Abstract
We analyzed prognostic factors and developed a prediction tool for exclusive locoregional (pelvic) recurrences after radical cystectomy for bladder cancer. We identified a subgroup of patients with the highest risk who might be best suited for clinical studies.
Background:
Limited information is available about the pattern of relapse after perioperative chemotherapy with radical cystectomy (RC) vs. RC alone in muscle-invasive bladder cancer.
Patients and Methods:
Data from 1082 patients of the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium database, treated from February 1990 to December 2013 at 27 centers in the United States, Europe, Israel, and Canada, were collected. Locoregional relapse was defined as any pelvic lymph node or soft tissue-only recurrences. Cumulative incidence methods were used to estimate time to locoregional relapse (TTRL). Cox regression analyses were performed and a nomogram for 12-month locoregional relapse-free survival (RFS) was developed. The nomogram was applied to an external data set (n = 1021).
Results:
A total of 517 patients (47.8%) developed a relapse: 177 (16.4%) exclusive locoregional relapse. In multivariable analyses, perioperative chemotherapy was associated with longer TTRL (P < .001). Other factors were nonurothelial histology (P = .013), pT-stage (P < .001), and surgical margins (P < .001). The concordance index of the model was 0.681 (95% bootstrapped confidence interval, 0.666–0.716). Risk group categories were obtained according to nomogram tertiles. Despite, overall, observed locoregional RFS in the validation cohort exceeding predicted results, for high-risk patients (80 points or less, lowest nomogram tertile) observed 12-month RFS was similar between development and validation cohorts (60.1% and 66.6%). The study is limited by its retrospective nature.
Conclusion:
In the largest study, to our knowledge, that analyzed locoregional recurrences after RC, we propose a risk prediction tool for exclusive locoregional failures that might be suitable for clinical studies. Patients best suited for adjuvant radiotherapy might be those within the lowest nomogram tertile. Prospective trials are needed to validate findings.
Keywords: Bladder cancer, Nomogram, Perioperative chemotherapy, Risk prediction, Urothelial carcinoma
Introduction
In patients with muscle-invasive bladder cancer (MIBC), radical cystectomy is the treatment of choice in most countries. For patients who are eligible to receive cisplatin-based neoadjuvant or adjuvant chemotherapy, a statistically significant improvement in relapse-free survival (RFS) is obtained, despite the fact that only neoadjuvant chemotherapy has yielded level 1 evidence for improvement in overall survival.1,2 However, almost half of patients who receive surgery develop a relapse within 5 years.3,4 Although risk factors for relapse after cystectomy have been extensively evaluated in the literature, there is still a paucity of data concerning the incidence of local or locoregional relapses in these patients.5–10 The proportion of patients who develop a lymph node or soft tissue recurrence within the pelvis limits might vary considerably across institutions and studies, and according to the number of risk factors, ranging between 5% and 40%.11 Risk factors that have been identified for locoregional relapse include: pathologic T-stage, surgical margin status, number of lymph nodes removed, and the presence of variant histologies. In addition, the quality of radical cystectomy (eg, extent of lymph node dissection) and administration of any neoadjuvant or adjuvant (ie, perioperative) chemotherapy might be relevant.
Limited information is available regarding the role of adjuvant radiotherapy to reduce the risk of pelvic relapses in patients who receive surgery.12 For this reason, the advantage from improved risk prediction of locoregional recurrences after radical cystectomy might be twofold: the possibility to inform prospective clinical trials aimed to deliver adjuvant radiotherapy to selected patients with the highest risk of locoregional recurrence, and the provision of benchmark locoregional RFS estimates to use when designing studies of definitive chemoradiation protocols or new drugs in the perioperative context.
In general, the issue of locoregional disease control is mainly believed to be important when the results of surgical vs. chemoradiation studies are retrospectively compared. Very limited risk prediction tools have been proposed thus far to differentiate recurrence patterns, and definition and timing of pelvic recurrences are not standardized.
Therefore, we aimed to provide a nomogram-based risk prediction of locoregional relapse in MIBC, including the assessment of neoadjuvant or adjuvant chemotherapy, after radical cystectomy. We hypothesized that the ability to predict locoregional recurrences at a landmark time point could be consistent across data sets, also resulting in additional aids to interpreting the results observed in the next nonrandomized phase II trials of perioperative chemotherapy or new agents and radical cystectomy vs. radical cystectomy alone.
Patients and Methods
Patient Selection
The Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) is a retrospective study encompassing individual patient-level data from individuals with muscle-invasive or advanced urothelial carcinoma (UC) or non-UC histology who have received systemic therapy during their disease course. This contemporary database includes data gathered from hospitals in the United States, Europe, Israel, and Canada. The RISC study was approved by the ethics committee at each participating institution.
In July 2017, data were extracted to select patients with the following characteristics: bladder primary, any tumor histology, and having undergone radical cystectomy. Surgical margins were defined positive whenever tumor infiltration was found at the time of pathologic examination or whenever bladder tumor or lymph nodes could not be macroscopically removed. Patients with missing information on surgical margins were excluded. The primary objective of the analysis was to evaluate the incidence and risk factors for locoregional recurrence. The latter was defined according to the European Association of Urology guidelines as any recurrence that takes place in soft tissues at the original surgical site or lymph nodes in the area of pelvic lymph node dissection.1 Data analysis was performed externally by a senior statistician (G.R.P.).
Statistical Analyses
Summary statistics were used to describe respondent characteristics and responses. The Kaplan—Meier method was used to estimate RFS (primary end point). The RFS time of each patient was computed as the interval between the date of surgery and the date of relapse or death for any reason, with censoring at the date of last follow-up in alive and relapse-free patients. Cumulative incidence methods were used to estimate time to locoregional relapse, which accounts for the competing risk of other types of relapse. Univariable and multivariable Cox regression analyses were performed to evaluate factors potentially prognostic of outcomes. Complete case analysis was performed, and no imputation was performed for missing data. A nomogram was developed to estimate the 12-month locoregional RFS, and 95% percentile confidence intervals (CIs) were constructed using 2000 bootstrap samples. Risk groups were defined according to nomogram tertiles. Calibration and prognostic ability was assessed comparing estimated vs. observed 12-month locoregional RFS. Concordance was measured using the concordance index (c-index). When the outcomes between chemotherapy-treated and untreated patients were evaluated, we re-sorted the 90-day and 180-day conditional landmark analyses, to remove the bias of early events. All tests were 2-sided and statistical significance was defined as P ≤ .05. Analyses were performed using SAS version 9.0 (SAS Institute Inc, Cary, NC) or R version 3.2.2 (https://cran.r-project.org/bin/windows/base/old/3.2.2).
Application of Nomogram to External Data Set
The nomogram was then applied to an external surgical data set from the Urological Research Institute (URI), San Raffaele Scientific Institute, Milano, Italy.5 From this data set, patients who received radical cystectomy for at least a muscle-invasive UC were selected, accounting for a total of 1021 patients. Expected 12-month survival was calculated on the basis of the nomogram for each patient and the observed 12-month survival was estimated using the Kaplan—Meier method. Regression models were used to evaluate the observed vs. expected 12-month locoregional RFS.
Results
Patient and Disease Characteristics, and Outcomes in the Development RISC Cohort
The study flow chart is presented in Figure 1. Of the 3024 registered cases, 1559 patients were initially identified. Of these patients, 477 were excluded because of missing information on the pT and pN stage, that we judged necessary to run a complete-case analysis on the most relevant prognostic factors, leaving 1082 patients (543 in the perioperative chemotherapy with radical cystectomy group and 539 in the cystectomy-alone group, treated from February 1990 to December 2013 at 27 contributing centers), who were suitable for analysis. Summary statistics, which describe patient, disease, and treatment characteristics, are presented in Table 1. The median follow-up of censored patients was 2 years. A total of 640 patients (59.1%) had a pT3 or pT4 stage, whereas 243 (22.4%) had a pN2 to pN3 stage. Surgical margins were positive in 139 cases (12.9%). A total of 517 patients (47.8%) developed a relapse, including 177 patients (16.4%), who developed a pelvic relapse only. Time to relapse, RFS, and overall survival results are shown in Supplemental Table 1 in the online version. The comparison between locoregional RFS estimates in chemotherapy-treated and untreated groups is provided in Supplemental Table 2 in the online version, with the use of 90-day and 180-day landmark analyses, and favored the multimodality therapy approach in both cases (P < .001 and P = .001, respectively).
Figure 1. Study Flow Chart, With Counts and Reasons for Patient Selection.
Abbreviations: RC = radical cystectomy; RISC = Retrospective International Study of Invasive/Advanced Cancer of the Urothellum
Table 1.
Patient and Disease Characteristics
| Characteristic | Statistic | RISC Data Set (n = 1082) |
San Raffaele Data Set (n = 1021) |
P |
|---|---|---|---|---|
| Median Age (Range), Years | 66 (32–93) | 68 (35–91) | .001 | |
| Male Sex, n (%) | 852 (78.7) | 853 (83.6) | .005 | |
| Smoking status | <.001 | |||
| Current smoker | 275 (25.4) | 148 (14.5) | ||
| Former smoker | 416 (38.5) | 289 (28.3) | ||
| Never smoker | 296 (27.4) | 133 (13.0) | ||
| Missing | 95 (8.8) | 451 (44.2) | ||
| Race | <.001 | |||
| Asian | 13 (1.2) | 0 (0) | ||
| Black | 40 (3.7) | 0 (0) | ||
| Hispanic | 15 (1.4) | 0 (0) | ||
| White | 995 (92.0) | 1021 (100) | ||
| Not stated | 7 (0.7) | 0 (0) | ||
| Other or mixed races | 12 (1.1) | 0 (0) | ||
| Ethnicity | <.001 | |||
| Hispanic or Latino | 85 (7.9) | 0 (0) | ||
| Other | 997 (92.1) | 1021 (100) | ||
| Median Charlson Comorbidity Index Score (Range) | 2 (0–13) | 1 (0–11) | <.001 | |
| Histology | <.001 | |||
| TCC | 802 (74.1) | 703 (68.9) | ||
| TCC with variants | 106 (9.8) | 196 (19.2) | ||
| Other (pure variant) | 120 (11.1) | 122 (12.0) | ||
| Missing | 54 (5.0) | 0 (0.0) | ||
| Clinical T stage | – | |||
| In situ, 0, 1 | 73 (6.7) | Not collected | ||
| 2 | 654 (60.4) | |||
| 3, 4 | 266 (24.6) | |||
| Missing | 89 (8.3) | |||
| Clinical N stage | – | |||
| 0 | 651 (60.2) | Not collected | ||
| 1 | 66 (6.1) | |||
| 2, 3 | 57 (5.3) | |||
| Missing | 308 (28.4) | |||
| Pathologic T stage | .089 | |||
| In situ, 0, 1 | 220 (20.4) | 211 (20.7) | ||
| 2 | 222 (20.5) | 178 (17.4) | ||
| 3 | 456 (42.1) | 421 (41.2) | ||
| 4 | 184 (17.0) | 211 (20.7) | ||
| Pathologic N stage | <.001 | |||
| 0 | 665 (61.5) | 625 (61.2) | ||
| 1 | 174 (16.1) | 112 (11.0) | ||
| 2 | 219 (20.2) | 241 (23.6) | ||
| 3 | 24 (2.2) | 43 (4.2) | ||
| Neoadjuvant Chemotherapy, Yes, n (%) | 316 (29.2) | 27 (2.6) | <.001 | |
| Adjuvant Chemotherapy, Yes, n (%) | 247 (22.8) | 155 (15.2) | <.001 | |
| Neoadjuvant or Adjuvant Chemotherapy, Yes, n (%) | 543 (50.2) | 179 (17.5) | <.001 | |
| Number of Lymph Nodes Removed | <.001 | |||
| 0 | 17 (1.6) | 11 (1.1) | ||
| 1–9 | 295 (27.3) | 129 (12.6) | ||
| >10 | 715 (66.1) | 881 (86.3) | ||
| Missing | 55 (5.1) | 0 (0.0) | ||
| Surgical Margins Positive, n (%) | 139 (12.9) | 98 (9.6) | .019 |
Abbreviations: RISC = Retrospective International Study of Invasive/Advanced Cancer of the Urothellum; TCC = transltional-cell carcinoma.
Results of the Cox Proportional Hazards Regression Models and Nomogram Development
Cox model results are shown in Table 2. In multivariable analyses, histology (P = .013), pT stage (P < .001), surgical margins (P < .001) and administration of perioperative chemotherapy (P < .001) were significantly associated with exclusive locoregional failures. In multivariable analyses on the basis of the 90-day and 180-day landmark, administration of perioperative chemotherapy remained significant for locoregional RFS (P = .007 and P = .004, respectively).
Table 2.
Univariable and Multivariable Logistic Regression Analyses for Exclusive Locoregiona Relapse Pattern (n = 1082)
| Univariable Analyses | Multivariable Analyses | ||||
|---|---|---|---|---|---|
| Characteristic | Statistic | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P |
| Age/Decade | 1.02 (1.01, 1.03) | .002 | |||
| Male vs. Female Sex | 0.84 (0.65, 1.09) | .19 | |||
| Smoking Status | .15 | ||||
| Current smoker | Reference | ||||
| Former smoker | 0.80 (0.60, 1.05) | ||||
| Never smoker | 1.01 (0.76, 1.36) | ||||
| Missing | 0.75 (0.46–1.20) | ||||
| Charlson Comorbidity Index, Continuous | 1.08 (1.03, 1.13) | <.001 | |||
| Histology | .014 | .013 | |||
| TCC | Reference | Reference | |||
| TCC with variants | 1.04 (0.70, 1.55) | 1.16 (0.78, 1.72) | |||
| Variant | 1.58 (1.16, 2.16) | 1.49 (1.09, 2.04) | |||
| Missing | 0.60 (0.28–1.28) | 0.48 (0.23, 1.02) | |||
| Pathologic T Stage | <.001 | <.001 | |||
| <2 | Reference | Reference | |||
| 2 | 2.15 (1.38–3.34) | 1.74 (1.12, 2.72) | |||
| 3, 4 | 4.22 (2.88–6.18) | 3.48 (2.37, 5.13) | |||
| Pathologic N Stage | <.001 | ||||
| 0 | Reference | ||||
| 1 | 1.27 (0.93–1.73) | ||||
| 2–3 | 1.74 (1.34–2.26) | ||||
| Positive vs. Negative Surgical Margins | 2.34 (1.76–3.09) | .018 | 1.78 (1.33, 2.36) | <.001 | |
| Number of Lymph Nodes Removed | .023 | ||||
| 0 | Reference | ||||
| 1–9 | 0.74 (0.38–1.48) | ||||
| ≥10 | 0.60 (0.31–1.17) | ||||
| Missing | 1.05 (0.49–2.27) | ||||
| Perioperative Chemotherapy, Yes vs. No | 0.48 (0.38, 0.60) | <.001 | 0.47 (0.38, 0.60) | <.001 | |
Abbreviation: TCC = transitional-cell carcinoma.
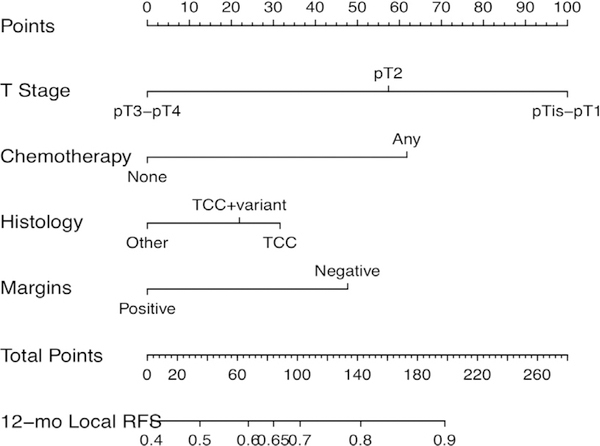
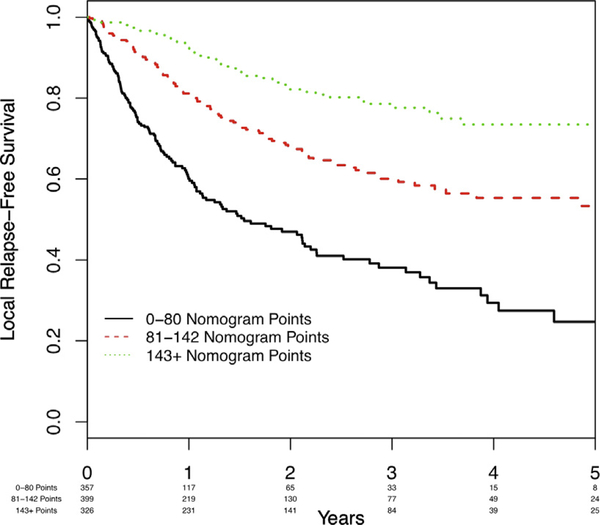
Then we constructed a nomogram for estimating the 12-month locoregional RFS probability (Figure 2). The c-statistic of the model was 0.681 (bootstrapped 95% CI, 0.666–0.716). Corresponding calibration plots are shown in Supplemental Figure 1A in the online version. Three risk categories were defined according to nomogram approximate tertiles. The corresponding RFS curves are shown in Figure 3.
Figure 2. Nomogram to Predict Individual Patient-Level 12-Month (12-mo) Locoregional Relapse-Free Survival (RFS).
Abbreviation: TCC = transitional-cell carcinoma.
Figure 3.
Kaplan–Meier Curves of Locoregional Relapse-Free Survival in the Development Cohort According to Nomogram Tertile
Of note, 357 patients had ≤ 80 nomogram points (corresponding to the lowest nomogram tertile). The predicted 12-month locoregional RFS for ≤80 points was 62%, so this group would be considered the highest-risk patients. In this group, the observed 12-month local RFS was 60.1% (95% CI, 53.7%−65.8%).
Use of Nomogram to Compare Predicted vs. Observed 12-Month Locoregional RFS in External Data Sets
The nomogram was applied to the external data set of URI-San Raffaele. Patient and disease characteristics and outcomes of this cohort are shown in Table 1 and Supplemental Table 3 in the online version. The median follow-up of this population was 1.8 years. Of note, the locoregional relapses in this group were much lower (n = 40; 3.9%). The c-index was 0.641 (96% CI, 0.6270.667). Observed 12-month locoregional RFS in the San Raffaele data set significantly exceeded the predicted results (Table 3). Corresponding calibration plots are shown in Supplemental Figure 1B in the online version.
Table 3.
Validation Analysis of Locoregional RFS, With Nomogram Points Segregated Into Tertiles
| Characteristic | Statistic | Hazard Ratio (95% CI) | P | C-Statistic (95% CI) |
|---|---|---|---|---|
| Nomogram Points | /10 points | 0.89 (0.87–0.91) | <.001 | 0.681 (0.666–0.716) |
| Locoregional RFS | n | Median Years (95% CI) | Expected 12-Month RFS, %a | Observed 12-Month RFS (%, 95% CI)a |
| 0–80 Points | 357 | 1.5 (1.1–2.2) | 62.0 | 60.1 (53.7–65.8) |
| 81–142 | 399 | 6.3 (3.5-NR) | 78.0 | 81.1 (76.4–85.0) |
| ≥143 | 326 | 8.1 (7.6-NR) | 90.0 | 92.5 (88.7–95.0) |
| Nomogram Points | /10 points | 0.91 (0.89–0.93) | <.001 | 0.641 (0.627–0.667) |
| Locoregional RFS | n | Median Years (95% CI) | Expected 12-Month RFS, %a | Observed 12-Month RFS, % (95% CI)a |
| 0–80 Points | 509 | 2.1 (1.6–2.4) | 62.0 | 66.6 (61.6, 71.1) |
| 81–142 | 283 | 7.1 (4.6–8.8) | 78.0 | 88.5 (84.0, 91.8) |
| ≥143 | 229 | 10.9 (8.3–14.8) | 90.0 | 92.3 (87.8, 95.2) |
Abbreviations: NR = not reached; RFS = relapse-free survival.
Expected 1-year estimates are on the basis of the nomogram estimates; median and observed 12-month RFS are on the basis of actual observed data, using the Kaplan–Meier estimator.
However, 509 patients in the URI-San Raffaele data set had 80 nomogram points or less: in this group of high-risk patients, the observed 12-month local RFS was 66.6% (95% CI, 61.6%−71.1%) and the observed 5-year local RFS was 35.6% (95% CI, 30.1%−41.2%). Conversely, the observed 12-month locoregional RFS was 90.2% (95% CI, 87.1%−92.6%) in patients with ≥81 nomogram points. RFS curves according to nomogram tertiles in validation cohort are shown in Supplemental Figure 2 in the online version.
Discussion
In this study we developed a nomogram to predict the 12-month locoregional RFS after radical cystectomy in patients with MIBC, to use as an aid to clinicians in daily practice and clinical study planning. Nomogram estimates were acceptably precise and externally validated to identify patients in the highest risk group. These patients are included in the lowest nomogram tertile (ie, having ≤79 points). This study adds information to the available literature relative to risk prediction models for locoregional relapses after radical cystectomy. Most noteworthy, our patient population is exclusively composed of patients who had received radical cystectomy for at least a muscle-invasive tumor, and the proportion of perioperative chemotherapy-treated patients was higher in the other studies.
The issue of local (pelvic) disease control with radical treatment in nonmetastatic UC represents an underappreciated problem in urologic oncology. In fact, very few reports address the issue of improving locoregional disease control, which might offer potential to optimize radical treatments with curative intent.
Several important limitations should be acknowledged in the present analysis. First, the present results recapitulate the substantial limitations that should be accounted for when developing prediction tools after radical cystectomy, mainly attributable to the source of the data. Several relapse risk prediction models have been offered to urologists to improve their abilities to assess patient prognosis and strengthen the prognosis-based decision-making after radical cystectomy.1 Although those analyses were endowed with the robustness of large samples, the inclusion of low-grade or non—muscle-invasive tumors, coupled with the availability of very few models on the basis of baseline precystectomy variables, limited the application of prediction models in the clinical management of MIBC.13,14 As a result, the therapeutic paradigm of MIBC has remained largely unchanged over the past few decades, and validated selection criteria for clinical trials (eg, trials of adjuvant radiotherapy) are lacking. Among the largest surgical data sets, the International Bladder Cancer Nomogram Consortium analyzed the oncologic outcomes of 9064 patients treated with radical cystectomy and lymphadenectomy, including 1550 patients with lymph node involvement.15 The authors developed a postoperative nomogram to predict the risk of recurrence. The pathologic tumor stage, grade, and node status were reported to have a direct correlation with the risk of recurrence. This association was confirmed in other nomograms developed in international series.16,17 However, close evaluation from these international appraisals aimed at collecting characteristics and outcomes of more advanced patients (eg, patients with at least muscle-invasive tumor receiving neoadjuvant or adjuvant chemotherapy) reveal that tumor grade becomes redundant and other patient-related factors, like age, Charlson Comorbidity Index, and the administration of perioperative chemotherapy might become significant in multivariable analyses. The characteristics of development and validation cohorts in our study have similar distribution of identified significant prognostic factors for locoregional RFS, with the only exception being represented by the proportion of patients who received perioperative chemotherapy, because that was much lower in the San Raffaele cohort. However, there might be several additional biases of patient selection that might have affected the observed outcomes, and such factors cannot be fully acknowledged retrospectively, even with application of advanced statistical methods, similar to what we have recently experienced in another context.18 Additionally, data in the RISC data set were mainly provided by oncologists, therefore it is likely that the RISC population suffered from an unavoidable enrichment in patients with the poorest outcome. This might the reason why the proportion of locoregional relapses was close to the upper bound of the range reported in the literature. Of note, the quality of surgical performance cannot be generalized. By definition, the RISC data set accounts for data from patients mainly treated in the community oncology practice, and centers that contributed cases were primarily those where patients received systemic therapies, not necessarily those where patients received surgery. It is likely that the outcomes obtained from a single referral center, used for validation purposes, cannot be applied to a mixed population of patients who received surgery from 27 different institutions worldwide. Despite there being a slight difference in the proportion of patients with positive surgical margins (12.9% in the RISC cohort vs. 9.6% in the validation cohort), 28.8% of patients in the RISC group had <10 lymph nodes removed vs. 13.7% in the validation group. Interestingly, the number of removed lymph nodes did not remain significant in multivariable analyses, but several other factors accounting for the quality of surgery and postsurgical recovery could not be addressed in this analysis. Additional unavoidable limitations are represented by the different type and timing of disease assessment during follow-up across the contributing centers, because the sensitivity of radiological imaging methods might partly account for different recurrence times and patterns.
The pattern of recurrences after definitive treatment has historically interested the radiation oncologists, and it is indeed an argument claiming a prospective comparison between surgical and bladder-sparing approaches. In the randomized, noninferiority trial of reduced high-dose volume vs. standard-volume radiation BC2001 (CRUK/01/004 study), the proportion of locoregional failures defined as pelvic lymph node recurrences approximated 4.5%, close to the proportion of locoregional failure in our validation cohort.19 However, important patient selection biases still apply in radiotherapy cohorts, primarily represented by the lower pT stage and by the selection of patients with limited clinical lymph node involvement or pure UC histology. For this reason any attempt to merge data sets or compare population-based data, as it was recently presented,20,21 is likely to retain important limitations that cannot be accommodated with the use of advanced statistics.
Some strengths should be acknowledged in our prognostic factor analyses as well. In particular, we were able to validate the same clinical factors that have been previously reported to be associated with locoregional failure from the validation cohort, and implemented them with the role of perioperative chemotherapy, which was under-represented in that population.5 Additionally, the use of our nomogram helped identify patients with the highest risk of developing locoregional relapse. For this group, we were able to obtain an external validation of the 12-month RFS risk of developing locoregional failure, although the nomogram was less precise for patients with lower risk. Indeed, high-risk patients might be regarded to as those who are best suited for an adjuvant radiotherapy approach, or for prospective studies comparing adjuvant radiotherapy with adjuvant chemotherapy or new systemic therapies. In the future, we will be able to observe the patterns of locoregional failures after perioperative immunotherapy strategies, because large adjuvant immunotherapy studies are currently recruiting participants (NCT02450331, NCT02632409, NCT03244384). In particular, we will observe whether use of new systemic therapies will modify the risk of locoregional relapse after radical cystectomy compared with the use of standard chemotherapy.
Conclusion
We analyzed the pattern of exclusive locoregional relapse after radical cystectomy and identified a group of patients with the nomogram-predicted highest risk, in the population with the largest number of events to our knowledge. Using our proposed nomogram might be useful for the design of adjuvant radiotherapy studies. In addition, perioperative chemotherapy was shown to provide enhanced locoregional RFS in patients who received surgery. Of course, the presence of unavoidable patient selection biases in retrospective data sets from different sources could not be avoided, and poor transferability of surgical results from single referral centers remains a major concern for similar multicentric data sets. This limitation is represented in this study by the lack of external validation of our nomogram estimates throughout the entire nomogram points, and prospective clinical trials are need.
Supplementary Material
Clinical Practice Points.
We analyzed prognostic factors and developed a prediction tool for exclusive locoregional (pelvic) recurrences after radical cystectomy for bladder cancer.
There is a subgroup of patients who receive surgery who yield the highest risk of developing pelvic relapses.
These patients are those with a nonurothelial histology, pathologic T3 to T4 stage, positive surgical margins, and have not received any perioperative chemotherapy.
These patients might be well suited for clinical studies, for example, with adjuvant radiotherapy alone or with concomitant systemic therapy.
Acknowledgments
These data were presented in part, as a poster at the Ninth European Multidisciplinary Meeting on Urological Cancers (EMUC17), November 16–19, 2017, Barcelona, Spain, the 2018 Genitourinary Cancers Symposium, February 7–10, 2018, San Francisco, California, and the 2018 European Association of Urology annual meeting, March 16–20, 2018, Copenhagen, Denmark.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental tables and figures accompanying this article can be found in the online version at https://doi.org/10.1016/jxlgc.2018.09.008.
References
- 1.Witijes AJ, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017; 71:462–75. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015; 16:76–86. [DOI] [PubMed] [Google Scholar]
- 3.Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 2006; 176:1354–61. [DOI] [PubMed] [Google Scholar]
- 4.Xylinas E, Cha EK, Sun M, et al. Risk stratification of pT1–3N0 patients after radical cystectomy for adjuvant chemotherapy counselling. Br J Cancer 2012; 107: 1826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moschini M, Shariat SF, Abufaraj M, et al. Predicting local failure after radical cystectomy in patients with bladder cancer: Implications for the selection of candidates at adjuvant radiation therapy. Urol Oncol 2017; 35:672 e1–6. [DOI] [PubMed] [Google Scholar]
- 6.Baumann BC, Guzzo TJ, He J, et al. A novel risk stratification to predict localregional failures in urothelial carcinoma of the bladder after radical cystectomy. Int J Radiat Oncol Biol Phys 2013; 85:81–8. [DOI] [PubMed] [Google Scholar]
- 7.Christodouleas JP, Baumann BC, He J, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer 2014; 120:1272–80. [DOI] [PubMed] [Google Scholar]
- 8.Cornu JN, Neuzillet Y, Hervé JM, et al. Patterns of local recurrence after radical cystectomy in a contemporary series of patients with muscle-invasive bladder cancer. World J Urol 2012; 30:821–6. [DOI] [PubMed] [Google Scholar]
- 9.Froehner M, Novotny V, Wirth MP, et al. External validation of a model to predict locoregional failure after radical cystectomy. Cancer 2014; 120:3584. [DOI] [PubMed] [Google Scholar]
- 10.Novotny V, Froehner M, May M, et al. Risk stratification for locoregional recurrence after radical cystectomy for urothelial carcinoma of the bladder. World J Urol 2015; 33:1753–61. [DOI] [PubMed] [Google Scholar]
- 11.Mari A, Campi R, Tellini R, et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: a comprehensive review of the literature. World J Urol 2018; 36:157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orre M, Lantorzeff I, Flechon A, et al. Adjuvant radiotherapy after radical cystectomy for muscle-invasive bladder cancer: a retrospective multicenter study. PLoS One 2017; 12:e0174978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19:666–75. [DOI] [PubMed] [Google Scholar]
- 14.Mitra AP, Skinner EC, Miranda G, et al. A precystectomy decision model to predict pathological upstaging and oncological outcomes in clinical stage T2 bladder cancer. BJU Int 2013; 111:240–8. [DOI] [PubMed] [Google Scholar]
- 15.International Bladder Cancer Nomogram Consortium. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006; 24:3967–72. [DOI] [PubMed] [Google Scholar]
- 16.Simone G, Bianchi M, Giannarelli D, et al. Development and external validation of nomograms predicting disease-free and cancer-specific survival after radical cystectomy. World J Urol 2015; 33:1419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Trapani E, Sanchez-Salas R, Gandaglia G, et al. A nomogram predicting the cancer-specific mortality in patients eligible for radical cystectomy evaluating clinical data and neoadjuvant cisplatinum-based chemotherapy. World J Urol 2016; 34:207–13. [DOI] [PubMed] [Google Scholar]
- 18.Necchi A, Lo Vullo S, Mariani L, et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJUInt 2018; 121:252–9. [DOI] [PubMed] [Google Scholar]
- 19.Huddart RA, Hall E, Hussain SA, et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: results of the BC2001 trial (CRUK/01/004). Int J Radiat Oncol Biol Phys 2013; 87:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017; 35:2299–305. [DOI] [PubMed] [Google Scholar]
- 21.Seisen T, Sun M, Lipsitz SR, et al. Comparative effectiveness of trimodal therapy versus radical cystectomy for localized muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2017; 72:483–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.