Abstract
Background: A potential evidence from previous epidemiological studies remains conflicting findings regarding the association between atrial fibrillation (AF) and dementia risk. We, therefore, carried out a meta-analysis of relevant studies to investigate the magnitude of the association between AF and dementia risk.
Methods: We performed a systematic literature search of PubMed, EMBASE, and Google Scholar for potential studies between January 1, 1990, and December 31, 2018, with no restriction on the publication language. All potential studies were independently assessed by two reviewers. We only included observational studies that calculated the odds ratio (OR)/hazards ratio (HR) for dementia associated with atrial fibrillation. We first assessed the heterogeneity among study-specific HRs using the Q statistic and I2 statistic. We then used the random-effects model to obtain the overall HR and its 95% CI for all studies. We also tested and corrected for publication bias by funnel plot–based methods. The quality of each study was assessed with the Newcastle Ottawa Scale.
Results: A total of 16 studies with 2,415,356 individuals, and approximately 200,653 cases of incidence dementia were included in this study. Patients with AF had a greater risk of incidence dementia than those without AF (random-effect hazard ratio HR: 1.36, 95% CI: 1.23–1.51, p < 0.0001; I2 = 83.58). Funnel plot and Egger test did not reveal significant publication bias. However, limitations of the study included high heterogeneity and varying degrees of confounder adjustment across individual studies.
Conclusion: This study serves as added evidence supporting the hypothesis that AF is associated with an increased risk of dementia. More studies are needed to establish whether optimal treatment of AF can reduce or mitigate the risk of dementia.
Keywords: atrial fibrillation, dementia, cardiac disease, cardiac arrhythmia, stroke, hypertension
Introduction
Atrial fibrillation (AF) is one of the most common cardiac arrhythmia (e.g., affecting approximately 2.7–6.1 million people in the United States of America) (January et al., 2014; Mou et al., 2018), and is characterized by irregular RR intervals and the absence of distinct P waves (European Heart Rhythm Association et al., 2010). The prevalence of AF is expected to rise further in the near future (Heeringa, 2010). AF has been traditionally considered to be a potential risk factor of ischemic stroke, thromboembolism, heart failure, myocardial infarction, and death (You et al., 2012; Piccini et al., 2013). Although a handful of epidemiological studies reported that the risk of vascular dementia always increases after an ischemic insult, there are still knowledge gaps about AF related cognitive decline and dementia beyond aging and stroke (Gorelick et al., 2011; Ding and Qiu, 2018). In a meta-analysis, Kalantarian et al. (2013) reported that AF is associated with a higher risk of cognitive impairment and dementia (~40%), with or without a history of clinical stroke. Similarly, Santangeli et al. (2012) mentioned that patients with AF had a 1.42-fold increased risk of dementia compared to patients without AF.
Dementia is characterized by cognitive and behavioral deficits and several potential factors such as increasing age, apolipoprotein E gene isoform 4 (APOEε4), diabetes, dietary factors, traumatic brain injury, and multiple environmental factors are associated with incidence and progression of dementia (Polidori et al., 2012; Banik et al., 2015; Islam et al., 2016). However, it is now well-established that the link between AF and dementia is more complicated than previously suspected. These two diseases shared common modifiable risk factors, such as lifestyle factors (e.g., physical activity, smoking, and alcohol consumption), cardio-metabolic risk factors (e.g., overweight, hypertension, diabetics, high cholesterol) and pathophysiological pathways (e.g., systematic inflammation, cerebral small vessel diseases, cerebral hypo-perfusion) (Qiu and Fratiglioni, 2015; Dietzel et al., 2017). In addition, evidence from in-vivo studies suggests that long-term AF diminishes cardiac output and may precede and/or promote chronic cerebral hypo-perfusion and hypoxia. Therefore, it impairs the clearance and enhance the accumulation of amyloid-β peptides collection in cerebral vessels, and thus ultimately increases the risk of Alzheimer's disease (AD) (Bell and Zlokovic, 2009; Ihara and Washida, 2018). Moreover, previous studies showed that inflammation markers like C-reactive protein, interleukin (IL)-2, IL-6, IL-8, and monocyte chemoattractant protein (MCP)-1 are always incorporated with hyper-coagulation and endothelial dysfunction that finally lead to AF-related thromboembolism formation (Friedrichs et al., 2011). However, a higher level of systemic inflammations is responsible for blood-brain barrier (BBB) damage and cerebral microstructural changes; thus, it ultimately contributes to cognitive decline and dementia in patients with AF (Takeda et al., 2014).
We herein report the results of a systematic review and meta-analysis of a higher number of epidemiological studies that explored the relationship between AF and dementia risk. Our aim was to gauge precisely the nature and magnitude of the association between AF and dementia risk. We also investigated dementia risk based on region, study design and duration of AF (≥5 years and <5 years). However, correctly evaluate the extent of the association between AF and dementia risk may have immense clinical inferences for the identification, prevention, and treatment of dementia.
Research Design and Methods
Registration of Review Protocol
The protocol for this systematic review was registered in advance with PROSPERO (International Prospective Register of Systematic Reviews, no. CRD42018117343).
Research Design
All the studies were included and excluded according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram recommended by the Cochrane library (Liberati et al., 2009). Since, only observational studies were considered for inclusion; we therefore considered the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for the meta-analysis of observational studies (Stroup et al., 2000; Poly et al., 2017; Wang et al., 2018) (Supplementary Table 1).
Literature Search
Initially, we systematically search in PubMed, Scopus, and Web of Science for relevant studies published between 1 January 1990 and 1 October 2018 using the free text term “atrial fibrillation” AND “dementia risk” OR “vascular dementia” OR “Alzheimer risk” (Supplementary Table 4). In addition, to ensure comprehensiveness, reference lists of retrieved studies and previous review articles were searched for additional relevant studies. Finally, widely used referencing software, EndNote X7 (Thomson Reuters) was applied to check duplication.
Study Selection Criteria
Two authors (MMI, TNP) who are expert in meta-analysis subsequently screened all the titles and abstracts for the studies included primarily in our meta-analysis. All the selected studies from this initial screening were then being considered for full-text review. Any disagreement during the screening stage was clarified by discussion with the other experts (HCY and CCW).
Studies were considered for inclusion in the meta-analysis if they fulfilled the following criteria:
-
(i). Types of studies
Observational design study such as cohort, case-control, or randomized control trial (RCT).
-
(ii). Types of participants
An adult (aged ≥ 18 years) with atrial fibrillation (AF).
Included studies that reported AF and dementia risk and AF inclusion was confirmed through ECG and physician's diagnosis (ICD-9/10 code), and other standard codes at baseline and each follow-up. Furthermore, for all individuals, it also needed to be confirmed that the presence and onset date of AF was prior to the baseline period and continued during the follow-up periods. A study considered all participants with AF at baseline to have prevalent AF, and also those who developed AF during the follow-up before the diagnosis of any kind dementia.
Included studies that reported AF and dementia risk and screened cognitive function using the Mini-Mental State Examination (MMSE) and also provided information regarding the dementia diagnosis based on the DSM-III revised or DSM-IV criteria validated 3-step procedure. We also included studies that examined vascular dementia and AD according to the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherch'e et l'Enseignement en Neurosciences (NINDS-AIREN) criteria, and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria, respectively.
Included studies that reported AF and dementia risk and dementia patients was identified by the International Classification of Diseases, Clinical Modification (ICD-9/10-CM) were also included in our study.
-
(iii). Type of intervention
AF patient for at least 1 year or longer. Any study with at least a 1 year follow-up period was considered for inclusion. A valid control group was healthy patients without AF and dementia.
-
(iv). Type of outcome
Primary outcome: Development of any kind of dementia in patients with AF.
Secondary outcome: Development of AD in patients with AF.
We excluded study that did not meet the following criteria: (1) study that was published as a case-report, editorial, review, or clinical trial; and (2) study that did not report dementia risk as their outcome.
Data Extraction
Data abstraction was conducted by MMI and TNP who used a predefined, standardized guidelines, and data collection procedures. They used the Review Manager software (RevMan-5) to check data accuracy. The following information was garnered from the included studies: (a) method: study design, data collection period, study duration, number of study centers and location, study setting, study protocol, inclusion and exclusion criteria; (b) participants: total number of participants, total number of dementia patients, mean age of participants, age range, gender, percentage of gender, diagnostic criteria; (c) interventions: intervention, comparison, concomitant medications, comorbidities; (d) outcome: primary and secondary findings. Afterward, MMI and TNP garnered the effect size data (HR with 95% CI) from all included observational studies. We resolved disagreements by discussing with the main investigator (YCL).
Assessment of Bias Risk
MMI and TNP utilized the Newcastle-Ottawa Scale (NOS) for evaluating the quality of each non-randomized study (cohort study). The NOS scale was used to address the participants selection, study comparability, and outcome or exposure assessment, with scores ranging from 6 to 9 (score 9 considered as no bias) (Stang, 2010) (Supplementary Table 2). Studies were then also assessed to be of good, fair, or poor quality (Donnelly et al., 2017). In addition, the methodological quality of the RCT was independently evaluated using the Cochrane collaboration's tool for measuring the potential risk of bias. The guidelines were also followed to determine whether trials took appropriate steps to minimize the risk of bias across six areas: (a) sequence generation, (b) allocation concealment, (c) blinding (study participants, personnel, and outcome), (d) incomplete outcome data, (e) selective outcome reporting, (f) other sources of bias. Finally, the risk of each study was classified into low, high, or unclear risk of bias (Higgins and Green, 2011). Moreover, the heterogeneity among study was determined by and statistic. Publication bias was also tested and corrected by the funnel plot-based method: Egger's regression test.
Subgroup Analyses
A comprehensive subgroup analyses was conducted with type of study design (cohort or RCT), continents (Europe or North America), and methodological quality (good or fair).
Statistical Analyses
The comprehensive meta-analysis software (CMA), version 3, was utilized to conduct statistical analyses. The overall hazard ratios (HRs) from individual studies were pooled using a random-effects model. Furthermore, forest plots were drawn to visually evaluate the results of pooling. AHR value > 1 indicates an increased risk of dementia, HR value 1 indicates no observed association, and HR value < 1 indicates a decreased risk of dementia. In case of statistical significance, p < 0.05. Furthermore, the heterogeneity in the results among the studies was calculated using the Higgins I2 which measures the percentage of the total variation across the included studies (Higgins and Thompson, 2002). The I2 was calculated as follows:
Here, Q is Cochran's heterogeneity statistic and df is the degree of freedom.
where w is the individual study weights and w*ES is the weighted effect size that is calculated by multiplying each effect size by the study weight. Negative values of I2 are considered equal to zero. The values of I2 therefore lies between 0 and 100%. A value of 0% indicates no observed heterogeneity. However, a value of I2 at 25–50%, 50–75%, and more than 75% is considered as mild, moderate, and severe heterogeneity (Higgins et al., 2003). Moreover, tau-squared (τ2) was also calculated to see the extent of variation among the effects observed in different studies (between-study variance). It represents the absolute value of the true variance (heterogeneity) (Deeks et al., 2008). The equation of calculating τ2 is given by:
A visual exploration of the funnel plot was shown to evaluate publication bias. A two-sided P < 0.05 was considered statistically significant.
Results
Study Screening
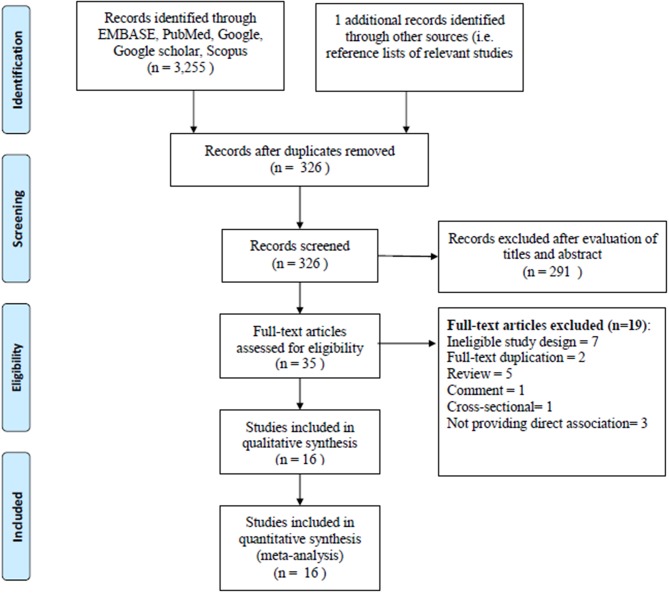
The literatures search of the electronic database yielded 3,256 articles. A total of 3,221 articles were excluded when we reviewed all the titles and abstracts; it is due to lack of adherence to our inclusion criteria. Thirty five articles were selected for the full-text revision and checked their reference lists for relevant articles, retrieving two additional articles. However, another 19 articles were excluded for non-adherence with the inclusion criteria. Furthermore, two articles were excluded for data overlap with another study. We also excluded three articles for not providing the associated risk of dementia with AF. Finally, 16 studies were included in our meta-analysis (Tilvis et al., 2004; Elias et al., 2006; Forti et al., 2006; Rastas et al., 2007; Peters et al., 2009; Bunch et al., 2010; Dublin et al., 2011; Marengoni et al., 2011; Marzona et al., 2012, 2016; Rusanen et al., 2014; de Bruijn et al., 2015; Liao et al., 2015; Singh-Manoux et al., 2017; Chen et al., 2018; Ding et al., 2018). The flow diagram of inclusion and exclusion information is presented in Figure 1.
Figure 1.
PRISMA flow diagram for study selection.
Study Characteristics
The study characteristics of the included 16 studies are presented in Table 1. Overall, 16 observational studies, comprising 2,415,356 individuals (200,653 dementia patients) were included in the meta-analysis. All the studies were published between 2004 (Tilvis et al., 2004) and 2018 (Chen et al., 2018). In this meta-analysis, 14 studies had a cohort study design (Tilvis et al., 2004; Elias et al., 2006; Forti et al., 2006; Rastas et al., 2007; Bunch et al., 2010; Dublin et al., 2011; Marengoni et al., 2011; Rusanen et al., 2014; de Bruijn et al., 2015; Liao et al., 2015; Marzona et al., 2016; Singh-Manoux et al., 2017; Chen et al., 2018; Ding et al., 2018) and two studies had an RCT design (Peters et al., 2009; Marzona et al., 2012). The mean age of patients for men and women was almost same; 20.43 to 100% of the sample population were men. The studies were conducted in diverse study populations with various comorbidities, and 10 studies included participants with type 2 diabetes; and hypertension (Supplementary Table 3). The minimum follow-up period ranged from 1.8 to 26.6 years. Moreover, most studies used standardized methods for identifying AF and dementia patients and had a low risk of bias. Most of the included studies were conducted in the Europe (Finland, Italy, Sweden, UK and Netherlands), four studies were carried out in the USA, and one in Asia (Taiwan). All the studies included AF and dementia patients based on a standard protocol.
Table 1.
Characteristics of included studies.
| References | Country | Study design | % of male | No. of pts. | No. of dementia | Inclusion criteria | Exclusion criteria | AF diagnosis | Dementia diagnosis | Follow-up period (years, months) | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tilvis et al. (2004) | Finland | Cohort | 26.39 | 629 | 629 | Random sample of pts born in 1904,1909, and 1914 | No specified | H&P, medical record | MMSE and CDR | 5 | HR = 2.88 (1.26–6.06) |
| Elias et al. (2006) | USA | Cohort | 100 | 1,011 | 952 | Male offspring of the Framingham heart study | Pts with dementia, prior stroke, females, decline examination | ECG, ECG-H, H&P | Neuropsychological tests approved by a panel of neurologists and neuropsychiatrists | 30 | HR = 4.01 (1.84–8.74) |
| Forti et al. (2006) | Italy | Cohort | 37 | 431 | 36 | Pts > 60 yrs. seeking medical advice for cognitive complaints | Pts with psychiatric disorder, Parkinson disease, epilepsy substance abuse | H&P | MMSE and neuropsychological tests | 4 | HR = 1.10 (0.40–3.03) |
| Rastas et al. (2007) | Finland | Cohort | 20.43 | 1,106 | 339 | Pts ≥ 85 y between 1991 and 1999 | Pts who died before examination | ECG, ECG-H, medical records | DSM-III, MMSE | 3.5 | HR = 0.86 (0.50–1.47) |
| Marengoni et al. (2011) | Sweden | Cohort | NR | 685 | 170 | Pts > 75 years | Pts with dementia, prior stroke | H&P, medical records, ICD codes | DSM-III Revised | 6 | HR = 0.9 (0.50–1.70) |
| Peters et al. (2009) | UK | RCT | 34.59 | 3,336 | 263 | Pts > 80 yrs. with hypertension | Pts with dementia | Not specified | DSM-IV | 1.8 | HR = 1.03 (0.62–1.72) |
| Bunch et al. (2010) | USA | Cohort | 60.1 | 37,025 | 1,535 | Pts included in the Intermountain Healthcare System database | Pts with dementia | ICD codes | ICD codes | 5 | HR = 1.36 (1.13–1.63) |
| Dublin et al. (2011) | USA | Cohort | 40 | 3045 | 572 | Pts ≥ 65 yrs. | Pts with dementia, prior stroke | ICD codes | DSM-IV | 6.8 | HR = 1.38 (1.10–1.73) |
| Marzona et al. (2012) | Italy | RCT | 70.3 | 31,506 | 890 | Pts ≥ 55 yrs., history of cardiovascular disease or diabetes with end-organ damage | Pts with heart failure, substantial valvular disease, or uncontrolled hypertension | ECG, medical records | MMSE | 4.7 | HR = 1.30 (1.14–1.49) |
| Rusanen et al. (2014) | Finland | Cohort | 37.6 | 1,510 | 127 | Pts of the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study, Pts aged 65 to 79 yrs | Not properly examined, not properly followed-up | ICD codes, MRI/CT | ICD codes, DSM-IV, MMSE | 7.8 | HR = 2.61 (1.05–6.47) |
| Liao et al. (2015) | Taiwan | Cohort | 55.9 | 665,330 | 56,901 | AF with age ≥ 20 years and no history of dementia for at least 1 year preceding study enrollment | Not specified | ICD codes | ICD codes | 15 | HR = 1.42 (1.39–1.44) |
| de Bruijn et al. (2015) | Netherlands | Cohort | 40.6 | 6,194 | 994 | Pts with proper identification of AF, proper follow-up | Not properly screened for dementia, had previously dementia, lack of follow-up, missing data on AF | EEG and MEANS | MMSE, GMSS, and DSM-III revised | 20 | HR = 1.33 (0.99–1.78) |
| Marzona et al. (2016) | Italy | Cohort | 47.48 | 1,627,631 | 134,837 | ≥65 yrs. pts with AF | Pts admitted to hospital for AF in the previous two years (2000–2001). Patients with incident dementia in the 2 years before entering the study | ICD codes | ICD codes | 10 | HR = 1.17 (1.12–1.23) |
| Singh-Manoux et al. (2017) | UK | Cohort | 84.3 | 10,308 | 912 | Pts with proper identification of AF and dementia, follow-up examination approximately every 5 years | No specified | ICD code and ECG | ICD code | 26.6 | HR = 1.87 (1.37–2.55) |
| Ding et al. (2018) | Sweden | Cohort | 37.1 | 2,685 | 399 | Pts aged >60 yrs. | Dementia at baseline and refused follow-up | ICD code | MMSE and DSM-III revised, NINDS-AIREN, NINCDS-ADRDA | 9 | HR = 1.88 (1.09–3.23) |
| Chen et al. (2018) | USA | Cohort | 44 | 22,924 | 1,157 | Pts aged >45 yrs., proper cognitive data | Dementia at baseline and refused follow-up | EEG and ICD code | DWRT, DSST, WFT, ARIC-NCS, MMSE, ICD code | 20 | HR = 1.23 (1.04–1.45) |
MEANS, Modular ECG Analysis System; ECG, Electrocardiography; MMSE, Mini-Mental State Examination; GMSS, Geriatric Mental State Schedule; DSM-III, Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised); ICD, International Classification of Diseases; CDR, Clinical Dementia Rating; NINDS-AIREN, National Institute of Neurological Disorders and Stroke and Association International pour la Recherch'e et l'Enseignement en Neurosciences; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; DWRT, the Delayed Word Recall Test; DSST, the Digit Symbol Substitution Test; WFT, the Word Fluency Test; NR, Not reported; RCT, Randomized controlled trials; H&P, History and physical examination.
Study Quality
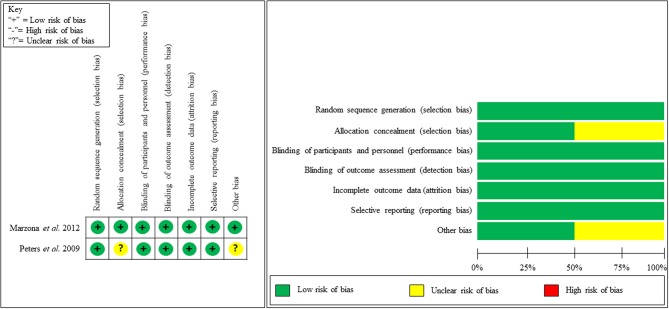
Our quality assessment of each observational study (cohort study) was conducted using the NOS, which is specifically used to assess the methodological quality of non-randomized studies. We evaluated methodological quality in addressing patients' selection, study comparability, and the assessment of outcome or exposure. We then categorized studies into good, fair, or poor quality. Nine studies received good quality, four studies received fair quality and one study received poor quality (Table 2). Figure 2 shows the study quality for RCTs.
Table 2.
Quality assessment using the Newcastle Ottawa Scale.
| Selection (S) | Comparability (C) | Outcome | Subtotal assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1a | 1b | 1 | 2 | 3 | S+ | Cand | E/Oand | Conclusion | |
| Cohort Studies | |||||||||||||
| Tilvis et al. (2004) | * | * | * | * | * | * | No | No | * | Good | Fair | Fair | Fair |
| Elias et al. (2006) | * | * | * | * | * | No | * | No | * | Good | Fair | Good | Fair |
| Forti et al. (2006) | * | No | * | * | * | No | * | No | * | Good | Fair | Good | Fair |
| Rastas et al. (2007) | * | * | * | No | * | * | * | No | No | Good | Fair | Fair | Fair |
| Marengoni et al. (2011) | * | No | * | No | No | No | * | No | No | Good | Poor | Fair | Poor |
| Bunch et al. (2010) | * | * | No | * | * | * | * | No | * | Good | Good | Good | Good |
| Dublin et al. (2011) | * | * | No | * | * | * | * | No | * | Good | Good | Good | Good |
| Rusanen et al. (2014) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
| de Bruijn et al. (2015) | * | * | * | * | * | * | * | * | No | Good | Good | Good | Good |
| Liao et al. (2015) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
| Marzona et al. (2016) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
| Singh-Manoux et al. (2017) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
| Chen et al. (2018) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
| Ding et al. (2018) | * | * | * | * | * | * | * | * | * | Good | Good | Good | Good |
Domain scored: 0–1 (Poor); 2 (Fair); 3+ (Good).
Domain scored: 0 (Poor); 1 (Fair); 2+ (Good).
Domain acceptable.
Figure 2.
Methodological quality assessment of RCT.
Meta-Analysis
Primary Analysis
Atrial fibrillation and dementia risk
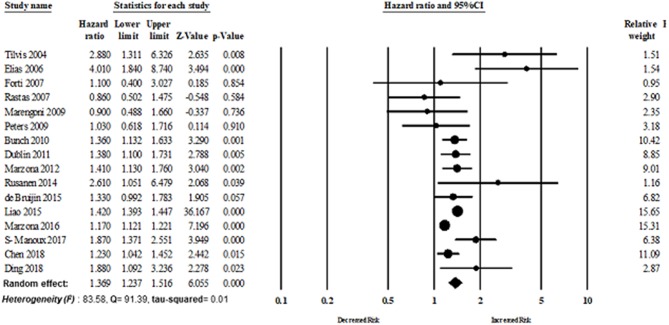
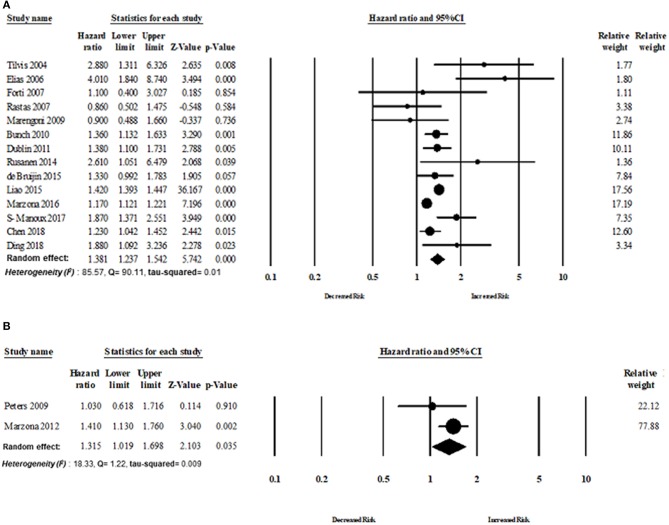
Sixteen studies evaluated the magnitude of the association between AF and dementia risk. The overall adjusted pooled HR of developing dementia was 1.36 (95% CI: 1.23–1.51, p < 0.0001; I2 = 83.58), in patients with AF. Figure 3 shows the overall risk of dementia patients with AF.
Figure 3.
Association between AF and dementia risk.
Secondary Analysis
Atrial fibrillation and AD risk
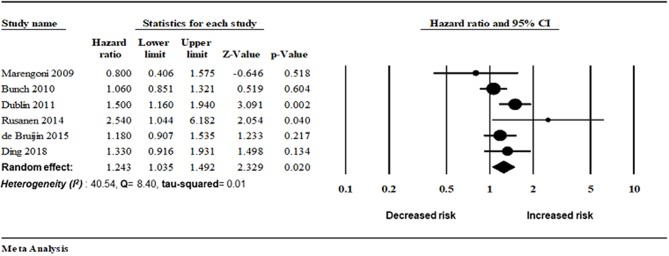
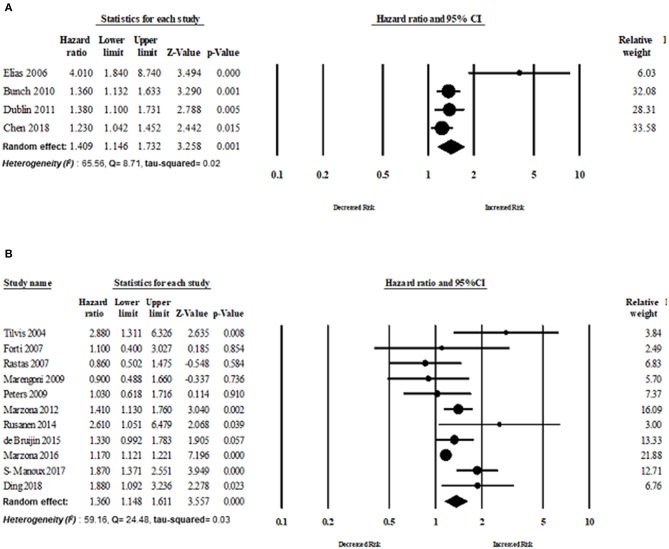
Six studies assessed the magnitude of the association between AF and AD risk. There was an increased risk of AD in patients with AF (random-effect HR: 1.24, 95% CI: 1.03–1.49, p = 0.02). However, lower heterogeneity was observed in this analysis (I2 = 40.54, Q = 8.40, tau2 = 0.019) (Figures 4, 5).
Figure 4.
Association between AF and Alzheimer disease risk.
Figure 5.
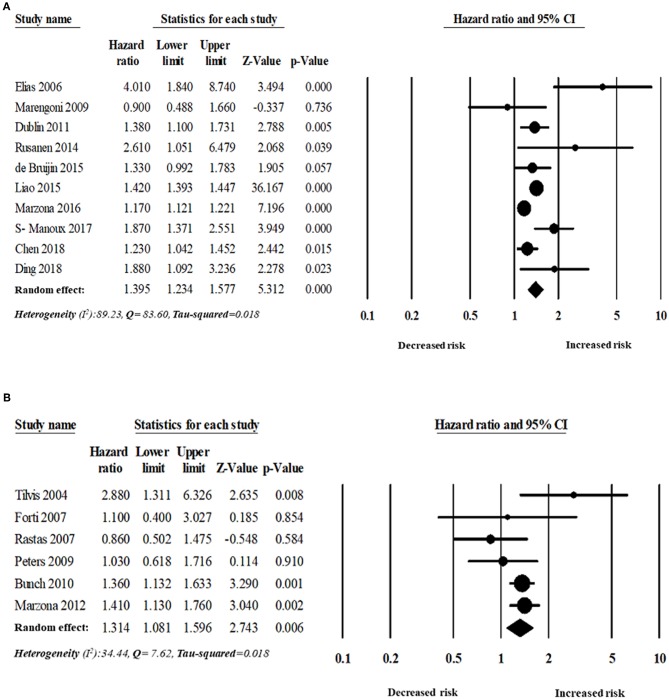
Risk of dementia in patients with AF based on follow-up period: (A) more than five years' follow-up period, (B) <5 years' follow-up period.
Subgroup analyses
The analyses were also stratified by the study design, region, and follow-up period. While stratified by the length of the follow-up period, the overall pooled HR was appeared to be higher in those studies with more than 5 years of follow-up (n = 10 studies). The overall adjusted pooled HR was 1.39 (95% CI:1.23–1.58, p < 0.0001) in the random effect model with higher heterogeneity (I2 = 89.23, Q = 83.60, tau2 = 0.018). However, the overall pooled risk of dementia was 31% for the study with <5 year of follow-up period (HR 1.31, 95% CI: 1.08–1.60, p = 0.006) in the random effect model with lower heterogeneity (I2 = 34.44, Q = 7.62, tau2 = 0.018).
Fourteen cohort studies and two RCTs evaluated the risk of dementia in patients with AF. The overall risk of dementia of cohort studies was HR 1.38 (95% CI: 1.23–1.55, p < 0.0001) in the random effect model with higher heterogeneity (I2 = 85.57, Q = 90.11, tau2 = 0.018). The overall risk of dementia of RCT studies was HR 1.31 (95% CI: 1.01–1.70, p = 0.03) in the random effect model with lower heterogeneity (I2 = 18.33, Q = 1.22, tau2 = 0.009) (Figure 6).
Figure 6.
Association between AF and dementia based on study design: (A) cohort study design, (B) randomized control trial.
Eleven studies published in Europe assessed the risk of dementia in patients with AF. The overall risk of dementia of studies published in Europe was HR 1.36 (95% CI: 1.14–1.61, p < 0.0001, I2 = 59.16, Q = 24.48, tau2 = 0.034). The overall risk of dementia of studies (n = 4) published in North America was HR 1.40 (95% CI: 1.14–1.73, p = 0.001, I2 = 65.56, Q = 8.71, tau2 = 0.026) (Figure 7).
Figure 7.
Risk of dementia based on region: (A) Europe, (B) North America.
However, the comparison was also stratified by good and fair methodological quality of study; the association of AF with dementia risk was significant in good methodological quality studies, but it appeared to be stronger in fair methodological quality studies (Supplementary Figure 1).
Publication bias
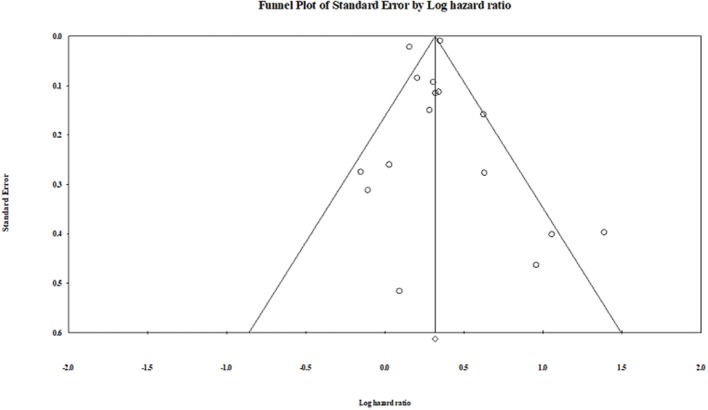
Publication bias was not observed in the pooled studies, or the subgroup analysis studies using the Egger test (Egger et al., 1997). The visual plot of the Egger regression test suggests that publication bias was unlikely (P = 0.90) (Figure 8).
Figure 8.
Funnel plot.
Discussion
Main Findings
Our meta-analysis provides evidence for a significant association between AF and risk of dementia. This meta-analysis involves a total of 16 unique observational studies with aggregate data on 2,415,356 adult individuals, and approximately 200,653 cases of dementia followed up over a median period of 7.3 years. We found that the presence of AF is associated with an increased risk of dementia (1.36-fold). In addition, when the analysis was stratified either by follow-up duration, study country, or study design, the association between AF and dementia risk appeared to be stronger in studies with a follow-up duration longer than 5 years, and in studies conducted in Asia compared with those conducted in North America and Europe countries. The risk of dementia in AF patients was higher in the cohort studies compared with the one found for the RCT studies. Most of the included studies used MMSE to assess cognitive function, and dementia was clinically diagnosed according to the DSM-IV criteria. Moreover, published studies also diagnosed vascular dementia and AD according to the NINDS-AIREN (National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherch'e et l'Enseignement en Neurosciences) criteria and the NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association) criteria, respectively.
Comparison With Previous Studies
A previous meta-analysis (Santangeli et al., 2012) evaluated the prospective relationship between AF and incident dementia based on only eight studies. In addition, they did not perform a subgroup analysis and did not provide any biological evidence regarding AF and dementia risk. Therefore, their analyses provide a less robust basis for decision and policy making. Kwok et al. (2011) also conducted a meta-analysis with 15 relevant studies and demonstrated that AF was associated with a significant increase in dementia, but their study also lacked subgroup analyses. In our meta-analysis, we included a higher number of studies and provided a comprehensive analysis to support the current evidence.
Biological Plausibility
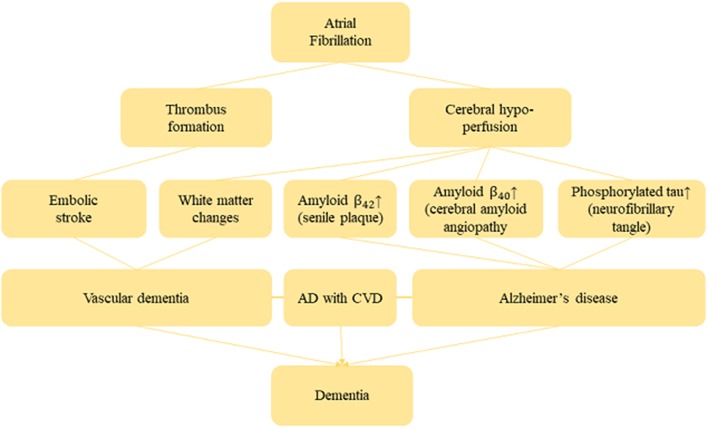
Several studies provided convincing evidence of the biological plausibility between AF and risk of dementia (Conway et al., 2004; Yaffe et al., 2014; Alosco et al., 2015). Indeed, AF may induce cerebral hypo-perfusion due to low cardiac output (Poggesi et al., 2015). However, cerebral hypo-perfusion exacerbates white matter change that is responsible for demyelination and axonal damage, and also leads to memory impairment (Shibata et al., 2004; Ihara and Tomimoto, 2011). In general, most vital organs in the human body including the brain have autoregulation mechanisms which maintain blood circulation even if cardiac output decreases (Akinyemi et al., 2013). However, this compensatory mechanism may deteriorate in a patient with long-term AF and thus lessen his cerebral circulation. Therefore, long-term cerebral hypo-perfusion induces senile plaques and amyloid angiopathy through β-(BACE 1) expression and γ-secretase. β- and γ-secretases then generates and aggregate β-amyloid peptide in the brain, which is always considered to be responsible for neuron death and AD (Pluta et al., 2013). Furthermore, senile plaques and cerebral amyloid angiopathy are formed due to Aβ42 with a higher aggregation ability which accumulates in the brain parenchyma, and Aβ40 with relatively lower aggregation ability accumulates in the cerebral blood vessels. Evidence from an in-vivo study shows that cerebral amyloid angiopathy (CA) triggers reduced blood supply in the brain, and also intensifies Aβ production due to dysfunction of the vascular smooth muscle (Niwa et al., 2002). It was also shown that CA hampers the Aβ clearance pathway, resulting in the accumulation of Aβ and generating a cycle of Aβ accumulation (Hawkes et al., 2011). Moreover, accumulation of Aβ causes neurofibrillary tangles which are mainly composed of phosphorylated tau, and thus finally promotes neuronal cell death (Hardy and Allsop, 1991). Yao et al. (2012) reported that chronic hypo-perfusion activates the tau phosphorylation enzymes glycogen synthase kinase-3 beta and cyclin-dependent kinase-5 which eventually induces the accumulation of Aβ. Figure 9 shows the plausible mechanism of AF and dementia risk.
Figure 9.
Biological mechanism between AF and dementia.
Public Health Implications
Our present meta-analysis of observational studies reported an evidence for the existence of a significant association between AF and risk of dementia. Inflammatory biomarkers and thromboembolism led to reduced and intermittent cerebral perfusion during arrhythmia or silent cerebral ischemia. It can cause chronic cerebral hypo-perfusion which is considered as a predictors of dementia (Jacobs et al., 2015). However, it should also be noted that oral anticoagulants (OACs) and a healthy lifestyle may reduce the risk of a dementia in patients with AF. Since patients with AF always tend to have a higher risk of strokes, they are consequently also at particular risk for cognitive decline, functional deficit and dependency. However, Ng et al. (2013) reported that novel anticoagulants, dabigatran, rivaroxaban, and apixaban are effective at reducing the absolute risk of stroke and are safer in elderly patients. Furthermore, an observational study provided interesting findings that included 2,685 dementia-free individuals from the Swedish National Study on Aging and Care in Kungsholmen, Stockholm. AF patients with OACs had a decreased risk of dementia (HR = 0.44, 95% CI: 0.18–0.92) compared to those patients without OACs (Ding et al., 2018). However, long-term anticoagulation therapy might raise the risk of intracranial bleeding. In addition, catheter ablation has emerged as a frontline therapy for improving symptoms, physical capacity, and quality of life in drug refractory AF patients. Bunch et al. (2011) enrolled 4,212 consecutive patients who underwent AF ablation, and 16,848 AF patients without ablation. AF ablation patients had a significantly lower risk of dementia (0.2% of the AF ablation patients) in comparison to AF patients without ablation (0.9% of the AF with no ablation) (Bunch et al., 2011). However, catheter ablation sometimes might itself lead to silent strokes and cognitive impairment (Shah et al., 2016). It is therefore important for the physicians to follow recommendations for performing ablation as well as management of AF patients before and after the procedure (Refaat et al., 2017). Furthermore, physicians should raise awareness of the risk of dementia in patients with AF, and recommend how to reduce the risk of dementia which could include the cessation of smoking, and the control of hypertension, obesity, diabetes, and sleep apnea. If any suspicion regarding cognitive decline or dementia is confirmed, physicians should conduct an objective assessment of cognitive function.
Strengths and Limitations
Our meta-analysis has several limitations that should be mentioned. Although a random-effect model was used in our meta-analysis, the findings of this current and comprehensive meta-analysis should be explained with caution because of their high heterogeneity (I2 > 75%) in the overall main analysis. It is feasible to summarize that high heterogeneity is observed due to differences in sample size, methodological quality, demographic, and ethno-racial characteristics of the study populations, various covariate assessments, different length of follow-up periods, and types of dementia. All these possible sources of statistical heterogeneity were then systematically scrutinized and evaluated using stratified analyses. However, higher heterogeneity was observed between the studies when the primary analysis was conducted but it is notable to mention that there was very low heterogeneity as well as a greater significant association between AF and dementia risk when we stratified our analyses according to study design (RCTs) and different continents (North America and Europe).
Despite these limitations, this current and updated meta-analysis has several strengths. This current study provides the most comprehensive evaluation between AF and dementia risk so far. These findings were obtained from a larger number of participants and dementia cases from 16 observational studies (overall pooled risk of non-randomized and randomized are almost similar, showing higher risk of dementia with AF patients). Moreover, it is notable to point out that we applied standardized risk estimates from all relevant studies to embrace a harmonious combination of estimates across studies. The greater number of dementia patient's inclusion, stratification according to study design, region, methodological quality, and follow-up duration provided rational statistical power to quantitatively evaluate the association between AF and dementia risk. Finally, some sort of reported bias of the included studies were not a concern in our analyses, as our extensive literature search and discussion with main investigator made it unlikely that any published article was missed, and visual inspection of funnel plots showed no publication bias.
Conclusion
The present comprehensive meta-analysis suggests that AF is associated with an increased risk of dementia. It is still unclear whether treatments of AF might lead the greater risk of dementia or not. Therefore, more biological studies are needed to find out their causality.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00305/full#supplementary-material
References
- Akinyemi R. O., Mukaetova-Ladinska E. B., Attems J., Ihara M., Kalaria R. N. (2013). Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer's disease and vascular dementia. Curr. Alzheimer Res. 10, 642–653. 10.2174/15672050113109990037 [DOI] [PubMed] [Google Scholar]
- Alosco M. L., Spitznagel M. B., Sweet L. H., Josephson R., Hughes J., Gunstad J. (2015). Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. Pacing Clin. Electrophysiol. 38, 178–186. 10.1111/pace.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik A., Brown R. E., Bamburg J., Lahiri D. K., Khurana D., Friedland R. P., et al. (2015). Translation of pre-clinical studies into successful clinical trials for Alzheimer's disease: what are the roadblocks and how can they be overcome? J. Alzheimer's Dis. 47, 815–843. 10.3233/JAD-150136 [DOI] [PubMed] [Google Scholar]
- Bell R. D., Zlokovic B. V. (2009). Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 118, 103–113. 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch T. J., Crandall B. G., Weiss J. P., May H. T., Bair T. L., Osborn J. S., et al. (2011). Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. 22, 839–845. 10.1111/j.1540-8167.2011.02035.x [DOI] [PubMed] [Google Scholar]
- Bunch T. J., Weiss J. P., Crandall B. G., May H. T., Bair T. L., Osborn J. S., et al. (2010). Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 7, 433–437. 10.1016/j.hrthm.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Chen L. Y., Norby F. L., Gottesman R. F., Mosley T. H., Soliman E. Z., Agarwal S. K., et al. (2018). Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J. Am. Heart Assoc. 7:e007301. 10.1161/JAHA.117.007301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D. S., Buggins P., Hughes E., Lip G. Y. (2004). Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am. Heart J. 148, 462–466. 10.1016/j.ahj.2004.01.026 [DOI] [PubMed] [Google Scholar]
- de Bruijn R. F., Heeringa J., Wolters F. J., Franco O. H., Stricker B. H., Hofman A., de Bruijn R. F., et al. (2015). Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 72, 1288–1294. 10.1001/jamaneurol.2015.2161 [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. P., Altman D. G. (2008). Analysing data and undertaking meta-analyses, in Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, eds Higgins J. P. T., Green S. (Chichester: John Wiley & Sons Ltd.), 243–296. [Google Scholar]
- Dietzel J., Haeusler K. G., Endres M. (2017). Does atrial fibrillation cause cognitive decline and dementia? EP Europace. 20, 408–419. 10.1093/europace/eux031 [DOI] [PubMed] [Google Scholar]
- Ding M., Fratiglioni L., Johnell K., Santoni G., Fastbom J., Ljungman P., et al. (2018). Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population-based study. Neurology 91, e1732–e1740. 10.1212/WNL.0000000000006456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Qiu C. (2018). Atrial fibrillation, cognitive decline, and dementia: an epidemiologic review. Curr. Epidemiol. Rep. 5, 252–261. 10.1007/s40471-018-0159-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K., Bracchi R., Hewitt J., Routledge P. A., Carter B. (2017). Benzodiazepines, Z-drugs and the risk of hip fracture: a systematic review and meta-analysis. PLoS ONE 12:e0174730. 10.1371/journal.pone.0174730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublin S., Anderson M. L., Haneuse S. J., Heckbert S. R., Crane P. K., Breitner J. C., et al. (2011). Atrial fibrillation and risk of dementia: a prospective cohort study. J. Am. Geriatr. Soc. 59, 1369–1375. 10.1111/j.1532-5415.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Smith G. D., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M. F., Sullivan L. M., Elias P. K., Vasan R. S., D'Agostino R. B., Seshadri S., et al. (2006). Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis. 15, 214–222. 10.1016/j.jstrokecerebrovasdis.2006.05.009 [DOI] [PubMed] [Google Scholar]
- European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Camm A. J., Kirchhof P., Lip G. Y., Schotten U., et al. (2010). Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31, 2369–2429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- Forti P., Maioli F., Pisacane N., Rietti E., Montesi F., Ravaglia G. (2006). Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Neurol. Res. 28, 625–629. 10.1179/016164106X130461 [DOI] [PubMed] [Google Scholar]
- Friedrichs K., Klinke A., Baldus S. (2011). Inflammatory pathways underlying atrial fibrillation. Trends Mol. Med. 17, 556–563. 10.1016/j.molmed.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Gorelick P. B., Scuteri A., Black S. E., Decarli C., Greenberg S. M., Iadecola C., et al. (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Allsop D. (1991). Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 12, 383–388. 10.1016/0165-6147(91)90609-V [DOI] [PubMed] [Google Scholar]
- Hawkes C. A., Härtig W., Kacza J., Schliebs R., Weller R. O., Nicoll J. A., et al. (2011). Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443. 10.1007/s00401-011-0801-7 [DOI] [PubMed] [Google Scholar]
- Heeringa J. (2010). Atrial Fibrillation: Is the Prevalence Rising? EP Europace. 12, 451–452. 10.1093/europace/euq056 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Green S. (eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions 5.1. 0. Wiley: The Cochrane Collaboration, 33–49. [Google Scholar]
- Higgins J. P., Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327:557. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Tomimoto H. (2011). Lessons from a mouse model characterizing features of vascular cognitive impairment with white matter changes. J. Aging Res. 2011:978761. 10.4061/2011/978761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Washida K. (2018). Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J. Alzheimer's Dis. 62, 61–72. 10.3233/JAD-170970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M., Iqbal U., Walther B., Atique S., Dubey N. K., Nguyen P. A., et al. (2016). Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology 47, 181–191. 10.1159/000454881 [DOI] [PubMed] [Google Scholar]
- Jacobs V., Cutler M. J., Day J. D., Bunch T. J. (2015). Atrial fibrillation and dementia. Trends Cardiovasc. Med. 25, 44–51. 10.1016/j.tcm.2014.09.002 [DOI] [PubMed] [Google Scholar]
- January C. T., Wann L. S., Alpert J. S., Calkins H., Cigarroa J. E., Cleveland J. C., Jr., et al. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 64, e1–e76. 10.1161/CIR.0000000000000041 [DOI] [PubMed] [Google Scholar]
- Kalantarian S., Stern T. A., Mansour M., Ruskin J. N. (2013). Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 158(Pt. 1), 338–346. 10.7326/0003-4819-158-5-201303050-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C. S., Loke Y. K., Hale R., Potter J. F., Myint P. K. (2011). Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology 76, 914–922. 10.1212/WNL.0b013e31820f2e38 [DOI] [PubMed] [Google Scholar]
- Liao J. N., Chao T. F., Liu C. J., Wang K. L., Chen S. J., Tuan T. C., et al. (2015). Risk and prediction of dementia in patients with atrial fibrillation—a nationwide population-based cohort study. Int. J. Cardiol. 199, 25–30. 10.1016/j.ijcard.2015.06.170 [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Qiu C., Winblad B., Fratiglioni L. (2011). Atrial fibrillation, stroke and dementia in the very old: a population-based study. Neurobiol. Aging 32, 1336–1337. 10.1016/j.neurobiolaging.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Marzona I., Baviera M., Vannini T., Tettamanti M., Cortesi L., Riva E., et al. (2016). Risk of dementia and death in patients with atrial fibrillation: a competing risk analysis of a population-based cohort. Int. J. Cardiol. 220, 440–444. 10.1016/j.ijcard.2016.06.235 [DOI] [PubMed] [Google Scholar]
- Marzona I., O'Donnell M., Teo K., Gao P., Anderson C., Bosch J., et al. (2012). Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. Can. Med. Assoc. J. 184, E329–E336. 10.1503/cmaj.111173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou L., Norby F. L., Chen L. Y., O'Neal W. T., Lewis T. T., Loehr L. R., et al. (2018). Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC Study (Atherosclerosis Risk in Communities). Circ. Arrhyth. Electrophysiol. 11:e006350. 10.1161/CIRCEP.118.006350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K. H., Hart R. G., Eikelboom J. W. (2013). Anticoagulation in patients aged≥ 75 years with atrial fibrillation: role of novel oral anticoagulants. Cardiol. Ther. 2, 135–149. 10.1007/s40119-013-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K., Kazama K., Younkin L., Younkin S. G., Carlson G. A., Iadecola C. (2002). Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am. J. Physiol. Heart Circ. Physiol. 283, H315–H323. 10.1152/ajpheart.00022.2002 [DOI] [PubMed] [Google Scholar]
- Peters R., Poulter R., Beckett N., Forette F., Fagard R., Potter J., et al. (2009). Cardiovascular and biochemical risk factors for incident dementia in the Hypertension in the Very Elderly Trial. J. Hypertens. 27, 2055–2062. 10.1097/HJH.0b013e32832f4f02 [DOI] [PubMed] [Google Scholar]
- Piccini J. P., Hammill B. G., Sinner M. F., Hernandez A. F., Walkey A. J., Benjamin E. J., et al. (2013). Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur. Heart J. 35, 250–256. 10.1093/eurheartj/eht483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R., Furmaga-Jabłonska W., Maciejewski R., Ułamek-Kozio,ł M., Jabłonski M. (2013). Brain ischemia activates β-and γ-secretase cleavage of amyloid precursor protein: significance in sporadic Alzheimer's disease. Mol. Neurobiol. 47, 425–434. 10.1007/s12035-012-8360-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggesi A., Inzitari D., Pantoni L. (2015). Atrial fibrillation and cognition: epidemiological data and possible mechanisms. Stroke 46, 3316–3321. 10.1161/STROKEAHA.115.008225 [DOI] [PubMed] [Google Scholar]
- Polidori M. C., Pientka L., Mecocci P. (2012). A review of the major vascular risk factors related to Alzheimer's disease. J. Alzheimer's Dis. 32, 521–530. 10.3233/JAD-2012-120871 [DOI] [PubMed] [Google Scholar]
- Poly T. N., Islam M. M., Walther B. A., Yang H. C., Nguyen P. A., Huang C. W., et al. (2017). Exploring the association between statin use and the risk of Parkinson's disease: a meta-analysis of observational studies. Neuroepidemiology 49, 142–151. 10.1159/000480401 [DOI] [PubMed] [Google Scholar]
- Qiu C., Fratiglioni L. (2015). A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 12:267. 10.1038/nrcardio.2014.223 [DOI] [PubMed] [Google Scholar]
- Rastas S., Verkkoniemi A., Polvikoski T., Juva K., Niinistö L., Mattila K., et al. (2007). Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke 38, 1454–1460. 10.1161/STROKEAHA.106.477299 [DOI] [PubMed] [Google Scholar]
- Refaat M. M., Ballout J., Mansour M. (2017). Ablation of atrial fibrillation in patients with congenital heart disease. Arrhyth. Electrophysiol. Rev. 6:191. 10.15420/2017.2017.15.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusanen M., Kivipelto M., Levälahti E., Laatikainen T., Tuomilehto J., Soininen H., et al. (2014). Heart diseases and long-term risk of dementia and Alzheimer's disease: a population-based CAIDE study. J. Alzheimer's Dis. 42, 183–191. 10.3233/JAD-132363 [DOI] [PubMed] [Google Scholar]
- Santangeli P., Di Biase L., Bai R., Mohanty S., Pump A., Cereceda Brantes M., et al. (2012). Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 9, 1761–1768. 10.1016/j.hrthm.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Shah A. D., Merchant F. M., Delurgio D. B. (2016). Atrial fibrillation and risk of dementia/cognitive decline. J. Atr. Fibrillation. 8:1353. 10.4022/jafib.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Ohtani R., Ihara M., Tomimoto H. (2004). White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 35, 2598–2603. 10.1161/01.STR.0000143725.19053.60 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A., Fayosse A., Sabia S., Canonico M., Bobak M., Elbaz A., et al. (2017). Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur. Heart J. 38, 2612–2618. 10.1093/eurheartj/ehx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Stroup D. F., Berlin J. A., Morton S. C., Olkin I., Williamson G. D., Rennie D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Takeda S., Sato N., Morishita R. (2014). Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front. Aging Neurosci. 6:171. 10.3389/fnagi.2014.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilvis R. S., Kähönen-Väre M. H., Jolkkonen J., Valvanne J., Pitkala K. H., Strandberg T. E. (2004). Predictors of cognitive decline and mortality of aged people over a 10-year period. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 59, M268–M274. 10.1093/gerona/59.3.M268 [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Tai P. A., Poly T. N., Islam M. M., Yang H. C., Wu C. C., et al. (2018). Increased risk of dementia in patients with antidepressants: a meta-analysis of observational studies. Behav. Neurol. 2018:5315098. 10.1155/2018/5315098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Vittinghoff E., Pletcher M. J., Hoang T. D., Launer L. J., Whitmer R., et al. (2014). Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 129, 1560–1567. 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z. H., Zhang J. J., Xie X. F. (2012). Enriched environment prevents cognitive impairment and tau hyperphosphorylation after chronic cerebral hypoperfusion. Curr. Neurovasc. Res. 9, 176–184. 10.2174/156720212801618974 [DOI] [PubMed] [Google Scholar]
- You J. J., Singer D. E., Howard P. A., Lane D. A., Eckman M. H., Fang M. C., et al. (2012). Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141, e531S–e575S. 10.1378/chest.141.4.1129b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.