Abstract
Background:
Attentional disruptions are common in PTSD, but findings across neuropsychological and neuroimaging studies have been variable. Few PTSD studies have investigated abnormalities in attention networks using a multi-modal imaging approach and attentional tasks that include emotionally-salient images. This study combined a behavioral task that included these images (emotional Stroop) with functional and structural neuroimaging (fMRI and diffusion tensor imaging; DTI) methods to comprehensively investigate attentional control abnormalities in a highly-traumatized civilian sample.
Methods:
48 traumatized women with and without PTSD received clinical assessments, fMRI and DTI. During fMRI, the Affective Stroop (AS), an attentional control task that includes emotionally-salient distractor images (trauma-relevant, positive, neutral) and variable task demands, was administered.
Results:
In response to more difficult AS trials, participants with PTSD demonstrated lower activation in the dorsal and rostral anterior cingulate cortex and greater activation in the insula. This group also showed comparatively poorer performance on positive AS distractor trials, even after adjusting for trauma exposure. Performance on these trials inversely correlated with structural integrity of the cingulum bundle and uncinate fasciculus.
Conclusions:
Even after adjusting for trauma exposure, participants with PTSD showed worse performance on an attentional control task in the context of emotional stimuli. They also showed relatively lower cognitive control network activation and greater salience network activation. Fronto-parietal and fronto-limbic white matter connectivity corresponded with AS performance. Our findings indicate that attentional control impairments in PTSD are most evident in the context of emotional cues, and are related to decrements in function and structure of cognitive control and salience networks.
Keywords: Attention, PTSD, fMRI, DTI, Structure, Cognition, Stroop
1. Introduction
Attentional disruptions are frequently experienced by traumatized people, and are reflected in several posttraumatic stress disorder (PTSD) symptoms: intrusive recollections or flashbacks of the trauma, an impaired ability to concentrate on desired tasks, and hypervigilance for trauma-related cues. Attention comprises three different processes: alerting, orienting, and executive control (Petersen and Posner, 2012). Among these, executive or attentional control may be the most amenable to modification; it functions under conscious awareness, making it a frequent target of treatments (Badura-Brack et al., 2015). Attentional control includes the ability to maintain focus on a given task and disregard irrelevant information, a process that is critical to many daily life activities.
Evidence for diminished attentional control in PTSD has emerged from studies that used neuropsychological tasks, including the Stroop paradigm (Scott et al., 2015; Stroop, 1935). The existing PTSD neuropsychological studies employing the Stroop task show some evidence for diminished attentional control in PTSD populations (Lagarde et al., 2010) via poorer task performance, as indicated by a recent meta-analysis (Cisler et al., 2011). However, effect sizes have been relatively small (d~.2) (Cisler et al., 2011), and some studies have not found between-group effects at all (Kanagaratnam and Asbjornsen, 2007; Lagarde et al., 2010; Vasterling et al., 2002). One potential reason relates to variations in PTSD symptom clusters between the different samples in these studies—there is evidence that these symptom clusters are differentially related to attentional control deficits (Grisanzio et al., 2018), with fearful arousal symptoms corresponding most with cognitive control problems.
Problems with attentional control in the context of emotion are likely to be related to dysfunction in two distinct neural circuits (Petersen and Posner, 2012; Posner and Petersen, 1990). The cingulo-opercular network includes the anterior cingulate cortex (ACC) and insula as well as the amygdala, and supports error monitoring and salience detection (frequently termed “salience network”). The fronto-parietal network, which includes the dorsal ACC, dorsolateral prefrontal (DLPFC) and inferior parietal cortex, supports the implementation of executive control and adjustment of attentional strategy. Increased cingulo-opercular and decreased fronto-parietal network function appears to characterize anxious populations in general (Sylvester et al., 2012).
However, neuroimaging studies of attention in traumatized populations indicate more mixed findings, both in terms of neural network response and behavior (Block and Liberzon, 2016). This may, in part, reflect methodological differences, including the types of stimuli employed in attentional control tasks (i.e., images vs words, trauma-relevant vs generally aversive stimuli), as well as heterogeneous samples. Studies using affective versions of the Stroop have indicated differential fronto-parietal and cingulo-opercular network activation in PTSD in response to emotional stimuli. One study of women with complex PTSD indicated higher dACC, dorsolateral prefrontal cortex (DLPFC), ventromedial prefrontal cortex (vmPFC) and anterior insula response to trauma-related words in civilians with complex PTSD on an emotional Stroop task, compared to both traumatized and healthy controls (Herzog et al., 2017). Participants with PTSD demonstrated poorer Stroop task performance, particularly when presented with trauma words. Using an image-based Affective Stroop (AS), Blair et al. (2007) observed decreased DLPFC activation in patients with PTSD in response to heightened task demands (Blair et al., 2007), but no significant differences in task performance were observed; aversive images used in the task were not trauma-specific. Building upon this work by examining the functional connectivity during this same task, White et al. (2015) observed that PTSD symptoms positively correlated with amygdala connectivity to the DLPFC and dACC in veterans in response to emotionally-valenced vs neutral images, particularly to trials with positive image distractors (White et al., 2015). The authors also found that PTSD symptoms correlated with increased functional connectivity between the amygdala, dACC and anterior insula (White et al., 2015). However, no group differences were observed in task performance, and their population did not meet clinical criteria for PTSD. Studies using other word or number-based emotional Stroop tasks have indicated higher dACC response (Bremner et al., 2004) but lower response in the rostral ACC (Bremner et al., 2004; Shin et al., 2001) in participants with PTSD versus controls; in these two studies, significant differences in task performance were not observed. These data indicate that salience network activation appears to be more consistently related to attentional control disruptions in PTSD, whereas the direction of fronto-parietal circuit involvement has been more variable. Only one such study observed significant differences in task performance (Herzog et al., 2017).
Decrements in the structural integrity of these neural pathways may contribute to the altered responses previously observed. In studies of depression, poorer microstructure of the cingulum bundle has been associated with increased interference on a Stroop task (Keedwell et al., 2016). This white matter tract connects brain regions in the attentional control network (ACC and parietal cortex) and is also a primary route of communication between prefrontal and entorhinal cortices. Similar findings with respect to cingulum integrity and Stroop task performance have been observed in schizophrenia (Kubicki et al., 2009). Our earlier studies suggest that the cingulum as well as the uncinate fasciculus, a fronto-limbic tract that connects the amgydala and vmPFC, are specifically compromised in PTSD (Daniels et al., 2013; Fani et al., 2015, 2012a, 2016). These tracts have been associated with treatment outcome as well (Kennis et al., 2015). However, little is known about how the structure of these pathways affects attentional control in PTSD.
To our knowledge, no studies to date have used a multi-modal approach, combining behavioral data (Affective Stroop task performance) with structural and functional MRI data to comprehensively investigate the nature of attentional control abnormalities in PTSD. We measured responses of traumatized participants with and without PTSD on the affective Stroop (administered during fMRI) to examine differences in attentional performance during neutral and emotional conditions, including both positive and trauma-relevant distractors in this task. We predicted that a PTSD diagnosis would be associated with poorer performance on the Affective Stroop (error rates and response times were used to assess attentional control), particularly in the presence of emotional stimuli. We expected decreased activation in the fronto-parietal/cognitive control network and increased cingulo-opercular/salience network activation in response to increased task demands. We hypothesized that poorer integrity of cingulum bundle (CB) and uncinate fasciculus (UF) pathways would be associated with poorer AS task performance in the presence of emotionally-valenced distractors. To assess for specificity of findings with respect to white matter, we included a third pathway, the medial forebrain bundle, in analyses. We conducted exploratory analyses to examine the relationships between task performance, neural response patterns and PTSD symptom dimensions.
2. Methods
2.1. Participants
A sample of 48 African-American women aged 22–61 (M = 38.7, SD = 11.2) was recruited from an NIH-funded study of attentional control in PTSD (MH101380) and received MRI. Individuals were approached in general medical clinics of a publicly-funded hospital serving low income individuals in inner-city Atlanta, Georgia. On average, nearly half of all participants had completed up to 12 years of schooling or the equivalent (GED), and had monthly incomes of less than $1000. Only 39% of the sample was employed. Participant characteristics are further detailed in Table 1.
Table 1.
Demographic and clinical characteristics.
| Trauma controls (n = 22) Mean (SD) |
PTSD (n = 26) Mean (SD) |
F | |
|---|---|---|---|
| Age | 40.7 (9.9) | 39.3 (12.8) | 0.2 |
| TEI lifetime trauma | 2.4 (1.3) | 4.2 (2.3) | 11.1 |
| TEI types of interpersonal trauma in adulthood | 2.2 (1.6) | 4.5 (2.3) | 14.8* |
| X2 | |||
| TEI childhood sexual, physical abuse endorsed | 18% | 53.8% | 7.1* |
| PSS total score | 3.1 (3.8) | 25.4 (11.3) | 77.9* |
| PSS re-experiencing | 1 (1.2) | 6.9 (3.9) | 45.5* |
| PSS avoidance | 0.5 (1) | 4 (1.8) | 64.6* |
| PSS hyperarousal | 1 (1.6) | 8.4 (3.7) | 77* |
| PSS anhedonia | 0.5 (0.7) | 4.1 (3) | 31.9* |
| BDI-II total score | 6.5 (10.1) | 20.8 (12.7) | 21.6* |
| No (%) | Yes (%) | X2 | |
| Currently employed | 50 | 70 | 2.7 |
| Currently married or domestic partnership | 27% | 19% | 2.8 |
| % | Fisher’s exact test | ||
| Education | 1.4 | ||
| <12th grade | 9.1 | 15.4 | |
| High school graduate/GED | 36.4 | 30.8 | |
| Some college/tech school | 36.4 | 42.3 | |
| College graduate | 9.1 | 3.8 | |
| Graduate school | 9.1 | 7.7 | |
| Monthly income | % | 7.3 | |
| $0–249 | 0 | 15.4 | |
| $250–499 | 18.2 | 3.8 | |
| $500–999 | 31.8 | 46.2 | |
| $1000–1999 | 31.8 | 15.4 | |
| $2000 + | 18.2 | 19.2 | |
TEI = Traumatic Events Inventory.
BDI-II = Beck Depression Inventory.
p < .01.
The eligibility criterion for participation in the current study included the ability to understand English (assessed by a study researcher) and willingness to provide informed consent. Participants were excluded if they had current neurological conditions or psychosis, as well as current psychotropic medication use. Participants were excluded from MRI on the basis of: claustrophobia; contra-indications to MRI scanning (e.g., metal implants); current bipolar disorder (as assessed with the MINI interview Sheehan et al., 1998); current substance or alcohol dependence or intoxication. Participants were given a urine drug test on the day of the scan and tested negative. Although handedness was not used as an inclusion/exclusion factor, a majority of participants were right handed (n = 42; 88%). After being enrolled in the study, one participant reported starting on psychotropic medications. Participants receiving MRI were asked to refrain from caffeine consumption on the day of the scan, given possible effects on BOLD signal and cognition, as shown previously (Koppelstaetter et al., 2010; Laurienti et al., 2002). The Institutional Review Board of Emory University and Grady Hospital Research Oversight Committee approved all study procedures.
2.2. MRI acquisition, image processing
Scanning was conducted on a research-dedicated Siemens 3-Tesla TIM-Trio scanner (12 channel head coil) at Emory University Hospital. Acquisition parameters and image pre-processing details are provided in the Supplement.
2.3. Clinical assessments
We administered the following assessments, detailed further in the Supplement. The Traumatic Events Inventory (TEI) was administered to measure lifetime trauma exposure. Given that the two diagnostic groups demonstrated significant differences in trauma exposure (see Table 1), overall TEI scores were submitted as covariates in statistical analyses. The Clinician Administered PTSD Scale (CAPS; DSMIV; administered to18 participants) and DSM5 (administered to 30 participants; Blake et al., 1995, Weathers et al., 2017) was administered to determine presence or absence of PTSD. Based on the CAPS, 26 participants met criteria for PTSD, whereas 22 did not meet PTSD criteria (Controls); all had experienced trauma. The PTSD Symptom Scale (PSS; (Foa et al., 1993) was administered on the day of the MRI scan, and subscale scores (re-experiencing, avoidance, hyperarousal, anhedonia) were used for correlational analyses with MRI data, given their similar time of administration. Participants in the control group had low PSS scores, with a mode of 0, a median score of 2 and mean = 3.4 (SD = 4). As expected, participants in the PTSD group had clinically significant PSS scores, with a mode of 25, a median score of 25 and mean = 25.4 (SD = 11.3) and scores were had a relatively even distribution across the symptom clusters.
2.4. fMRI task: affective stroop
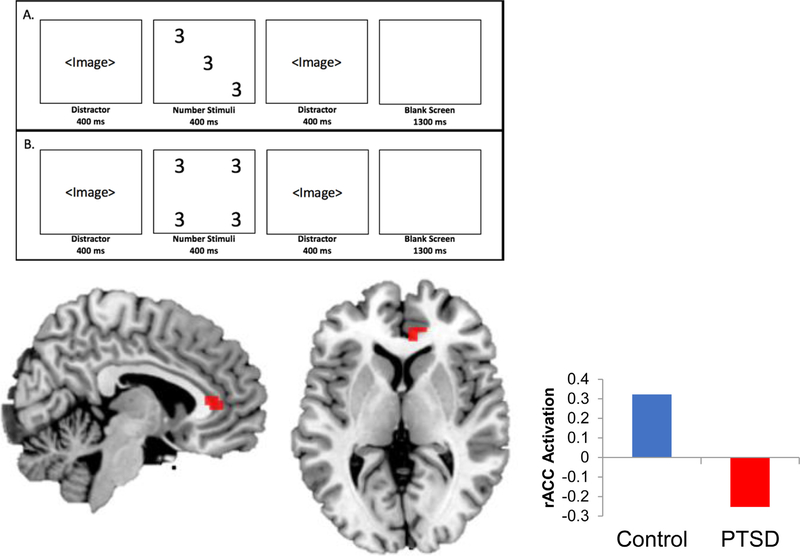
The Affective Stroop (AS) is a measure of attentional control that has been detailed in earlier studies (Blair et al., 2007, 2012b; White et al., 2015), further described in the Supplement. Participants rapidly identify, via button-press, the number of numbers in a given display (3, 4, 5, or 6) while ignoring distractor images (trauma-relevant, positive, and neutral scenes) that appear prior to and following each number stimulus (Fig. 1a and b). The distractor images are identical. In some of these trials, the number of numbers is inconsistent with the actual number displayed, posing a heightened cognitive challenge. In addition, the task includes trials with no cognitive demands (“view only” trials). Error rates and response times were recorded and analyzed.
Fig. 1.
Response to number incongruent (b) vs number congruent (a) Affective Stroop trials, voxelwise p < .001. Controls demonstrated higher activation in the dorsal and rostral ACC (shown in red, MNIx,y,z = 9,35,7) in response to higher Affective Stroop task demands.
2.5. fMRI data analysis
To examine blood-oxygen-level dependent (BOLD) signal change to task stimuli, a first-level, fixed-effects analysis was conducted. Onset times for each task condition were entered into a general linear model, convolved with a hemodynamic response function and linear contrasts between conditions were estimated. Subject-specific motion parameters were also included in the model as effects of non-interest. Given our interest in examining neural response and task performance under high vs low attentional demands, and in accordance with earlier studies using this task (Blair et al., 2012b; White et al., 2015), our primary analysis involved contrasting number incongruent versus number congruent trials. Events included the distractor and number stimuli. Since our primary objective was to examine differences in performance between PTSD and traumatized controls, between-group analyses were conducted at a whole brain, voxel-wise level, for this contrast. Where between-group differences in task performance were observed, we performed correlational analyses including all participants; PSS subscale scores were entered as subject-level regressors. Regions of interest included the ACC (dorsal and rostral), DLPFC, superior parietal cortex, insula, and amygdala, similar to prior studies (White et al., 2018). Results were considered significant at a whole brain statistical threshold of p < .001, with cluster-level FWE correction at a threshold of p < .05 conducted with these a priori specified regions of interest, as recommended to account for error inflation due to multiple comparisons (Woo et al., 2014). Contrast parameters were extracted from clusters showing significant between-group differences and were used in analyses with behavioral data. To confirm that findings were within established networks of interest, we used Neurosynth (www.neurosynth.org; Yarkoni et al., 2011) to conduct a meta-analysis using cognitive control and salience network terms to identify regions consistently related to these networks.
2.6. DTI data processing and probabilistic tractography
To examine structural connectivity, probabilistic fiber tracking was conducted with PROBTRACKX implemented in FSL, detailed further in the Supplement. Masks of the CB and UF were created using the JHU White Matter Tractography Atlas (Mori et al., 2005) and used as an anatomical waypoint for these paths; a separate exclusion mask was created to eliminate the likelihood of pathways in irrelevant white matter tracts, gray matter regions and CSF. The medial forebrain bundle (MFB) was included in analyses to assess for specificity of findings. To create probabilistic streamlines for MFB pathways, we selected the nucleus accumbens and orbitofrontal cortex as seed regions using the Harvard-Oxford Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html. Example probabilistic paths for the UF and CB are provided in Fig. 3c and d. Fractional anisotropy (FA) was used as our measure of tract integrity, given that earlier studies have indicated it to be a reliable assessment of microstructural integrity of white matter fibers (Fox et al., 2011). Data from 8 participants were discarded due to motion artifacts or unusable tracts (included anatomically unfeasible, deviated projections), leaving a sample size of 40; this sample of participants was used in all Affective Stroop and DTI data analyses. This sub-group of participants was also similar to the overall sample in terms of age (M = 38.4, SD = 11.3), PTSD symptoms (PSS total: M = 16.1, SD = 14.1), and trauma exposure (TEI lifetime trauma: M = 4.7, SD = 2.6).
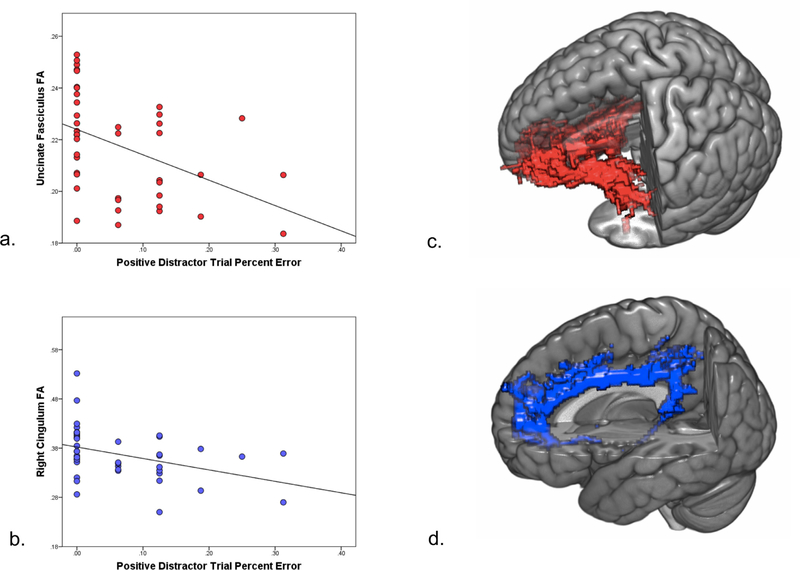
Fig. 3.
White matter integrity and performance on positive Affective Stroop distractor trials in PTSD. Errors on positive number congruent distractor trials were inversely correlated with microstructure of the uncinate fasciculus (a) and right cingulum (b). Example probabilistic tracts of the uncinate fasciculus (c) and right cingulum (d).
2.7. Affective number stroop data analysis
Multivariate analyses of covariance were conducted to examine between-group differences in performance on cognitive measures (AS: error rate and response time) after accounting for the effects of trauma exposure (TEI), given between-group differences and associations found previously with test performance (Palmer et al., 1997); data were analyzed for the 40 participants with both fMRI and DTI data. PTSD diagnosis was the grouping variable, outcome variables included performance on the AS. Where group differences were observed, we also examined associations between PTSD symptom clusters and AS task performance in the entire sample. Finally, we examined correlations of task performance with structural connectivity of cingulum, uncinate fasciculus, and medial forebrain bundle pathways; using IBM SPSS version 24, partial correlations were conducted with mean FA values of all tracts and AS performance variables, controlling for trauma exposure (TEI), given its possible effects on white matter integrity (Fani et al., 2012a; Lebel et al., 2012; Ly et al., 2013; Wang et al., 2010). A threshold of p < .05, two-tailed, was used to define statistical significance; Sidak correction was applied to adjust for error due to multiple comparisons (p < .006).
3. Results
3.1. Affective stroop task performance
3.1.1. PTSD vs controls
We examined effects of emotion and task condition on differences in accuracy and response time in the sample using two 3 (emotion: positive, trauma-relevant, neutral) × 2 (task condition: number congruent, number incongruent) repeated measures ANCOVAs, with trauma exposure (TEI) as a covariate. We observed a significant emotion by task condition by TEI interaction (F1,37 = 3.6), p =.04), a significant emotion by diagnostic group interaction (F1,37 = 5, p =.01), as well as a significant emotion by task condition by diagnostic group interaction (F1,37 = 4.1, p =.02). Participants with PTSD demonstrated significantly poorer performance (more errors) on positive number congruent AS distractor trials (Cohen’s d = 0.65). Controls had average error rates of approximately 4%, whereas participants with PTSD demonstrated average error rates of 9% (see Table 2). No other main effects or interactions were observed. With respect to response time, a main effect of task condition was observed (F1,37 = 23, p < .001), with slower response times observed in response to number incongruent trials. However, no other main effects or interactions were observed with respect to response time.
Table 2.
Affectivestroop performance.
| Controls (n = 22) Mean (SD) |
PTSD (n = 26) Mean (SD) |
F | |
|---|---|---|---|
| Percent error | |||
| Trial type | |||
| Trauma-relevant, number congruent | 8 (10.6) | 11.8 (12.2) | 1.2 |
| Positive, number congruent | 3.7 (8.3) | 9.2 (8.6) | 7.4* |
| Neutral, number congruent | 6.3 (9.4) | 8.4 (10.3) | 0.4 |
| Trauma-relevant, number incongruent | 9.7 (10.9) | 12.5 (13.1) | 0.6 |
| Positive, number incongruent | 6.8 (9.2) | 12.5 (15.3) | 0.7 |
| Neutral, number incongruent | 6.2 (8.4) | 11.5 (12.2) | 1.9 |
| Overall errors | 6.9 (8.2) | 11.5 (10) | 1.6 |
| Response time | |||
| Trauma-relevant, number congruent | 871.2 (160.6) | 942.4 (147.4) | 1.4 |
| Positive, number congruent | 856.1 (164.9) | 926.4 (132.8) | 1.9 |
| Neutral, number congruent | 861 (170.5) | 914 (130) | 1.2 |
| Trauma-relevant, number incongruent | 928.1 (157) | 1010.5 (137.7) | 2.1 |
| Positive, number incongruent | 906.7 (135.2) | 996.7 (163.5) | 2.3 |
| Neutral, number incongruent | 914.5 (139.2) | 997.3 (143.7) | 3.2 |
| Overall response time | 889.6 (148.6) | 968.8 (135.9) | 1.9 |
p < .05.
3.1.2. PTSD symptom clusters
In the group as a whole, a significant correlation was observed between PTSD hyperarousal symptoms and performance on neutral number incongruent distractor trials (r = 0.36, p = .03), with a marginally significant correlation observed with trauma-relevant number congruent trials (r = 0.32, p = .05). Anhedonic PTSD symptoms also correlated with performance on neutral number incongruent distractor trials (r = 0.34, p = .03) and trauma-relevant number congruent distractor trials (r = 0.32, p = .05).
Response times for all conditions were normally distributed in the sample, as indicated by Shapiro-Wilk tests (all ps > .25). Hyperarousal was significantly associated with longer response times on trauma-relevant number congruent (r = 0.39, p = .02), positive number congruent (r = 0.34, p = .04), trauma-relevant number incongruent (r = 0.42, p = .01), positive number incongruent (r = 0.38, p = .02) and neutral number incongruent (r = 0.45, p < .01) distractor trials. Anhedonic PTSD symptoms correlated with response time on traumarelevant number congruent (r = 0.37, p = .02), trauma-relevant number incongruent (r = 0.43, p < .01), positive number incongruent (r = 0.35, p = .03) and neutral number incongruent (r = 0.42, p < .01) distractor trials.
3.2. fMRI analyses
3.2.1. PTSD vs controls
In response to AS number incongruent vs number congruent trials (increased attentional demands), PTSD participants demonstrated lesser activation in the dorsal ACC (Brodmann Area; BA 32, MNIx,y,z = 9,23,34; Table 3) and rostral ACC (BA 24, MNIx,y,z = 9,35,7) as compared to controls; the dACC cluster fell within the cognitive control network as defined by Neurosynth. For this contrast, participants with PTSD demonstrated heightened response in the insula (BA 13, MNIx,y,z = −39,−37,22). No other significant between-group differences in activation were found at the p < .001 statistical threshold between groups for all contrast conditions.
Table 3.
Anatomical locations of voxel-wide BOLD response to Affective Stroop distractor trials (p < .001, uncorrected).
| Brodmann area | x | y | z | Cluster size (mm3) |
|
|---|---|---|---|---|---|
| Number incongruent vs number congruent trials | |||||
| Trauma controls ≥ PTSD | |||||
| Anterior Cingulate Gyrus (dACC) | 32 | 9 | 23 | 34 | 8 |
| Superior frontal gyrus | 6 | −6 | 29 | 58 | 12 |
| Medial frontal gyrus | 8 | −6 | 41 | 40 | 6 |
| Anterior cingulate gyrus (rACC) | 24 | 9 | 35 | 7 | 7 |
| PTSD ≥ Trauma controls | |||||
| Insula | 13 | −39 | −37 | 22 | 5 |
| −33 | −37 | 16 | |||
| Precuneus | 7 | 15 | −70 | 46 | 7 |
| Positive vs Neutral trials | |||||
| PTSD ≥ Trauma controls | |||||
| Middle temporal gyrus | 21 | 54 | 5 | −23 | 7 |
| PTSD Re-experiencing symptoms | |||||
| Positive correlation | |||||
| Middle temporal gyrus | 21 | 54 | 2 | −20 | 8 |
| Midbrain | 0 | −13 | −5 | 10 | |
| Anterior cingulate cortex (dACC) | 24 | 0 | 23 | 22 | 7 |
| PTSD avoidance symptoms | |||||
| Positive correlation | |||||
| Parahippocampal gyrus | 34 | 9 | −10 | −23 | 33 |
| Uncus | 28 | 18 | −7 | −26 | |
| Cerebellum | −24 | −46 | −11 | 8 | |
| Precentral gyrus | 6 | 45 | −13 | 28 | 8 |
| Brainstem | 0 | −13 | −5 | 5 | |
| Orbitofrontal gyrus | 11 | 12 | 50 | 20 | 7 |
| PTSD hyperarousal symptoms | |||||
| Positive correlation | |||||
| Middle cingulate cortex | 24 | 0 | −19 | 34 | 12 |
| Uncus | 34 | 15 | −7 | −23 | 8 |
3.2.2. PTSD symptom clusters
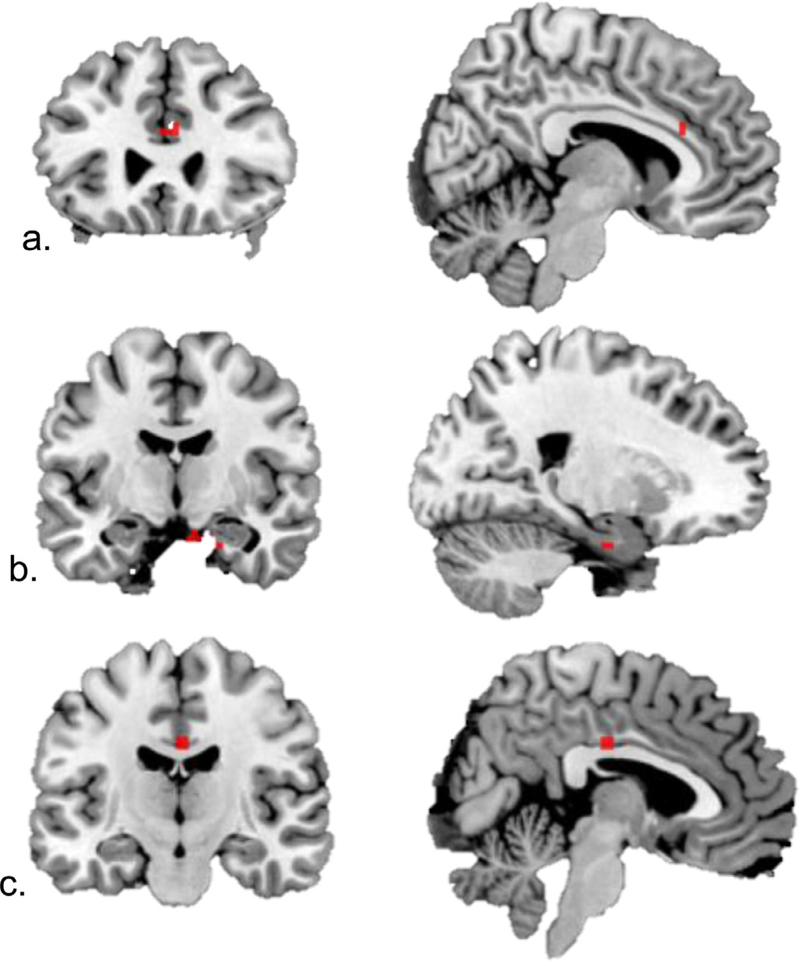
As planned, regression analyses were conducted with the AS condition that had yielded between-group performance differences: positive versus neutral images. In response to positive vs neutral images, re-experiencing symptoms were associated with greater dACC activation (Brodmann area 24; MNIx,y,z = 0,23,22; Fig. 2a), whereas avoidance was positively correlated with activation in the parahippocampal gyrus (MNIx,y,z = 9, −10, −23; Fig. 2b). Hyperarousal symptoms were not significantly correlated with activation in specified ROIs, but were positively correlated with activation in the uncus (BA 34, MNIx,y,z = 15, −7, −23) and middle cingulate gyrus (BA 24, MNIx,y,z = 0,−19,34; Fig. 2c). No significant patterns of activation were observed for correlations with anhedonic symptoms for this condition.
Fig. 2.
Correlations between PTSD symptom clusters and response to positive vs neutral Affective Stroop distractor trials, voxelwise p < .001. (a) Reexperiencing positively correlated with dACC (MNIx,y,z = 0,23,22) activation. (b) Avoidance positively correlated with right parahippocampal gyrus (MNIx,y,z = 9,−10,−23) activation. (c). Hyperarousal correlated with middle cingulate cortex (MNIx,y,z = 0,−19,34) activation.
3.3. White matter analyses
After accounting for trauma exposure, uncinate fasciculus FA was negatively associated with errors on positive number congruent trials (r = −0.44, p = .005; Fig. 3a, c) and trauma-relevant number incongruent trials (r = −0.46, p = .002). These relationships remained significant after removing trauma covariates (r = −0.44, p = .004 for positive number congruent trials and r = −0.46, p = .002 for trauma-relevant number incongruent trials). Right cingulum FA was also negatively correlated with performance on positive number congruent trials (r = −0.42, p = .01) and neutral number incongruent trials (r = −0.33, p = .04); however, these findings did not survive Sidak correction. After removing trauma covariates, right cingulum FA remained negatively correlated with performance on positive number congruent trials (r = −0.39, p = .01; Fig. 3b, d) and trauma-relevant number incongruent trials (r = −0.35, p = .03). After controlling for depression symptoms (BDI total score), all ps < .05 but were not less than the Sidak-corrected threshold. No other significant associations were observed between white matter integrity and task performance. Participants with PTSD showed lower right cingulum FA as compared to controls (F1,41 = 6.6, p = .01), replicating our earlier findings (Fani et al., 2012a, 2016).
4. Discussion
We administered an attentional control task with variable attentional demands and both neutral and emotionally-salient distractor stimuli to examine the precise nature of attentional control abnormalities in PTSD. We investigated potential differences in performance and neural response, as well as white matter connectivity. Together, our data indicate abnormalities in cognitive control and salience networks in participants with PTSD. As compared to traumatized controls, we found that participants with PTSD demonstrated similar task performance in the context of neutral stimuli, but performed more poorly in the context of emotional AS distractor stimuli, as we had predicted. Specifically, those with PTSD made more errors on positive AS distractor trials and demonstrated lower activation in cognitive control (fronto-parietal) and higher activation in salience networks in the context of higher AS task demands. Further, task performance on these AS positive distractor trials was correlated with structural integrity of the cingulum bundle (CB), a primary fronto-parietal white matter connection, and the uncinate fasciculus (UF), a fronto-limbic white matter fiber implicated in various social and emotional processes (Johnson et al., 2011; Zappala et al., 2012). Re-experiencing, avoidance, and hyperarousal symptom clusters were correlated with distinctly different patterns of BOLD activation to positive AS stimuli. Hyperarousal and anhedonic PTSD symptoms were associated with poorer performance on neutral and emotion AS trials.
When faced with higher attentional demands on the AS, the PTSD group demonstrated lower activation in the dorsal and rostral ACC, regions involved with regulation of response on attentional tasks. The dACC has been implicated in top-down control more generally, and rACC involvement appears to be more specific to emotional task conditions (Banich et al., 2009). Decreased dACC activation during affective attentional processing has been observed in anxious populations (Blair et al., 2012a), and lower dACC and rACC response have been linked to PTSD in meta-analyses (Etkin and Wager, 2007). The rACC is implicated in performance monitoring (Ridderinkhof et al., 2004)—during emotional Stroop task performance, this region is more active when attentional demands are increased (Etkin et al., 2006). In healthy individuals, increased rACC corresponds with the ability to successfully identify erroneous responding and improve subsequent performance (Ridderinkhof et al., 2004). Volume of the rACC (Bryant et al., 2008) and increased response in the rACC (Bremner et al., 2017; Peres et al., 2011) and dACC (Malejko et al., 2017) has also been associated with successful treatment of PTSD. Similar to prior Stroop studies (Bremner et al., 2004; Shin et al., 2001), our findings indicate that participants with PTSD demonstrated under-engagement of the rACC during emotional Stroop task performance—unlike these studies, we also observed a difference in task performance. Participants with PTSD performed more poorly than controls on AS trials that included positive images. In the context of conditions with higher attentional demands, they also demonstrated comparatively higher insula activation. Given the role of the insula in salience detection, and the frequent observation of insula hyper-activation in PTSD (Hughes and Shin, 2011), it is likely that activation on this salience network disrupted the appropriate function of fronto-parietal networks, precluding their ability to exert cognitive control successfully.
We were somewhat surprised to find that attentional performance was disrupted by positive, rather than trauma-relevant, distractor images in PTSD—however, studies of other traumatized populations have observed similar findings with respect to positive images, including one using the AS task (White et al., 2015). Emotionally-salient images, including those with positive valence, have been found to disrupt attention in anxious (Chen et al., 2012, 2014; Taylor et al., 2010) and traumatized populations, such as our own (Fani et al., 2011). Disruptions in cognitive control circuits have also been observed in children with PTSD in response to positive images (Jatzko et al., 2006). Increasingly, disrupted behavioral and neural response to appetitive stimuli are being observed as manifestations of PTSD (Nawijn et al., 2015). People with high/frequent exposure to past trauma and adversity may have a complex relationship with positive images—these scenes may be incongruous with the realities and expectations of this population, and as such, can disrupt attentional focus.
Poorer white matter connections extending from the ACC were likely to play a role in producing these attentional decrements—task performance on positive emotion distractor trials corresponded with UF, and to a lesser degree, right CB integrity. The UF is an association fiber extending from ventromedial prefrontal (vmPFC) regions to the amygdala, permitting bi-directional communication. Integrity of this tract has been associated with Stroop task performance (He et al., 2016; Schulte et al., 2012); more broadly, it is involved in emotion regulation (d’Arbeloff et al., 2018; Zheng et al., 2018). Disruptions in UF integrity have been linked to mood, anxiety, and trauma-related disorders (Lindner et al., 2018; Jenkins et al., 2016; Koch et al., 2017; Liao et al., 2014). Taken together, alterations in this fronto-limbic pathway appear to influence attentional control in the context of emotion in people with PTSD.
Task performance was also linked to integrity of the CB, a large, c-shaped white matter tract extending from prefrontal (orbitofrontal and ACC) to limbic (parahippocampal) regions. In addition to connecting fronto-parietal control network areas, the limbic aspects of this tract access emotion processing regions. Integrity of the CB has been associated with performance on cognitive control/response inhibition tasks in healthy (Takahashi et al., 2010; Yamamoto et al., 2015) and psychiatric populations (Keedwell et al., 2016; Takei et al., 2009). Stroop performance, particularly in the context of positive distractors (Keedwell et al., 2016), has been associated with CB integrity, indicating the functional consequences of these white matter decrements. Other studies have observed that CB integrity is linked to performance on tasks of response conflict and sustained attention (Takahashi et al., 2010; Yamamoto et al., 2015). Injury to this path has been linked to deficits on tasks of executive control e.g., (Cohen et al., 1999; Janer and Pardo, 1991; Kim et al., 2003), and reduced emotional responsivity (Cohen et al., 2001). In light of this corpus of data, the CB is thought to play a role in modulating attentional control in the context of emotion (Bubb et al., 2018). We have previously observed how decrements in cingulum structure and connectivity characterize PTSD (Fani et al., 2012a, 2016) and related functions, including extinction learning (Fani et al., 2015). The present findings extend our earlier work, elaborating on how these decrements play a role in dysfunctional attentional control in this disorder.
Re-experiencing symptoms were associated with increased response in the dACC to positive versus neutral distractor trials. These findings are aligned with earlier studies showing PTSD-specific increases in dACC response to trauma-related stimuli more generally (Hayes et al., 2012) and to emotional stimuli embedded in cognitive tasks (Fani et al., 2012b; Herzog et al., 2017; White et al., 2015). After successful treatment, individuals with PTSD have shown differences in dACC response (Malejko et al., 2017) and improved performance during emotional, but not neutral Stroop task conditions (Thomaes et al., 2012), further suggesting that attentional control measures that include emotional stimuli are more sensitive to treatment effects. We did not find group-wise differences in amygdala activation, similar to other studies of PTSD using the same task (Blair et al., 2007, 2013; White et al., 2018). This suggests that the AS measure is a better probe of attention network function vs basic emotion processing/fear network function in traumatized populations. This task can also provide meaningful multimodal data in treatment outcome research.
Symptoms of avoidance and hyperarousal were predominantly associated with activation in regions involved with salience detection, including limbic (parahippocampal) and the mid-cingulate gyrus, in response to positive images. Hyperarousal was also correlated with poorer AS task performance, particularly on trauma-relevant and neutral trials. Given the other relationships observed between integrity of the UF, CB and AS task performance, it is possible that avoidance and hyperarousal clusters emerge as a result of abnormalities within salience and cognitive control networks. Traumatized people with more fearful arousal and avoidance symptoms may show attentional control deficits in the context of emotion, heightened limbic activation and under-activation in attention control regions. Other studies have observed specific associations between hyperarousal and attentional control deficits (Grisanzio et al., 2018). This could indicate that these specific clinical and cognitive features of PTSD (hyperarousal and attentional control deficits, both of which are transdiagnostic features) may have shared neural substrates.
Although participants with PTSD demonstrated poorer performance on emotion-related AS trials, they demonstrated relatively similar performance on neutral trials. These findings highlight the fact that PTSD-related deficits in attention may be most visible in emotional contexts, and similarly illustrate the value of using attentional tasks that incorporate emotional stimuli in research and clinical settings. Given that they can accommodate images, tasks such as the AS are most useful in PTSD studies and are both sensitive enough to detect attentional disruptions and equipped to identify the nature of these disruptions in various traumatized populations.
Our findings not only reveal specificity in attentional control dysfunction and PTSD symptoms, but also shed light on attractive targets for PTSD treatment. Greater ACC response at pre-treatment has been shown to predict treatment response (Malejko et al., 2017). As a hub of attention and salience networks, the ACC is an excellent target for neuromodulation. Cognitive control can be trained in PTSD, as has been shown recently in a study that observed improved performance after training with an affective working memory task (Schweizer et al., 2017; others have proposed the use of web-based cognitive training (Fine et al., 2018). Mindfulness-based treatments, which involve training attentional focus to a sensory stimulus while monitoring emotional states, has been shown to improve response in fronto-parietal attentional control networks; a recent study found increased DLPFC and dACC response, as well as improved AS performance, in participants who completed 6 weeks of mindfulness treatment compared to those who received an active control condition (Allen et al., 2012).
The limitations of this study include the fact that the majority of our sample had experienced multiple traumas, which can influence cognition, brain structure and function. However, PTSD-specific effects remained even after accounting for trauma exposure in statistical analyses. We also used two different versions of the CAPS to diagnose PTSD, which introduces a source of variance to the findings, and included one participant with psychotropic medication use, a factor that may influence results. Given our cross-sectional study design, we were also unable to determine the onset of attentional control abnormalities and their temporal relationship to trauma—this is worthy of examination in future prospective designs with more heterogenous and expansive sample sizes.
In conclusion, we found that PTSD was characterized by impaired attentional control in emotional contexts, and that these disruptions were associated with abnormal fronto-parietal and fronto-limbic network function and structural integrity, as well as increased salience network activation. Our data show a mechanism through which attentional control problems may emerge in PTSD, and highlight the importance of using emotionally-salient tasks in PTSD assessment and intervention research.
Supplementary Material
Funding and Acknowledgments
This work was primarily supported by National Institutes of Mental Health (MH101380 to NF, MH092576 to TJ). Support was also received from the Emory Medical Care Foundation and American Psychological Association, Society for Clinical Neuropsychology, award 36496. We wish to thank Amrita Kaimal, Saankari Challa, Jenani Srijeyanthan, Michaela Desrosiers, Katrina Conrad, and Nayan Tiwary for their assistance with data collection, as well as the contributions of our research participants and numerous research volunteers for the Grady Trauma Project.
Footnotes
Disclosure
The authors have no financial conflicts of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2019.04.098.
References
- Allen M, Dietz M, Blair KS, van Beek M, Rees G, Vestergaard-Poulsen P, Lutz A,Roepstorff A, 2012. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci 32, 15601–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, McDermott TJ, Pine DS, Bar-Haim Y, 2015. Effect of attention training on attention bias variability and PTSD symptoms: randomized controlled trials in Israeli and U.S. combat veterans. Am. J. Psychiatry 172, 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W, 2009. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav. Rev. 33, 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Blair JR, Pine DS, 2012a. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol. Psychiatry 72, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ, 2007. Modulation of emotion by cognition and cognition by emotion. Neuroimage 35, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Scaramozza M, Mondillo K, Pine DS, Charney DS, Blair RJ, 2012b. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol. Med. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Scaramozza M, Mondillo K, Pine DS, Charney DS, Blair RJ, 2013. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol. Med. 43, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, 1995. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Block SR, Liberzon I, 2016. Attentional processes in posttraumatic stress disorder and the associated changes in neural functioning. Exp. Neurol. 284, 153–167. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Mishra S, Campanella C, Shah M, Kasher N, Evans S, Fani N, Shah AJ, Reiff C, Davis LL, Vaccarino V, Carmody J, 2017. A pilot study of the effects of mindfulness-based stress reduction on post-traumatic stress disorder symptoms and brain response to traumatic reminders of combat in operation enduring freedom/operation iraqi freedom combat veterans with post-traumatic stress disorder. Front Psychiatry 8, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS, 2004. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry 55, 612–620. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Whitford TJ, Kemp A, Hughes G, Peduto A, Williams LM, 2008. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J. Psychiatry Neurosci. 33, 142–146. [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C, Aggleton JP, 2018. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav. Rev. 92, 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NT, Clarke PJ, MacLeod C, Guastella AJ, 2012. Biased attentional processing of positive stimuli in social anxiety disorder: an eye movement study. Cogn. Behav. Ther. 41, 96–107. [DOI] [PubMed] [Google Scholar]
- Chen NT, Clarke PJ, Watson TL, Macleod C, Guastella AJ, 2014. Biased saccadic responses to emotional stimuli in anxiety: an antisaccade study. PLoS One 9, e86474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Wolitzky-Taylor KB, Adams TG Jr., Babson KA, Badour CL, Willems JL, 2011. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin. Psychol. Rev. 31, 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H, 1999. Impairments of attention after cingulotomy. Neurology 53, 819–824. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H, 2001. Emotional and personality changes following cingulotomy. Emotion 1, 38–50. [DOI] [PubMed] [Google Scholar]
- d’Arbeloff TC, Kim MJ, Knodt AR, Radtke SR, Brigidi BD, Hariri AR, 2018. Microstructural integrity of a pathway connecting the prefrontal cortex and amygdala moderates the association between cognitive reappraisal and negative emotions. Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M, 2013. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depress. Anxiety 30, 207–216. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Bradley-Davino B, Ressler KJ, McClure-Tone EB, 2011. Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognit Ther. Res. 35, 57–67. [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T, 2015. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 64, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ, 2012a. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 37, 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, Bradley B, Ressler KJ, 2016. Structural and functional connectivity in posttraumatic stress disorder: associations with Fkbp5. Depress. Anxiety 33, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Kamkwalala A, Jovanovic T, 2012b. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 42, 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine NB, Achituv M, Etkin A, Merin O, Shalev AY, 2018. Evaluating web-based cognitive-affective remediation in recent trauma survivors: study rationale and protocol. Eur. J. Psychotranmatol. 9, 1442602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO, 1993. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Traum. Stress 6, 459–473. [Google Scholar]
- Fox RJ, Sakaie K, Lee JC, Debbins JP, Liu Y, Arnold DL, Melhem ER, Smith CH, Philips MD, Lowe M, Fisher E, 2011. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR Am. J. Neuroradiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Rashed Ahmed AP, Samara Z, Williams LM, 2018. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry 75, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM, 2012. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Stefan M, Terranova K, Steinglass J, Marsh R, 2016. Altered white matter microstructure in adolescents and adults with bulimia nervosa. Neuropsychopharmccology 41, 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog JI, Niedtfeld I, Rausch S, Thome J, Mueller-Engelmann M, Steil R, Priebe K, Bohus M, Schmahl C, 2017. Increased recruitment of cognitive control in the presence of traumatic stimuli in complex PTSD. Eur. Arch. Psychiatry Clin. Neurosci. [DOI] [PubMed] [Google Scholar]
- Hughes KC, Shin LM, 2011. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev. Neurother. 11, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer KW, Pardo JV, 1991. Deficits in selective attention following bilateral anterior cingulotomy. J. Cogn. Neurosci 3, 231–241. [DOI] [PubMed] [Google Scholar]
- Jatzko A, Schmitt A, Demirakca T, Weimer E, Braus DF, 2006. Disturbance in the neural circuitry underlying positive emotional processing in post-traumatic stress disorder (PTSD). An fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 256, 112–114. [DOI] [PubMed] [Google Scholar]
- Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, Ajilore O, Langenecker SA, 2016. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin. 12, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing-Cobbs L, 2011. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J. Int. Neuropsychol Soc. 17, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagaratnam P, Asbjornsen AE, 2007. Executive deficits in chronic PTSD related to political violence. J. Anxiety Disord. 21, 510–525. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Doidge AN, Meyer M, Lawrence N, Lawrence AD, Jones DK, 2016. Subgenual cingulum microstructure supports control of emotional conflict. Cereb. Cortex 26, 2850–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, van Rooij SJ, Tromp do PM, Fox AS, Rademaker AR, Kahn RS, Kalin NH, Geuze E, 2015. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology 40, 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chang JW, Koo MS, Kim JW, Suh HS, Park IH, Lee HS, 2003. Anterior cingulotomy for refractory obsessive-compulsive disorder. Acta Psychiatr. Scand 107, 283–290. [DOI] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2017. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J. Psychiatry Neurosci. 42, 160129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Kolbitsch C, Mottaghy FM, Felber SR, Jaschke WR, Krause BJ, 2010. Caffeine and cognition in functional magnetic resonance imaging. J. Alzheimers Dis. 20 (Suppl 1), S71–S84. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Niznikiewicz M, Connor E, Ungar L, Nestor P, Bouix S, Dreusicke M, Kikinis R, McCarley R, Shenton M, 2009. Relationship between white matter integrity, attention, and memory in schizophrenia: a diffusion tensor imaging study. Brain Imaging Behav. 3, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde G, Doyon J, Brunet A, 2010. Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Res. 177, 144–149. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM, 2002. Dietary caffeine consumption modulates fMRI measures. Neuroimage 17, 751–757. [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352. [DOI] [PubMed] [Google Scholar]
- Liao M, Yang F, Zhang Y, He Z, Su L, Li L, 2014. White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 14, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner P, Flodin P, Larm P, Budhiraja M, Savic-Berglund I, Jokinen J, Tiihonen J, Hodgins S, 2018. Amygdala-orbitofrontal structural and functional connectivity in females with anxiety disorders, with and without a history of conduct disorder. Sci. Rep. 8, 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Canu E, Xu G, Oh J, McLaren DG, Dowling NM, Alexander AL, Sager MA, Johnson SC, Bendlin BB, 2013. Midlife measurements of white matter microstructure predict subsequent regional white matter atrophy in healthy adults. Hum. Brain Mapp.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko K, Abler B, Plener PL, Straub J, 2017. Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: a systematic literature review. Front. Psychiatry 8, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, Van Zijl PC, 2005. MRI Atlas of Human White Matter. Amsterdam, the Netherlands [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M, 2015. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev. 51, 189–204. [DOI] [PubMed] [Google Scholar]
- Palmer LK, Armsworth M, Swank PR, Bush G, Frantz C, Copley J, 1997. The neuropsychological sequelae of chronically psychologically traumatized children. Arch. Clin. Neuropsychol 12, 379–380. [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, Moreira-Almeida A, Lederman H, 2011. Police officers under attack: resilience implications of an fMRI study. J. Psychiatr. Res. 45, 727–734. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI, 2012. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, 1990. The attention system of the human brain. Ann. Rev. Neurosci. 13, 25–42. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS, 2004. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 56, 129–140. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A, 2012. White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia 50, 2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Samimi Z, Hasani J, Moradi A, Mirdoraghi F, Khaleghi M, 2017. Improving cognitive control in adolescents with post-traumatic stress disorder (PTSD). Behav. Res. Ther. 93, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, Schweinsburg BC, 2015. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 141, 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 20, 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL, 2001. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Stroop JR, 1935. Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662. [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ, 2012. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N, 2010. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci. Lett. 477, 72–76. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K, 2009. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr. Res. 114, 119–127. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Bomyea J, Amir N, 2010. Attentional bias away from positive social information mediates the link between social anxiety and anxiety vulnerability to a social stressor. J. Anxiety Disord 24, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, van Balkom AJ, Smit JH, Veltman DJ, 2012. Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol. Med. 42, 2337–2349. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr., Sutker PB, 2002. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology 16, 5–14. [DOI] [PubMed] [Google Scholar]
- Wang HH, Zhang ZJ, Tan QR, Yin H, Chen YC, Wang HN, Zhang RG, Wang ZZ, Guo L, Tang LH, Li LJ, 2010. Psychopathological, biological, and neuroimaging characterization of posttraumatic stress disorder in survivors of a severe coalmining disaster in China. J. Psychiatr. Res. 44, 385–392. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP, 2017. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Costanzo ME, Blair JR, Roy MJ, 2015. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a traumaexposed sample. Neuroimage Clin. 7, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Costanzo ME, Thornton LC, Mobley AM, Blair JR, Roy MJ, 2018. Increased cognitive control and reduced emotional interference is associated with reduced PTSD symptom severity in a trauma-exposed sample: a preliminary longitudinal study. Psychiatry Res. Neuroimaging 278, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD, 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kushima I, Kimura H, Hayashi A, Kawano N, Aleksic B, Iidaka T, Ozaki N, 2015. White matter microstructure between the pre-SMA and the cingulum bundle is related to response conflict in healthy subjects. Brain Behav. 5, e00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–U95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappala G, Thiebaut de Schotten M., Eslinger PJ, 2012. Traumatic brain injury and the frontal lobes: what can we gain with diffusion tensor imaging? Cortex 48, 156–165. [DOI] [PubMed] [Google Scholar]
- Zheng KZ, Wang HN, Liu J, Xi YB, Li L, Zhang X, Li JM, Yin H, Tan QR, Lu HB, Li BJ, 2018. Incapacity to control emotion in major depression may arise from disrupted white matter integrity and OFC-amygdala inhibition. CNS Neurosci. Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.