content, and Zn bioavailability,9 and relative bioavailability values (RBV) of ZnO have been reported from 39% to 93%.19 Such variability in RBV is a potential concern for commercial diets in which inadvertently feeding a high RBV of ZnO could lead to excessive Zn accumulation and overload.
Zn toxicity commonly manifests as reduced rate of gain, anorexia, gastroenteritis, and lameness.24 Given the lower bioavailability of ZnO, chronic toxicity, or overloading, is far more likely than acute toxicosis. Hepatic accumulation of Zn is cumulative over time with continued exposure. Our study reveals a clear linear association between the level of supplemental ZnO in the diet and hepatic, pancreatic, and serum Zn concentrations. Although overt toxicosis was not observed, pigs fed the highest concentration of ZnO had increased frequency of pancreatic apoptosis as well as enlarged, often vacuolated, acinar cell cytoplasm compared to control pigs and those fed common U.S. industry concentrations of Zn in the first 3 wk post-weaning. This is similar to a study in which 4,000 ppm of ZnO did not induce overt toxicosis but did reduce ADG.22 Such findings suggest that the margin of safety for administration of pharmacologic concentrations of ZnO in feed may be lower than what is perceived, and shorter durations of administration may be warranted to reduce the potential for accumulation. Additionally, the significant reductions in serum Cu and Se concentrations as well as hepatic Se stores in our study reveal the potential for significant trace mineral imbalances associated with excessive ZnO feeding and the potential for the development of deficiencies in these minerals with continued feeding of high-dose Zn beyond 3 wk.
Reference values for Zn toxicity are difficult to define given the relatively high tolerance of swine for Zn and the vagueness of clinical signs, such as poor growth and inappetence. Reference texts indicate that Zn toxicity can be associated with liver concentrations >500 ppm wet weight and that values are considered high when >200 ppm.24 This aligns well with published reports in which pigs receiving 3,000 ppm ZnO for 4 wk or 4,000 ppm ZnO for 2 wk showed no signs of toxicity, and their mean hepatic Zn concentrations did not exceed 400 ppm.4,18
We found mean hepatic Zn concentrations >500 ppm only in pigs receiving ZnO at double, or more than double, the inclusion rate typical for U.S. feeding practices, and those pigs were the only ones to develop a high degree of pancreatic acinar cell apoptosis. Pancreatic acinar cell apoptosis has also been reported in Sprague Dawley rats exposed to chronic high concentrations of ZnO.17
Although a majority of pigs in a group may tolerate pharmacologic concentrations of dietary Zn in post-weaning diets, and increases in overall ADG for the group may be observed, individual pigs within the population may not tolerate such high concentrations and may become overloaded with Zn very quickly. These Zn-overloaded pigs may fall behind the group and are likely to be selected for diagnostic evaluation to detect etiologies related to their poor growth. Our data give specific parameters that are indicative of Zn overload and will assist veterinary diagnosticians in determining if poor-doing pigs may be over-supplemented with Zn. One limitation of our study is that the pigs were housed and fed individually. This may have masked group behavior dynamics leading to exclusion of weak pigs from the feeder as well as ear biting and other social stressors that may further reduce growth rates of affected pigs.
Supplemental Material
Supplemental material, DS1_VET_10.1177_0300985819852138 for Zinc overload in weaned pigs: tissue accumulation, pathology, and growth impacts by Eric R. Burrough, Carson De Mille and Nicholas K. Gabler in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary material: Supplementary material for this article is available online.
ORCID iD: Eric R. Burrough  https://orcid.org/0000-0003-4747-9189
https://orcid.org/0000-0003-4747-9189
References
- 1. Abdel-Mageed AB, Oehme FW. A review of the biochemical roles, toxicity and interactions of zinc, copper and iron: I. Zinc. Vet Hum Toxicol 1990;32:34–39. [PubMed] [Google Scholar]
- 2. Amasheh M, et al. Barrier effects of nutritional factors. Ann N Y Acad Sci 2009;1165:267–273. [DOI] [PubMed] [Google Scholar]
- 3. Bondzio A, et al. Feeding low or pharmacological concentrations of zinc oxide changes the hepatic proteome profiles in weaned piglets. PLoS One 2013;8:e81202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlson MS, et al. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J Anim Sci 1999;77:1199–1207. [DOI] [PubMed] [Google Scholar]
- 5. Davin R, et al. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J Anim Physiol Anim Nutr (Berl) 2013;97(Suppl. 1):6–12. [DOI] [PubMed] [Google Scholar]
- 6. De Lisle RC, et al. Metallothionein is a component of exocrine pancreas secretion: implications for zinc homeostasis. Am J Physiol 1996;271:C1103–C1110. [DOI] [PubMed] [Google Scholar]
- 7. Dębski B. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol J Vet Sci 2016;19:917–924. [DOI] [PubMed] [Google Scholar]
- 8. Djoko KY, et al. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 2015;290:18954–18961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards HM, Baker DH. Bioavailability of zinc in several sources of zinc oxide, zinc sulfate, and zinc metal. J Anim Sci 1999;77:2730–2735. [DOI] [PubMed] [Google Scholar]
- 10. Hahn JD, Baker DH. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci 1993;71:3020–3024. [DOI] [PubMed] [Google Scholar]
- 11. Hedemann MS, et al. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J Anim Sci 2006;84:3310–3320. [DOI] [PubMed] [Google Scholar]
- 12. Hill GM, et al. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J Anim Sci 2000;78:1010–1016. [DOI] [PubMed] [Google Scholar]
- 13. Hill GM, et al. A copper deficiency in neonatal pigs induced by a high zinc maternal diet. J Nutr 1983;113:867–872. [DOI] [PubMed] [Google Scholar]
- 14. Janczyk P, et al. Effect of high dietary zinc oxide on the caecal and faecal short-chain fatty acids and tissue zinc and copper concentration in pigs is reversible after withdrawal of the high zinc oxide from the diet. J Anim Physiol Anim Nutr (Berl) 2015;99(Suppl. 1):13–22. [DOI] [PubMed] [Google Scholar]
- 15. Jensen-Waern M, et al. Dietary zinc oxide in weaned pigs—effects on performance, tissue concentrations, morphology, neutrophil functions and faecal microflora. Res Vet Sci 1998;64:225–231. [DOI] [PubMed] [Google Scholar]
- 16. Katouli M, et al. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol 1999;87:564–573. [DOI] [PubMed] [Google Scholar]
- 17. Kim YR, et al. Toxicity of 100 nm zinc oxide nanoparticles: a report of 90-day repeated oral administration in Sprague Dawley rats. Int J Nanomedicine 2014;9(Suppl. 2):109–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martínez MM, et al. Dietary pharmacological or excess zinc and phytase effects on tissue mineral concentrations, metallothionein, and apparent mineral retention in the newly weaned pig. Biol Trace Elem Res 2005;105:97–115. [DOI] [PubMed] [Google Scholar]
- 19. Mavromichalis I, et al. Growth-promoting efficacy in young pigs of two sources of zinc oxide having either a high or a low bioavailability of zinc. J Anim Sci 2000;78:2896–2902. [DOI] [PubMed] [Google Scholar]
- 20. McClain CJ. The pancreas and zinc homeostasis. J Lab Clin Med 1990;116:275–276. [PubMed] [Google Scholar]
- 21. National Research Council. Nutrient Requirements of Swine. 11th revised ed. Washington, DC: National Academies Press, 2012. [Google Scholar]
- 22. Poulsen HD. Zinc oxide for weanling piglets. Acta Agric Scand A Anim Sci 1995;45:159–167. [Google Scholar]
- 23. Poulsen HD, Larsen T. Zinc excretion and retention in growing pigs fed increasing levels of zinc oxide. Livest Prod Sci 1995;43:235–242. [Google Scholar]
- 24. Puls R. Mineral Levels in Animal Health: Diagnostic Data. Clearbrook, BC: Sherpa International, 1994. [Google Scholar]
- 25. Reese DE, Miller PS. Nutrient deficiencies and excesses. In: Zimmerman JJ, et al., eds. Diseases of Swine. 10th ed. Ames, IA: Wiley-Blackwell, 2012:923–937. [Google Scholar]
- 26. Rincker MJ, et al. Effects of dietary zinc and iron supplementation on mineral excretion, body composition, and mineral status of nursery pigs. J Anim Sci 2005;83:2762–2774. [DOI] [PubMed] [Google Scholar]
- 27. Schell TC, Kornegay ET. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine, Zn-lysine, or ZnSO4. J Anim Sci 1996;74:1584–1593. [DOI] [PubMed] [Google Scholar]
- 28. Surjawidjaja JE, et al. Growth inhibition of enteric pathogens by zinc sulfate: an in vitro study. Med Princ Pract 2004;13:286–289. [DOI] [PubMed] [Google Scholar]
- 29. Wedekind KJ, et al. Bioavailability of zinc from inorganic and organic sources for pigs fed corn-soybean meal diets. J Anim Sci 1994;72:2681–2689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_VET_10.1177_0300985819852138 for Zinc overload in weaned pigs: tissue accumulation, pathology, and growth impacts by Eric R. Burrough, Carson De Mille and Nicholas K. Gabler in Journal of Veterinary Diagnostic Investigation