Abstract
Renal impairment is associated with a poor prognosis in patients with multiple myeloma (MM), and more treatment options are needed. The pharmacokinetics of elotuzumab, a humanized IgG1 monoclonal antibody, combined with lenalidomide and dexamethasone, is not significantly different between patients with MM with and without renal impairment, suggesting that elotuzumab might be administered without dose adjustment for renal function.
Introduction:
The present study evaluated the pharmacokinetics and safety of elotuzumab, a humanized IgG1 monoclonal antibody against signaling lymphocyte activation molecule-F7, combined with lenalidomide and dexamethasone, in patients with multiple myeloma (MM) and renal impairment.
Patients and Methods:
Patients with MM and normal renal function (NRF) (creatinine clearance [CrCl] ≥ 90 mL/min), severe renal impairment (SRI) (CrCl < 30 mL/min, not requiring dialysis), or end-stage renal disease (ESRD) (requiring dialysis) were enrolled in this open-label, phase Ib study. Elotuzumab (10 mg/kg), lenalidomide (5–25 mg), and dexamethasone (40 mg) were administered in 28-day cycles until disease progression or unacceptable toxicity developed. The primary endpoint was single-dose elotuzumab pharmacokinetics.
Results:
A total of 26 patients (median age, 63 years) were treated (NRF, n = 8; SRI, n = 9; ESRD, n = 9). The median baseline CrCl was 105 mL/min (range, 84–146 mL/min) for those with NRF and 26 mL/min (range, 15–33 mL/min) for those with SRI. Twenty-three patients (89%) had received previous therapy (median, 2 regimens; range, 1–7). Treatment was discontinued in 6 patients with NRF, 4 with SRI, and 5 with ESRD, primarily because of disease progression. The mean elotuzumab serum concentrations were comparable across groups (n = 23). No statistically significant differences were observed in the maximum observed serum concentration, area under the concentration–time curve from time 0 to the last quantifiable serum concentration, or area under the concentration–time curve from time 0 to infinity when the SRI and ESRD groups were compared with the NRF group (P >.05). All patients had > 1 adverse event (AE). Of the 8 patients with NRF, 9 with SRI, and 9 with ESRD, 7,8, and 7 experienced grade 3 to 4 AEs. The overall response rates were 75% in the NRF, 67% in the SRI, and 56% in the ESRD groups.
Conclusion:
The results of the present study support the use of elotuzumab for the treatment of patients with MM and renal dysfunction without dose adjustment.
Keywords: Creatinine clearance, End-stage renal disease, Glomerular filtration rate, Monoclonal antibody, SLAMF7
Introduction
Renal impairment is a common comorbidity associated with multiple myeloma (MM), with ≤ 50% of patients affected during the course of their disease1 and 10% requiring dialysis.2 In most patients with MM, renal impairment is due to the overproduction of monoclonal free light chains, which causes cast nephropathy (also known as myeloma kidney).1,3 Renal impairment is associated with poor outcomes4 and is an important prognostic factor in MM. The median survival of patients with MM and renal failure has been reported to be 19.5 months compared with 40.4 months for patients without renal failure.5 Furthermore, the reversal of renal impairment in patients with MM has been associated with an improved prognosis and longer overall survival (OS).2,6
Advances in therapy, including the use of immunomodulatory drugs (IMiDs) (eg, lenalidomide, thalidomide, and pomalidomide), proteasome inhibitors (eg, bortezomib), and autologous stem cell transplantation, have greatly improved the life expectancy of patients with MM, including those with impaired renal function.7,8 Continuous lenalidomide and dexamethasone in newly diagnosed patients has demonstrated a median progression-free survival (PFS) of 25.5 months and an OS at 4 years of 59%.9 The 1- and 3-year PFS have also been shown to be superior in patients newly treated with lenalidomide and dexamethasone compared with patients treated with placebo and dexamethasone (78% and 52% vs. 52% and 32%, respectively).10 Furthermore, the overall response and very good partial response (VGPR) rates were 78% and 63% with lenalidomide and dexamethasone and 48% and 16% with placebo and dexamethasone, respectively. An overall response rate (ORR) of 64% was reported for patients with MM and impaired renal function treated with lenalidomide combined with high-dose dexamethasone, with improvements in renal function reported in 72% of patients with MM and mild-to-moderate renal impairment.11 However, because lenalidomide is excreted primarily through the kidney, the half-life of the drug increases and drug clearance decreases linearly with the severity of kidney impairment. Thus, dose adjustments are required according to the creatinine clearance (CrCl).11,12 Dimopoulos et al11 reported that a dose reduction of lenalidomide or interruption because of adverse events (AEs) was necessary in 22% of patients with MM and mild or no renal impairment, 40% of patients with MM and moderate renal impairment, and 38% of patients with MM and severe renal impairment (SRI). Furthermore, patients with SRI treated with lenalidomide plus dexamethasone have been shown to have shorter OS compared with patients with mild or no renal impairment.11 Also, the response rate has been shown to decline with severity of renal impairment.13 To improve the outcome of patients with MM and renal impairment, new alternative efficacious and well-tolerated treatment options are necessary.
Elotuzumab is a humanized IgG1 immunostimulatory monoclonal antibody targeted against signaling lymphocyte activation molecule-F7 (SLAMF7; also referred to as CS1), a glycoprotein expressed on myeloma and natural killer cells but not on normal tissues.14 Through both direct activation and engagement of natural killer cells, elotuzumab selectively targets and kills SLAMF7-expressing myeloma cells with minimal effects on normal tissue.15 A phase I study assessing the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics of elotuzumab (dose range, 0.5–20 mg/kg every 2 weeks) demonstrated that elotuzumab was generally well tolerated at doses sufficient to achieve consistent SLAMF7 saturation (10 or 20 mg/kg).16 No objective responses were seen in this single-agent phase I trial. However, 27% of patients achieved disease stabilization. A phase Ib-II study investigating the safety and efficacy of elotuzumab combined with lenalidomide and dexamethasone demonstrated an ORR of 82% in phase Ib,17 which compared favorably with the historical response rate of 60% with lenalidomide and dexamethasone alone in patients with relapsed or refractory MM (RRMM).18,19 Moreover, in phase II of the study, an ORR of 84% and PFS of 29 months were observed, and treatment was generally well tolerated, with no dose-limiting toxicities reported.20 In the randomized, open-label phase III ELOQUENT-2 study, patients treated with elotuzumab plus lenalidomide and dexamethasone demonstrated an ORR of 79% compared with an ORR of 66% for patients treated with lenalidomide and dexamethasone. A median PFS of 19.4 months versus 14.9 months was observed in the elotuzumab arm and lenalidomide/dexamethasone arm, respectively.21 Bortezomib significantly enhanced elotuzumab activity in a preclinical model,22 and a phase II, randomized, proof-of-concept study demonstrated a median PFS of 9.7 months for patients receiving elotuzumab combined with bortezomib and dexamethasone versus 6.9 months for patients receiving bortezomib and dexamethasone.23
To determine whether elotuzumab could be safely administered with lenalidomide and dexamethasone in patients with renal impairment, the present phase Ib study was conducted to evaluate the PK and safety of elotuzumab combined with lenalidomide and dexamethasone in patients with MM and various levels of renal function (normal renal function [NRF], SRI, and end-stage renal disease [ESRD]).
Patients and Methods
Study Design
The present study was a phase Ib, multicenter, open-label study (ClinicalTrials.gov identifier, ) of elotuzumab combined with lenalidomide and dexamethasone in patients with MM and NRF (CrCl ≥ 90 mL/min), SRI (CrCl <30 mL/min and not requiring dialysis), and ESRD (requiring dialysis). The present study was conducted in accordance with Good Clinical Practice, as defined by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, and the ethical principles of the European Union Directive and the US Code of Federal Regulations. All patients (or, where necessary, legal guardians) provided written, informed consent before participation. The present study was conducted at 8 sites across the United States, with patients enrolled from January 2012 to October 2013. The cutoff for data analysis was June 30, 2014.
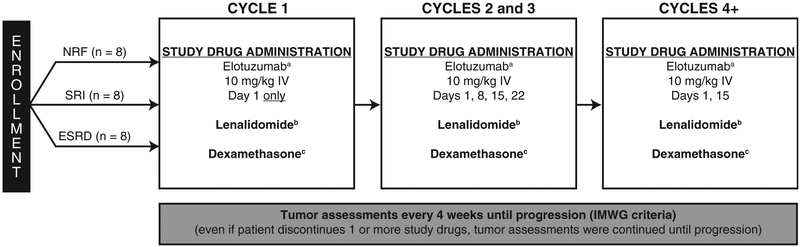
Treatment was administered in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent. The overall study design is shown in Figure 1. During each cycle, elotuzumab was administered intravenously (I.V.) at a dose of 10 mg/kg. Lenalidomide was administered according to renal function (NRF, 25 mg orally [p.o.] once daily; SRI, 15 mg p.o. every 48 hours; ESRD, 5 mg p.o. once daily). Dexamethasone was administered at a dose of 40 mg p.o. on the weeks without elotuzumab infusion and at 8 mg I.V. plus 28 mg p.o. on the weeks with elotuzumab infusion. A premedication regimen was administered 30 to 90 minutes before elotuzumab administration and consisted of an H1 blocker (diphenhydramine; 25–50 mg, or equivalent), an H2 blocker (ranitidine; 50 mg, adjusted for renal failure, or equivalent), and acetaminophen (650–1000 mg). Patients were given daily aspirin, low-molecular-weight heparin, or warfarin, as clinically indicted, for thromboembolic prophylaxis. Tumor assessment was performed every 4 weeks until progression, death, or discontinuation from the study, in accordance with the International Myeloma Working Group (IMWG) criteria.24
Figure 1.
Study Design Showing the Number of Patients Planned for Enrollment. aPremedication With an H1 Blocker (Diphenhydramine, 25–50 mg, or Equivalent), an H2 Blocker (Ranitidine, 50 mg, Adjusted for Renal Failure, or Equivalent), and Acetaminophen (650–1000 mg) Was Required 30–90 Minutes Before Elotuzumab Administration. bIn All Patients, Lenalidomide Was Given Daily for 21 Days of a 28-day Cycle: Normal Renal Function (NRF), 25 mg Orally (p.o.) Once Daily; Severe Renal Impairment (SRI), 15 mg p.o. Every 48 Hours; End-Stage Renal Disease (ESRD), 5 mg p.o. Once Daily. cWeeks Without Elotuzumab: 40 mg p.o.; Weeks With Elotuzumab 8 mg Intravenously (I.V.) Plus 28 mg p.o.
Abbreviation: IMWG = International Myeloma Working Group.
Inclusion and Exclusion Criteria
All patients met the following criteria: age ≥ 18 years; documented evidence of symptomatic newly diagnosed MM or RRMM; NRF, SRI, or ESRD; Eastern Cooperative Oncology Group performance status of ≤ 2; evaluable or measurable disease as defined by the IMWG.24 Previous lenalidomide treatment was permitted if patients had not discontinued lenalidomide because of grade ≥ 3 treatment-related AEs.
The key exclusion criteria included previous or concurrent malignancy, monoclonal gammopathy of unknown significance, Waldenstrom’s macroglobulinemia, or smoldering myeloma; active plasma cell leukemia; acute renal failure owing to readily reversible causes; significant cardiac disease; and previous therapy with elotuzumab or any IMiD (including pomalidomide), except for previous thalidomide or lenalidomide (as defined in the inclusion criteria).
Study Population
The safety population included all patients who had received ≥ 1 dose of the study treatment. The PK population included all patients who had received ≥ 1 dose of elotuzumab and had stable renal function, determined by 2 creatinine measurements ≥ 24 hours apart and within a 14-day screening period. To ensure stable renal function, patients with a significant change in renal function during cycle 1 (ie, level of renal impairment improved or worsened in relation to enrollment category) were excluded.
Study Objectives
The primary objective was to assess the effect of SRI and ESRD on the single-dose PK of elotuzumab. The secondary objective was to assess the safety of elotuzumab combined with lenalidomide and dexamethasone in patients with MM, with or without SRI or ESRD. Other exploratory objectives included the efficacy of elotuzumab combined with lenalidomide and dexamethasone in patients with SRI or ESRD and assessment of the degree and rapidity of renal function improvement in patients with SRI.
Assessments
During cycle 1, blood samples were collected before and at 10 points after elotuzumab administration to evaluate elotuzumab single-dose PK. The PK assessments included the maximum observed serum concentration (Cmax), area under the concentration–time curve from time 0 to the last quantifiable serum concentration [AUC(O–T)L area under the concentration–time curve from time 0 to infinity [AUC(INF)], and total body clearance (CLT). Additional samples were collected from patients with ESRD immediately before and after dialysis. Elotuzumab serum concentrations were assessed using a validated enzyme-linked immunosorbent assay. Throughout the study, blood samples were collected before elotuzumab administration for the detection of antidrug antibodies and assessed using a validated enzyme-linked immunosorbent assay. The first sample was collected on day 1 of cycle 1 and on day 1 of all subsequent cycles.
AE data were gathered through spontaneous reporting or open-ended questioning, examination, or evaluation. All serious adverse events (SAEs) that occurred within 60 days of discontinuation of dosing or within 30 days of the last visit were reported.
For the efficacy assessments, the ORR (defined as a partial response or better) was evaluated every 4 weeks from the date of the first dose of the study drug using the IMWG response assessment criteria. The criteria proposed by Dimopoulos et al25 were used to define the degree of renal response in the SRI group. A minor renal response was defined as sustained improvement of baseline CrCl of < 15 mL/min to 15 to 29 mL/min or improvement of the baseline CrCl of 15 to 29 mL/min to 30 to 59 mL/min.25
Statistical Analysis
PK parameters were determined using WinNonlin, version 5.2 or higher (Pharsight Corporation, Mountain View, CA). Analysis of variance was performed on log-transformed AUC(O–T), AUC(INF), and Cmax, with the renal function group as a fixed effect to assess the effect of renal impairment on elotuzumab PK.
Results
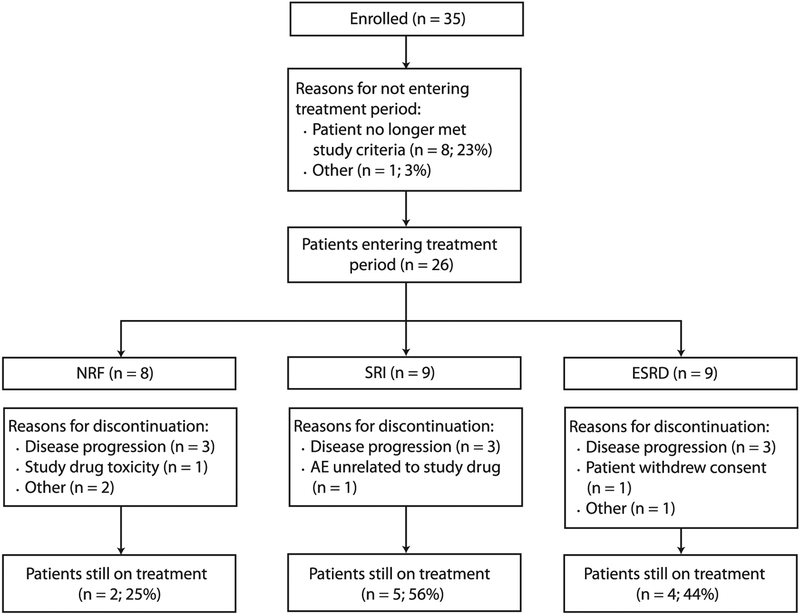
A total of 35 patients were enrolled. Of the 35 patients, 26 (74%) were treated with elotuzumab combined with lenalidomide and dexamethasone. However, 9 (26%) did not receive treatment because they no longer met the study criteria (n = 8) or at the decision of the investigator (n = 1; Figure 2). At the data cutoff point, 11 patients (42%) were still receiving treatment (2 with NRF [25%], 5 with SRI [56%], and 4 with ESRD [44%]), and 15 (58%) had discontinued the study. The most common reason for discontinuation was disease progression (Figure 2). One patient in the NRF group was withdrawn from the study because of study drug toxicity (infusion-related reaction).
Figure 2.
Patient Disposition Flow Diagram. Nine Patients Did Not Enter the Treatment Period After Enrollment for the Following Reasons: Platelet Count Too Low, No Longer Met Criteria for Severe Renal Impairment (SRI), Ineligibility, Best Response Achieved Was Not at Least a Partial Response, Progression With Previous Lenalidomide Therapy, Previous Lenalidomide Exposure Was Discontinued Because of a Grade 3 Adverse Event (AE), Lenalidomide Was Discontinued Because of a Grade 3 AE, Creatinine Clearance of 52 mL/min, and at the Decision of the Investigator (n = 1 for All)
Abbreviations: ESRD = end-stage renal disease; NRF = normal renal function.
The baseline demographics are listed in Table 1. The patients in the SRI group were slightly older, and a greater proportion of patients in the ESRD group were black or African American and had stage III disease (according to the International Staging System). A total of 23 patients (89%) had received previous therapy for MM, which included bortezomib (81%), thalidomide (42%), and lenalidomide (35%). Three patients (12%) had newly diagnosed MM, and 21 (81%) had RRMM. A greater proportion of patients in the ESRD group had disease refractory to their most recent line of therapy (NRF, 38%; SRI, 22%; and ESRD, 56%).
Table 1.
Baseline Characteristics
| Characteristic | NRF (n = 8) | SRI (n = 9) | ESRD (n = 9) |
|---|---|---|---|
| Age (years) | |||
| Median | 59.5 | 75.0 | 56.0 |
| Range | 42–68 | 63–87 | 39–80 |
| Female sex | 3 (38) | 3 (33) | 4 (44) |
| Race | |||
| White | 8 (100) | 6 (67) | 1 (11) |
| Black or African American | 0 | 2 (22) | 8 (89) |
| Asian | 0 | 1 (11) | 0 |
| Creatinine clearance (mL/min) | NAa | ||
| Mean | 105 | 26 | |
| Range | 84–146 | 15–33 | |
| ISS disease stage | |||
| I | 1 (13) | 2 (22) | 2 (22) |
| II | 3 (38) | 4 (44) | 1 (11) |
| III | 3 (38) | 3 (33) | 6 (67) |
| NR | 1 (13) | 0 | 0 |
| Disease status | |||
| Newly diagnosed | 1 (13) | 2 (22) | 0 |
| Refractory | 3 (38) | 2 (22) | 5 (56) |
| Relapsed | 3 (38) | 4 (44) | 4 (44) |
| Unknownb | 1 (13) | 1 (11) | 0 |
| Cytogenetics | |||
| del(17p) | |||
| Yes | 1 (13) | 2 (22) | 1 (11) |
| No | 7 (88) | 7 (78) | 8 (89) |
| t(14;16) | |||
| Yes | 1 (13) | 1 (11) | 0 |
| No | 5 (63) | 8 (89) | 8 (89) |
| NR | 2 (25) | 0 | 1 (11) |
| t(4; 14) | |||
| Yes | 1 (13) | 1 (11) | 0 |
| No | 6 (75) | 8 (89) | 9 (100) |
| NR | 1 (13) | 0 | 0 |
| Patients with ≥ 1 previous line of therapy | 7 (88) | 7 (78) | 9 (100) |
| Previous regimensc | |||
| Median | 2 | 2 | 2 |
| Range | 1–3 | 1–6 | 1–7 |
| Patients with previous therapies | |||
| Bortezomib | 5 (63) | 7 (78) | 9 (100) |
| Thalidomide | 3 (38) | 3 (33) | 5 (56) |
| Lenalidomide | 5 (63) | 2 (22) | 2 (22) |
Data presented as n (%), unless otherwise noted.
Abbreviations: ESRD = end-stage renal disease; ISS = International Staging System; NA = not available; NR = not reported; NRF = normal renal function; SRI = severe renal impairment.
Creatinine clearance was not required for patients in the ESRD group.
Refractory status to most recent line of therapy unknown.
Number of previous regimens applicable only to patients with relapsed or refractory disease.
The patients received a median of 6.5 (range, 2–23), 16.0 (range, 2–25), and 9.0 (range, 2–25) treatment cycles in the NRF, SRI, and ESRD groups, respectively. The median duration of treatment for the NRF, SRI, and ESRD groups was 5.4 months (range, 1.0–21.7 months), 15.5 months (range, 1.4–23.7 months), and 8.1 months (range, 1.0–23.0 months) for elotuzumab; 5.7 months (range, 0.5–21.8 months), 7.2 months (range, 0.3–21.6 months), and 8.6 months (range, 0.7–22.8 months) for lenalidomide; and 5.6 months (range, 1.0–21.7 months), 15.5 months (range, 1.4–23.8 months), and 8.1 months (range, 1.0–23.1 months) for dexamethasone, respectively. The median duration of treatment was lower for the NRF group than for the SRI and ESRD groups because patients discontinued treatment < 6 months from study initiation owing to infusion reaction in 1, physician’s request for 2, and progressive disease after an initial response of stable disease and a partial response in 1 each.
With regard to the relative dose intensity, 5 patients (63%) in the NRF group, 6 (67%) in the SRI group, and 5 (56%) in the ESRD group received ≥ 90% of the planned elotuzumab dose. Two patients in each group (NRF, 25%; SRI, 22%; ESRD 22%) received 80% to < 90% of the planned elotuzumab doses. Two patients (25%) in the NRF group, 4 (44%) in the SRI group, and 3 (33%) in the ESRD group received ≥ 90% of the planned lenalidomide dose. Two patients (25%) in the NRF group, 4 (44%) in the SRI group, and 1 (11%) in the ESRD group received ≥ 90% of the planned dexamethasone dose.
Elotuzumab PK
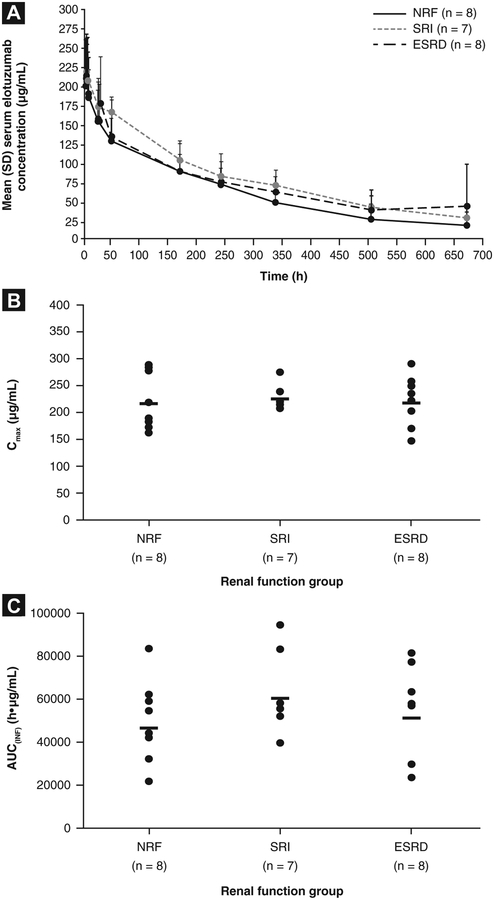
The mean elotuzumab serum concentration profiles were comparable across all treatment groups after single-dose administration in cycle 1 (Figure 3A). No statistically significant differences were observed in Cmax, AUC(O–T)> or AUC(INF) between the SRI and ESRD groups and the NRF group (Table 2; Figure 3B and C).
Figure 3.
Elotuzumab Pharmacokinetics (PK) Values Stratified by Renal Functiona: (A) Elotuzumab Serum Concentration Profiles Over Time From Initial Elotuzumab Doseb; (B) Maximum Observed Serum Concentration (Cmax); (C) Area Under the Concentration-Time Curve From Time 0 to Infinity [AUC(INF)]. aThree Patients Were Excluded From the PK Summary Statistics Because of a Dosing Error (End-Stage Renal Disease [ESRD] Group, n = 1), Estimated Glomerular Filtration Rate (eGFR) Outside the Value Limit Range (Severe Renal Impairment [SRI] Group, n = 1), and Limited Samples or Biologically Implausible Time Corresponding to Cmax (Tmax) at 672 Hours (SRI Group, n = 1). bMean 48-hour Dialysis Values Were Excluded in 1 Patient
Abbreviations: NRF = normal renal function; SD = standard deviation.
Table 2.
Elotuzumab PK Parameters
| Renal Function Groupa | Adjusted Geometric Mean (90% Cl) | Geometric Mean (% CV) | ||
|---|---|---|---|---|
| Cmax (μg/mL) | AUC(0–T) (μg.h/mL) | AUC(INF) (μg.h/mL) | CLT (mL/h/kg) | |
| NRF | 217 (192–245; n = 8) | 39,559 (32,635–47,953; n = 8) | 46,401 (36,221–59,442; n = 8) | 0.22 (46; n = 8) |
| SRI | 226 (198–257; n = 7) | 50,080 (40,769–61,518; n = 7) | 60,225 (46,238–78,522; n = 7) | 0.17 (28; n = 7) |
| ESRD | 218 (193–246; n = 8) | 45,937 (37,896–55,684; n = 8) | 51,227 (39,310–66,756; n = 8) | 0.20 (54; n = 8) |
| SRI vs. NRF (%) | 104 (87–125; P = .704) | 127 (96–168; P = .164) | 130 (90–187; P =.228) | NA |
| ESRD vs. NRF (%) | 100 (85–119; P = .965) | 116 (89–152; P = .355) | 110 (77–159; P = .642) | NA |
Abbreviations: AUC(0-T)= area under the concentration-time curve from time 0 to the last quantifiable serum concentration (calculated by log- and linear-trapezoidal summations); AUC(INF) = area under the concentration-time curve from time 0 to infinity; Cl = confidence interval; CLT = total body clearance; Cmax = maximum observed serum concentration; CV = coefficient of variation; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; NRF = normal renal function; PK = pharmacokinetics; SRI = severe renal impairment; Tmax = time corresponding to Cmax
Three patients were excluded from the PK summary statistics because of a dosing error (ESRD group, n = 1), being outside the range of eGFR value limits (SRI group, n = 1), and having limited samples/biologically implausible Tmax at 672 hours (SRI group, n = 1).
Minor differences in AUC(INF) were observed among the groups. A trend was seen toward a greater AUC(INF) in the SRI and ESRD groups than in the NRF group, although these differences were not statistically significant. In an exploratory analysis, relatively smaller differences in AUC(INF) were observed among the 3 groups after the exclusion of 3 patients (2 in the NRF group and 1 in the ESRD group) who had developed antidrug antibodies on day 1 of cycle 2. The mean AUC(INF) after exclusion of these 3 patients was 53,062, 60,255, and 56,093 μg.h/mL for the NRF, SRI, and ESRD groups, respectively. The mean elotuzumab CLT values were similar among the 3 groups (Table 2).
Safely
All patients experienced ≥ 1 AE. A summary of AEs occurring in ≥ 3 patients in any 1 study group is provided in Table 3. Fatigue, diarrhea, back pain, constipation, and anemia were the most frequently reported AEs (Table 3). Overall, 3 of 26 patients (12%) experienced grade 2 infusion reactions (1 in the NRF group and 2 in the ESRD group).
Table 3.
Adverse Events in ≥ 3 More Patients in Any Study Groupa
| Preferred Term | NRF (n = 8) | SRI (n = 9) | ESRD (n = 9) | |||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Total patients with AE | 8 (100) | 7 (88) | 9 (100) | 8 (89) | 9 (100) | 7 (78) |
| Musculoskeletal and connective tissue disorders | 8 (100) | 1 (13) | 7 (78) | 1 (11) | 5 (56) | 1 (11) |
| Back pain | 3 (38) | 0 | 3 (33) | 1 (11) | 4 (44) | 0 |
| Muscle spasms | 3 (38) | 0 | 1 (11) | 0 | 1 (11) | 0 |
| Gastrointestinal disorders | 7 (88) | 0 | 7 (78) | 1 (11) | 7 (78) | 1 (11) |
| Constipation | 6 (75) | 0 | 3 (33) | 0 | 1 (11) | 0 |
| Diarrhea | 2 (25) | 0 | 5 (56) | 1 (11) | 4 (44) | 1 (11) |
| Nausea | 2 (25) | 0 | 1 (11) | 0 | 3 (33) | 0 |
| Stomatitis | 1 (13) | 0 | 4 (44) | 0 | 1 (11) | 0 |
| General disorders and administration-site conditions | 7 (88) | 2 (25) | 7 (78) | 0 | 9 (100) | 1 (11) |
| Fatigue | 6 (75) | 2 (25) | 4 (44) | 0 | 6 (67) | 0 |
| Pyrexia | 4 (50) | 0 | 1 (11) | 0 | 4 (44) | 0 |
| Malaise | 3 (38) | 0 | 1 (11) | 0 | 0 | 0 |
| Peripheral edema | 1 (13) | 0 | 4 (44) | 0 | 4 (44) | 0 |
| Blood and lymphatic system disorders | 6 (75) | 4 (50) | 6 (67) | 4 (44) | 6 (67) | 4 (44) |
| Neutropenia | 3 (38) | 2 (25) | 1 (11) | 1 (11) | 1 (11) | 1 (11) |
| Anemia | 2 (25) | 0 | 5 (56) | 2 (22) | 3 (33) | 0 |
| Thrombocytopenia | 1 (13) | 0 | 4 (44) | 1 (11) | 3 (33) | 1 (11) |
| Infections and infestations | 6 (75) | 2 (25) | 6 (67) | 1 (11) | 4 (44) | 3 (33) |
| Skin and subcutaneous tissue disorders | 6 (75) | 0 | 6 (67) | 1 (11) | 4 (44) | 0 |
| Hyperhidrosis | 4 (50) | 0 | 1 (11) | 0 | 0 | 0 |
| Rash | 2 (25) | 0 | 4 (44) | 0 | 1 (11) | 0 |
| Pruritus | 1 (13) | 0 | 0 | 0 | 3 (33) | 0 |
| Metabolism and nutrition disorders | 5 (63) | 2 (25) | 7 (78) | 3 (33) | 6 (67) | 3 (33) |
| Hyperglycemia | 3 (38) | 1 (13) | 3 (33) | 2 (22) | 2 (22) | 1 (11) |
| Hypokalemia | 1 (13) | 0 | 3 (33) | 1 (11) | 3 (33) | 0 |
| Decreased appetite | 0 | 0 | 2 (22) | 0 | 3 (33) | 0 |
| Hypocalcemia | 0 | 0 | 3 (33) | 0 | 3 (33) | 2 (22) |
| Nervous system disorders | 5 (63) | 0 | 7 (78) | 4 (44) | 4 (44) | 0 |
| Peripheral neuropathy | 2 (25) | 0 | 1 (11) | 0 | 3 (33) | 0 |
| Syncope | 0 | 0 | 4 (44) | 4 (44) | 0 | 0 |
| Psychiatric disorders | 5 (63) | 0 | 2 (22) | 0 | 3 (33) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 5 (63) | 2 (25) | 5 (56) | 0 | 3 (33) | 1 (11) |
| Cough | 4 (50) | 0 | 2 (22) | 0 | 1 (11) | 0 |
| Dyspnea | 3 (38) | 1 (13) | 2 (22) | 0 | 2 (22) | 1 (11) |
| Cardiac disorders | 4 (50) | 0 | 0 | 0 | 2 (22) | 1 (11) |
| Eye disorders | 3 (38) | 0 | 2 (22) | 1 (11) | 1 (11) | 0 |
| Investigations | 2 (25) | 0 | 7 (78) | 2 (22) | 3 (33) | 2 (22) |
| Increased blood creatinine | 0 | 0 | 5 (56) | 1 (11) | 1 (11) | 1 (11) |
| Injury, poisoning, and procedural complications | 1 (13) | 0 | 4 (44) | 0 | 2 (22) | 0 |
| Renal and urinary disorders | 1 (13) | 0 | 3 (33) | 1 (11) | 0 | 0 |
| Vascular disorders | 1 (13) | 0 | 4 (44) | 3 (33) | 2 (22) | 1 (11) |
Data presented as n (%).
Abbreviations: AE = adverse event; ESRD = end-stage renal disease; NRF = normal renal function; SRI = severe renal impairment.
No grade 5 events occurred.
AEs led to treatment discontinuation in 4 patients (1 in the NRF group [13%] and 3 in the SRI group [33%]). In the NRF group, discontinuation was because of an infusion-related reaction in 1 patient. In the SRI group, discontinuation was because of a soft tissue infection, increased blood creatinine level and psychiatric disorder (agitation), and a skin and subcutaneous tissue disorder (drug eruption) in 1 patient each. The AEs leading to discontinuation were grade 2 and 3 in severity and were considered to be related to study treatment, except for the elevation in the blood creatinine level.
SAEs were reported in 3 patients (38%) in the NRF group (all grade 3 or 4), 5 (56%) in the SRI group (grade 3 or 4 in 3 [33%]), and 7 (78%) in the ESRD group (grade 3 or 4 in 6 [67%]). Pneumonia (NRF group, n = 1; ESRD group, N = 1) and upper respiratory tract infection (SRI group, n = 1; ESRD group, n = 1) were the most common grade 3 and 4 SAEs. No deaths occurred.
Efficacy
Elotuzumab combined with lenalidomide and dexamethasone resulted in overall responses in 6 (75%), 6 (67%), and 5 (56%) patients in the NRF, SRI, and ESRD groups, respectively (Table 4). A VGPR or better was observed in 3 (38%), 5 (56%), and 1 (11%) patients in the NRF, SRI, and ESRD groups, respectively.
Table 4.
Efficacy Stratified by Renal Function
| Treatment Response | NRF (n = 8) | SRI (n = 9) | ESRD (n = 9) |
|---|---|---|---|
| Best overall response | |||
| Stringent complete response | 0 | 1 (11) | 0 |
| Complete response | 2 (25) | 0 | 0 |
| Very good partial response | 1 (13) | 4 (44) | 1 (11) |
| Partial response | 3 (38) | 1 (11) | 4 (44) |
| Minor response | 0 | 3 (33) | 2 (22) |
| Stable disease | 1 (13) | 0 | 1 (11) |
| Progressive disease | 0 | 0 | 0 |
| Not evaluable | 1 (13) | 0 | 1 (11) |
| ORR (%; 95% Cl) | 6 (75; 35–97) | 6 (67; 30–93) | 5 (56; 21–86) |
| Renal responsea | 0 | 2 (22) | 0 |
Data presented as n (%), unless otherwise noted.
Abbreviations: CI = confidence interval; ESRD = end-stage renal disease; NRF = normal renal function; ORR = overall response rate; SRI = severe renal impairment.
Minor renal response.
A minor renal response, based on the estimated CrCl values, was observed in 2 patients (22%) in the SRI group (Table 4). These occurred in 1 patient with newly diagnosed MM and 1 patient with relapsed MM. No patient achieved a complete or partial renal response across all groups.
Discussion
The findings from the present phase Ib study indicate that renal dysfunction (SRI or ESRD) does not significantly affect elotuzumab PK when administered in combination with lenalidomide and dexamethasone. These data suggest that elotuzumab 10 mg/kg, combined with lenalidomide and dexamethasone, can be administered safely to patients with MM and SRI or ESRD without the need to adjust the dose of elotuzumab according to renal function. A trend toward a greater AUC(INF) was observed in patients with renal dysfunction compared with patients with NRF. However, slight differences in elotuzumab PK exposure seem unlikely to affect clinical efficacy. The presence of antidrug antibodies can affect the PK of biologic compounds.26 The exclusion of 3 patients positive for antidrug antibodies resulted in a smaller difference in the AUC(INF) among the 3 patient cohorts; however, additional investigation would be required to confirm the effect of antidrug antibodies on the systemic clearance of elotuzumab. Furthermore, based on PK analyses performed in 375 patients across elotuzumab trials, no effect on the glomerular filtration rate (range, 4.58–124 mL/min/1.73 m2) has been observed after elotuzumab administration (Bristol-Myers Squibb; data on file). The absence of a relationship between renal function and elotuzumab PK is consistent with renal physiology, because the large size of elotuzumab (approximately 144 kDa) is expected to prevent it from being filtered through the glomerulus and eliminated by the kidney.27 The mean serum elotuzumab concentrations in the pre- and postdialysis blood samples were comparable, demonstrating that elotuzumab is not likely to be extracted to a significant extent in the dialysate.
The tolerability of elotuzumab combined with lenalidomide and dexamethasone across all classes of renal function is consistent with what has been previously described for lenalidomide plus dexamethasone alone. Treatment discontinuation in the present study most commonly resulted from disease progression, with AEs leading to treatment discontinuation in 1 patient with NRF and 3 patients with SRI. Moreover, grade 2 infusion reactions were only experienced by 3 patients: 1 in the NRF group and 2 in the ESRD group, suggesting that the rate of infusion-related reactions did not appear increased in patients with compromised renal function.
Efficacy was evaluated in an exploratory manner owing to the small sample size in the present study. However, the ORR in the NRF group was consistent with that previously reported for elotuzumab combined with lenalidomide and dexamethasone.17 As reported in other studies of patients with renal impairment, ORR was progressively lower for patients with SRI and ESRD.11,13 However, a VGPR or better was observed in ≥ 1 patient across all groups. Furthermore, a minor improvement in renal function was observed in 2 patients with SRI, 1 of whom had relapsed disease. The ORRs in the present study compared favorably with previously published rates for lenalidomide and dexamethasone (present study, 75%, 67%, and 56% for NRF, SRI, and ESRD, respectively, compared with 67%, 60%, and 49% for patients with NRF, SRI, and moderate/severe renal function, respectively, for lenalidomide and dexametheasone13). However, the small number of patients in the present study precluded any definitive comparison. In a large randomized trial, the addition of elotuzumab to lenalidomide and dexamethasone improved the overall response rates by 13% compared with lenalidomide and dexamethasone alone, an incremental benefit similar to that seen in the present study.21 Together, these findings support the feasibility of using elotuzumab combined with lenalidomide and dexamethasone in patients with MM and renal impairment and demonstrate that dose adjustments for elotuzumab are not required in this patient population.
Conclusion
The results of the present study support the use of elotuzumab without dose adjustment for the treatment of patients with MM and renal dysfunction. Elotuzumab combined with lenalidomide and dexamethasone was tolerated and efficacious for the treatment of patients with MM and renal dysfunction, including ESRD, and might be a new therapy option for this patient population. Ongoing phase III studies are investigating the efficacy of elotuzumab combined with lenalidomide and dexamethasone in patients with RRMM (ELOQUENT-2 study; ClinicalTrials.gov identifier, ) or newly diagnosed or previously untreated MM (ELOQUENT-1 study; ClinicalTrials.gov identifier, ).
Clinical Practice Points.
Renal impairment affects ≤ 50% of patients with MM during the course of the disease and is associated with a poor prognosis.
New treatment options are required for the treatment of patients with MM and renal impairment.
Elotuzumab, a humanized IgG1 monoclonal antibody targeted against SLAMF7, in combination with lenalidomide and dexamethasone, may be used without elotuzumab dose adjustment (but with a lenalidomide dose adjustment) for renal function in patients with MM.
Renal function does not significantly affect the PK of elotuzumab when administered with lenalidomide and dexamethasone.
Elotuzumab, combined with lenalidomide and dexamethasone, is well tolerated in patients with MM, regardless of their renal function.
Elotuzumab, combined with lenalidomide and dexamethasone, can be a promising new therapy for the treatment of patients with MM and renal dysfunction.
Acknowledgments
Many thanks to all the patients, their families, and the investigators who participated in the present study. Elotuzumab was developed in a partnership between Bristol-Myers Squibb and AbbVie Biotherapeutics and is approved for use in combination with lenalidomide/dexamethasone in patients with multiple myeloma who have received one to three prior therapies. Bristol-Myers Squibb was involved in the study design; collection, analysis, and interpretation of data; and decision to submit the report for publication. The present analysis was supported by research funding from Bristol-Myers Squibb. Writing and editorial assistance were provided by Sarah Addison, PhD, of Caudex, funded by Bristol-Myers Squibb.
Disclosure
J.B. has received research funding from Bristol-Myers Squibb, Janssen, Onyx, Novartis, Takeda, Acetylon Pharmaceuticals, Array BioPharma, and AbbVie. S.J. has received honoraria from Bristol-Myers Squibb, Celgene, Novartis, and Sanofi-Aventis. J.Z. has been an Advisory Board member for Celgene and Bristol-Myers Squibb. J.L.K. has worked as a consultant for Celgene, Janssen, Millennium, Novartis, Onyx, and Spectrum; received research funding from Celgene, Merck, Novartis, and Onyx; and received honoraria from Celgene, Janssen, Millennium, Novartis, Onyx, and Spectrum. M.G., A.T., M.L., E.B., and P.P. are employees and share owners of Bristol-Myers Squibb. R.V. has received research funding from Celgene and Onyx and honoraria from Array, Celgene, Janssen, Millennium, Novartis, Onyx, and Sanofi. The remaining authors declare that they have no competing interests.
References
- 1.Clark AD, Shetty A, Soutar R Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev 1999; 13:79–90. [DOI] [PubMed] [Google Scholar]
- 2.Blade J, Fernandez-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998; 158:1889–93. [DOI] [PubMed] [Google Scholar]
- 3.Leung N, Behrens J. Current approach to diagnosis and management of acute renal failure in myeloma patients. Adv Chronic Kidney Dis 2012; 19:297–302. [DOI] [PubMed] [Google Scholar]
- 4.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia 2008; 22:842–9. [DOI] [PubMed] [Google Scholar]
- 5.Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 2007; 48:337–41. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000; 65:175–81. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol 2014; 25:195–200. [DOI] [PubMed] [Google Scholar]
- 8.Uttervall K, Duru AD, Lund J, et al. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One 2014; 9:e101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 2014; 371:906–17. [DOI] [PubMed] [Google Scholar]
- 10.Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood 2010; 116:5838–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer 2010; 116:3807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Christoulas D, Roussou M, et al. Lenalidomide and dexamethasone for the treatment of refractory/relapsed multiple myeloma: dosing of lenalidomide according to renal function and effect on renal impairment. Eur J Haematol 2010; 85:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Klein U, Neben K, Hielscher T, et al. Lenalidomide in combination with dexamethasone: effective regimen in patients with relapsed or refractory multiple myeloma complicated by renal impairment. Ann Hematol 2011; 90:429–39. [DOI] [PubMed] [Google Scholar]
- 14.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14:2775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins SM, Bakan CE, Swartzel GD, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013; 62:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012; 120:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30:1953–9. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007; 357: 2123–32. [DOI] [PubMed] [Google Scholar]
- 19.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 2007; 357: 2133–42. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Jagannath S, Moreau P, et al. Final results for the 1703 phase 1b/2 study of elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood 2014; 124:302. [Google Scholar]
- 21.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373:621–31. [DOI] [PubMed] [Google Scholar]
- 22.van Rhee F, Szmania SM, Dillon M, et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther 2009; 8:2616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowiak A, Offidani M, Pegourie B, et al. A randomized, open-label phase 2 study of bortezomib and dexamethasone with or without elotuzumab in patients with relapsed/refractory multiple myeloma. Haematologica 2015; 100(suppl 1):9 (abstract). [Google Scholar]
- 24.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20:1467–73. [DOI] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 2010; 28:4976–84. [DOI] [PubMed] [Google Scholar]
- 26.Sailstad JM, Amaravadi L, Clements-Egan A, et al. A white paper–consensus and recommendations of a global harmonization team on assessing the impact of immunogenicity on pharmacokinetic measurements. AAPS J 2014; 16:488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol 2012; 52:54S–62S. [DOI] [PubMed] [Google Scholar]