Abstract
Background:
The Pharmacokinetics of Methotrexate (MTX) has been reported to show significant inter-subject variability. MTX is metabolized by SHMT1 and transported by OATP1B1 and OATP1B3 both of which show genetic polymorphisms. The non-genetic and genetic factors may influence the pharmacokinetics of MTX.
Objective:
This study aimed to determine the pharmacokinetic parameters of MTX in Chinese patients and to investigate the effect of various non-genetic factors and genetic variants of OATP1B1, OATP1B3 on MTX’s pharmacokinetics.
Methods:
MTX concentration and clinical characteristics data were collected from 71 rheumatoid arthritis patients. For each patient, SLC19A1, SHMT1, OATP1B1, and OATP1B3 genotyping were tested. Population pharmacokinetic analysis was performed by Nonlinear Mixed-Effect Modeling (NONMEM). MTX pharmacokinetic properties analysis was executed using the one-compartment pharmacokinetic model which incorporated first-order conditional estimation methods with interaction. Besides, the impact of genetic factors and demographic factors on MTX disposition were explored.
Results:
All the genotypes of steady-state plasma concentrations and OATP1B1 rs4149056, OATP1B1 rs2306283, and OATP1B3 rs7311358 were determined. The detected blood drug concentration reached the standard. Genotypes were all measured. At the same time, the population pharmacokinetic model of methotrexate was obtained CL(L•h-1) =8.25× e0.167× SNP (SNP: SLCO1B1 388A/A=3; SLCO1B1 388A/G=2; SLCO1B1 388G/G=1); V(L)= 32.8; Ka(h-1)=1.69.
Conclusion:
In our study, it was showed that OATP1B1-388 G>A SNP had a significant effect on CL/F. The factor should be considered when determining MTX dosing. However, prospective studies with a large number of participants are needed to validate the results of this study.
Keywords: Rheumatoid arthritis, methotrexate, organic anion-transporting polypeptides (OATPs), nonlinear mixed-effect modeling (NONMEM), pharmacogenetics, population pharmacokinetics
1. INTRODUCTION
Rheumatoid Arthritis (RA) is a long-term, progressive disease that affects joints. Its mean features are inflammation at the synovial joints, which can cause pain, swelling, and ultimately irreversible joint destruction. The disease leads to functional joint impairment, decreased quality of life, unemployment, and increased mortality [1]. Timely and effective disease control is expected to prevent joint injury, which can greatly change the course of the disease and improve the outcome of RA.
1.1. Methotrexate
Methotrexate (MTX), a folic acid analog belonging to the disease-modifying anti-rheumatic drug class, is the most common drug for the control of RA in clinical therapeutic. MTX is the core drug in the majority combination therapies. Besides, it is the gold standard for evaluating the therapeutic effect of new anti-rheumatic treatment [2]. MTX is used to treat other types of inflammatory arthritis [3]. According to Quantitative Standard Monitoring of Patients with Rheumatoid Arthritis (QUEST-RA), which now includes more than 30 countries, 83% of RA patients choose MTX therapy, compared to 23% for any biological agent [4]. However, even in patients with normal liver function and renal function, the pharmacokinetics model of low-dose MTX is highly variable and hard to predict. Morgan et al. [5] reported that approximately 10% of patients are unresponsive to MTX, and a further 20% of patients discontinue treatment because of adverse effects. Although the prediction of the pharmacokinetics of MTX is critical to patient safety and efficacy, we have not yet been able to predict whether a patient will be a non-responder or develop into adverse effects requiring discontinuation in the early stages of treatment.
Inter-individual differences of MTX pharmacokinetics can be due to many factors, including the genetic variation of transporters and metabolic enzymes [6]. Considering the relationship between drug concentration and drug effect is crucial for the determining of Pharmacodynamics (PD) [7]. The extent of drug transport through the cellular membranes is determined by Pharmacokinetics (PK), and to a certain extent, will also be affected by membrane transporters [8]. Membrane transporters’ functional activity and expression level to decide the amount of MTX entering or exiting a particular organization, is possibly affected by genetic polymorphisms [7]. Although the bioavailability of MTX is relatively high, changes in dosing dose and route of administration alter the bioavailability [8].
1.2. Organic Anion-Transporting Polypeptides
Organic Anion-transporting Polypeptides (OATPs) play an essential role in the disposition of a vast number of drugs including MTX. MTX’s liver uptake and plasma clearance depend on some critical factors: OATP1B1, OATP1B3, and OATP2B1, which are highly expressed in hepatocytes sinusoidal membrane. As a result, the alteration of OATP1B functional activity may have a meaningful impact on the PK of drugs transported by them [9].
SLCO/Slco genes encode OATPs, several sequence vibration and Single-nucleotide Polymorphism (SNP) are found in SLCO1B1 which are responsible for encoding OATP1B1. Besides, some of them are associated with changing transportation activity in vivo and in vitro. Studies have shown that c.521T>C SNP is related to a significant decrease in the in vitro uptake activity of the OATP1B1 substrate [10].
According to the global analysis of genetic variation of SLCO1B1, it is found that the allele frequency of the c.521T>C variant is approximately 14% in the Chinese Han population [10]. Another common functional SLCO1B1 mutant, the allele frequency of the c.388A>G SNP is 73.4% in the Chinese Han population. The mutation rates of OATP1B1 388A and 521T in white Americans were 30% and 14%, respectively [10]. Therefore, studies are needed to elucidate the effects of c.388A>G and c.521T>C SNP on drug pharmacokinetics in humans [11].
SLCO1B3, which plays a crucial role in hepatocyte uptake of MTX from the blood, is a known uptake transporter for MTX and is located in hepatocytes basolateral (sinusoidal) membrane. Several SLCO1B3 polymorphisms have been reported previously [12-15]. A previous study reported that SLCO1B3 c.334 T > G played no role in affecting transport activity, but c.699 G > A is considered to be controversial depending on the substrate [15]. Therefore, one locus that was known to be high in linkage disequilibrium scores was selected in our study.
1.3. Population Pharmacokinetics
The Population Pharmacokinetic (PPK) study method can quantitatively estimate the inter-patient variability of pharmacokinetic responses, the internal variability of patients, and clarify the impact of demographic, clinical, and genetic factors on pharmacokinetics. Meanwhile, PPK is suited for constructing pharmacokinetic models in a relatively large number of subjects, even if each subject has only sparse samples. Few studies show that SLCO1B genetic polymorphism has an impact on the pharmacokinetics of MTX.
This study aimed to establish a PPK model of MTX in the Chinese population and assess the impact of SLCO1B1 and SLCO1B3 genetic polymorphisms on MTX pharmacokinetics. Our secondary objective was to evaluate the bioequivalence of MTX formulations by using the constructed PPK model.
2. METHODS
2.1. Patients and Data
Seventy-one patients with rheumatoid arthritis who were treated with low-dose MTX therapy were enrolled in this study to clarify the population pharmacokinetics of MTX. All patients underwent a comprehensive medical examination, determined their weight and height, besides, calculated body surface area. All subjects get blood biochemical examination of Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), total Bilirubin (BUN), Gamma-glutamyl Transpeptidase (GGT), C-reactive Protein (CRP), Creatinine Clearance (Ccr), serum creatinine, and Glomerular Filtration Rate (GFR) was calculated by Cockcroft-Gault formula. They also performed the blood routine examination to determine Hemoglobin (HGB), Red Blood Cell Counts (RBC), red blood cell-specific volume (HCT), Erythrocyte Sedimentation Rate (ESR), and erythrocyte Mean Corpuscular volume (MCY). Above clinical laboratory examinations were accomplished by the Department of Laboratory Medicine at Peking University First Hospital. Based on the information recorded in the electric medical record system, we collected the patients’ demographic basic information and blood sample pathological information. Moreover, the combined using drugs (including folic acid treatment, prednisolone, hydroxychloroquine, calcitriol, leflunomide) were recorded. This study was ratified by the local ethics committee, and the physician informed the patients about the research content.
2.2. Determination of MTX Concentration
We used Aminopterin as an internal standard to analyze MTX concentration by validated Liquid Chromatography-Mass Spectrometry detection method. The calibration curve of methotrexate is linear with 0.5-200 ng/mL linear range, 0.5ng/mL the lower limit of quantification. The Relative Standard Deviations (RSD) of intra and inter precisions were less than 15%, and the accuracies were within ± 15% for methotrexate.
2.3. Genotype Analysis
Extracted DNA from 5 mL blood samples using the TIANamp® DNA Blood DNA kit (TIANgen, Beijing, China) following the manufacturer’s instructions. Identification of OATP1B1 rs4149056, OATP1B1 rs2306283, and OATP1B3 rs7311358. We detected the genotypes of each subject at the OATP1B1 and OATP1B3 alleles by using the Sanger sequencing method as previously described. The sequencing profile was 95°C for 15s followed by 35 cycles at 95°C for 15 seconds, 50°C for 5 seconds, and 60°C for 90 seconds. According to the Hardy-Weinberg Equilibrium (HWE), we used the Chi-square test to determine the genotype distribution.
2.4. Population Pharmacokinetic Modeling
The pharmacokinetic characteristics of methotrexate are described by one-compartment and two-compartment model. We used the nonlinear mixed effects modeling tool NONMEM (version 7.3, ICON Development Solutions) to conduct the modeling analysis. It is assumed that the model parameters follow a lognormal distribution, and the inter-individual variability of each structural parameter is modeled in NONMEM as follows (equation 1):
Pi= PTV × Exp(ηi) (1)
Where Pi represents parameters for the ith individual, PTV is the typical values of the parameters, and ηi are random variables with zero mean and variance of ω2.
The residual variability was modeled using proportional error, as shown in equation 2.
Cij = Cpred,ij × (1+ εprop,ij) (2)
Where Cij is the jth observation for the ith subject, Cpred, ij is the jth predicted value for the ith subject, εprop,ij is the proportional portions of intra-individual variability with means of zero and variances of σprop2.
The model was established based on the First-order Conditional Estimation (FOCE) method with η-ε interaction, and likelihood ratio tests, parameter rationalities and residual analysis were used as the basis to select models. A decrease in Objective Function Value (OFV) >6.63 (p=0.01, x2 distribution with degree of freedom=1) was considered statistically significant.
The covariate analysis was performed after the base model was determined. Drew scatter plots with parameters to explore the relationship between covariates and parameters which reckoned from the determined base model and potential covariates (including sex, age, weight, height, BSA, BUN, MCV, CR, eGFR, HGB, HCT, RBC, ALT, AST, GGT, Ccr, CRP, ESR, folic acid, lef, hcq, Caltrate, pred, Rocaltrol, and genotype). We applied the Stepwise Covariate Model (SCM) module in Perl-speaks-NONMEM software (version 3.4.2, Uppsala University, Sweden) to screen and identify covariate.
Covariate relationships as a linear function, exponential function, and power function were assessed for continuous covariates, and a stepwise approach was used to evaluate covariate effects. On the basis of the statistical criteria, the covariate is considered to be applicable to the model when the reduction of OFV (p=0.05, χ2 distribution with one degree of freedom) is more than 3.84 in the forward step and the rise of OFV (p=0.01, χ2 distribution with one degree of freedom) is less than 6.63 in the backward step.
We used bootstrap resampling technique when validating models. The advantage is that the calculation of certain statistics with a nonparametric bootstrap method does not depend on the assumption of the sample distribution. The basic idea is to use the Monte Carlo method repeated sampling with replacement in the sample, thus forming a self-sampling. Once the self-sampling reaches a certain frequency, it forms a statistical distribution, and the original sample statistics can then be estimated with a semi-empirical method. Typical parameters of the final models simulated the concentration profiles and evaluated the model’s predictive performance by comparing with observed data.
3. RESULTS
3.1. Patient Characteristics
The patients provided 85 MTX blood concentration points which were available for analysis. Table 1 and Table 2 depict the main characteristic and genotype of the patients, respectively. In the 71 subjects analyzed, the minor allele frequencies of OATP1B1 rs2306283 A>G was 23.94%, OATP1B1 rs4149056 T>C was 7.74%, and OATP1B3 rs7311358 A > G was 21.83%. Genotype frequencies were in HWE.
Table 1. Characteristics of the included patients.
| Characteristic | Number of Patients/Mean (SD) |
|---|---|
| Number of patients | 71 |
| Number of concentrations | 85 |
| GEND(Male/Female) | 60/11 |
| WT(kg) | 59.37(10.74) |
| AGE(y) | 48.01(15.24) |
| HT(m) | 1.61(0.06) |
| BSA(m2) | 1.6(0.16) |
| LEF(0/1) | 54/17 |
| FOLIC(0/1) | 25/46 |
| HCQ(0/1) | 48/23 |
| CAL(0/1) | 48/23 |
| PRE(0/1) | 60/11 |
| BUN(mmol/L) | 4.9(1.28) |
| RBC(109) | 4.11(0.75) |
| GGT(U/L) | 21.66(15.8) |
| ESR(mm/h) | 21.52(19.09) |
| RS2306283(1/2/3) | 42/23/6 |
| RS4149056(2/3) | 11/60 |
| RS7311358(1/2/3) | 2/26/43 |
Table 2. Gene frequency.
| Gene | SNP | Allele Genotype | Genotype Frequency n (%) | MAF/% | HWE |
|---|---|---|---|---|---|
| OATP1B1 | rs2306283 | G/A | GG 43 (59.72%) AG 22 (30.99%) AA 6 (8.45%) |
23.94 (A) |
0.209 |
| OATP1B1 | rs4149056 | T/C | TC 11 (15.49%) TT 60 (84.51%) |
7.74 (C) |
0.479 |
| OATP1B3 | rs7311358 | G/A | AA 42(59.15%) AG 27(38.02%) GG 2(2.82%) |
21.83 (G) |
0.336 |
| SHMT1 | rs1979277 | G/A | AG 14(19.72%) GG 57(80.28%) |
9.86 (A) |
0.357 |
| SLC19A1 | rs1051266 | G/A | AA 17(23.94%) AG 35(49.30%) GG 19(26.76%) |
48.59 (A) |
0.911 |
3.2. Population Pharmacokinetics
Fig.A-DVIPRED and Fig.A-DVPRED depict the base model and shows that the mean population of apparent Clearance(CL/F) was 5.98L/h and V/F was 32.8L. It was found that RBC, OATP1B1 rs4149056 T>C, OATP1B1 rs2306283 A>G, and SLC19A1 rs1051266 G>A have an impact on CL/F in the forward inclusion. Therefore, these covariates were included in the full model.
Fig.
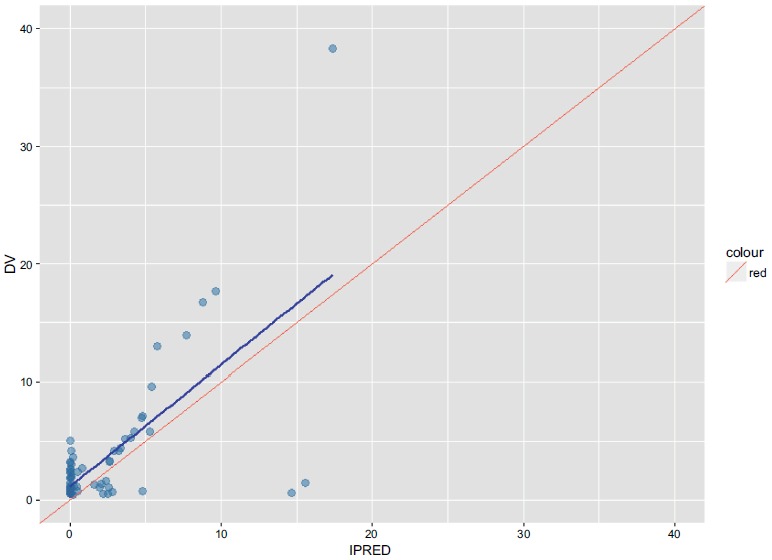
A-DVIPRED (Base Model).
Individual predicted concentrations versus observed concentrations (Base Model).
DV Observed methotrexate concentrations.
IPRED Individual predicted concentrations.
Fig.
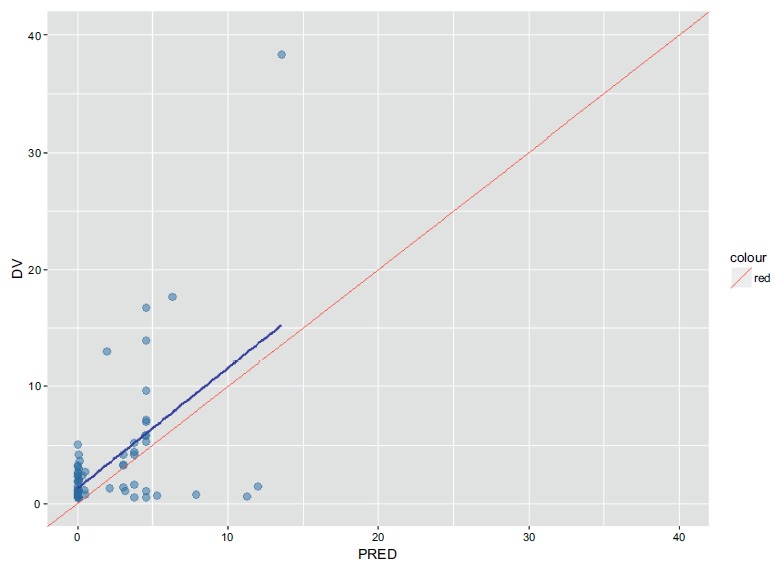
A-DVPRED (Base Model).
Population predicted concentrations versus observed concentrations. (Base Model).
DV Observed methotrexate concentrations.
PRED population predicted concentrations.
3.3. Population Pharmacokinetic Modeling
The backward deletion results showed that RBC, OATP1B1 rs4149056 T>C, and SLC19A1 rs1051266 G>A did not reach a significance level; Consequently, OATP1B1 rs2306283 A>G remained as a significant covariate in the final model. Table 3 summarizes the backward detection results. The final model was described by equation followed:
RS230 = 1:CL = 7.75 × e0.167 × 0.805
RS230 = 2:CL = 7.75 × e0.167 × 0.647
RS230 = 3:CL = 7.75 × e0.167 × 1
V = 32.8 KA = 1.69 F = 0.704
These covariates failed to found to have an impact on V/F. Parameters reckoned from the final model are described in Table 3, and the goodness-of-fit plots are shown in Figs. (1, 2, 3 and 4). There was no significant deviation showed between the observed concentration and predicted concentration, and the final model was considered to be able to describe the data adequately.
Table 3. The final parameter estimates and 95% confidence interval from the bootstrap analysis. Trying Times:500, Success Times:499, Fail Times:1, Success rate:99.8%.
| Parameter | NONMEM Parameter | Bootstrap Median | Bootstrap 95% CI |
|---|---|---|---|
| θ1 CL/F (L/h) | 7.75 | 8.04 | (6.02, 32.19) |
| θ2 V/F (L) | 32.8 FIX | 32.8 FIX | - |
| θ3 Ka (h/L) | 1.69 FIX | 1.69 FIX | - |
| θ4 F | 0.704 FIX | 0.704 FIX | - |
| θ5 RS2306283 on CL | 0.805 | 0.760 | (0.188, 1.051) |
| θ6 RS2306283 on CL | 0.647 | 0.589 | (0.147, 0.915) |
| ωCL | 0.167 FIX | 0.167 FIX | - |
| ωv | 0 FIX | 0 FIX | - |
| ωKa | 0 FIX | 0 FIX | - |
| ωF | 0 FIX | 0 FIX | - |
| σ1 (pro) | 0.713 | 0.693 | (0.412, 0.888) |
| σ2 (add) | 2.83 | 2.65 | (1.52, 3.98) |
NONMEM Parameter: parameter estimated by NONMEM; Bootstrap Median: median estimated by Bootstrap; Bootstrap 95% CI: 95% confidence of median in Bootstrap.
CL/F, apparent clearance; V/F: apparent volume of distribution; Ka: absorption rate constant;
F: bioavailability; RS2306283: gene of RS2306283; ωCL: inter-individual variability of CL;
ωV: inter-individual variability of V; σ1 (pro): proportional error; σ2 (add): additive error.
a From 499 runs with a successful minimization and successful covariance.
b 2.5th and 97.5th percentiles of the bootstrap parameter estimates.
Fig. (1).
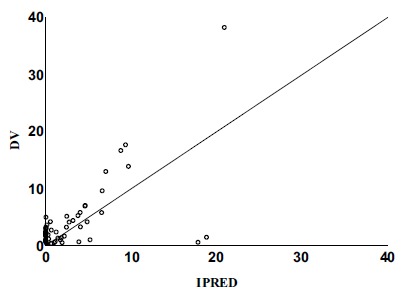
Individual predicted concentrations versus observed concentrations (Final Model).
DV Observed methotrexate concentrations.
IPRED Individual predicted concentrations.
Fig. (2).
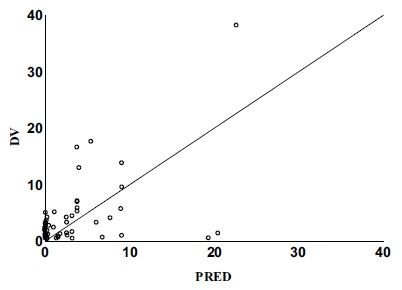
Population predicted concentrations versus observed concentrations. (Final Model).
DV Observed methotrexate concentrations.
PRED population predicted concentrations.
Fig. (3).
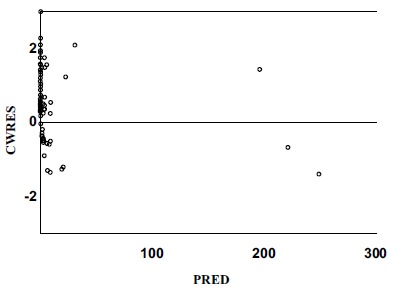
Conditional weighted residuals versus population predicted concentrations. (Final Model).
CWRES Conditional weighted residuals.
PRED Population predicted concentrations.
Fig. (4).
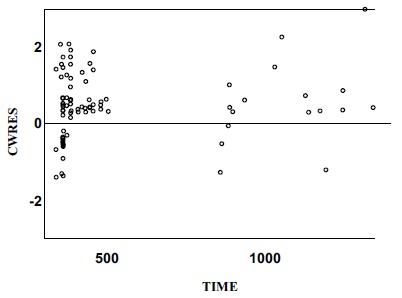
Conditional weighted residuals versus time. (Final Model).
CWRES: Conditional weighted residuals.
3.4. Model Evaluation
The relationship between the observed concentration and the population-model-predicted concentration / the individual-model-predicted concentration was demonstrated in the final model diagnostic plots, which indicated model and data fits well (Figs. 1 and 2). The plots of conditional weighted residuals versus population model-predicted concentration and conditional-weighted residuals versus time were symmetrically distributed and were mostly within three units of the null ordinate, indicating a good fit of the model to the data (Figs. 3 and 4).
From 500 keeps running of the bootstrap, 499 runs were minimal effectively with a fruitful covariance step and were fused in the analysis. The outcomes of the bootstrap analysis are displayed in Table 3. The 95% CI of all parameters from NONMEM was like the range acquired from the bootstrap approach, aside from V/F. Consequently, the estimated parameters and their 95% CI acquired by using NONMEM can be regarded reliable.
4. DISCUSSION
This study is the first to investigate the effects of OATP1B1 on MTX pharmacokinetics in Chinese adult patients with rheumatoid arthritis using population pharmacokinetics analysis. The impact of OATP1B1 and OATP1B3 polymorphisms on MTX pharmacokinetics was also investigated.
Fitting of the methotrexate compartment model is often based on a one-compartment or two-chamber model [16-18]. Although the one-compartment model may underestimate the peak concentration, the data points in this study are sparse. In this case, using a two-compartment model for fitting will result in model instability and large deviations in pharmacokinetic parameters. Moreover, according to our research results, a more complex model does not optimize the results. Therefore, according to the literature [18] and the situation that the concentration of blood drug concentration is less, the preliminary selection of the one-compartment model is the basic model for the pharmacokinetic study of the test population. The one-compartment model explained MTX pharmacokinetics.
The estimated CL/F we acquired is coherent with the value gotten from former MTX PPK study (6.53-7.0 L/h) [17, 18]. For instance, Bannwarth et al. mentioned in a review that the body clearance of MTX is 4.8-7.8 L/h [19]. Consequently, the data were fitted to the one-compartment model with first-order absorption and elimination rules. The CL/F estimated parameters are similar to the previous MTX PPK parameters.
Because of the final regression model, MTX CL/F diminished by 32.3% in patients carrying the OATP1B1-388AG and 17.8% in patients carrying the OATP1B1 -388GG genotypes paralleled with those carrying the OATP1B1-388 AA gene, suggesting the OATP1B1 transporters’ activity is relatively low in these groups of patients. The results obtained in this study are consistent with a previous study which reported that patients carried the OATP1B1-388AG or GG genotype showed a lower CL/F than those carrying the AA genotype. At the same time, in patients with the SLCO1B1-388AG and AA genotypes, MTX dose-corrected trough concentrations were significantly lower than those of the GG genotype. Therefore, it is speculated that the rs2306283 A will increase the OATP1B1 activity. The increase of the liver uptake thus promotes its clearance and lowers the plasma concentration.
At present, studies on this site are more common in statins [20]. Changes in the AUC of several statins vary from 1.2- to 3.2-fold are highly dependent on the impact of SLCO1B1 genotype [21, 22]. In vivo studies reported that the rs2306283 polymorphism is related to the enhanced transport activity of pravastatin by OATP1B1 [23], which is consistent with the results presented in this paper. Nakai et al. reported in the article that several polymorphisms of this gene alter the transportation activity [24]. The OATP1B1 transport activity of several statins, including pravastatin, can be reduced by more than 50% by the 388A>G polymorphism [21]. Nevertheless, the influence of the polymorphisms on the pharmacokinetics of MTX used in RA in these studies was not investigated. However, the relevant mechanisms still need to be further studied.
Membrane transporters are now considered to be essential determinants of drug transmembrane convey. Organic anion transporting polypeptides constituent a family of influx transporters expressed in different tissues significant for pharmacokinetics. OATP1B1 and OATP1B3, which are highly expressed in hepatocytes sinusoidal membrane, can advance MTX’s liver uptake.
All OATP proteins have twelve trans-membrane domains, and 388A>G polymorphism is present in one of the extracellular loops [25]. This polymorphism changes the amino acid asparagine to aspartic acid, which in turn increases the overall negative charge of the protein. Substrate recognition or binding is the first step in the transportation of any molecule through a membrane. Specific substrate recognition by all OATPs is usually high molecular weight molecules with steroid nucleus [26].
Non-alcoholic fatty liver disease refers to extensive accumulation of fat in the liver, excluding alcohol and other definite liver damage factors. The morbidity of non-alcoholic fatty liver disease ranges from 15% to 40%, which is widely variable in different parts of the world [27]. Non-alcoholic fatty liver disease can change the expression of multiple OATP subfamily, including OATP 1A4, 1B1, 1B2, 2B1 [28, 29]. Besides, non-alcoholic steatohepatitis is associated with increased the expression level of OATP1B1, OATP1B3 [30]. Hence, patients with non-alcoholic fatty disease or non-alcoholic steatohepatitis should be treated more carefully when they are taking MTX to avoid liver damage or renal damage. In future research, we also need to explore the MTX PPK in patients with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis.
This study has some limitation. First, methotrexate is mainly excreted by the kidneys. After 24 hours of taking methotrexate, 80% of methotrexate is eliminated from the body through urine as it is. Animal experiments [31] and clinical observations [32] showed that the elimination half-life of methotrexate was significantly prolonged even if the reduction of renal function was not apparent. Moreover, Ran Guoxia [17] and C. Godfrey [16] thought that creatinine clearance, which represents the renal function, has a significant effect on overall clearance. However, this study did not show that clearance significantly (P<0.05) affected the overall clearance of MTX. The reason may be that 71 patients were collected in this study. All of them were regularly reviewed during medication, and creatinine clearance was well controlled. The collected data showed that their creatinine clearance values were closer. Moreover, the influence of SLCO1B1 *5 and SLCO1B1 *15 haplotypes (521T→C) could not be identified, which may be due to the small-scale subjects enrolled in this study and low allele frequencies in the study data. Finally, most of the blood samples were collected on trough concentration; accordingly, the remaining pharmacokinetic parameters (including Ka and the IIV of V/F and Ka) could not be estimated.
Despite these limitations, this study provides evidence for the impact of SLCO1B1-388 A>G polymorphisms on MTX pharmacokinetics. Patients carrying SLCO1B1 AG or GG have lower CL/F of MTX. Hence, patients carrying SLCO1B1 AG or GG should be treated a lower dose of MTX therapy. The formula derived from this study may be instructive for the use of initial MTX dose in those patients.
CONCLUSION
In this study, it was observed that OATP1B1-388 G>A SNP had a significant impact on CL/F. The factor should be considered when determining MTX dosing. However, prospective studies with a large sample size subjects are needed to validate the results of this study.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethics approval and consent to participate was approved by Peking University First Hospital Clinical Research Ethics Committee, and the approval number is 2013[672].
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONSENT FOR PUBLICATION
The physician informed the patients about the research content.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research was supported by National Natural Science Foundation of China (No.81373487).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Visser K.H.D., Dougados M. Methotrexate in rheumatoid arthritis: Experience and recommendations from the 3E initiative. Int. J. Clin. Rheumatol. 2009;4:239–243. doi: 10.2217/ijr.09.13. [DOI] [Google Scholar]
- 2.Tugwell P., Bennett K., Gent M. Methotrexate in rheumatoid arthritis. Indications, contraindications, efficacy, and safety. Ann. Intern. Med. 1987;107(3):358–366. doi: 10.7326/0003-4819-107-2-358. [DOI] [PubMed] [Google Scholar]
- 3.Stamp L.K., Roberts R.L. Effect of genetic polymorphisms in the folate pathway on methotrexate therapy in rheumatic diseases. Pharmacogenomics. 2011;12(10):1449–1463. doi: 10.2217/pgs.11.86. [DOI] [PubMed] [Google Scholar]
- 4.Sokka T., Kautiainen H., Toloza S., Mäkinen H., Verstappen S.M., Lund Hetland M., Naranjo A., Baecklund E., Herborn G., Rau R., Cazzato M., Gossec L., Skakic V., Gogus F., Sierakowski S., Bresnihan B., Taylor P., McClinton C., Pincus T., QUEST-RA Group QUEST-RA: Quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann. Rheum. Dis. 2007;66(11):1491–1496. doi: 10.1136/ard.2006.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan C., Lunt M., Brightwell H., Bradburn P., Fallow W., Lay M., Silman A., Bruce I.N. Contribution of patient related differences to multidrug resistance in rheumatoid arthritis. Ann. Rheum. Dis. 2003;62(1):15–19. doi: 10.1136/ard.62.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian H., Cronstein B.N. Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 2007;65(3):168–173. [PubMed] [Google Scholar]
- 7.Rowland M.T.T. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 4th ed. PA: Wolters Kluwer; 2011. [Google Scholar]
- 8.Inoue K., Yuasa H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab. Pharmacokinet. 2014;29(1):12–19. doi: 10.2133/dmpk.DMPK-13-RV-119. [DOI] [PubMed] [Google Scholar]
- 9.Durmus S., Lozano-Mena G., Van Esch A., Wagenaar E., Van Tellingen O., Schinkel A.H. Preclinical mouse models to study human OATP1B1- and OATP1B3-mediated drug-drug interactions in vivo. Mol. Pharm. 2015;12(12):4259–4269. doi: 10.1021/acs.molpharmaceut.5b00453. [DOI] [PubMed] [Google Scholar]
- 10.Pasanen M.K., Neuvonen P.J., Niemi M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics. 2008;9(1):19–33. doi: 10.2217/14622416.9.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Xu L.Y., He Y.J., Zhang W., Deng S., Li Q., Zhang W.X., Liu Z.Q., Wang D., Huang Y.F., Zhou H.H., Sun Z.Q. Organic anion transporting polypeptide-1B1 haplotypes in Chinese patients. Acta Pharmacol. Sin. 2007;28(10):1693–1697. doi: 10.1111/j.1745-7254.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith N.F., Marsh S., Scott-Horton T.J., Hamada A., Mielke S., Mross K., Figg W.D., Verweij J., McLeod H.L., Sparreboom A. Variants in the SLCO1B3 gene: Interethnic distribution and association with paclitaxel pharmacokinetics. Clin. Pharmacol. Ther. 2007;81(1):76–82. doi: 10.1038/sj.clpt.6100011. [DOI] [PubMed] [Google Scholar]
- 13.Hamada A., Sissung T., Price D.K., Danesi R., Chau C.H., Sharifi N., Venzon D., Maeda K., Nagao K., Sparreboom A., Mitsuya H., Dahut W.L., Figg W.D. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin. Cancer Res. 2008;14(11):3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith N.F., Acharya M.R., Desai N., Figg W.D., Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol. Ther. 2005;4(8):815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 15.Gong I.Y., Kim R.B. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet. 2013;28(1):4–18. doi: 10.2133/dmpk.DMPK-12-RV-099. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey C., Sweeney K., Miller K., Hamilton R., Kremer J. The population pharmacokinetics of long-term methotrexate in rheumatoid arthritis. Br. J. Clin. Pharmacol. 1998;46(4):369–376. doi: 10.1046/j.1365-2125.1998.t01-1-00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi G.T.Y., Li H. Population pharmacokinetics of methotrexate in patients with rheumatoid arthritis. Zhongguo Lin Chuang Yao Li Xue Za Zhi. 2001;17:125–128. [Google Scholar]
- 18.Zhang C.G.J., Li Y. Population pharmacokinetic study of 329 children with acute lymphoblastic leukemia treated with high-dose methotrexate. J. Exp. Hematol. 2008;16:106–110. [PubMed] [Google Scholar]
- 19.Bannwarth B., Péhourcq F., Schaeverbeke T., Dehais J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin. Pharmacokinet. 1996;30(3):194–210. doi: 10.2165/00003088-199630030-00002. [DOI] [PubMed] [Google Scholar]
- 20.Romaine S.P., Bailey K.M., Hall A.S., Balmforth A.J. The influence of SLCO1B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J. 2010;10(1):1–11. doi: 10.1038/tpj.2009.54. [DOI] [PubMed] [Google Scholar]
- 21.Tirona R.G., Leake B.F., Merino G., Kim R.B. Polymorphisms in OATP-C: Identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 2001;276(38):35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 22.Pasanen M.K., Neuvonen M., Neuvonen P.J., Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet. Genomics. 2006;16(12):873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 23.Maeda K., Ieiri I., Yasuda K., Fujino A., Fujiwara H., Otsubo K., Hirano M., Watanabe T., Kitamura Y., Kusuhara H., Sugiyama Y. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin. Pharmacol. Ther. 2006;79(5):427–439. doi: 10.1016/j.clpt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Nakai D., Nakagomi R., Furuta Y., Tokui T., Abe T., Ikeda T., Nishimura K. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J. Pharmacol. Exp. Ther. 2001;297(3):861–867. [PubMed] [Google Scholar]
- 25.Michalski C., Cui Y., Nies A.T., Nuessler A.K., Neuhaus P., Zanger U.M., Klein K., Eichelbaum M., Keppler D., Konig J. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J. Biol. Chem. 2002;277(45):43058–43063. doi: 10.1074/jbc.M207735200. [DOI] [PubMed] [Google Scholar]
- 26.Hagenbuch B., Meier P.J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003;1609(1):1–18. doi: 10.1016/S0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., Li X., Chien N., Wei M., Ogawa E., Zhao C., Wu X., Stave C.D., Henry L., Barnett S., Takahashi H., Furusyo N., Eguchi Y., Hsu Y.C., Lee T.Y., Ren W., Qin C., Jun D.W., Toyoda H., Wong V.W., Cheung R., Zhu Q., Nguyen M.H. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 28.Clarke J.D., Cherrington N.J. Genetics or environment in drug transport: The case of organic anion transporting polypeptides and adverse drug reactions. Expert Opin. Drug Metab. Toxicol. 2012;8(3):349–360. doi: 10.1517/17425255.2012.656087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditzel E.J., Li H., Foy C.E., Perrera A.B., Parker P., Renquist B.J., Cherrington N.J., Camenisch T.D. Altered hepatic transport by fetal arsenite exposure in diet-induced fatty liver disease. J. Biochem. Mol. Toxicol. 2016;30(7):321–330. doi: 10.1002/jbt.21796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke J.D., Novak P., Lake A.D., Hardwick R.N., Cherrington N.J. Impaired N-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease. Liver Int. 2017;37(7):1074–1081. doi: 10.1111/liv.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami Y., Yamazaki K., Sakauchi N., Ogasawara H., Yamashita N., Masuda T., Tauchi K. A one-month repeated oral dose toxicity study of methotrexate in unilaterally nephrectomized rats. J. Toxicol. Sci. 1998;23(Suppl. 5):681–699. doi: 10.2131/jts.23.SupplementV_681. [DOI] [PubMed] [Google Scholar]
- 32.Bressolle F., Bologna C., Kinowski J.M., Sany J., Combe B. Effects of moderate renal insufficiency on pharmacokinetics of methotrexate in rheumatoid arthritis patients. Ann. Rheum. Dis. 1998;57(2):110–113. doi: 10.1136/ard.57.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


