Abstract
Allogeneic hematopoietic stem cell transplant (AHSCT) outcomes data of older AML/myelodysplastic syndrome (MDS) patients are limited. We retrospectively evaluated consecutive patients ⩾60 years old with AML/MDS who underwent AHSCT between January 2005 and December 2014. The primary objectives were to determine nonrelapse mortality (NRM), relapse, relapse-free survival (RFS) and overall survival (OS) at 1 year post AHSCT. A total of 159 patients underwent AHSCT with a median age of 64 (range, 60–75) years. Of these, 103 patients (65%) had AML and 56 patients (35%) had MDS. At 1 year post AHSCT, grade III–IV acute GvHD and chronic GvHD occurred in 20.8% (95% confidence interval (CI), 14.9–27.5%) and 54.1% (95% CI, 46.0–61.5%) of patients, respectively. NRM, RFS, relapse rate and OS at 1 year post AHSCT were 25.3% (95% CI, 18.8–32.3%), 53.3% (95% CI, 46.1–61.7%), 21.4% (95% CI, 15.4–28.1%) and 56.4% (95% CI, 49.2–54.7%), respectively. High disease risk index was associated with poor RFS, OS and higher relapse rate (P < 0.03), whereas non-thymoglobulin-based GvHD prophylaxis, higher comorbidity index (⩾3) and MDS were associated with higher NRM (P < 0.03). Importantly, age did not have an adverse effect on NRM, relapse, RFS and OS. AHSCT was well tolerated. Hence, older age alone should not be considered a contraindication to AHSCT.
INTRODUCTION
AML and myelodysplastic syndrome (MDS) are clonal diseases involving hematopoietic stem cells with a median age of onset of 67 years and 70 years, respectively.1 Older patients with AML and MDS represent a discrete group of patients with a different disease biology.2 Various disease- and patient-related factors account for poor outcomes. These include higher frequency of unfavorable cytogenetics, lower incidence of favorable cytogenetics, increased anthracyclines resistance secondary to multidrug resistance phenotype (MDR1) and P-glycoprotein (gp170) chemotherapy efflux pump, increased proportion of secondary AML arising from MDS, multiple comorbid conditions, poor performance status and limited availability of related donors because of advanced age.2,3 Moreover, age-related pharmacokinetic and pharmacodynamic changes and weakened immunity result in poor tolerability to chemotherapy, and predispose them to increased morbidity and mortality.4 Therefore, odds of achieving CR1 and long-term leukemia-free survival with conventional chemotherapy among these patients are 55–80% and 10–15%, respectively.5,6
Allogeneic hematopoietic stem cell transplantation (AHSCT) remains a successful treatment modality for both diseases, especially with intermediate- and high-risk category.7 However, toxicities associated with myeloablative conditioning regimen often preclude older patients the opportunity to use potentially lifesaving modality.8–10 A phase III study demonstrated lower relapse rate and superior relapse-free survival (RFS) with myeloablative conditioning regimen but it was offset by higher transplant-related mortality.11 Reduced-intensity conditioning (RIC) regimen is another alternative that is well tolerated and offers comparable disease-free survival and nonrelapse mortality (NRM).12–15 It is well recognized that patient selection may be partially responsible for success of RIC. Despite favorable outcomes of RIC transplant in older patients, a common misperception of higher NRM among older patients still prevails among physicians. Thus, the number of older patients referred for AHSCT evaluation remains small. Essentially all older patients are excluded from prospective trials because of arbitrary age cutoff of 50–55 years, and thus data on AHSCT in older patients are limited. Moreover, the available trials have showed inconclusive associations between age and transplant outcomes.16–20 To better define this, we conducted a retrospective review of patients ⩾ 60 years of age who received AHSCT for AML and MDS at our institution.
MATERIALS AND METHODS
We conducted a retrospective study of patients ⩾ 60 years old who underwent AHSCT for AML and MDS between January 2005 and December 2014 at Karmanos Cancer Institute. Patients with cord blood or haploidentical transplantation were excluded to avoid heterogeneity. This study was approved by Wayne State University institutional review board.
The Karmanos Cancer Institute Blood and Marrow Transplant Program database was used. Demographic and transplant details for all patients were collected. Disease status at transplant was grouped into ‘complete remission’ (CR), ‘not complete remission’ (non-CR) and ‘untreated’ (URx) groups. Non-CR group included progressive disease, primary induction failure, relapse and stable disease. Patients with AML were further classified into low, intermediate, high and very high disease risk index (DRI) based on cytogenetics at diagnosis (NCCN (National Comprehensive Cancer Network) categories) and disease status at transplant.21 MDS patients were grouped into 4 groups (low, intermediate-1, intermediate-2 and high) based on IPSS (International Prognostic Scoring System) criteria.22 Comorbidity index (CI) was calculated using HSCT-CI formula.23 Patients’ records were reviewed to determine acute and chronic GvHD and followed till the last follow-up or death.
Outcome measures
The primary end points were to determine NRM, relapse rate, RFS and overall survival (OS) at 1 year post AHSCT. The secondary objectives were to estimate cumulative incidence and severity of acute GvHD (aGvHD) and chronic GvHD (cGvHD) at 1 year, GvHD-free relapse-free survival (GRFS), length of stay and readmission rate in the first 100 days following transplant.
Preparative regimen
The choice of preparative regimen was determined by the treating physician. Full-intensity regimen included: IV busulfan 130 mg/m2 daily for 4 days (days −6 to −3) and IV fludarabine 30 mg/m2 daily for 5 days (days −6 to −2). RIC regimen included IV busulfan 130 mg/m2 daily for 2 days (days −6 and −5), IV fludarabine 30 mg/m2 daily for 5 days (days −6 to −2) and TBI 200 cGy (day 0). The actual dose of busulfan delivered was adjusted to target the daily area under the curve for busulfan of 5000 μmol ×min.
GvHD prophylaxis
The GvHD prophylaxis was selected by the treating physician. Rabbit antithymocyte globulin (ATG) at a total dose of 4.5 mg/kg was given in divided doses (day −3: 0.5 mg/kg; day −2: 1.5 mg/kg; and day −1: 2.5 mg/kg). Tacrolimus was IV administered (0.03 mg/kg/day) starting on day −3 and tapered starting around day +100 in the absence of active GvHD with a goal of tapering off completely by day +180. Sirolimus was given on day −3 with a 12 mg loading oral dose, followed by 4 mg daily beginning on day −2 onwards. Mycophenolate was initiated at 15 mg/kg twice daily from day −3 and stopped at day +30.
Supportive care
All patients received standard antimicrobial prophylaxis consisting of fluoroquinolone, fluconazole and acyclovir. G-CSF (5 μg/kg) was started at day +6 until engraftment. CMV was monitored weekly by blood PCR. Patients with PCR titers >1000 copies/ml were treated with ganciclovir. Patients received Pneumocystis prophylaxis with double strength trimethoprim/sulfamethoxazole twice weekly started on day +30 post transplant. Weekly EBV PCR surveillance was started on day +20 and continued until day +120 post transplant. Patients with EBV PCR>1000 genome copies/ml were treated with one dose of rituximab. Additional doses were used if no decline in viral load was seen following the first dose.
Statistical analysis
Baseline patient characteristics were summarized using count and percentage for categorical variables and median and range for continuous variables. Patient characteristics were compared between two diagnosis groups (AML and MDS) and two age groups (age ⩽65 and >65 years), respectively. Kruskal–Wallis tests were used to compare age groups for continuous variables. Fisher’s exact tests were used to compare age groups for categorical variables. The length of hospital stay was calculated as the time from the date of admission before transplant to the date of discharge post transplant. OS was calculated as the time from the date of transplant to death from any cause. Patients who were alive were censored at the date of last observation. RFS was calculated as the time from the date of transplant to the date of relapse or death from any cause. Patients who were alive without relapse were considered censored at the date of last observation. Composite end point of GRFS was calculated as the time from the date of transplantation to the date of grade III–IV aGvHD, cGvHD requiring treatment, relapse or death from any cause.24 Patients who were alive without grade III–IV aGvHD, grade E1–E3 cGvHD, relapse and death were censored at the date of last observation. Kaplan–Meier estimates were used to summarize the distribution of OS, RFS and GRFS. The cumulative incidences of acute and chronic GvHD were calculated with relapse or death without GvHD as competing risks. When calculating the cumulative incidence of grade III–IV aGvHD, the events of grade I–II aGvHD were ignored. The cumulative incidences of relapse and NRM were calculated with death without relapse for relapse and disease relapse for NRM, respectively, as competing risks. Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between seven prior chosen predictors (age at transplant, diagnosis, transplant type, DRI, comorbidity, conditioning regimen, GvHD prophylaxis and CMV match) and survival benefit (GRFS, RFS and OS). For relapse and NRM, the proportional subdistribution hazards regression model in competing risks was used for univariable and multivariable analyses with the seven predetermined covariates. The proportional hazards assumption was assessed and no violation was found. In addition, the covariate age was further separated into two groups (age ⩾ 60 and ⩽65, >65 and ⩽75) and used for the univariable and multivariable Cox and subdistribution proportional hazards regression models. Note that because of the retrospective nature of this study, observed power is determined completely by the P-value of each analysis.25
RESULTS
Baseline characteristics
A total of 159 patients underwent AHSCT. Of these, 103 (65%) patients had AML and 56 (35%) had MDS (Table 1). Of the AML patients, 67 (42%) had de novo and 36 (23%) had secondary AML. The patients with secondary AML had previous MDS,16 myeloproliferative disorders,13 and therapy-related AML.7 The patients were divided into 2 groups: ⩽65 years and >65 years old. More patients in ⩽65 years of age were untreated (19% vs 4%) and underwent 7/8 transplant (17% vs 4%), whereas patients with > 65 years of age had increased use of RIC regimen (87% vs 60%) and non-thymoglobulin-based GvHD prophylaxis (62% vs 42%) (Supplementary Table S1).
Table 1.
Baseline patient characteristics
| AML (N= 103) | MDS (N= 56) | All (N= 159) | Signif | |
|---|---|---|---|---|
| Age, year-median (range) | 65 (60–75) | 63 (60–72) | 64 (60–75) | 0.013 |
| Sex–no. (%) | 0.221 | |||
| Female | 38 (37) | 15 (27) | 53 (33) | |
| Male | 65 (63) | 41 (73) | 106 (67) | |
| Race–no. (%)* | 0.543 | |||
| Caucasian | 101 (98) | 55 (98) | 156 (98) | |
| Black/Others | 2 (2) | 0 (0) | 2 (1) | |
| Diagnosis–no. (%) | ||||
| AML–no. (%) | ||||
| De novo | 67 (65) | — | 67 (42) | |
| Secondary | 36 (35) | — | 36 (23) | |
| MDS–no. (%) | ||||
| RCUD | — | 1 (2) | 1 (1) | |
| RAEB-1 | — | 13 (23) | 13 (8) | |
| RAEB-2 | — | 20 (36) | 20 (13) | |
| RARS | — | 5 (9) | 5 (3) | |
| RCMD | — | 15 (27) | 15 (9) | |
| Therapy-related MDS | — | 2 (4) | 2 (1) | |
| IPSS score–no. (%) | ||||
| 0 | — | 2 (4) | 2 (1) | |
| 0.5–1 | — | 21 (38) | 21 (13) | |
| 1.5–2 | — | 22 (39) | 22 (14) | |
| >2.5 | — | 4 (7) | 4 (3) | |
| Disease status at transplant-no. (%) | <0.001 | |||
| Complete remission | 61 (59) | 3 (5) | 64 (40) | |
| Not complete remission | 36 (35) | 37 (66) | 73 (46) | |
| Untreated | 6 (6) | 16 (29) | 22 (14) | |
| Comorbidity index-median (range)$ | 3 (0–8) | 3 (0–7) | 3 (0–8) | 0.652 |
| Disease risk index–no. (%)$ | 0.172 | |||
| Low | 1 (1) | 0 (0) | 1 (1) | |
| Intermediate | 41 (40) | 20 (36) | 61 (38) | |
| High | 44 (43) | 31 (55) | 75 (47) | |
| Very high | 15 (15) | 3 (5) | 18 (11) | |
| Duration from diagnosis to transplant–no. (%) | 0.822 | |||
| 0–3 Months | 17 (17) | 8 (14) | 25 (16) | |
| >3 Months | 86 (83) | 48 (86) | 134 (84) | |
| Admit KPS–no. (%) | > 0.99 | |||
| ⩾80 | 46 (45) | 25 (45) | 71 (45) | |
| < 80 | 57 (55) | 31 (55) | 88 (55) | |
| Transplant type–no. (%) | 0.022 | |||
| Allogeneic related | 27 (26) | 25 (45) | 52 (33) | |
| Allogeneic unrelated | 76 (74) | 31 (55) | 107 (67) | |
| HLA match–no. (%) a | 0.627 | |||
| 8/8 | 87 (84) | 50 (89) | 137 (86) | |
| 7/8 | 14 (14) | 6 (11) | 20 (13) | |
| Donor/recipient CMV serotype–no. (%)b | 0.168 | |||
| −/− | 30 (29) | 19 (34) | 49 (31) | |
| −/+ | 32 (31) | 20 (36) | 52 (33) | |
| +/− | 11 (11) | 9 (16) | 20 (13) | |
| +/+ | 28 (27) | 7 (12) | 35 (22) | |
| Donor/recipient sex matching–no. (%)c | 0.351 | |||
| Female/female | 12 (12) | 3 (5) | 15 (9) | |
| Female/male | 26 (25) | 13 (23) | 39 (25) | |
| Male/female | 24 (23) | 11 (20) | 35 (22) | |
| Male/male | 37 (36) | 28 (50) | 65 (41) | |
| ABO matching–no. (%)a | 0.868 | |||
| Match | 52 (50) | 29 (52) | 81 (51) | |
| Mismatch | 50 (48) | 26 (46) | 76 (48) | |
| Conditioning regimen–no. (%) | 0.475 | |||
| Full intensity | 34 (33) | 15 (27) | 49 (31) | |
| Reduced intensity | 69 (67) | 41 (73) | 110 (69) | |
| BMT year–no. (%) | 0.218 | |||
| 2005–2009 | 30 (29) | 22 (39) | 52 (33) | |
| 2010–2014 | 73 (71) | 34 (61) | 107 (67) | |
| GvHD prophylaxis–no. (%) | 0.510 | |||
| Thymoglobulin based | 51 (50) | 31 (56) | 82 (52) | |
| Non-thymoglobulin based | 52 (51) | 25 (45) | 77 (48) |
Abbreviations: BMT = bone marrow transplant; IPSS = International Prognostic Scoring System; MDS = myelodysplastic syndrome; RAEB-1 = refractory anemia with excess blasts-1; RAEB-2 = refractory anemia with excess blasts-2; RARS = refractory anemia with ringed sideroblasts; RCMD = refractory cytopenia with multilineage dysplasia; RCUD = refractory cytopenia with unilineage dysplasia; Signif = significance.
Data are not available for 2 patients.
Data are not available for 3 patients.
Data are not available for 5 patients.
Data are not available for 1 patient.
Data are not available for 4 patients.
Bold and Italic numbers stand for statistical significant numbers.
The median time from diagnosis to AHSCT was 154 (range, 16–3716) days. The median length of hospitalization following transplant was 26 (range, 19–112) days and half of patients (52%) were readmitted within the first 100 days.
Engraftment and GvHD
The median times to neutrophil and platelet engraftment were 11 (range, 7–22) days and 16 (range, 0–675) days post AHSCT, respectively. Primary graft failure was observed in 2 patients and both required second transplantations, whereas secondary graft failure was seen in one patient at day +233 post AHSCT.
In all, 65 patients (41%) developed grade II–IV aGvHD, with a cumulative incidence of 39.7% (95% confidence interval (CI), 32–47.2) at 1 year. The median time to development of grade II–IV aGvHD was 32 (95% CI, 28–37) days. Of these patients, 51 (78%) were 8/8 HLA matched, and 13 patients (20%) were 7/8 matched. Thirty-five patients (22%) developed grade III–IV aGvHD, with a cumulative incidence of 20.8% (95% CI, 14.9–27.5) at 1 year (Figure 1a). Of these patients, 26 (74%) were 8/8 matched and 9 patients (26%) were 7/8 matched. Two patients developed aGvHD beyond day +100 (Supplementary Table S2). Seventy-six patients developed cGvHD, with a cumulative incidence of 54.1% (95% CI, 46–61.5) at 1 year (Figure 1b). The cumulative incidence of extensive cGvHD was 39.8% (95% CI, 32.1–47.4). The median time to development of both limited and extensive cGvHD was 165 (95% CI, 145–194) days. Mild, moderate and severe extensive cGvHD were noted in 26 (16%), 25 (16%) and 17 (11%) patients, respectively, and bronchiolitis obliterans was developed in 19 patients.
Figure 1.
(a) Cumulative incidence of grade III–IV aGvHD after transplantation with disease relapse or death without grade III–IV aGvHD as competing risks. (b) Cumulative incidence of cGvHD after transplantation with disease relapse or death without cGvHD as competing risks.
Post transplant infections
Seventy patients (44%) developed blood stream infections. Single Gram-positive, single Gram-negative and polymicrobial infections were noticed in 23, 17 and 30 patients, respectively. Fifty-six patients (35%) had CMV reactivation. Of these, 8 patients developed CMV disease: 7 had gastrointestinal disease, and 1 had both gastrointestinal and lung disease. EBV reactivation was noticed in 35 patients (22%); however, only 4 patients required rituximab therapy with successful resolution following treatment. No patients developed EBV-related post-transplant lymphoproliferative disorder (PTLD) or sinusoidal obstruction syndrome. Clostridium difficile colitis was noticed in 41 patients (26%) and Aspergillus sp. infection was found in 10 patients (6%). Seventeen patients (11%) developed respiratory syncytial virus infection, of which 4 patients required IVIg and ribavirin.
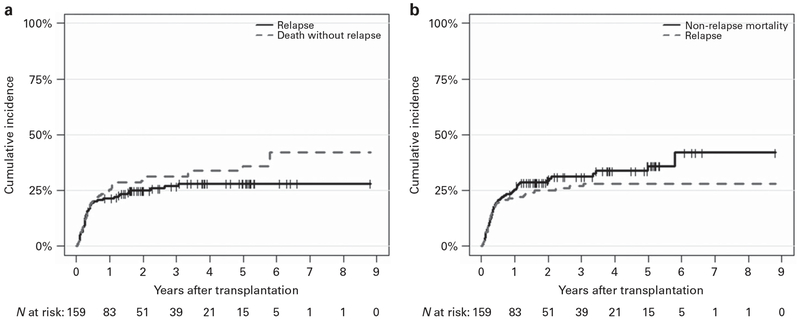
Disease progression
Forty-two patients had disease progression, with a cumulative incidence of 21.4% (95% CI, 15.4–28.1) at 1 year (Figure 2a). Of these patients, 10 (76%) received full-intensity conditioning regimen and 32 patients (24%) had RIC regimen. The median time to progression was 101 days (range, 12–1120). Twenty-one patients had disease progression within +100 days, 13 patients progressed between +100 days and 1 year and 8 patients progressed beyond 1 year. The relapses differed significantly by donor type that is, 67% had an unrelated donor compared with 33% with a related donor. Four patients received donor lymphocytes infusions and three underwent second transplantation. Twenty patients developed post-transplant large granular lymphocytosis. The multivariate analysis demonstrated AML, high and very high DRI and higher CI (⩾3) (P < 0.004) to be associated with higher relapse rate. There was no difference in relapse in patients ⩽ 65 and >65 years of age (Supplementary Table S3).
Figure 2.
(a) Cumulative incidence of relapse after transplantation with death without relapse as a competing risk. (b) Cumulative incidence of NRM after transplantation with disease relapse as a competing risk.
Non-relapse mortality
The cumulative incidence of NRM at 1 year post AHSCT was 25.3% (95% CI, 18.8–32.3) (Figure 2b). The factors associated with higher NRM in multivariate analysis were MDS, higher CI (⩾3) and non-thymoglobulin-based GVHD prophylaxis (P < 0.03); however, age, donor type, DRI, conditioning regimen and CMV serotype had no impact on NRM (Supplementary Table S3).
RFS, GRFS and OS
The median follow-up of living patients was 3.3 (95% CI, 2.51–3.87) years. At 1 year, the cumulative incidence of RFS was 53.3% (95% CI, 46.1–61.7) and GRFS was 35%. OS probability at 1 year was 56.4% (95% CI, 49.2–54.7) (Figure 3). The median OS was 1.6 (95% CI, 0.936–4.997) years. On multivariate analysis, patients with high and very high DRI had worse RFS (P = 0.017), GRFS (P = 0.021) and poor OS (P = 0.032) compared with patients with low or intermediate DRI, whereas a favorable trend was noticed with thymoglobulin-based GvHD prophylaxis. No difference in RFS, GRFS and OS was observed between patients ⩽65 and >65 years of age (Supplementary Table S4).
Figure 3.
OS, RFS and GRFS estimates.
Eighty-nine patients died at a median of 135 (range, 15–2118) days post AHSCT. The causes of death were recurrence of leukemia (45%), multiorgan failure (24%), aGvHD (18%), cGvHD (12%), graft rejection (2%) and new malignancy (1%).
DISCUSSION
Management of older AML and MDS patients is challenging and there is no consensus regarding optimal induction chemotherapy and consolidation AHSCT. This is a critical issue as the number of older AML and MDS patients is expected to increase with an aging population. Our study demonstrated that AHSCT was well tolerated in older patients and older age had no impact on NRM, relapse rate, RFS, GFRS and OS. Compared with expected outcomes without AHSCT in this age group, this group of selected patients did much better.5,6 Like previous studies, an increased number of older patients underwent AHSCT in recent years.26,27 However, the proportion of older patients referred for AHSCT remains limited, often because of unfounded bias regarding appropriate age for transplant.
Our study revealed an acceptable rate of grade III–IV aGvHD.28,29 However, a higher rate of cGvHD was noticed that was consistent with previous studies.16,30 The higher cGvHD could possibly be attributed to more patients who underwent peripheral stem cells and more use of unrelated donors because of unavailability of sibling donors.16,31,32 Devine et al.33 reported relatively lower rates of acute and chronic GvHD with the incorporation of higher dose of rabbit thymoglobulin (7.5 vs 4.5 mg/kg). We observed a favorable effect of thymoglobulin-based GvHD prophylaxis on NRM, whereas positive trend on RFS and OS was noted. These results could most likely be attributed to a reduction of incidence and/or severity of GVHD as noticed in prior studies.34–36
Despite higher use of RIC and T cell-depleting agents, relatively lower relapse rate was noticed in our study. We predominantly used busulfan/fludarabine/TBI as RIC regimen compared with fludarabine/busulfan or fludarabine/melphalan used in the BMT CTN (Blood and Marrow Transplant Clinical Trials Network) study by Scott et al.37 We recently presented our experience with AML/MDS patients and found no difference in RFS and OS between myeloablative regimen and TBI containing RIC regimen.38 In our opinion, addition of TBI might have further increased effectiveness of busulfan/fludarabine and lowered relapse rate. The relapse rate was lower compared with the study of Devine et al.33 Probably, the relatively lower dose of thymoglobulin in our study might have impacted relapse rate. Soiffer et al.39 observed higher relapse rate (51% vs 38%), poor RFS (25% vs 39%) and OS (38% vs 46%) with ATG in RIC regimen AHSCT. However, in this study, rabbit ATG was given at a dose of 7 mg/kg, whereas we utilized rabbit ATG at a dose of 4.5 mg/kg. We think that higher dose of ATG could be responsible for higher relapse rate, posttransplant lymphoproliferative disease (PTLD) and poor RFS. Similar associations between ATG dose and relapse were noted in previous studies. In a study by Deeg et al.,40 optimal GvHD benefit was noticed with thymoglobulin 4.5 to 6 mg/kg with no increased risk of relapse or EBV reactivation. Furthermore, improved NRM and infection rates were observed with reduced dose of thymoglobulin.41 Our study demonstrated higher CI as one of the adverse factors for relapse and NRM. The higher CI is associated with increased risk of GvHD and eventually higher NRM as seen in previous studies.29,42,43
High and very high DRI have emerged as important factors predicting higher relapse rate and poor RFS, GRFS and OS in our study. Our study showed significant survival benefit in patients with intermediate/low DRI, consistent with studies by Koreth et al.7 and Fukuda and colleagues.44 One of the impediments in determining AHSCT eligibility of older patients is donor availability. Often, finding a related donor becomes difficult as potential donors are old and comorbidities exclude them from stem cell donation. Fukuda and colleagues44 and Lim et al.45 noticed lower incidence of NRM among related donor transplants; however, we observed no effect of donor type on transplant outcomes including NRM. These results imply that limited availability of matched related donor should not be a barrier to AHSCT. Previous studies have revealed better NRM and OS in older AML patients undergoing AHSCT with CR1.44,46 In contrast, our study included patients who were not in complete remission. Despite this, no negative impact on NRM, relapse rate or RFS was observed.
Our observed GRFS rate is comparable to the study of Holtan et al.24 They noted donor type, age, DRI and year of transplant to be independent predictors for GRFS. Our study supported the effect of DRI on GRFS, whereas age and donor type had no impact on GRFS.
Our study is retrospective and limited to a single center. However, presence of higher median CI (⩾3) and high to very high DRI in more than half of the patients speak against any selection bias in our study.
In conclusion, our results indicate that biologic age alone should not be the only criterion in determining transplant eligibility. Instead, a careful consideration should be given to patient characteristics including comorbid conditions, and disease characteristics including cytogenetic abnormalities, and disease status at transplant. Moreover, the beneficial effect of thymoglobulin-based GvHD regimen on NRM stipulates its potential role in this patient population. Because of the nature of disease, prompt decision should be made to refer these patients for AHSCT consideration to achieve better outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The study data were presented at the American Society of Hematology (ASH) annual meeting 2016. Only abstract of the study was published in Blood journal supplement issue in December 2016 and only abstract is available online (Google scholar).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
REFERENCES
- 1.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control 2008; 19: 379–390. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE et al. Age and acute myeloid leukemia. Blood 2006; 107: 3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamdani H, Santos CD, Konig H. Treatment of acute myeloid leukemia in elderly patients-a therapeutic dilemma. J Am Med Dir Assoc 2016; 17: 581–587. [DOI] [PubMed] [Google Scholar]
- 4.Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood 2015; 125: 767–774 [DOI] [PubMed] [Google Scholar]
- 5.Baudard M, Marie JP, Cadiou M, Viguie F, Zittoun R. Acute myelogenous leukaemia in the elderly: retrospective study of 235 consecutive patients. Br J Haematol 1994; 86: 82–91. [DOI] [PubMed] [Google Scholar]
- 6.Estey EH. How I treat older patients with AML. Blood 2000; 96: 1670–1673. [PubMed] [Google Scholar]
- 7.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009; 301: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahn JY, Labopin M, Schattenberg A, Reiffers J, Willemze R, Zittoun R et al. Allogeneic bone marrow transplantation for acute leukemia in patients over the age of 40 years. Acute Leukemia Working Party of the European Group for Bone Marrow Transplantation (EBMT). Leukemia 1997; 11: 416–419. [DOI] [PubMed] [Google Scholar]
- 9.Zikos P, Van Lint MT, Frassoni F, Lamparelli T, Gualandi F, Occhini D et al. Low transplant mortality in allogeneic bone marrow transplantation for acute myeloid leukemia: a randomized study of low-dose cyclosporin versus low-dose cyclosporin and low-dose methotrexate. Blood 1998; 91: 3503–3508. [PubMed] [Google Scholar]
- 10.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol 2005; 23: 3439–3446. [DOI] [PubMed] [Google Scholar]
- 11.Pasquini MC, Logan B, Wu J, Devine S, Porter DL, Maziarz RT et al. Results of a Phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood 2015; 126: LBA-8. [Google Scholar]
- 12.Farag SS, Maharry K, Zhang MJ, Perez WS, George SL, Mrozek K et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant 2011; 17: 1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Daly A, Lipton JH, Hasegawa W, Chun K, Kamel-Reid S et al. Non-myeloablative stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia in patients 60 years or older. Biol Blood Marrow Transplant 2005; 11: 764–772. [DOI] [PubMed] [Google Scholar]
- 14.Wong R, Giralt SA, Martin T, Couriel DR, Anagnostopoulos A, Hosing C et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood 2003; 102: 3052–3059. [DOI] [PubMed] [Google Scholar]
- 15.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005; 105: 1810–1814. [DOI] [PubMed] [Google Scholar]
- 16.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol 2010; 28: 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JE, Anasetti C, Appelbaum FR, Schoch G, Gooley TA, Hansen JA et al. Unrelated donor marrow transplantation for myelodysplasia (MDS) and MDS-related acute myeloid leukaemia. Br J Haematol 1996; 93: 59–67. [DOI] [PubMed] [Google Scholar]
- 18.Arnold R, de Witte T, van Biezen A, Hermans J, Jacobsen N, Runde V et al. Unrelated bone marrow transplantation in patients with myelodysplastic syndromes and secondary acute myeloid leukemia: an EBMT survey. European Blood and Marrow Transplantation Group. Bone Marrow Transplant 1998; 21: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood 2002; 99: 1943–1951. [DOI] [PubMed] [Google Scholar]
- 20.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004; 104: 579–585. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012; 120: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088. [PubMed] [Google Scholar]
- 23.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 2001; 55: 19–24. [Google Scholar]
- 26.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2016; 22: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 2015; 94: 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chevallier P, Szydlo RM, Blaise D, Tabrizi R, Michallet M, Uzunov M et al. Reduced-intensity conditioning before allogeneic hematopoietic stem cell transplantation in patients over 60 years: a report from the SFGM-TC. Biol Blood Marrow Transplant 2012; 18: 289–294. [DOI] [PubMed] [Google Scholar]
- 29.Aoki J, Kanamori H, Tanaka M, Yamasaki S, Fukuda T, Ogawa H et al. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am J Hematol 2016; 91: 302–307. [DOI] [PubMed] [Google Scholar]
- 30.Koreth J, Aldridge J, Kim HT, Alyea EP 3rd, Cutler C, Armand P et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant 2010; 16: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eapen M, Logan BR, Confer DL, Haagenson M, Wagner JE, Weisdorf DJ et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant 2007; 13: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huisman C, Meijer E, Petersen EJ, Lokhorst HM, Verdonck LF. Hematopoietic stem cell transplantation after reduced intensity conditioning in acute myelogenous leukemia patients older than 40 years. Biol Blood Marrow Transplant 2008; 14: 181–186. [DOI] [PubMed] [Google Scholar]
- 33.Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol 2015; 33: 4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W, Smith D, Mitchell A et al. Antithymocyte globulin (thymoglobulin), tacrolimus, and sirolimus as acute graft-versus-host disease prophylaxis for unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1734–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pidala J, Tomblyn M, Nishihori T, Ayala E, Field T, Fernandez H et al. ATG prevents severe acute graft-versus-host disease in mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson R, Shapiro JF, Perkins JB, Kim J, Nishihori T, Pidala J et al. Sirolimus, tacrolimus and antithymocyte globulin as GVHD prophylaxis in HLA-mismatched unrelated donor hematopoietic cell transplantation: a single institution experience. Bone Marrow Transplant 2015; 50: 1487–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 2017; 35: 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albabtain A, Kim S, Uberti J, Ratanatharathorn V, Deol A, Ayash LJ et al. A retrospective comparison of outcomes in AML/MDS patients undergoing allogeneic stem cell transplant with reduced intensity and myeloablative conditioning regimens. Biol Blood Marrow Transplant 23: S258. [Google Scholar]
- 39.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 2011; 117: 6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant 2006; 12: 573–584. [DOI] [PubMed] [Google Scholar]
- 41.Hamadani M, Blum W, Phillips G, Elder P, Andritsos L, Hofmeister C et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2009; 15: 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood 2014; 124: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keller JW, Andreadis C, Damon LE, Kaplan LD, Martin TG, Wolf JL et al. Hematopoietic cell transplantation comorbidity index (HCT-CI) is predictive of adverse events and overall survival in older allogeneic transplant recipients. J Geriatr Oncol 2014; 5: 238–244. [DOI] [PubMed] [Google Scholar]
- 44.Kurosawa S, Yamaguchi T, Uchida N, Miyawaki S, Usuki K, Watanabe M et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant 2011; 17: 401–411. [DOI] [PubMed] [Google Scholar]
- 45.Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol 2010; 28: 405–411. [DOI] [PubMed] [Google Scholar]
- 46.Michelis FV, Messner HA, Atenafu EG, Kim DD, Kuruvilla J, Lipton JH et al. Benefit of allogeneic transplantation in patients age >/ = 60 years with acute myeloid leukemia is limited to those in first complete remission at time of transplant. Biol Blood Marrow Transplant 2014; 20: 474–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.