Abstract
Microtubule (MT)-targeting agents are highly successful drugs as chemotherapeutic agents, and this is attributed to their ability to target MT dynamics and interfere with critical cellular functions, including, mitosis, cell signaling, intracellular trafficking, and angiogenesis. Because MT dynamics vary in the different stages of the cell cycle, these drugs tend to be the most effective against mitotic cells. While this class of drug has proven to be effective against many cancer types, significant hurdles still exist and include overcoming aspects such as dose limited toxicities and the development of resistance. Newer generations of developed drugs attack these problems and alternative approaches such as the development of dual tubulin and kinase inhibitors are being investigated. This approach offers the potential to show increased efficacy and lower toxicities. This review covers different categories of MT-targeting agents, recent advances in dual inhibitors, and current challenges for this drug target.
Keywords: chemotherapeutics, dual inhibitors, kinases, multidrug resistance, tubulin
1 ∣. INTRODUCTION
1.1 ∣. Microtubule dynamics as an anticancer drug target
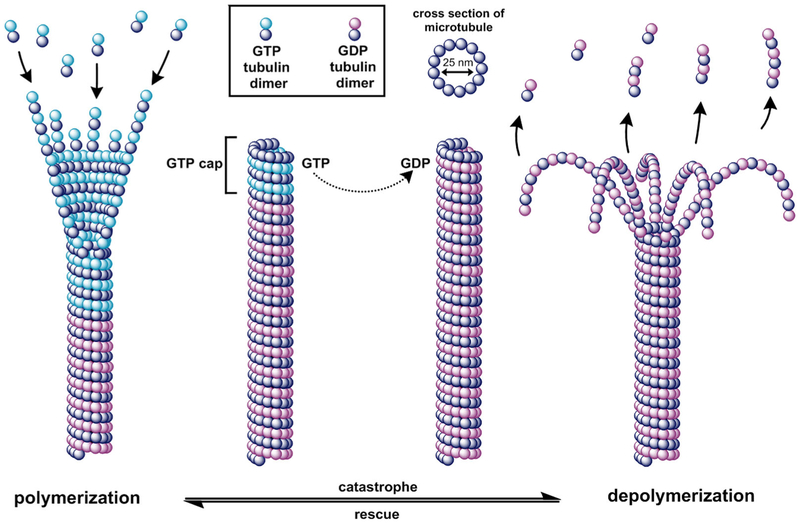
Microtubules (MTs) are key components of the cellular cytoskeleton and have essential roles in proliferation, intracellular trafficking, migration, and mitosis. MTs are composed of α and β tubulin protein heterodimers that bind in a head-to-tail fashion and form cylindrical polymeric tubes of 13 protofilaments that measure approximately 25 nm in diameter.1,2 MTs undergo stochastic phases of growth and shrinkage in a phenomenon termed “dynamic instability.” Dynamic instability includes the rates of polymerization and depolymerization and the frequencies of the transition between polymerization to depolymerization (“catastrophe”), and from depolymerization to polymerization (“rescue”).1 Another characteristic of MTs involves “treadmilling” or the net change in growth on one end and shrinkage on the opposite end of the protofilament. Each α and β tubulin subunit can accommodate one molecule of guanosine-5′-triphosphate (GTP); the α bound-GTP is not hydrolyzed or exchanged, but the GTP bound to the β tubulin can be hydrolyzed after being merged into the polymeric tubulin.3 MTs cycle through stages of adding GTP-tubulin dimers to their growing end, hydrolyzing GTP to guanosine diphosphate (GDP) in the MT protofilament, and dissociating the GDP dimer from the MT, though the conformations affiliated with each of these mechanical states are complex. Free GTP-tubulin dimers naturally have a 12-degree kink at the intradimer surface but convert to a straight, expanded conformation when they bind to the MT end, introducing strain energy into the MT lattice.4,5 Once the GTP is hydrolyzed to GDP, there is a conformational change and an accompanying relaxation in strain energy.6 Figure 1 shows the cycling between MT polymerization and depolymerization states. In the polymerization state, MTs adopt a straight structure, stabilized through lateral interactions, but hydrolyzed GDP-tubulin in the depolymerization state bow outward from the protofilament.7 It is generally accepted that a GTP rich “cap” on the end of the MT will allow it to stabilize and grow, however, when the cap is lost through hydrolysis to GDP, the core of the unstable protofilament rapidly shortens as the GDP-tubulin subunits are released from the MT ends.1,8
FIGURE 1.
Microtubule dynamic instability. Conformational changes of microtubules during catastrophe and rescue phases. During polymerization, GTP-tubulin is added to the growing end of the microtubule. During depolymerization, GTP is hydrolyzed to GDP and GDP protofilaments peel away from the microtubule and are released. A GTP rich cap stabilizes the microtubule and acts as a primer for tubulin polymerization. GDP, guanosine diphosphate; GTP, guanosine-5′-triphosphate [Color figure can be viewed at wileyonlinelibrary.com]
Both polymerizing and depolymerizing MTs are observed within a cell population, supporting that dynamics are an intrinsic property of the polymer, and while the exact biomechanics of the phenomenon is not completely understood, dynamic instability is linked to the hydrolysis of GTP.2,3,9 MT dynamics are also governed by a variety of regulator proteins and mechanisms, and they function both spatially and temporally.10 Furthermore, they are essential for successful mitotic events governed by mitotic spindles and mitotic organizing centers. Mitotic spindle MTs are significantly more dynamic than the interphase cytoskeleton MT networks. The mitotic spindles radiate outward from centrioles and attach to the centromeres of sister chromatids in dividing cells.11 This is followed by simultaneous addition of tubulin at the kinetochore and is accompanied by a comparable rate of tubulin loss at the opposite poles.12,13 If a chromosome is unable to achieve bipolar attachment to the spindle, the cell is unable to continue through the cell cycle and is blocked in the metaphase, eventually to succumb to apoptosis.14
Interfering with MT dynamics is an attractive anticancer strategy, and many drugs employing this tactic are therapeutically effective in a wide range of malignancies. Mitotic arrest in the G2/M phase, a hallmark of MT-targeting agents, is thought to occur through the perturbation of mitotic spindle machinery and failure to pass mitotic checkpoints. There are several sites on the tubulin heterodimer to which MT-targeting agents can bind, the most common being the vinca alkaloid, taxane, colchicine, and laulimalide binding sites. These drugs are generally divided into one of two classes; stabilizing agents, which enhance polymerization, or destabilizing agents, which inhibit tubulin polymerization.
1.2 ∣. Stabilizing agents
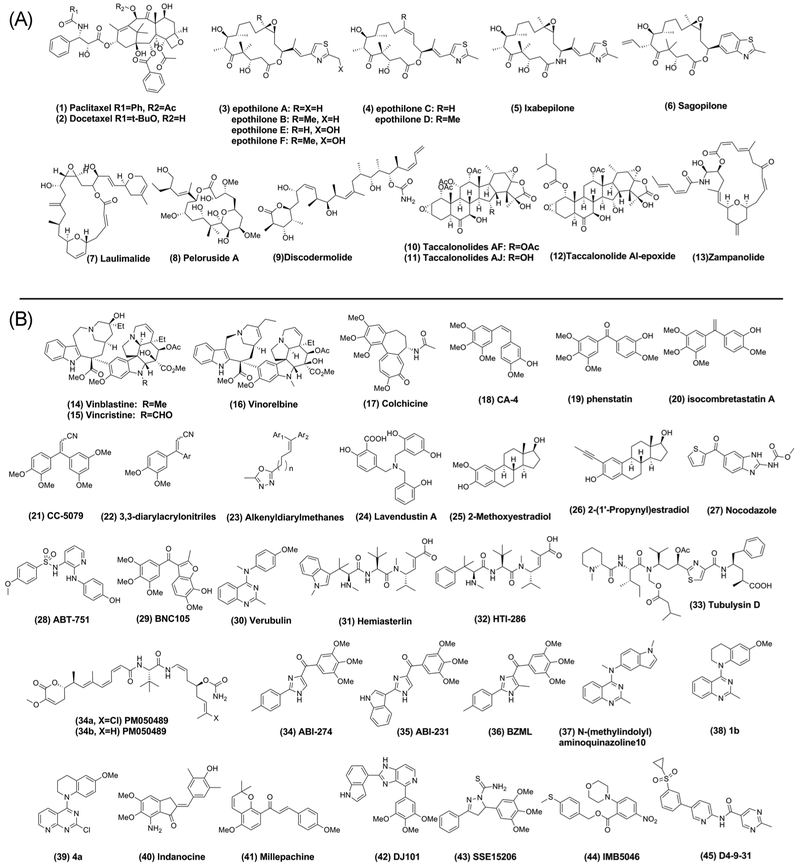
Stabilizing agents are able to promote polymeric tubulin structures even when the unfavorable GDP molecule is bound and are more effective than endogenous MT-associated proteins at stabilizing the MT.15,16 There are several drug categories within this class, including taxanes, epothilones, and laulimalide binding site agents.16,17 Representative drugs in this class can be found in Figure 2A.
FIGURE 2.
Chemical structures of microtubule targeting agents. A, Microtubule stabilizing compounds. B, Microtubule destabilizing compounds
1.2.1 ∣. Taxanes
The taxane binding site is located at the lumen of the β tubulin subunit in the intact heterodimer. Taxanes use the H6/H7 loop on β tubulin as a hinge to translocate from an intermediate position on the outside of the MT to the final binding site on the lumen of the MT.18 This motion is supported by a hydrogen bond in Ser277 in the M loop present in most tubulin isoforms. However, in the class βIII isoform, this Ser277 is replaced by an arginine residue, and overexpression of the βIII tubulin isoform is associated with resistance to taxanes.18,19 The first stabilizing agent identified was paclitaxel (Taxol) (1) and was approved by the FDA in 1992 and has been available clinically since 1996.20 Docetaxel (Taxotere) (2), a semisynthetic analog of paclitaxel, is another early generation taxane that was FDA approved for breast cancer in 1996, non–small-cell lung cancer in 1999, metastatic hormone-refractory prostate cancer in 2004, and head and neck cancer in 2006.21 While these drugs achieved much success in treating various cancers, the development of drug resistance limits their efficacy in many cases. Another issue hindering the clinical efficacy of taxanes is their limited solubility. Formulations such as Cremaphor EL and polysorbate are vehicles that allow for the solubilization of hydrophobic drugs but can induce a range of negative biological side effects in patients.22 Development of Abraxane, also known as paclitaxel protein-bound, is a delivery method that has mitigated this problem to some degree. High toxicity, immunosuppression, and peripheral neuropathy that accompany long-term use of taxanes prove that there is still a great need to develop agents with higher specificity, improved efficacy, and minimal off-target toxicities for this class of drugs.23
1.2.2 ∣. Epothilones
Epothilones make up another class of tubulin targeting agents that are distinct from taxanes, though they share a common binding site. While epothilones compete with paclitaxel for β tubulin binding, their common pharmacophore differs in that each class exploits the binding pocket in an independent manner and involves distinct amino acids.24,25 Epothilones, originally isolated from the myxobacterium Sorangium cellulosum, are more amenable to synthetic modifications than taxanes, more potent in taxane-resistant and sensitive cell lines, and are poor substrates for P-glycoprotein (P-gp) drug efflux pumps.26 Epothilones contain a macrolide ring with a methyl thiazole side chain, and those that are naturally occurring are characterized as either epoxides (epothilone A, B, E, and F) (3) or olefins (epothilone C and D) (4).27 Semisynthetic compounds have been developed to improve their pharmacologic properties and biological activities. For example, Ixabepilone (5), a second-generation derivative of Epothilone B, is less susceptible to degradation by carboxylesterase and has increased water solubility. It was the first epothilone derivative to be FDA approved for cancer treatment.25 Sagopilone (6) is the first fully synthetic analog of Epothilone B and has reached phase II clinical trials.28,29
1.2.3 ∣. Laulimalide binding site agents
Another binding site on tubulin is the laulimalide binding site. This site was discovered when it was observed that laulimalide (7) possessed a similar mode of action as taxanes but was completely unable to inhibit the binding of [3H] paclitaxel or Flutax-2, paclitaxel derivative to tubulin, confirming that it did not compete with the taxane binding site.30,31 Peloruside A (8) also directly binds to tubulin without competing with paclitaxel but can be displaced by laulimalide, supporting that Peloruside A shares a binding site with laulimalide.32,33 Both laulimalide and Peloruside A synergistically enhanced MT assembly induced by stabilizing agents, including discodermolide (9), dictyostatin, paclitaxel, Epothilones A and B, eleutherobin, and cyclostreptin.32 Furthermore, laulimalide and Peloruside A stabilize the M loop at the taxoid binding site, which contributes to crosstalk and explains the observed synergism between these agents and taxane site ligands.34
1.3 ∣. Destabilizing agents
The other class of MT-targeting agents are the destabilizing agents and include drugs that bind to the vinca alkaloid binding site and colchicine binding site. Their major mechanism of action (MOA) is to interfere with tubulin dynamics as opposed to just reducing overall polymerization. Selected destabilizing agents are shown in Figure 2B.
1.3.1 ∣. Vinca alkaloids
Vinca alkaloids are a natural product or semisynthetic extraction derivative from the periwinkle plant, Catharanthus roseus.35 The major vincas in clinical use are vinblastine (14), vincristine (15) and semisynthetic derivatives vinorelbine (16), vindesine, and vinflunine. The last two are only approved for use in Europe while the rest are FDA approved in the United States. In addition to their MT depolymerizing effects, vincas have also been shown to increase oxidized glutathione, alter lipid metabolism and membrane lipid content, elevate cAMP, and inhibit cAMP phosphodiesterase.36 Vincas bind near the exchangeable GTP site and inhibit GTP to GDP hydrolysis and GDP exchange, causing a change in the tubulin dimer from the straight conformation (which is favored for polymerization) to the curved conformation.23 Maytansinoids and halichondrins also induce depolymerization of MTs at the vinca site, while dolastatins, spongistatins, and cryptophycins bind to the “peptide site” of the vinca binding domain.23,37 Though vincas are among the most commonly used MT-targeting agents, they also are susceptible to the development of drug-resistant mechanisms and toxic side effects.
1.3.2 ∣. Colchicine binding site agents
The final major category of tubulin inhibitors is colchicine binding site agents. Colchicine (17), from the meadow saffron Colchicum autumnale L., was the first agent discovered in this group as a tubulin depolymerizing agent. The colchicine binding domain is located at the interface between the α and β tubulin dimer. When colchicine binds in the binding domain, it displaces the M loop and then the bound colchicine-tubulin complex becomes incorporated into the MT and interferes with the formation of lateral contacts at the end of protofilaments. This causes steric clashes and the tubulin can no longer maintain a straight conformation.38 As the concentration of colchicine increases, more lateral contracts are broken, ultimately leading to destabilization of MTs. Colchicine and colchicine binding site inhibitors are, therefore, able to suppress tubulin dynamics at lower concentrations and induce destabilization of MTs at higher concentrations. Colchicine itself is an approved drug for the treatment of gout and has implications in other inflammatory conditions such as familial Mediterranean fever pericarditis and Behçet’s disease. However, there are currently no colchicine binding site agents that have been approved for cancer therapy due to limitations such as poor drug solubility, a narrow therapeutic window, and harsh side effects including neurotoxicity. Despite these shortcomings, colchicine binding site agents have several advantages over other tubulin agents targeting different binding sites. First, drugs binding to the colchicine binding pocket may overcome mechanisms of drug resistance, which will be discussed herein. Also, colchicine binding site inhibitors can target tumor vasculature and prevent the formation of new blood vessels or disrupt existing microvessels.39,40 For these reasons, the colchicine binding site is an attractive target for the development of chemotherapeutic drugs and numerous scaffolds are being investigated.
Combretastatins are naturally occurring stilbenoid phenols isolated from the bark of Combretum caffrum.41 Combretastatin A-4 (CA-4) (18) demonstrates cell cycle–dependent arrest, potent antiangiogenic activity, and cytotoxicity by apoptosis rather than necrosis.42,43 The water-soluble phosphate prodrug, CA-4P, phenostatin (19) and many other CA-4 derivatives have been developed to optimize its potency, vascular disrupting potential, drug-like properties, and anticancer efficacy.44 Based on the knowledge gained from combretastatin and phenostatin, 1,1-diarylethene isomers of combretastatin A were developed called isocombretastatin A (20).45 CC-5079 (21), an isocombretastatin A analog, was determined to be an inhibitor of tubulin polymerization and tumor necrosis factor α (TNF-α). It showed cell cycle arrest in G2/M phase, increased phosphorylation of G2/M checkpoint proteins, and stimulated apoptosis. Additionally, its effect on TNF-α production also diminished phosphodiesterase type 4 enzymatic activity.46 CC-5079 was tested as a mixture of E and Z isomers, demonstrating a need for methods that would allow stereoselective synthesis and isolation of the E and Z isomers so that stereochemically pure forms can be obtained. The structure–activity relationship (SAR) of the two isomers of 3,3-diarylacrylonitriles (22) was explored by utilizing the Stille cross-coupling reaction, and it was determined that both were effective tubulin polymerization inhibitors, though the Z isomer was more potent.47 Alkenyldiarylmethanes (23) are a class of nonnucleoside reverse transcriptase inhibitors that are structurally related to CC-5079, and several compounds in this class also demonstrate potent tubulin destabilization.48 Along the same lines, Lavendustin A (24) derivatives, which inhibit protein-tyrosine kinases, inhibited tubulin polymerization.49,50 Extensive SAR investigation evaluating the conformations of Lavendustin A derivatives that resemble the trans-stilbene structure of the kinase inhibitor piceatannol and the cis-stilbene structure of the CA-4 revealed that conformational restriction of the bridge did not diminish biological activity.49,50
2-Methoxyestradiol (2-ME) (25), a metabolite of estradiol-17β, suppresses MT dynamics at the colchicine binding site, induces G2/M phase arrest, inhibits blood vessel formation at several stages in the angiogenic process, and has potent anticancer activity.51,52 It inhibits tubulin assembly in a concentration-dependent manner, and higher concentrations are needed to depolymerize MTs. In addition to showing efficacy in a variety of cancer types (breast, ovarian, lung, prostate, and colorectal carcinomas), 2-ME is active against estrogen-dependent and -independent cancerous cells. In addition to disrupting MT dynamics, 2-ME also acts through triggering the apoptotic cascade via reactive oxygen species, superoxide dismutase, and nitric oxide synthase.53 Exploration to optimize structure, function, and bioavailability was reported and modifications made include, substitutions on the A-D rings, homologation, aromatization, incorporating a variety of substituents to the C2 position, and substitution of the 17 hydroxy with alkyl and ethynyl groups.54 For example, Cushman et al55 evaluated the effect on cytotoxicity and polymerization inhibition of synthetic modifications at the C2 position to generate estradiol derivatives, and they delineated structural requirements to achieve antitubulin actions. While all compounds showed cytotoxic activity against 55 human cancer cell lines, only E-3′-hydroxy-1′-propenyl, 2′-hydroxyethoxy, ethynyl, and 1′-propynyl maintained destabilizing activity. The most potent and strongest depolymerizing compound, 2-(1′-Propynyl)estradiol (26), demonstrated significant in vivo anticancer activity in a hollow fiber animal model. The Cushman group also sought a solution to the problem of metabolic inactivation, similar to the in vivo inactivation and clearance of β-estradiol and other steroid hormones.56 This is primarily a result of oxidation by 17β-hydroxysteroid dehydrogenase of the alcohol at the C17-position of estradiol to estrone and also from the conjugation of the hydroxyls at the C3 and C17 positions to form a sulfate or glucuronide.57 They discovered that the 17α-methyl moiety of 17α-methyl-β-estradiol, 2-propynyl-17α-methyl estradiol, 2-ethoxy-17α-methyl estradiol, and 2-ethoxy-17-(1′-methylene) estra-1,3,5(10)-triene-3-ol were expected to reduce the deactivating oxidative metabolism of the 17-hydroxyl group while maintaining equal or greater cytotoxicity and tubulin inhibiting potency as the parental 2-ME.56 Another issue regarding 2-ME in biological systems is low solubility, so the Cushman group investigated a method to prepare soluble prodrugs that could be converted to 2-ME.58 A variety of derivatives were examined and they determined that 2-ME 3-phosphate, though it did not achieve significant bioavailability, did show dose-dependent tumor growth inhibition in the Lewis lung carcinoma metastasis model when dosed by intraperitoneal injection.58 These studies underscore the importance of drug properties, pharmacokinetics, and contribute to the knowledge of scaffold modifications for optimal drug design.
Nocodazole (27) is another natural product that inhibits tubulin polymerization by binding to the colchicine binding site as a reversible inhibitor.59 It also binds with varying affinities to the different tubulin isotypes, with the weakest affinity for βIII tubulin.60 Nocodazole did not advance through clinical trials due to toxic side effects, however, it is frequently used in cell culture to block mitosis and as a reference compound for colchicine binding site agents.15,61 Several colchicine binding site agents have made it to clinical trials including ABT-751 (28), BNC105 (29), and verubulin (30).36,62 One of the hallmarks of colchicine binding site agents is that they accommodate a wide range of structurally diverse scaffolds, and there is still much room for improvement to limit toxicities, improve solubility, and increase efficacies. Future directions for colchicine binding site agents aim to optimize structural based design, utilize targeting strategies, and increase specificity.
2 ∣. MECHANISMS OF MULTIDRUG RESISTANCE
One of the major hurdles that continue to limit current anticancer agents is the development of drug resistance. Multidrug resistance (MDR) is the simultaneous resistance to a number of structurally and functionally unrelated chemotherapeutic drugs and is a substantial obstacle impeding the success of anticancer agents.63 Many cancers initially respond well to chemotherapy early on during treatment but subsequently develop acquired resistance, and more than 90% of patients with metastatic cancer fail to respond to or relapse from chemotherapeutics.64 For these reasons, rigorous efforts have been devoted to elucidating both inherent and acquired MDR mechanisms over the past few decades. Although a complete understanding of MDR is still far from being uncovered, two major mechanisms have been proposed: noncellular and cellular mechanisms.
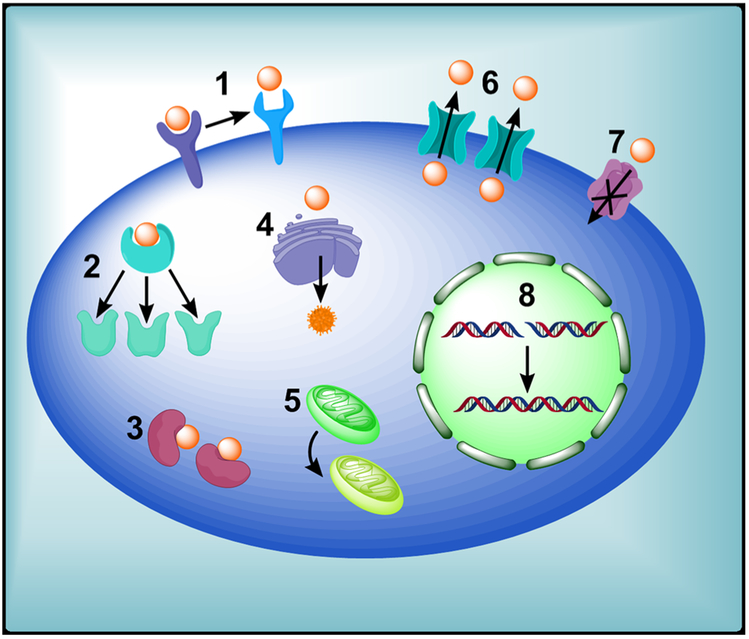
Noncellular drug resistance is the inherent capacity of tumor cells to survive chemotherapy. It is always mediated by tumor microenvironment and is typically associated with solid tumors having unique properties, such as heterogeneous tumor vasculature,65 high interstitial fluid pressure,66 increased presence of noncycling tumors caused by insufficient supply of nutrients and oxygen, as well as acidic environment.67-69 Cellular MDR mechanisms arise inside solid tumors. Compared with noncellular MDR mechanisms, cellular MDR mechanisms are more important for novel drug development. Some examples in which cellular mechanisms are modified to escape drug effect include: elevated DNA repair, increased drug metabolism, altered apoptotic pathways to bypass drug targets, loss or change of drug target proteins, and increased efflux of anticancer drugs (eg, altered activities of membrane transporters such as ATP-binding cassette [ABC] transporters)70,71 (Figure 3). One example in which cancer cells can alter surface receptors is represented by signaling kinases targeting epidermal growth factor receptor (EGFR) family members. Mutations can occur when these kinases are constitutively active in some cancers, such as human EGFR2, which is overexpressed by as much as 30% in breast cancer patients, and rapidly develop resistance to EGFR ligands.72 Another instance involving altered drug target is from topoisomerase II inhibitors. Resistant human leukemia cell lines can confer resistance to amsacrine through a single point mutation and nullify the stabilizing action of this topoisomerase inhibitor on the DNA.73 Metabolic changes in drugs that promote drug degradation are also critical to MDR. For instance, overexpression of glutathione, an important metabolic antioxidant, promotes MDR by converting drugs to drug conjugates or by generating inactive metabolites that expel as waste.74 Mutation of key apoptotic regulators is another primary reason for cellular MDR. Tumor suppressor protein p53 is crucial to many physiological processes, especially in regulating apoptosis. Several studies have shown that mutation of p53 and overexpression of BAX, an antiapoptotic protein in the Bcl-2 family, are involved in MDR.70,75 DNA repair after drug-induced damage also has a significant role in drug resistance and can counteract the chemotherapeutic action of some agents. In the case of platinum-based chemotherapeutics such as cisplatin, nucleotide excision repair and homologous recombination are the main pathways responsible.76 Another mechanism of conferred MDR is through downregulation of proapoptotic ion channels or upregulation of antiapoptotic ion transporters. Reduction of proapoptotic plasma membrane ion channels K+, Cl−, and Ca2+, which are responsible for apoptotic volume decrease, a hallmark of apoptosis, is implicated in MDR development.77
FIGURE 3.
Drug-resistance mechanisms in tumor cells. 1, Loss or change of surface receptor. 2, Mutations in drug targets. 3, Enzymatic deactivation. 4, Altered drug metabolism. 5, Change in apoptotic pathways. 6, Increased drug efflux. 7, Decreased drug influx (solute carriers). 8, Increased DNA repair [Color figure can be viewed at wileyonlinelibrary.com]
Among all the MDR mechanisms, the increase of drug efflux mediated by ABC transporters is the most significant factor leading to MDR. Many studies demonstrated that the ABC transporters comprise seven distinct classes of structurally related membrane proteins including ABCB, ABCC, and ABCG. Overexpression of ABC transporters in tumor cells promotes MDR by reducing sufficient accumulation of anticancer drugs through increasing drug efflux. P-gp, encoded by the ABCB1/ MDR1 gene, is the best-characterized transporter protein mediating drug resistance and many drugs (eg, docetaxel, paclitaxel, doxorubicin, etoposide, vinblastine, vincristine, and teniposide) are susceptible to resistance incurred from elevated P-gp.75,78,79 P-gp overexpression was reported to lead to failure of chemotherapy in various cancers, such as ovarian, lung, breast, and acute myeloid leukemia cancer.80 ABCC1/multidrug resistance-associated protein 1 (MRP1) is responsible for transporting organic anions and phase II metabolic products and causes resistance to anticancer agents. It was first shown to be overexpressed in a doxorubicin-resistant cell line, but also accommodates a wide range of substrates in addition to doxorubicin, including anthracyclines, epipodophyllotoxins, vinca alkaloids, and camptothecins.81 Finally, ABCG2/breast cancer resistant protein (BCRP) is a well-defined efflux transporter first identified in a breast cancer cell line selected for resistance to mitoxantrone. Similar to the other MDR transporters, BCRP serves as an efflux pump for many diverse scaffolds of xenobiotics, including 7-ethyl-10-hydroxycamptothecin (SN-38),82 tyrosine kinase inhibitors,83 methotrexate,75,84 and is involved in drug resistance in colon, breast cancer, and gastric carcinomas.85,86
2.1 ∣. Strategies to overcome MDR
Strategies to overcome inherent or acquired drug resistance are essential to the treatment of cancers. Using inhibitors of ABC transporters is one of the common approaches to overcome P-gp-mediated MDR, rendering tumor cells more sensitive to chemotherapeutics. Some strategies for overcoming MDR include developing novel anticancer drugs that are not the substrates of P-gp, suppressing the MDR-related genes through destruction of messenger RNAs (mRNAs) by using microRNA and RNA interference,87,88 decreasing intracellular GSH levels via hammerhead ribozymes against glutamate cysteine ligase or l-buthionine-(S,R)-sulfoxime to repress glutathione synthesis,89,90 targeting P-gp to reverse MDR with anti-P-gp monoclonal antibodies (eg, MRK16 and MRK17), and using nanotechnology to deliver anticancer drugs to specific targets efficiently.91 Development of novel drugs that overcome MDR continues to be a major area of research in drug discovery and development. Herein, we briefly summarize some strategies in developing tubulin inhibitors that overcome MDR.
2.1.1 ∣. Taxane binding site
Paclitaxel and docetaxel, members of the taxane family, are chemotherapeutics used to treat patients with various solid tumors, including ovarian, breast, head and neck, lung, and prostate cancers. However, severe adverse effects and development of MDR have largely compromised the use of paclitaxel and docetaxel. In addition to transporter-mediated resistance, overexpression of class III β tubulin isoform also limits the efficacy of many taxanes. Differences between βI tubulin, the most commonly represented and constitutively expressed isotype, and βIII tubulin are limited to substitution within only a 13 amino acid region, where βIII have an Arg277 instead of the Ser277 present in βI tubulin.92 This substitution prevents stable paclitaxel binding and creates more dynamic MTs, minimizing the polymerization-inducing activity of taxanes and increasing resistance to stabilization.93 Creation of newer generations of taxanes that are poor substrates of P-gp such as cabazitaxel, drug combination therapy, and drug conjugates for tumor-targeted delivery are all strategies that attempt to address the issue of MDR and develop more effective treatments.21
Ixabepilone (BMS-247550), an epothilone derivative, was first marketed under the trade name Ixempra for the treatment of drug-resistant metastatic breast cancer. The efficacy of ixabepilone in resistant tumors may result from its low susceptibility to alteration in tubulin isotypes, tubulin mutations, and overexpression of P-gp/MDR1 drug. In a phase II clinical trial of metastatic breast cancer patients treated with 40 mg/m2 ixabepilone as a 3-hour infusion every 3 weeks, ixabepilone demonstrated promising antitumor activity and had an acceptable safety profile.94,95 Taccalonolides are taxol-like MT stabilizing agents isolated from plants of the genus Tacca that increase the density of cellular MTs, block mitotic progression, and induce apoptosis. Similar to ixabepilone, taccalonolides can circumvent MDR mechanisms due to their low susceptibility to P-gp overexpression and tubulin alteration-mediated resistance.96,97 Recently, new taccalonolides AF and AJ were obtained and demonstrated potent IC50 values of 23 and 4 nmol/L in HeLa cell lines. Taccalonolides AF (10) and AJ (11) were generated from their parent taccalonolides A and B, by epoxidizing the C22–C23 double bond. This simple epoxidation dramatically increased the potency over 200- and 700-fold, respectively.98 Due to the unique structural characteristics and mechanism of taccalonolides, Susan Mooberry’s group modified the precursor structure and reported on a semisynthetic derivative, taccalonolide AI (12), which showed an IC50 below 1 nM.99,100 These studies are particularly informative for the synthesis of taccalonolide derivatives. Due to their strong cytotoxicity and unique mechanisms of covalent bonding, taccalonolide semisynthetic derivatives are a very promising generation of irreversible tubulin inhibitor for resolving the drug resistance dilemma. Combination therapies have been investigated to reduce blood toxicity and combat MDR. One example of this is by the combination treatment of zampanolide (13), a stabilizing agent that covalently interacts with the taxane luminal site, and daunomycin.101 Zampanolide was active against chemoresistant cells overexpressing P-gp and paired treatment displayed synergistic killing without increased in vitro hematopoietic toxicity.102
2.1.2 ∣. Vinca alkaloid binding site
Hemiasterlin (31), a natural product, is a member of tripeptides derived from marine sponges. Hemiasterlin and its analog HTI-286 (32) bind to a vinca-peptide site in tubulin, disrupt normal MT dynamics, and trigger the cell apoptotic process.103 Total synthesis of hemiasterlin and its analogs have been accomplished and the SAR studies have been explored. HTI-286 showed potent activity with an IC50 of about 2 to 5 nM against a panel of tumor cell lines and was less sensitive to the P-gp drug transporter pump than current anti-MT agents including paclitaxel, docetaxel, vinorelbine, and vinblastine.104 Additionally, resistance to HTI-286 was not detected in cells overexpressing the multixenobiotic resistance drug pump. However, the side effects of HTI-286 included neutropenia, hair loss, and pain, which ultimately terminated phase II trials. Further optimization is needed to address the current limitations of anticancer therapies and side effects.
Tubulysins, originally isolated from myxobacterial cultures, are tetrapeptides composed of d-methylpipecolate, l-isoleucine, l-tubuvaline, and l-tubuphenylalanine residues.105 Tubulysin D (33) demonstrated the most potent activity, exceeding other marketed chemotherapeutics such as Ixabepilone, vinblastine, and paclitaxel, by 20- to 1000-fold. The MOA of tubulysins resembled dolastatin-10 and hemiasterlin, which bind to the vinca domain site, arrest cancer cells in the G2/M phase, and trigger subsequent cell apoptosis.106,107 Tubulysins are highly active in MDR cell lines that either overexpress P-gp pumps or have tubulin mutations, suggesting an alternative solution to treating MDR cancers.
PM050489 (34a) and PM060184 (34b), polyketides isolated from marine natural products that are able to be totally synthesized, are highly potent tubulin binding agents.108 The binding of these compounds is inhibited by vinca alkaloids, though they possibly bind at a distinct site and weakly induce tubulin self-association, and PM060184 is undergoing clinical trials.109 PM060184 is able to overcome P-gp mediated resistance in vivo.110 Peripheral neuropathy was the main dose-limiting toxicity observed in a phase 1 study of PM060184 in patients with advanced solid tumors, which is consistent with tubulin inhibitors.111 Further efforts to optimize the dosing regimen and reduce toxicity are underway for additional clinical trials.
2.1.3 ∣. Colchicine binding site
While MT-targeting agents often face shortcomings due to the development of resistance, colchicine binding site agents have several advantages over other classes of tubulin inhibitors. First, many drugs in this class are less susceptible to MDR mechanisms that limit the efficacy of so many other tubulin inhibitors.62,112 For example, one of the main mechanisms of drug resistance is due to the overexpression of class III β tubulin that alters the conformation of the taxane binding site but does not confer resistance to colchicine binding site agents.113 Humans have at least seven expressed β tubulin isoforms, and alterations in their expression are linked to acquired drug resistance.113 Furthermore, paclitaxel-resistant mutants often show elevated sensitivity to drugs that bind to alternative tubulin sites, such as colchicine site agents.113 There is also evidence that the efficacy of the vinca drug, vinorelbine, is reduced when βIII tubulin is overexpressed, whereas colchicine binding site agents were unaffected, suggesting that that the resistance from βIII tubulin isoforms are binding site specific.93,114 This offers an advantage for chemotherapeutic drugs targeting the colchicine binding site.
To address conventional MDR to many MT-inhibiting agents, there have been numerous efforts to develop colchicine binding site inhibitors because of their therapeutic advantages over taxanes and vinca alkaloids. Recently, 2-aryl-4-benzoyl-imidazoles ABI-274 (34) and ABI-231 (35) were discovered by Miller and Li’s group and they specifically target the colchicine binding site.115,116 Currently, ABI-231, as VERU-111, is under clinical trials in men with advanced castration resistant prostate cancer (). The compounds exhibited similar potency to MDR cells compared with corresponding parent cells.117 They also demonstrated synergistic action through the combination of ABI-274 and vemurafenib to overcome vemurafenib-acquired resistance in BRAF (V600E) melanoma. The combination treatment effectively decreased the levels of phosphorylated and total AKT, activated the apoptosis cascade, and reduced cleaved caspase-3, and cleaved PARP.118 These contributions offer new strategies to alleviate the problem of drug resistance and they provide practical implications for the further investigation of colchicine binding site agents. Bai et al117 reported a similar colchicine binding site inhibitor, BZML (36), that showed potent cytotoxic activity against both A549 and A549/taxol-resistant cells. Mechanistic studies showed that BZML decreased P-gp expression at the protein and mRNA levels. Cell morphology changes and the expression of apoptosis-related proteins denoted that BZML-induced mitotic catastrophe to A549/taxol-resistant cells in a p53-independent, apoptotic-like pathway, whereas BZML-caused apoptosis to A549 cells.117 Verubulin (MPC-6827) (30) is a quinazoline derivative that showed potent antineoplastic activities against diverse tumors and promoted apoptosis in both sensitive and MDR cancer cell phenotypes.119 Other verubulin analogs have been developed in an attempt to maintain potency and avoid resistance, and these compounds have reported vascular disrupting activities and low IC50 values against various cancer cells.120 Mahal et al120 reported an analog of verubulin bearing an indole moiety N-(methylindolyl)aminoquinazoline10 (37), which showed good vascular disrupting effects with low nanomolar IC50 values ranging from 0.4 to 5.8 nM against several cell lines. Additionally, some other tetrahydroquinoline analogs of verubulin 1b (38) were designed and synthesized by Wang’s group.121 Recently, Banerjee et al122 reported compound 4a (39), an analog of verubulin, which exhibited potent activity with low nanomolar IC50 values of about 6 nM. X-ray crystal structures confirmed the binding of compound 4a to the colchicine site, and it significantly reduced taxane-resistant PC-3/TxR prostate cancer tumor proliferation in a xenograft model. Other colchicine binding site inhibitors investigated include indanocine (40), millepachine (41), DJ101 (42), SSE15206 (43), IMB5046 (44), D4-9-31(45), and their respective synthetic derivatives. Indanocine is a derivative of indanone and a selective inducer of apoptosis to stationary multidrug-resistant cancer cells. One approach to eradicate tumor cells involved combination therapy with indanocine, which was selectively cytotoxic to noncycling, multidrug-resistant cells, and other chemotherapeutic drugs, which targeted abnormalities of cell cycle tumors.123 Jianhong et al124 reported the ability of millepachine to evade MDR in an A2780CP (P-gp overexpressing) model and demonstrated that it irreversibly interacted with the colchicine site of β tubulin and retained full activity toward MDR cells. DJ101 targeted the colchicine binding site, which was confirmed through X-ray crystallography, potently reduced cell viability in MDR cell lines, and also significantly inhibited tumor growth in taxane-resistant prostate cancer xenografts without causing toxicity to the mice.125,126 SSE15206 also circumvented drug resistance in KB-V1 and A2780-Pac-Res cell lines that overexpressed P-gp.127 IMB5046 was not a P-gp substrate and displayed potent cytotoxicity against a panel of tumor cell lines and multidrug-resistant cell lines that were resistant to colchicine, vincristine, and paclitaxel treatment.128 D4-9-31, a pyridine-pyrimidine amide, showed strong efficacy in isogenic paclitaxel-resistant breast cancer cells and also evaded P-gp-mediated drug efflux.129 Another major mechanism of drug resistance to tubulin inhibitors that is a critical clinical concern is due to the overexpression of certain β tubulin isoforms. As stated above, humans have at least seven expressed β tubulin isoforms, and alterations in their expression is linked to acquired drug resistance.113 Studies have shown that overexpression of class III β tubulin reduces the efficacy of taxanes and vinca alkaloids, but drugs that target the colchicine binding site may circumvent resistance.93
Further efforts in evaluating novel tubulin inhibitors will help elucidate the drug resistance mechanisms and thus guide drug design that can overcome inherent or acquired drug resistance. In addition, the discovery of tubulin inhibitors with different inhibition mechanisms (such as targeting two pathways with one compound) will open alternative avenues to bypass the problem of drug resistance. Another strategy involves utilizing antibody-drug conjugates of tubulin inhibitors for targeted drug delivery to circumvent MDR, improve the therapeutic window, and limit toxicities.130 While significant strides have been made to address MDR, resistance is often multifactorial and heterogeneous. Therefore, efforts to gain insight into tumor dependence and the relationship of the individual drug resistance effectors may assist in combating the complex phenomenon of MDR.131
3 ∣. TUBULIN KINASE DUAL INHIBITION IN ANTICANCER TREATMENT
There have been tremendous efforts in developing numerous natural and synthetic small molecules capable of binding to and affecting MT dynamics.39,116,121,122,125,126,132 However, these MT-targeting agents offer broad diversity in terms of their chemical structures and tubulin protein binding sites, suggesting a potential affinity to bind to proteins other than tubulin.39,133-138 Likewise, many cellular kinases play crucial roles in diverse cellular processes, including cell growth as well as differentiation.139 These findings led to intensive research in kinase modulators resulting in the identification of many kinase inhibitors as anticancer agents.139,140 Many kinase inhibitors have also been shown to modulate MTs and affect their functions as well.141-143 These dual inhibitors, in many cases, through their multiple MOAs, have been observed to cause substantially enhanced apoptosis of cancer cells and strongly overcome cancer cell resistance compared with their single inhibitor counterparts.143-149 Additionally, numerous tubulin/kinase dual inhibitors have been observed to demonstrate substantially lower toxicities to normal cells with significantly higher maximum tolerated dose (MTD), overcoming one of the major limitations of single-tubulin inhibitor-based therapeutic agents.147,150,151 In the event of dual inhibition, it is difficult to understand which MOA is responsible for the underlying cell toxicity and cell cycle arrest, given that both kinase and tubulin inhibitors are responsible for either phenotype.140,142,144,145,147 In recent years, cell cycle arrest analysis at G0/G1 phase and at G2/M phase as well as image analysis of cellular phenotypes has provided insight into a drug candidate’s dual MOAs.140,152 The numerous physiological aspects of cancer cells has provided opportunities for added advantages of drugs with multiple MOAs, and consequently has shifted modern anticancer drug discovery toward the identification of multitargeted chemotherapeutic agents. In this section of the review, we cover recently reported tubulin/kinase dual inhibitors as well as some combination therapy showing significantly improved therapeutic outcomes in contrast to single inhibitor counterparts.
3.1 ∣. Recent examples of kinases showing dual inhibition against tubulin
3.1.1 ∣. Vascular endothelial growth factor receptor 2 kinases
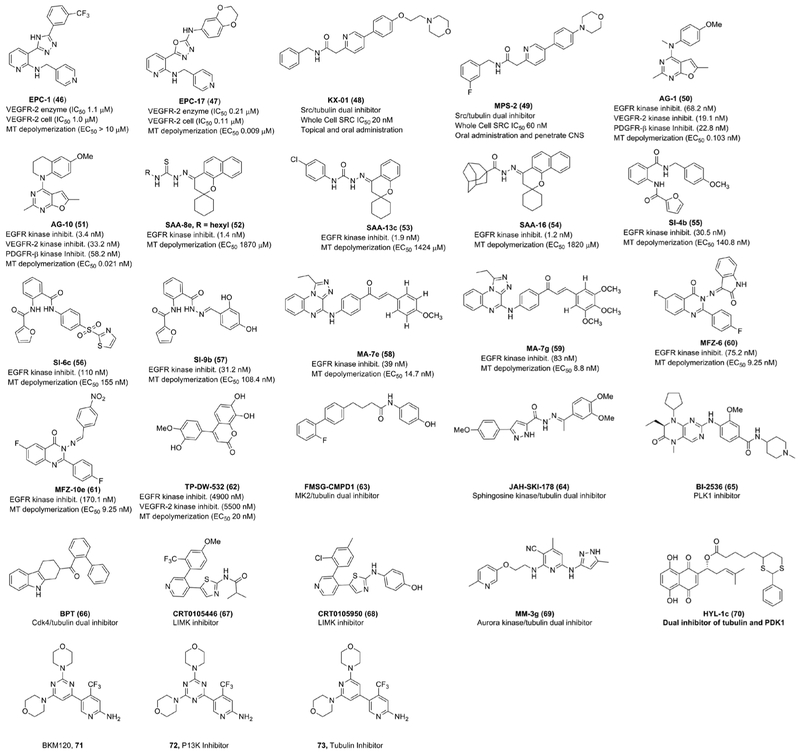
Chekler et al144 have developed dual inhibitors of vascular endothelial growth factor receptor 2 (VEGFR-2) kinase and tubulin, capable of inhibiting both tumor growth and tumor vasculature with enhanced synergistic antitumor responses in animal studies than respective inhibitors alone. They started with the triazole lead enhancer of polycomb homolog 1 (EPC-1) (46; Figure 4), weak VEGFR kinase inhibitor and obtained the oxadiazole EPC-17 (47; Figure 4) as the potent dual inhibitor of VEGFR-2 as well as tubulin polymerization. They confirmed the potent cell cycle arrest capability of EPC-2 at the G2/M phase was from the inhibition of tubulin polymerization since it was screened against the kinases, known to affect the G2/M transition, with very weak activity at the micromolar range. The authors demonstrated that the EPC-2 has a high MTD of 100 mg/kg when dosed once a week and lower toxicities than paclitaxel. Finally, EPC-2 was administered orally to the mice with human breast cancer (MDA-MB435LM2) and it exhibited significantly higher efficacy and substantially lower toxicities than paclitaxel while keeping tumor progression stable over the course of the treatment.
FIGURE 4.
Recently developed kinase inhibitors with secondary tubulin inhibitory activity or ability to render strong drug synergistic effects with microtubule targeting agents [Color figure can be viewed at wileyonlinelibrary.com]
3.1.2 ∣. SRC kinases
Smolinski et al145 have described two recently developed dual inhibitors, KX-01 (48) and MPS-2 (49), shown in Figure 4. KX-01 is a small molecule dual inhibitor of SRC kinase and MT polymerization.147,153,154 There are a number of SRC inhibitors that failed to demonstrate clinical efficacy while undergoing breast cancer treatments.147,155-157 On the contrary, KX-01 has shown promising preclinical results, through a dual MOAs, in several breast cancer cell lines with the potential to overcome the therapeutic limitations of current SRC inhibitors.158-160 Recently, Kim et al147 revealed the underlying MOA for KX-01 using triple-negative breast cancer (TNBC) cell lines. The authors selected three KX-01 sensitive TNBC cell lines (MDA-MB-231, MDA-MB-468, and BT-549) and a KX-01 resistant TNBC cell line (Hs578T) for the detailed in vitro studies. The authors observed an expected reduction in p-SRC in KX-01 sensitive TNBC cell lines upon exposure to KX-01, suggesting KX-01 inhibits SRC activity and its downstream proteins. The authors also demonstrated that KX-01 inhibited cell migration and metastasis in vitro in sensitive TNBC cell lines at a concentration of 20 nmol/L through suppression of SRC phosphorylation. Additionally, they showed that KX-01 sensitive TNBC cell lines reduced P-AKT, p-ERK, and p-Stat3 upon exposure to KX-01, suggesting KX-01 has strong antitumor efficacy against a broad range of TNBC cell lines through inhibition of SRC. However, unlike previously reported SRC inhibitors, the authors found KX-01 was able to induce cell cycle arrest at the G2/M phase in a dose-dependent manner in KX-01 sensitive cell lines. The G2/M phase cell cycle arrest along with an abnormal MT polymerization phenomenon was found to be very prominent at 100 nM KX-01 concentration, suggesting that this concentration of KX-01 is optimal for MT depolymerization. These findings suggest that KX-01 has dual inhibitory activity at 100 nM or higher. Additionally, KX-01 did not affect normal cells when nonmalignant MCF10A cells were treated with KX-01. Furthermore, the authors also demonstrated the ability of KX-01 to overcome paclitaxel resistance in a taxol-resistant MCF-7 cell line. Finally, the authors showed the ability of KX-01 to delay tumor growth in a KX-01 sensitive MDA-MB-231 breast cancer xenograft model. The compound KX-01 is currently undergoing phase-2 clinical trial and it would potentially be the first in class for the treatment of actinic keratosis (AK). It has demonstrated great promise due to its significantly higher efficacy and lower toxicity than existing therapeutic agents. Likewise, a second SRC/tubulin dual inhibitor, MPS-2, is currently undergoing a phase 1 clinical trial for the treatment of glioblastoma. It has been observed to completely penetrate the brain, is orally bioavailable, making it potentially the first in a class therapeutic candidate with enhanced efficacy against glioblastoma exhibiting multiple MOAs.
3.1.3 ∣. Receptor tyrosine kinases
Recently, Gangjee et al146,161,162 have reported furopyrimidine analogues, AG-1 (50; Figure 4) and AG-10 (51; Figure 4) as dual inhibitors of receptor tyrosine kinases (RTKs) and tubulin polymerization. The authors started with the lead AG-1, that they previously reported on, and made several conformationally restricted analogues and discovered that compound AG-10 was the most potent dual inhibitor in the series. The authors have demonstrated that conformational restriction in AG-10 renders significant enhancement in EGFR inhibition as well as in the MT inhibitory activity. AG-10 obtained optimal balance in both RTK as well as tubulin inhibitory activity resulting in improved efficacy in diverse cancer cell lines.
Abdelatef et al163 have synthesized a series of spiro-chromene derivatives and tested them against three human cancer cell lines, MCF-7, HT-29, and A549. Three of the designed compounds, SAA-8e (52), SAA-13c (53), and SAA-16 (54), have shown improved anticancer activity in low single digit micromolar range than two selected multikinase inhibitors, sorafenib and erlotinib. Further investigation demonstrated that all three designed compounds possess moderate tubulin polymerization, EGFR as well as BRAF inhibitory activity providing insight into the MOAs to design effective anticancer therapeutic candidates.
Ihmaid et al164 have designed and synthesized a series of anthranilate diamide derivatives, SI-4b (55), SI-6c (56), and SI-9b (57), with high potency in single digit nanomolar range against two breast cancer cell lines (MCF-7 and MDA-MB-231). Additional biological assays demonstrated that the compounds exhibit strong EGFR tyrosine kinase inhibition ranging from 30 to 80 nM as well as strong tubulin polymerization inhibition ranging from 87 to 400 nM. Hence, a dual MOAs contributes to the tremendous cytotoxicity of these compounds.
Alswah et al165 reported a series of triazoloquinoxaline-chalcone derivatives, MA-7e (58) and MA-7g (59), with moderate antiproliferative activity (1.65-35 μM) against three human cancer cell lines (MCF-7, HCT-116, and HEPG-2). The authors have demonstrated that two of the compounds (MA-7e and MA-7g) exert strong EGFR tyrosine kinase inhibitory activity (39-83 nM) as well as moderate tubulin polymerization inhibitory activity (8-14 μM).
Zayed et al166 designed and synthesized a series of quinazolinone derivatives, MFZ-6 (60) and MFZ-10e (61), with cytotoxicity ranging from 0.28 to 36.6 μM against two human breast cancer cell lines (MCF-7 and MDA-MBA-231). Some of the most potent compounds, including MFZ-6 and MFZ-10e have shown higher cytotoxicity against two selected breast cancer cell lines than gefitinib, a known EGFR kinase inhibitor. Either of the MFZ-6 and MFZ-10e has demonstrated excellent EGFR as well tubulin polymerization inhibitory activity compared with the control gefitinib attributing enhanced cytotoxic activity to the dual inhibitory actions.
Recently, Peng et al167 have reported a benzochromenone derivative, TP-DW532 (62), with promising MOAs against EGFR, VEGFR as well as tubulin polymerization. This candidate demonstrated moderate inhibitory ability of EGFR and VEGFR in lower single digit micromolar range in addition to the ability to dose dependently inhibit tubulin polymerization ranging from 20 to 40 μM. Finally, this compound demonstrated ability to dose dependently suppress angiogenesis both in vitro and in vivo.
3.1.4 ∣. MAPK-activated protein kinase 2
Gurgis et al142 recently demonstrated that the observed cytotoxicity of the known non-ATP competitive MK2 (MAPK-activated protein kinase 2) inhibitor, FMSG-CMPD1 (63), against a panel of glioblastoma cells is independent of MK2 inhibitory MOA. The authors demonstrated that FMSG-CMPD1 induces significant cell cycle arrest in G2/M phase and destabilizes polymerized MTs. The authors compared CMPD1, MK2 knocked down mice, and ATP competitive MK2 inhibitor III (MK2i) to find FMSG-CMPD1 is able to induce selective killing of cancer cells. However, MK2 knockdown and MK2i did not affect the viability of glioblastoma cells. This example also demonstrates that tubulin is one of the common nonkinase targets of many kinase inhibitors.
3.1.5 ∣. Sphingosine kinase
Recently, Hengst et al143 have demonstrated that one of the known Sphingosine kinase inhibitors (SKIs) JAH-SKI-178 (64) induces apoptosis in acute myeloid leukemia (AML) cell lines through MOAs, namely (a) blocking sphingosine kinases (SphK1 and 2) as well as (b) disrupting MT network. This synergistic effect significantly enhances the therapeutic efficacy of SKI-178 against a number of AML mouse models. Additionally, it is safe and well tolerated in diverse AML mouse models, bolstering ongoing demand of developing multitarget anticancer therapeutic agents.
3.1.6 ∣. Polo-like kinase
Polo-like kinase 1 (PLK1), which is crucial for tumor progression through mitosis, is known to frequently overexpressed in different cancers.168 Continuous overexpression of PLK1 has been linked with enhanced proliferation and poor prognosis in many tumors resulting in reduced survival.168 Thus, PLK1 has been identified as an important therapeutic target in cancer chemical biology. Constant research has led to the development of many PLK1 inhibitors, with a few exhibiting promising results in early clinical trials.168 For example, Fulda et al169 demonstrated that one of the PLK1 inhibitors, BI-2536 (65), and cotreatment with vincristine, a MT destabilizer, synergistically induced significantly enhanced apoptosis in different sarcoma cells as compared with either single drug treatment. The authors confirmed synergistic drug interactions by calculating combination index (combination index < 0.9). Similar observations have been reported when BI-2536 was used in combination with other MT destabilizers, including vinblastine as well as vinorelbine. However, the same was not found true when used in combination with MT stabilizers, like taxol, or doxorubicin. The authors also reported that BI-2536/vincristine treatment did not synergistically induce apoptosis in nonmalignant fibroblasts or myoblasts, suggesting tumor selectivity to some extent. Their findings support that PLK1 kinase and tubulin dual inhibition induces manifold increased apoptosis and significantly reduced long-term clonogenic survival.
3.1.7 ∣. Cyclin-dependent kinase 4
The marine natural product fascaplysin is known to be one of the most potent cyclin-dependent kinase 4 (CDK4) specific inhibitors.150,170 However, fascaplysin exhibits high toxicities due to its DNA intercalating properties.150,171 As of recent, Mahale et al150 identified a nonplanar tryptoline analogue, BPT (66), as potent CDK4 D1 enzyme specific inhibitor and did not demonstrate DNA binding properties, primarily due to its planar structure. BPT has shown cytotoxicity across p53-positive, p53-negative, pRb-positive, and pRb-negative cell lines with IC50< 1 μM, suggesting that antitumor efficacy is independent of tumor suppressor proteins, namely p53 and pRb. The authors also observed that BPT induces apoptosis in SV40 transformed mouse embryonic hepatic cells human fibroblasts, but not in untransformed cells, suggesting selectivity in cell killing. However, unlike a true CDK4 inhibitor, BPT induces cell cycle arrest predominantly at the G2/M phase. Although BPT blocks the G0/G1 phase only partially, it blocks G2/M phase cycle overwhelmingly at a lower concentration, suggesting a dual MOAs. The authors have also demonstrated that the G2/M phase cycle arrest capability is independent of p53. In continuation, the authors have exhibited the ability of BPT to inhibit polymerization of tubulin in vitro. BPT induced a significant reduction in colony formation in both p53-positive and p53-negative cancer cells. In vivo, BPT exhibited strong antitumor activity at 10 times less than the reported MTD (1000 mg/kg) in HCT-116 and NCI-H460. It is noteworthy that dual inhibitor BPT has significantly lower toxicity than either fascaplysin or tubulin inhibitors alone. In summary, the authors identified a significantly less toxic CDK4/tubulin dual inhibitor with promising efficacy in both in vitro and in vivo models and, therefore, holds potential to be considered for further clinical development.
3.1.8 ∣. Lim kinases 1 and 2
Lim kinases 1 and 2 (LIMK1 and LIMK2) have been well known to regulate cytoskeletal dynamics through modulating cofilin activity and, therefore, have an impact on cancer proliferation, survival, and metastasis.172,173 LIMK activity has also been shown to contribute to the resistance of several chemotherapeutic agents as well as ionizing radiation.174-176 Inhibition of LIMK has resulted in a reversal of drug resistance and enhancement of drug sensitivity.175,177 These observations have sparked growing efforts to develop LIMK inhibitors as potential anticancer therapeutic agents as single agents or in combination with other chemotherapeutics. The extensive research to identify LIMK inhibitors has led to a number of small molecules LIMK inhibitors with strong anticancer efficacy against diverse cancer cells, including breast, pancreatic, prostate, leukemia, and glioma among others.172,178-182 Recently, it has been discovered that LIMK1 and LIMK2 modulate MT dynamics and, hence, mitosis.172 Mardilovich et al172 have made great strides in validating the importance of inhibiting LIMK and modulating MT structure. They demonstrated that two known LIMK inhibitors, CRT0105466 (67) and CRT0105950 (68), in combination with the MT-destabilizing agent, vincristine, synergistically enhanced apoptosis in A549 cancer cells as compared with vincristine alone. The authors observed significant differences in MT spindle structure and organization in combination treatment of vincristine and either of the LMK inhibitors with increasing concentrations of LMK inhibitor. This abnormality in MT spindle structure and organization attributes to the enhanced cell cycle arrest achieved in the combination treatment as compared with the MT-targeting drug alone. These findings are in concert with the previous findings showing elevated LMK2 expression in vincristine-resistant neuroblastoma cells. However, LMK2 knockdown led to abnormal mitotic spindle formation and sensitization of neuroblastoma cells to vincristine or vinblastine. These findings direct modern research toward finding dual LMK/tubulin inhibitors in a single molecule capable of inducing enhanced apoptosis as well as efficiently overcoming LMK mediated resistance to MT-targeting agents.
3.1.9 ∣. Aurora kinase
Aurora kinases (AKs), one of the serine/threonine protein kinases, has been known to be involved in several mitotic events.183-185 Out of three know homologues of AKs in human, Aurora A (A2K) and Aurora B (A1K) kinases are abundantly present in several cells, while Aurora C kinase (A3K) is overexpressed only in testis.186 Over recent years, AKs A and B have emerged as attractive targets for cancer treatment and sparked tremendous efforts to develop small molecule AK inhibitors.187-189 A number of recently developed AK inhibitors have shown promising efficacy on a variety of tumor types in clinical trials. For example, Nerviano Medical science’s Danusertib (PHA-739358) is a pan-AK inhibitor that has shown excellent efficacy on chronic myeloid leukemia treatment. Takeda developed Alisertib (MLN-8237), a selective Aurora A inhibitor, that demonstrates tremendous efficacy on small-cell lung cancer (SCLC) treatment.183 Encouraged by these clinical data, there has been a rapidly growing interest in developing structurally diverse AK inhibitors.188,189 For another example, Morioka et al183 identified a 2,3-diamino-3-cycano-pyridyl analogue, MM-3g (69), as dual inhibitor of AK and tubulin polymerization. Compound MM-3g demonstrated strong AK inhibitor activity and outstanding protein kinase selectivity to AK as compared with 66 other kinases. Additionally, compound MM-3g exhibited excellent abilities to inhibit tubulin polymerization in vitro and ability to greatly permeate cell membrane with brilliant PK profile. Finally, MM-3g has shown strong efficacy in HCT-116 subcutaneous mouse model at a dose concentration of 30 mg/kg.
3.1.10 ∣. Pyruvate dehydrogenase kinase
Lately, Hong-Yan Lin et al190 have identified eighteen α-lypolic acyl shikonin ester derivatives (HYL-1c, 70) with an aim to provide access to dual inhibitors of tubulin polymerization and pyruvate dehydrogenase kinase 1 (PDK1) through molecular docking aided drug discovery campaign. The goal was to find compounds with ability to block both mitosis and glycolysis by keeping tubulin polymerization as well as PDK1 mediated signals from occurring. One of the compounds, 70, in the series showed moderate activity (IC50 ~3 μM, Hela) against cervical cancer and inhibited tumor growth in Hela xenograft model in dose-dependent manner. Further pharmacological investigations demonstrated that the compound is not only able to induce cell cycle arrest in G2/M phase, but capable of exhibiting PDK1 inhibitory activity and, thus, forces the cells to undergo apoptosis by promoting enhanced aerobic metabolism.
3.1.11 ∣. Phosphoinositide 3-kinase
Buparlisisb (BKM120, 71) is known to be one of the frontline phosphoinositide 3-kinase (PI3K) inhibitors for the treatment of cancer.191 However, it is also known to interfere with MT polymerization promoting dual inhibitory MOA. Recently, Bohnacker et al191 have derived two BKM120 analogues (72 and 73) that deviate from the parent by a single atom. These minor changes led to separate dual action of BKM120 resulting in the discovery of new selective inhibitors of P13K (72) and tubulin polymerization (73), respectively. X-ray cocrystal structure mode of binding of these inhibitors suggests that antiproliferative activity of BKM120 primarily relies upon the cytotoxicity through MT depolymerization as opposed to inhibition of P13K. Their findings are in contrast with the generally accepted mode of action of BKM120 family proving insight into the development of next generation of selective P13K inhibitors as well as combination therapy.
4 ∣. MT-TARGETING AGENTS’ POTENTIATION OF IMMUNE RESPONSE AND IMPLICATIONS IN CANCER
4.1 ∣. Dendritic cells: Induction of maturation and T-cell priming
In addition to their antimitotic effects, MT-targeting agents can also affect many nonmitotic mechanisms, including eliciting an immune response following the disruption of intact MTs. The perturbance of MTs from antitubulin agents can then induce anti-inflammatory responses and leukocyte action.192 For example, dendritic cells (DC) are antigen-presenting cells and are important regulators in the initiation of innate and adaptive immunity. While they have long been heralded for their defensive action to foreign pathogens, they are more recently being appreciated for their role in the regulation of antitumor response. However, tumor-altered DC differentiation can interfere with their ability to potentiate an immune response.193 This can lead to DCs and their precursors accumulating in the tumor microenvironment and exacerbate the immunosuppressive action of the tumor. Therefore, intervening and converting the immature DCs to mature antigen-presenting cells is an important target for immune-therapy based cancer treatment.194 MT-destabilizing agents including ansamitocin P3, dolastatin, and antibody-drug conjugates containing dolastatin-10 analogs have been shown to induce functional DC maturation and activation, and also increase the capacity of priming antigen-specific CD4+ and CD8+ T cells.195,196 It has also been reported that other classes of MT-destabilizing agents can activate DCs, unlike MT stabilizing agents which only invoked minimal activation in murine cells and caused no observed effect in DCs.194 Another group also investigated the effects of MT-destabilizing agents on DC maturation and T-cell activation in relation to immunogenic tumor cell death. They reported that dendritic cell vaccines pulsed with colchicine- or 2-phenyl-4-quinolone analog-treated cell lysates mitigated tumor growth, revealed cytotoxic T-lymphocyte activity in tumors, and extended survival of treated mice inflicted with tumor xenografts.197
4.2 ∣. Inflammation
Inflammation can play a role in malignant transformation, cancer progression, and pathogenesis in numerous tissues. For example, inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease contribute to the development of colon cancer, because chronic inflammation can endorse the production of tumors. It has been reported that the use of nonsteroidal anti-inflammatory drugs lowers mortality caused by sporadic colon cancers, representing a link between cancer and inflammation.198-200 The interaction of tumor cells with a variety of aspects within the tumor microenvironment, including macrophages, B and T cells, mast cells, fibroblasts, myofibroblasts, and extracellular matrix components, has been shown to propagate tumor progression. Furthermore, tumors are capable of secreting cytokines, chemokines, growth factors, and proteases to remodel the stroma microenvironment.201 Inflammation is driven by these soluble factors from the tumor cells or from cells recruited to the tumor microenvironment such as macrophages and mast cells, which can then drive tumor cell growth, interfere with differentiation, and increase cancer cell survival. The elevated influx of angiogenic cytokines from the surrounding immune cells to the tumor microenvironment can also contribute to tumor metastasis.202 Since levels of cytokines such as TNF-α, interleukin-8 (IL-8), IL-6, and VEGF are often elevated in the serum of colorectal carcinoma patients, they represent potential anticancer therapeutic targets.203,204 Oncogenic signaling pathways are also activated by cytokines that induce nuclear factor κB (NF-κB) signaling and turn on oncogenic mediators that promote inflammation. 201 While aberrantly expressed inflammation is linked to cancer promotion and disease progression, targeting proapoptotic signals from an inflammatory immune response to induce apoptosis of tumor cells could utilize these targets in anticancer treatment. Cell death imposed by an immune response can be from cell killing by cytotoxic T cells and natural killer cells, and can also be stimulated by apoptotic ligands, such as TNF-α. There have been reports on MT-targeting agents sensitizing cells to TNF-α and other inflammatory apoptotic ligands.205,206 AK301, a colchicine binding site tubulin inhibitor, was found to cause treated cells to express elevated levels of TNF receptor 1 (TNFR1) on the cell surface and increase caspases-8, -9, and -3 activations in the presence of TNF-α. It also induced Fas- and tumor necrosis ligand–dependent apoptosis.205 Interestingly, it caused greater TNF-α sensitization than other tubulin binding agents, including, colchicine, nocodazole, and vincristine, though it achieved a lower degree of tubulin polymerization inhibition from tubulin binding assays. Nonetheless, these studies explore the relationship between MTs and apoptotic ligand-sensitizing agents and how these agents can exploit immune response elements for cancer therapy. This may be particularly useful for colon or other cancers that have high levels of inflammatory cell infiltrate or following treatment with an immune stimulant or vaccines.
Colchicine, which is historically used in the treatment of gout, has been shown to induce an anti-inflammatory response through destabilization of MTs, which subsequently interferes with the assembly of the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome.207 Activation of the NLRP3 inflammasome within macrophages is commonly implicated in innate immune inflammatory response.207 NLRP3 uses a mitochondrially bound adapter protein (apoptosis-associated speck-like protein) containing a caspase recruitment domain to recruit caspase-1 to the complex, followed by autocatalytic processing and activation, ultimately facilitating the cleavage of proinflammatory cytokines IL- 1β and IL-18 to their activated forms.208 The generation of optimal sites for activation of the NLRP3 inflammasome, correct localization, and its direct activation is mediated by MTs, and MT disrupting agents block inflammasome activation.209
4.3 ∣. Polyploidy and micronucleation
Another way that MT-targeting agents may provoke an immune response is related to their ability to cause polyploidization.210-212 This can happen through a process called mitotic slippage, where cells arrested in the M phase can enter interphase without undergoing proper chromosome segregation and cytokinesis, resulting in tetraploid multinucleated cells.213 An increase in the number of chromosome sets (>4) has been shown to elevate endoplasmic reticulum stress and cause translocation of calreticulin to the cell surface. This exposure promotes phagocytosis of stressed cells by macrophages and dendritic cells of the immune system and stimulates immunosurveillance.212
MT-targeting agents induce mitotic arrest via disruption of the mitotic spindle, and interestingly, they have achieved greater success than mitosis-specific inhibitors. This may be in part attributed to their multifaceted roles in other interphase functions including intracellular trafficking, migration, angiogenesis, and cell signaling, suggesting that their anticancer efficacy is due to their roles in both mitotic and nonmitotic events.214,215 A relatively new theory as to why tubulin targeting agents, particularly those in the taxane class, have achieved greater clinical success than mitosis-specific inhibitors is their capacity to promote multiple micronuclei.216 These form from chromosome segregation errors during mitosis and recruit a nuclear envelope during telophase which does not incorporate into the main nucleus. This phenomenon happens when cells are treated with subtoxic drug concentrations that fail to induce complete mitotic arrest. These micronuclei formed from double-stranded DNA breaks can initiate the activation of cyclic GMP-AMP synthase (cGAS) inflammatory signaling.217 While this may only occur in a small population of dividing cells, it can have substantial implications on whole tumor regression through amplified inflammatory activation. Micronucleated cells can accumulate within the tumor and are not cleared by phagocytosis in the same manner as apoptotic cells. Fragmented DNA is exported through nuclear pores into the cytoplasm and binds to the cytosolic DNA sensor cGAS. cGAS consequently turns on the endoplasmic reticulum membrane protein STING through the synthesis of 2′3′-cGAMP.218 This activation triggers the transcription factors NF-κB and IRF3 through the kinases IKK and TBK1, eventually leading to the activation of inflammation. The cGAS-STING pathway activation has been shown to restrain oncogenes and increase proinflammatory genes in cancer cells.219 Ultimately, cGAS-STING–dependent inflammatory signaling can play a role in antitumor drug response.
5 ∣. FUTURE DIRECTIONS/CONCLUSIONS
Because of their major role in mitosis and other critical cellular processes, targeting MTs is an excellent strategy for developing anticancer agents. While some MT-targeting agents that exploit the taxane and vinca alkaloid binding site have been FDA approved for cancer treatment, there are still many hurdles to overcome to find a more satisfactory treatment. Several techniques have been used to improve drug properties and limit toxicity such as delivery via nanoparticle formulations and antibody-drug conjugation. Targeting the colchicine binding site on tubulin may circumvent many MDR mechanisms affiliated with taxanes and vinca alkaloids, such as overexpression of drug efflux pumps and alterations in β tubulin isoforms. Although there are currently no marketed anticancer agents that target the colchicine binding site, immense efforts have been made to advance small molecules in this class. Additionally, drugs targeting this site often are effective as vascular disrupting agents and can accommodate a large range of structurally diverse scaffolds. While these drugs show promise for the future of anticancer therapeutics, poor solubility, metabolic stability, and dose-limiting neural and cardiotoxicities continue to be major issues limiting their clinical potential. It is also essential to consider taking advantage of dual inhibitors of tubulin and kinases. Clearly understanding their mechanisms of action will be of utmost importance, especially as personalized medicine evolves.140 The emergence of dual kinase/tubulin inhibitors has provided several therapeutic benefits, as they may improve efficacy by targeting multiple pathways and reduce the risk of drug resistance since cells would need to overcome multiple mechanisms. While the search for acceptable compounds targeting MTs continues, newer generations of tubulin inhibitors will need to optimize efficacy, capitalize on combination therapies, and effectively escape the shortcomings of current anticancer drugs.
Acknowledgments
Funding information
NIH/NCI, Grant/Award Number: R01CA148706 and R01CA193609
List of Abbreviations:
- 2-ME
2-methoxyestradiol
- ABC
ATP-binding cassette
- ABI
2-aryl-4-benzoyl-imidazole
- AK
actinic keratosis
- AKs
aurora kinases
- AML
acute myeloid leukemia
- BCRP
breast cancer resistant protein
- CA-4
combretastatins A-4
- CDK4
cyclin-dependent kinase 4
- CIK
cytokine-induced killer cells
- c-GAS
cyclic GMP-AMP synthase
- DC
dendritic cells
- DU-145/TxR
docetaxel-resistant DU-145
- EGFR
epidermal growth factor receptor
- GDP
guanosine diphosphate
- GTP
guanosine-5′-triphosphate
- LIMK1
Lim kinases 1
- MDR
multidrug resistance
- MDR1
multidrug resistance protein 1
- MK2
MAPK-activated protein kinase 2
- MK2i
MK2 inhibitor III
- MOAs
mechanism of actions
- MRP1
multidrug resistance-associated protein 1
- MT
microtubule
- MTD
maximum tolerated dose
- NLRP3
NOD-like receptor pyrin domain containing 3
- PARP
poly (ADP-ribose) polymerase
- PC-3/TxR
paclitaxel-resistant PC-3
- PI3K
phosphoinositide 3-kinase
- PDKs
pyruvate dehydrogenase kinases
- P-gp
P-glycoprotein
- PLK1
polo-like kinase 1
- SAR
structure-activity relationship
- SphK
sphingosine kinase
- SW620/Ad300
P-gp overexpressing SW620
- TNBC
triple-negative breast cancer
- TNF-α
tumor necrosis factor α
- TNFR
tumor necrosis factor receptor
Biography
Kinsie E Arnst is currently a graduate research assistant serving in Dr Wei Li’s lab at the University of Tennessee Health Science Center (Memphis, TN, United States), since she joined in 2014. She received her BS degrees in both biology and biochemistry from Houston Baptist University in Houston, Texas. She is pursuing her doctorate in Pharmaceutical Sciences with a concentration in bioanalysis of small-molecule anticancer agents. The focus of her research is on efficacy and mechanism of action studies for tubulin compounds, particularly those which target the colchicine binding site, to improve potency and overcome multidrug resistance in cancer. She has published six peer-reviewed papers and one book chapter. She shares a close collaboration with Dr. Duane Miller’s lab and continues to analyze compounds developed at UTHSC.
Souvik Banerjee is a Research Associate in the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center (UTHSC; Memphis, TN, United States). He is a member of the American Chemical Society as well as the Royal Society of Chemistry (MRSC). Additionally, he served as the Secretary and News Letter Editor of Memphis Local Section of the American Chemical Society. Dr Banerjee earned his PhD in Organic Chemistry from the University of Southern Mississippi in 2013. Before his PhD study, Dr Banerjee worked as a Scientist in the Biocon Biopharmaceuticals, a leading biotechnology company in India from 2005-2008. Following his completion of PhD study, he went through 3 years of postdoctoral training in Medicinal Chemistry at the UTHSC under the mentorship of Dr Duane D Miller. Upon completion of his postdoctoral training, he joined the College of Pharmacy at the UTHSC as a Research Associate in Medicinal Chemistry since February 2017. His research in Organic Chemistry as well as in Medicinal Chemistry has resulted in a number of peer-reviewed papers and a pending US patent. His research is mainly focused on anticancer drug discovery that includes Autotaxin inhibitors, tubulin polymerization inhibitors, lysophosphatidic acid receptors (LPARs) antagonists, estrogen receptor beta agonists, and somatostatin peptide analogs consisting of unnatural amino acids.
Hao Chen obtained his PhD in organic synthesis from Sichuan University (Chengdu, China) in 2008 under the supervision of Prof Qing Jiang. During his Sr Organic Chemist and Research Associate in Chengdu Chempartner of ShangPharma and Sichuan University, he worked on design and synthesis of heterocyclic compounds, nucleic acid, peptide, and carbohydrate chemistry, as well as medicinal chemistry, drug discovery, and targeted cancer therapy candidate antibody-drug conjugates (ADCs). He is currently a postdoctoral fellow in the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center (Memphis, TN, United States). He is now working on the synthesis and bioactivity of colchicine binding site inhibitors and tubulin inhibitor–based antibody-drug conjugate strategies for cancer therapy.
Shanshan Deng graduated from China Pharmaceutical University in 2013 with a BS degree in pharmacy, and then obtained her MS degree in 2016 from the School of Pharmaceutical Sciences at China Pharmaceutical University with a concentration in pharmacokinetics. She is currently a graduate student in the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center (Memphis, TN, United States). Her research focuses on evaluating new generations of tubulin inhibitors that interact with the colchicine binding site as potential agents for more effective treatment of metastatic breast cancer.
Dong-Jin Hwang is currently a Research Associate in the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center (UTHSC; Memphis, TN, United States). He received his BS degree in Chemistry at Cheongju University in 1992 and went on to obtain his MS from the Department of Chemistry, Chungbuk National University. In 2002, he graduated with his PhD degree from the Hanyang University with Korea Institute of Science and Technology (KIST) Academia Collaborative Education PhD program in Seoul, Korea. He then did postdoctoral research on: selective androgen receptor modulators (SARMs) from 2002 to 2007 at the UTHSC. In 2007 he joined GTX, Inc and served as a scientist until 2011. This was followed by postdoctoral work at the University of Minnesota, Department of Medicinal Chemistry (Minnesota). He then returned to the Department of Pharmaceutical Sciences at UTHSC as a Research Associate. His main interests are in discovery of new small drug molecules and the role of drug design and synthesis for treating human diseases including cancers. He is working on Selective Androgen Receptor Degraders (SARD) for resistant prostate cancer, GPRC6A, and tubulin inhibitors for future drug development.
Dr Wei Li is currently a Professor of Pharmaceutical Sciences at the University of Tennessee Health Science Center (UTHSC; Memphis, TN, United States), a member of the West Cancer Center, Director of UTHSC College of Pharmacy Drug Discovery Center, and the Faculty Director of the Shared Analytical Instruments at UTHSC. He obtained his BS degree in chemistry from the University of Science and Technology of China in 1992. After a 2-year study at the Dalian Institute of Chemical Physics, the Chinese Academy of Sciences, he went to Columbia University and obtained his PhD degree in chemistry in 1999. He has been working at UTHSC College of Pharmacy since 1999. His research focus is medicinal chemistry and small molecule drug discovery. He has published over 140 peer-reviewed papers, three book chapters, and is an inventor of five issued US patents and a number of related patents in other countries. Dr Li has been actively serving as a grant reviewer for a number of US government, state, and foreign funding agencies. He is also a frequent reviewer for journal articles and is serving as an editorial board member for Current Medicinal Chemistry, Genes and Diseases, Molecules, and Acta Pharmaceutica Sinica B.
Dr Duane D Miller is currently Professor Emeritus in the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center (UTHSC; Memphis, TN, United States). He obtained his BS degree in Pharmacy at Kansas University in 1966, and his interest in research was stimulated as a National Science Foundation Undergraduate Research Fellow. He was an NIH Fellow while at the University of Washington and obtained his PhD in Medicinal Chemistry in 1969. He next joined The Ohio State University Faculty in 1969, where he became Professor and Chairman of the Division of Medicinal Chemistry and Pharmacognosy in 1982. He moved to the University of Tennessee, Health Science Center in 1992 and was the Van Vleet Endowed Chair and Professor. Dr Miller has published over 430 publications and 16 book chapters and has given 360 presentations at national and international meetings and has over 400 patents and patent applications. Dr Miller along with Dr Jim Dalton discovered the first new nonsteroidal selective androgen receptor modulators (SARMs) and reported on them in 1998. Our recent research has focused on discovery and development of new agents for unmet medical condition such as resistant prostate cancer, inflammation, breast cancer, and advanced melanoma.
REFERENCES
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. [DOI] [PubMed] [Google Scholar]
- 2.Sept D Microtubule polymerization: one step at a time. Curr Biol. 2007;17(17):R764–R766. [DOI] [PubMed] [Google Scholar]
- 3.Horio T, Murata T. The role of dynamic instability in microtubule organization. Front Plant Sci. 2014;5:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouhard GJ, Rice LM. Microtubule dynamics: an interplay of biochemistry and mechanics. Nat Rev Mol Cell Biol. 2018;19(7):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouhard GJ, Rice LM. The contribution of alphabeta-tubulin curvature to microtubule dynamics. J Cell Biol. 2014;207(3):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, Nogales E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157(5):1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HW, Nogales E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature. 2005;435(7044):911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. [DOI] [PubMed] [Google Scholar]
- 9.Vale RD, Coppin CM, Malik F, Kull FJ, Milligan RA. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994;269(38):23769–23775. [PubMed] [Google Scholar]
- 10.Heald R, Nogales E. Microtubule dynamics. J Cell Sci. 2002;115(Pt 1):3–4. [DOI] [PubMed] [Google Scholar]
- 11.Jordan MA, Horwitz SB, Lobert S, Correia JJ. Exploring the mechanisms of action of the novel microtubule inhibitor vinflunine. Semin Oncol. 2008;35(suppl 3):S6–S12. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Zhang D. Kinetochore fibre dynamics outside the context of the spindle during anaphase. Nat Cell Biol. 2004;6(3):227–231. [DOI] [PubMed] [Google Scholar]
- 13.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111(3):1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816–825. [PubMed] [Google Scholar]
- 15.Amos LA. What tubulin drugs tell us about microtubule structure and dynamics. Semin Cell Dev Biol. 2011; 22(9):916–926. [DOI] [PubMed] [Google Scholar]
- 16.Field Jessica J, Diaz José F, Miller John H. The binding sites of microtubule-stabilizing agents. Chem Biol. 2013; 20(3):301–315. [DOI] [PubMed] [Google Scholar]
- 17.Ranade AR, Higgins L, Markowski TW, et al. Characterizing the epothilone binding site on β-tubulin by photoaffinity labeling: identification of β-tubulin peptides TARGSQQY and TSRGSQQY as targets of an epothilone photoprobe for polymerized tubulin. J Med Chem. 2016;59(7):3499–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman H, Huzil JT, Luchko T, Ludueña RF, Tuszynski JA. Identification and characterization of an intermediate taxol binding site within microtubule nanopores and a mechanism for tubulin isotype binding selectivity. J Chem Inf Model. 2009;49(2):424–436. [DOI] [PubMed] [Google Scholar]
- 19.Mozzetti S, Ferlini C, Concolino P, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11(1):298–305. [PubMed] [Google Scholar]
- 20.Field Jessica J, Díaz José F, Miller John H. The binding sites of microtubule-stabilizing agents. Cell Chem Biol. 2013;20(3):301–315. [DOI] [PubMed] [Google Scholar]
- 21.Ojima I, Lichtenthal B, Lee S, Wang C, Wang X. Taxane anticancer agents: a patent perspective. Expert Opin Ther Pat. 2016;26(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. [DOI] [PubMed] [Google Scholar]
- 23.Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev. 2011;31(3):443–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone a on α,β-tubulin by electron crystallography. Science. 2004;305(5685):866–869. [DOI] [PubMed] [Google Scholar]
- 25.Brogdon CF, Lee FY, Canetta RM. Development of other microtubule-stabilizer families: the epothilones and their derivatives. Anticancer Drugs. 2014;25(5):599–609. [DOI] [PubMed] [Google Scholar]
- 26.Cheng KL, Bradley T, Budman DR. Novel microtubule-targeting agents—the epothilones. Biol Targets Thera. 2008;2(4):789–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumoleau P, Coudert B, Isambert N, Ferrant E. Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol. 2007;18(suppl 5):v9–v15. [DOI] [PubMed] [Google Scholar]
- 28.DeConti RC, Algazi AP, Andrews S, et al. Phase II trial of sagopilone, a novel epothilone analog in metastatic melanoma. Br J Cancer. 2010;103(10):1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow PK, Divers S, Provencher L, et al. Phase II study evaluating the efficacy and safety of sagopilone (ZK-EPO) in patients with metastatic breast cancer that has progressed following chemotherapy. Breast Cancer Res Treat. 2010;123(3):837–842. [DOI] [PubMed] [Google Scholar]
- 30.Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59(3):653–660. [PubMed] [Google Scholar]
- 31.Pryor DE, O'Brate A, Bilcer G, et al. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41(29):9109–9115. [DOI] [PubMed] [Google Scholar]
- 32.Hamel E, Day BW, Miller JH, et al. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol Pharmacol. 2006;70(5):1555–1564. [DOI] [PubMed] [Google Scholar]
- 33.Gaitanos TN, Buey RM, Díaz JF, et al. Peloruside A does not bind to the taxoid site on beta-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res. 2004;64(15):5063–5067. [DOI] [PubMed] [Google Scholar]
- 34.Prota AE, Bargsten K, Northcote PT, et al. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew Chem Int Ed Engl. 2014;53(6):1621–1625. [DOI] [PubMed] [Google Scholar]
- 35.Moudi M, Go R, Yien CY, Nazre M. Vinca alkaloids. Int J Prev Med. 2013;4(11):1231–1235. [PMC free article] [PubMed] [Google Scholar]
- 36.Bates D, Eastman A. Microtubule destabilising agents: far more than just antimitotic anticancer drugs. Br J Clin Pharmacol. 2017;83(2):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SM, Meng LH, Ding J. New microtubule-inhibiting anticancer agents. Expert Opin Invest Drugs. 2010; 19(3):329–343. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2008;28(1):155–183. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee S, Hwang D-J, Li W, Miller D. Current advances of tubulin inhibitors in nanoparticle drug delivery and vascular disruption/angiogenesis. Molecules. 2016;21(11):1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji YT, Liu YN, Liu ZP. Tubulin colchicine binding site inhibitors as vascular disrupting agents in clinical developments. Curr Med Chem. 2015;22(11):1348–1360. [DOI] [PubMed] [Google Scholar]
- 41.Hamel E Antimitotic natural products and their interactions with tubulin. Med Res Rev. 1996;16(2):207–231. [DOI] [PubMed] [Google Scholar]
- 42.Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li LY, Smulson ME. Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4. Cancer Res. 1998;58(20): 4510–4514. [PubMed] [Google Scholar]
- 43.Young SL, Chaplin DJ. Combretastatin A4 phosphate: background and current clinical status. Expert Opin Invest Drugs. 2004;13(9):1171–1182. [DOI] [PubMed] [Google Scholar]
- 44.Zheng S, Zhong Q, Mottamal M, et al. Design, synthesis, and biological evaluation of novel pyridine-bridged analogues of combretastatin-A4 as anticancer agents. J Med Chem. 2014;57(8):3369–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Álvarez R, Álvarez C, Mollinedo F, Sierra BG, Medarde M, Peláez R. Isocombretastatins A: 1,1-diarylethenes as potent inhibitors of tubulin polymerization and cytotoxic compounds. Bioorg Med Chem. 2009;17(17):6422–6431. [DOI] [PubMed] [Google Scholar]
- 46.Zhang LH, Wu L, Raymon HK, et al. The synthetic compound CC-5079 is a potent inhibitor of tubulin polymerization and tumor necrosis factor-alpha production with antitumor activity. Cancer Res. 2006;66(2):951–959. [DOI] [PubMed] [Google Scholar]
- 47.Fang Z, Song Y, Sarkar T, et al. Stereoselective synthesis of 3,3-diarylacrylonitriles as tubulin polymerization inhibitors. J Org Chem. 2008;73(11):4241–4244. [DOI] [PubMed] [Google Scholar]
- 48.Cullen MD, Sarkar T, Hamel E, et al. Inhibition of tubulin polymerization by select alkenyldiarylmethanes. Bioorg Med Chem Lett. 2008;18(2):469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu F, Hamel E, Lee DJ, Pryor DE, Cushman M. Synthesis, anticancer activity, and inhibition of tubulin polymerization by conformationally restricted analogues of lavendustin A. J Med Chem. 2003;46(9):1670–1682. [DOI] [PubMed] [Google Scholar]
- 50.Mu F, Lee DJ, Pryor DE, Hamel E, Cushman M. Synthesis and investigation of conformationally restricted analogues of lavendustin A as cytotoxic inhibitors of tubulin polymerization. J Med Chem. 2002;45(21):4774–4785. [DOI] [PubMed] [Google Scholar]
- 51.Kamath K, Okouneva T, Larson G, Panda D, Wilson L, Jordan MA. 2-Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerizing microtubules. Mol Cancer Ther. 2006;5(9):2225–2233. [DOI] [PubMed] [Google Scholar]
- 52.Pribluda VS, Gubish ER Jr., Lavallee TM, Treston A, Swartz GM. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19(1-2):173–179. [DOI] [PubMed] [Google Scholar]
- 53.Kumar BS, Raghuvanshi DS, Hasanain M, et al. Recent advances in chemistry and pharmacology of 2-methoxyestradiol: an anticancer investigational drug. Steroids. 2016;110:9–34. [DOI] [PubMed] [Google Scholar]
- 54.Peyrat JF, Brion JD, Alami M. Synthetic 2-methoxyestradiol derivatives: structure-activity relationships. Curr Med Chem. 2012;19(24):4142–4156. [DOI] [PubMed] [Google Scholar]
- 55.Cushman M, Mohanakrishnan AK, Hollingshead M, Hamel E. The effect of exchanging various substituents at the position of 2-methoxyestradiol on cytotoxicity in human cancer cell cultures and inhibition of tubulin polymerization. J Med Chem. 2002;45(21):4748–4754. [DOI] [PubMed] [Google Scholar]
- 56.Edsall AB, Mohanakrishnan AK, Yang D, et al. Effects of altering the electronics of 2-methoxyestradiol on cell proliferation, on cytotoxicity in human cancer cell cultures, and on tubulin polymerization. J Med Chem. 2004; 47(21):5126–5139. [DOI] [PubMed] [Google Scholar]
- 57.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57(2):237–257. [DOI] [PubMed] [Google Scholar]
- 58.Edsall AB, Agoston GE, Treston AM, et al. Synthesis and in vivo antitumor evaluation of 2-methoxyestradiol 3-phosphate, 17-phosphate, and 3,17-diphosphate. J Med Chem. 2007;50(26):6700–6705. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang H, Gigant B, et al. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2016;283(1):102–111. [DOI] [PubMed] [Google Scholar]
- 60.Xu K, Schwarz PM, Ludueña RF. Interaction of nocodazole with tubulin isotypes. Drug Dev Res. 2002;55(2):91–96. [Google Scholar]
- 61.Attia SM. Molecular cytogenetic evaluation of the mechanism of genotoxic potential of amsacrine and nocodazole in mouse bone marrow cells. J Appl Toxicol. 2013;33(6):426–433. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012;29(11):2943–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017;8(48):84559–84571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera E, Lee J, Davies A. Clinical development of ixabepilone and other epothilones in patients with advanced solid tumors. Oncologist. 2008;13(12):1207–1223. [DOI] [PubMed] [Google Scholar]
- 65.Sevick EM, Jain RK. Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer Res. 1989;49(13):3506–3512. [PubMed] [Google Scholar]
- 66.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–3051. [PubMed] [Google Scholar]
- 67.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4): 265–283. [DOI] [PubMed] [Google Scholar]
- 68.Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013;13(3):326–346. [DOI] [PubMed] [Google Scholar]
- 69.Demant EJF, Sehested M, Jensen PB. A model for computer simulation of P-glycoprotein and transmembrane delta pH-mediated anthracycline transport in multidrug-resistant tumor cells. Biochim Biophys Acta. 1990;1055(2):117–125. [DOI] [PubMed] [Google Scholar]
- 70.Oh K, Baik H, Lee A, Oh Y, Youn Y, Lee E. The reversal of drug-resistance in tumors using a drug-carrying nanoparticular system. Int J Mol Sci. 2009;10(9):3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z, Shi T, Zhang L, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett. 2016;370(1):153–164. [DOI] [PubMed] [Google Scholar]
- 72.Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinds M, Deisseroth K, Mayes J, et al. Identification of a point mutation in the topoisomerase II gene from a human leukemia cell line containing an amsacrine-resistant form of topoisomerase II. Cancer Res. 1991;51(17):4729–4731. [PubMed] [Google Scholar]
- 74.Schröder CP, Godwin AK, O’Dwyer PJ, Tew KD, Hamilton TC, Ozols RF. Glutathione and drug resistance. Cancer Invest. 1996;14(2):158–168. [DOI] [PubMed] [Google Scholar]
- 75.Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur J Pharm Sci. 2016;89:20–30. [DOI] [PubMed] [Google Scholar]
- 76.Bonanno L, Favaretto A, Rosell R. Platinum drugs and DNA repair mechanisms in lung cancer. Anticancer Res. 2014;34(1):493–501. [PubMed] [Google Scholar]
- 77.Hoffmann EK, Lambert IH. Ion channels and transporters in the development of drug resistance in cancer cells. Philos Trans R Soc London Ser B. 2014;369(1638):20130109–20130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Triller N, Korošec P, Kern I, Košnik M, Debeljak A. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54(2):235–240. [DOI] [PubMed] [Google Scholar]
- 79.Baran Y, Gür B, Kaya P, Ural AU, Avcu F, Gündüz U. Upregulation of multi drug resistance genes in doxorubicin resistant human acute myelogeneous leukemia cells and reversal of the resistance. Hematology. 2007;12(6):511–517. [DOI] [PubMed] [Google Scholar]
- 80.Mechetner E, Kyshtoobayeva A, Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4(2):389–398. [PubMed] [Google Scholar]
- 81.Sun YL, Patel A, Kumar P, Chen ZS. Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 2012;31(2):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawabata S, Oka M, Shiozawa K, et al. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280(5):1216–1223. [DOI] [PubMed] [Google Scholar]
- 83.Ozvegylaczka C, Cserepes J, Elkind N, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat. 2005;8(1-2):15–26. [DOI] [PubMed] [Google Scholar]
- 84.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. [DOI] [PubMed] [Google Scholar]
- 85.Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83(8):1084–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hasanabady MH, Kalalinia F. ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J Biosci. 2016;41(2):313–324. [DOI] [PubMed] [Google Scholar]
- 87.Li H, Zhou S, Li T, et al. Suppression of BCRP expression and restoration of sensitivity to chemotherapy in multidrug-resistant HCC cell line HEPG2/ADM by RNA interference. Hepatogastroenterology. 2012;59(119): 2238–2242. [DOI] [PubMed] [Google Scholar]
- 88.Zhang G, Wang Z, Qian F, Zhao C, Sun C. Silencing of the ABCC4 gene by RNA interference reverses multidrug resistance in human gastric cancer. Oncol Rep. 2015;33(3):1147–1154. [DOI] [PubMed] [Google Scholar]
- 89.Li M, Zhang Z, Yuan J, Zhang Y, Jin X. Altered glutamate cysteine ligase expression and activity in renal cell carcinoma. Biomed Rep. 2014;2(6):831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979;254(16):7558–7560. [PubMed] [Google Scholar]
- 91.Dong X, Mumper RJ. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine. 2010;5(4):597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferlini C, Raspaglio G, Mozzetti S, et al. The seco-taxane IDN5390 is able to target class III beta-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65(6):2397–2405. [DOI] [PubMed] [Google Scholar]
- 93.Stengel C, Newman SP, Leese MP, Potter BVL, Reed MJ, Purohit A. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer. 2010;102(2):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmes FA, Walters RS, Theriault RL, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991;83(24):1797–1805. [DOI] [PubMed] [Google Scholar]
- 95.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28(20):3256–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89(13):917–931. [DOI] [PubMed] [Google Scholar]
- 97.Risinger AL, Jackson EM, Polin LA, et al. The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008;68(21):8881–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Risinger AL, Peng J, Chen Z, Hu L, Mooberry SL. Potent taccalonolides, AF and AJ, inform significant structure-activity relationships and tubulin as the binding site of these microtubule stabilizers. J Am Chem Soc. 2011;133(47):19064–19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng J, Risinger AL, Li J, Mooberry SL. Synthetic reactions with rare taccalonolides reveal the value of C-22,23 epoxidation for microtubule stabilizing potency. J Med Chem. 2014;57(14):6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng J, Risinger AL, Fest GA, et al. Identification and biological activities of new taccalonolide microtubule stabilizers. J Med Chem. 2011;54(17):6117–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Field JJ, Pera B, Calvo E, et al. Zampanolide, a potent new microtubule-stabilizing agent, covalently reacts with the taxane luminal site in tubulin alpha,beta-heterodimers and microtubules. Chem Biol. 2012;19(6):686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pera B, Calvo-Vidal MN, Ambati S, et al. High affinity and covalent-binding microtubule stabilizing agents show activity in chemotherapy-resistant acute myeloid leukemia cells. Cancer Lett. 2015;368(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krishnamurthy G, Cheng W, Lo MC, et al. Biophysical characterization of the interactions of HTI-286 with tubulin heterodimer and microtubules. Biochemistry. 2003;42(46):13484–13495. [DOI] [PubMed] [Google Scholar]
- 104.Loganzo F, Discafani CM, Annable T, et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63(8):1838–1845. [PubMed] [Google Scholar]
- 105.Sasse F, Sieinmetz H, Heil J, Höfle G, Reichenbach H. Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli. Production, isolation, physico-chemical and biological properties. J Antibiot. 2000;53(9):879–885. [DOI] [PubMed] [Google Scholar]
- 106.Khalil MW, Sasse F, Lünsdorf H, Elnakady YA, Reichenbach H. Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. ChemBioChem. 2006;7(4):678–683. [DOI] [PubMed] [Google Scholar]
- 107.Steinmetz H, Glaser N, Herdtweck E, Sasse F, Reichenbach H, Höfle G. Isolation, crystal and solution structure determination, and biosynthesis of tubulysins—powerful inhibitors of tubulin polymerization from myxobacteria. Angew Chem Int Ed Engl. 2004;43(37):4888–4892. [DOI] [PubMed] [Google Scholar]
- 108.Martín MJ, Coello L, Fernández R, et al. Isolation and first total synthesis of PM050489 and PM060184, two new marine anticancer compounds. J Am Chem Soc. 2013;135(27):10164–10171. [DOI] [PubMed] [Google Scholar]
- 109.Pera B, Barasoain I, Pantazopoulou A, et al. New interfacial microtubule inhibitors of marine origin, PM050489/PM060184, with potent antitumor activity and a distinct mechanism. ACS Chem Biol. 2013;8(9):2084–2094. [DOI] [PubMed] [Google Scholar]
- 110.Martínez-Díez M, Guillén-Navarro MJ, Pera B, et al. PM060184, a new tubulin binding agent with potent antitumor activity including P-glycoprotein over-expressing tumors. Biochem Pharmacol. 2014;88(3):291–302. [DOI] [PubMed] [Google Scholar]
- 111.Elez E, Gomez-Roca C, Soto Matos-Pita A, et al. First-in-human phase I study of the microtubule inhibitor plocabulin in patients with advanced solid tumors. Invest New Drugs. 2018. In Press. 10.1007/s10637-018-0674-x [DOI] [PubMed] [Google Scholar]
- 112.Wu X, Wang Q, Li W. Recent advances in heterocyclic tubulin inhibitors targeting the colchicine binding site. Anticancer Agents Med Chem. 2016;16(10):1325–1338. [DOI] [PubMed] [Google Scholar]
- 113.Huzil JT, Chen K, Kurgan L, Tuszynski JA. The roles of beta-tubulin mutations and isotype expression in acquired drug resistance. Cancer Inform. 2007;3:159–181. [PMC free article] [PubMed] [Google Scholar]
- 114.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67(19):9356–9363. [DOI] [PubMed] [Google Scholar]
- 115.Wang Z, Chen J, Wang J, et al. Novel tubulin polymerization inhibitors overcome multidrug resistance and reduce melanoma lung metastasis. Pharm Res. 2012;29(11):3040–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen J, Ahn S, Wang J, et al. Discovery of novel 2-aryl-4-benzoyl-imidazole (ABI-III) analogues targeting tubulin polymerization as antiproliferative agents. J Med Chem. 2012;55(16):7285–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bai Z, Gao M, Zhang H, et al. BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett. 2017;402:81–92. [DOI] [PubMed] [Google Scholar]
- 118.Wang J, Chen J, Miller DD, Li W. Synergistic combination of novel tubulin inhibitor ABI-274 and vemurafenib overcome vemurafenib acquired resistance in BRAFV600E melanoma. Mol Cancer Ther. 2014;13(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasibhatla S, Baichwal V, Cai SX, et al. MPC-6827: a small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007;67(12):5865–5871. [DOI] [PubMed] [Google Scholar]
- 120.Mahal K, Resch M, Ficner R, Schobert R, Biersack B, Mueller T. Effects of the tumor-vasculature-disrupting agent verubulin and two heteroaryl analogues on cancer cells, endothelial cells, and blood vessels. ChemMedChem. 2014;9(4):847–854. [DOI] [PubMed] [Google Scholar]
- 121.Wang XF, Guan F, Ohkoshi E, et al. Optimization of 4-(N-cycloamino)phenylquinazolines as a novel class of tubulin-polymerization inhibitors targeting the colchicine site. J Med Chem. 2014;57(4):1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Banerjee S, Arnst KE, Wang Y, et al. Heterocyclic-fused pyrimidines as novel tubulin polymerization inhibitors targeting the colchicine binding site: structural basis and antitumor efficacy. J Med Chem. 2018;61(4):1704–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leoni LM, Hamel E, Genini D, et al. Indanocine, a microtubule-binding indanone and a selective inducer of apoptosis in multidrug-resistant cancer cells. J Natl Cancer Inst. 2000;92(3):217–224. [DOI] [PubMed] [Google Scholar]
- 124.Jianhong Y, Wei Y, Yamei Y, et al. The compound millepachine and its derivatives inhibit tubulin polymerization by irreversibly binding to the colchicine-binding site in beta-tubulin. J Biol Chem. 2018;293(24):9461–9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hwang D-J, Wang J, Li W, Miller DD. Structural optimization of indole derivatives acting at colchicine binding site as potential anticancer agents. ACS Med Chem Lett. 2015;6(9):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arnst KE, Wang Y, Hwang D-J, et al. A potent, metabolically stable tubulin inhibitor targets the colchicine binding site and overcomes taxane resistance. Cancer Res. 2017;78:265–277. [DOI] [PubMed] [Google Scholar]
- 127.Manzoor S, Bilal A, Khan S, et al. Identification and characterization of SSE15206, a microtubule depolymerizing agent that overcomes multidrug resistance. Sci Rep. 2018;8(1):3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng YB, Gong JH, Liu XJ, et al. A novel nitrobenzoate microtubule inhibitor that overcomes multidrug resistance exhibits antitumor activity. Sci Rep. 2016;6:31472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siddiqui-Jain A, Hoj JP, Cescon DW, Hansen MD. Pharmacology and in vivo efficacy of pyridine-pyrimidine amides that inhibit microtubule polymerization. Bioorg Med Chem Lett. 2018;28(5):934–941. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, Lin Z, Arnst K, Miller D, Li W. Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules. 2017;22(8):1281–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konieczkowski DJ, Johannessen CM, Garraway LA. A convergence-based framework for cancer drug resistance. Cancer Cell. 2018;33(5):801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao M, Ahn S, Wang J, et al. Discovery of 4-aryl-2-benzoyl-imidazoles as tubulin polymerization inhibitor with potent antiproliferative properties. J Med Chem. 2013;56(8):3318–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discovery. 2010;9(10):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prota AE, Bargsten K, Diaz JF, et al. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc Natl Acad Sci USA. 2014;111(38):13817–13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaur R, Kaur G, Gill RK, Soni R, Bariwal J. Recent developments in tubulin polymerization inhibitors: an overview. Eur J Med Chem. 2014;87:89–124. [DOI] [PubMed] [Google Scholar]
- 136.Mangiatordi GF, Trisciuzzi D, Iacobazzi R, et al. Automated identification of structurally heterogeneous and patentable antiproliferative hits as potential tubulin inhibitors. Chem Biol Drug Des. 2018;92(1):1161–1170. [DOI] [PubMed] [Google Scholar]
- 137.Sampson VB, Vetter NS, Zhang W, et al. Integrating mechanisms of response and resistance against the tubulin binding agent Eribulin in preclinical models of osteosarcoma. Oncotarget. 2016;7(52):86594–86607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Pandey MK, Gowda K, Sung S, et al. A novel dual inhibitor of microtubule and Bruton’s tyrosine kinase inhibits survival of multiple myeloma and osteoclastogenesis. Exp Hematol. 2017;53:31–42. [DOI] [PubMed] [Google Scholar]
- 139.Wu P, Nielsen TE, Clausen MH. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today. 2016;21(1):5–10. [DOI] [PubMed] [Google Scholar]
- 140.Tanabe K Microtubule depolymerization by kinase inhibitors: unexpected findings of dual inhibitors. Int J Mol Sci. 2017;18(12):2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Basilico C, Pennacchietti S, Vigna E, et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin Cancer Res. 2013;19(9):2381–2392. [DOI] [PubMed] [Google Scholar]
- 142.Gurgis F, Åkerfeldt M, Heng B, et al. Cytotoxic activity of the MK2 inhibitor CMPD1 in glioblastoma cells is independent of MK2. Cell Death Discov. 2015;1:15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hengst J, Dick T, Sharma A, et al. SKI-178: a multitargeted inhibitor of sphingosine kinase and microtubule dynamics demonstrating therapeutic efficacy in acute myeloid leukemia models. Cancer translational medicine. 2017; 3(4):109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chekler ELP, Kiselyov AS, Ouyang X, et al. Discovery of dual VEGFR-2 and tubulin inhibitors with in vivo efficacy. ACS Med Chem Lett. 2010;1(9):488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smolinski MP, Bu Y, Clements J, et al. Discovery of novel dual mechanism of action Src signaling and tubulin polymerization inhibitors (KX2-391 and KX2-361). J Med Chem. 2018;61(11):4704–4719. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Raghavan S, Ihnat M, et al. The design, synthesis and biological evaluation of conformationally restricted 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multi-targeted receptor tyrosine kinase and microtubule inhibitors as potential antitumor agents. Bioorg Med Chem. 2015;23(10):2408–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim S, Min A, Lee K-H, et al. Antitumor effect of KX-01 through inhibiting Src family kinases and mitosis. Cancer Res Treat. 2017;49(3):643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mangiatordi GF, Trisciuzzi D, Alberga D, et al. Novel chemotypes targeting tubulin at the colchicine binding site and unbiasing P-glycoprotein. Eur J Med Chem. 2017;139:792–803. [DOI] [PubMed] [Google Scholar]
- 149.Mahapatra DK, Bharti SK, Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur J Med Chem. 2015;98:69–114. [DOI] [PubMed] [Google Scholar]
- 150.Mahale S, Bharate SB, Manda S, et al. Antitumour potential of BPT: a dual inhibitor of cdk4 and tubulin polymerization. Cell Death Dis. 2015;6(5):e1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bohnacker T, Prota AE, Beaufils F, et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat Commun. 2017;8:14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanabe K Image-based compound profiling reveals a dual inhibitor of tyrosine kinase and microtubule polymerization. Sci Rep. 2016;6:25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anbalagan M, Ali A, Jones RK, et al. Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts. Mol Cancer Ther. 2012;11(9):1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Antonarakis ES, Heath EI, Posadas EM, et al. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2013;71(4):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puls LN, Eadens M, Messersmith W. Current status of Src inhibitors in solid tumor malignancies. Oncologist. 2011;16(5):566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elias D, Ditzel HJ. The potential of Src inhibitors. Aging. 2015;7(10):734–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gucalp A, Sparano JA, Caravelli J, et al. Phase II trial of saracatinib (AZD0530), an oral src-inhibitor for the treatment of patients with hormone receptor negative metastatic breast cancer. Clin Breast Cancer. 2011;11(5):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anbalagan M, Sheng M, Fleischer B, et al. Dual Src kinase/pretubulin inhibitor KX-01, sensitizes ERα-negative breast cancers to tamoxifen through ERα reexpression. Mol Cancer Res. 2017;15(11):1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor α positive breast cancer. Breast Cancer Res Treat. 2012;132(2):391–409. [DOI] [PubMed] [Google Scholar]
- 160.Anbalagan M, Carrier L, Hangauer D, Hangauer D, Rowan B. KX-01, a novel Src kinase inhibitor directed towards the peptide substrate site, induces robust apoptosis and synergizes with tamoxifen and chemotherapy in breast cancer. Cancer Res. 2009;69(suppl 24):6104–6104. [Google Scholar]
- 161.Zhang X, Raghavan S, Ihnat M, et al. The design and discovery of water soluble 4-substituted-2,6-dimethylfuro[2,3-d] pyrimidines as multitargeted receptor tyrosine kinase inhibitors and microtubule targeting antitumor agents. Bioorg Med Chem. 2014;22(14):3753–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Devambatla RKV, Choudhary S, Ihnat M, Hamel E, Mooberry SL, Gangjee A. Design, synthesis and preclinical evaluation of 5-methyl-N4-aryl-furo[2,3-d]pyrimidines as single agents with combination chemotherapy potential. Bioorgan Med Chem Lett. 2018;28(18):3085–3093. [DOI] [PubMed] [Google Scholar]
- 163.Abdelatef SA, El-Saadi MT, Amin NH, Abdelazeem AH, Omar HA, Abdellatif KRA. Design, synthesis and anticancer evaluation of novel spirobenzo[h]chromene and spirochromane derivatives with dual EGFR and B-RAF inhibitory activities. Eur J Med Chem. 2018;150:567–578. [DOI] [PubMed] [Google Scholar]
- 164.Ihmaid S, Ahmed H, Zayed M. The design and development of potent small molecules as anticancer agents targeting EGFR TK and tubulin polymerization. Int J Mol Sci. 2018;19(2):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alswah M, Bayoumi A, Elgamal K, Elmorsy A, Ihmaid S, Ahmed H. Design, synthesis and cytotoxic evaluation of novel chalcone derivatives bearing triazolo[4,3-a]-quinoxaline moieties as potent anticancer agents with dual egfr kinase and tubulin polymerization inhibitory effects. Molecules. 2018;23(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zayed M, Ahmed S, Ihmaid S, Ahmed H, Rateb H, Ibrahim S. Design, synthesis, cytotoxic evaluation and molecular docking of new fluoroquinazolinones as potent anticancer agents with dual EGFR kinase and tubulin polymerization inhibitory effects. Int J Mol Sci. 2018;19(6):1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peng T, Wu J, Tong L, et al. Identification of DW532 as a novel anti-tumor agent targeting both kinases and tubulin. Acta Pharmacol Sin. 2014;35:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strebhardt K Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discovery. 2010;9:643–660. [DOI] [PubMed] [Google Scholar]
- 169.Hugle M, Belz K, Fulda S. Identification of synthetic lethality of PLK1 inhibition and microtubule-destabilizing drugs. Cell Death Differ. 2015;22(12):1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shafiq MI, Steinbrecher T, Schmid R. FASCAPLYSIN as a specific inhibitor for CDK4: insights from molecular modelling. PLOS One. 2012;7(8):e42612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hamilton G Cytotoxic effects of fascaplysin against small cell lung cancer cell lines. Mar Drugs. 2014;12(3): 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mardilovich K, Baugh M, Crighton D, et al. LIM kinase inhibitors disrupt mitotic microtubule organization and impair tumor cell proliferation. Oncotarget. 2015;6(36):38469–38486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med. 2007;85(6):555–568. [DOI] [PubMed] [Google Scholar]
- 174.Gamell C, Schofield AV, Suryadinata R, Sarcevic B, Bernard O. LIMK2 mediates resistance to chemotherapeutic drugs in neuroblastoma cells through regulation of drug-induced cell cycle arrest. PLOS One. 2013;8(8):e72850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Q, Jiao D, Hu H, et al. Downregulation of LIMK1 level inhibits migration of lung cancer cells and enhances sensitivity to chemotherapy drugs. Oncol Res Featur Preclin Clin Cancer Therap. 2012;20(11):491–498. [DOI] [PubMed] [Google Scholar]
- 176.Croft DR, Crighton D, Samuel MS, et al. p53-mediated transcriptional regulation and activation of the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway promotes cell survival. Cell Res. 2011;21(4):666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Po’uha ST, Shum MSY, Goebel A, Bernard O, Kavallaris M. LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene. 2009;29:597–607. [DOI] [PubMed] [Google Scholar]
- 178.Yin Y, Zheng K, Eid N, et al. Bis-aryl urea derivatives as potent and selective lim kinase (Limk) inhibitors. J Med Chem. 2015;58(4):1846–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mashiach-Farkash E, Rak R, Elad-Sfadia G, et al. Computer-based identification of a novel LIMK1/2 inhibitor that synergizes with salirasib to destabilize the actin cytoskeleton. Oncotarget. 2012;3(6):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goodwin NC, Cianchetta G, Burgoon HA, et al. Discovery of a type III inhibitor of LIM kinase 2 that binds in a DFG-out conformation. ACS Med Chem Lett. 2015;6(1):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li R, Doherty J, Antonipillai J, et al. LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clin Exp Metastasis. 2013;30(4):483–495. [DOI] [PubMed] [Google Scholar]
- 182.Rak R, Haklai R, Elad-Tzfadia G, Wolfson HJ, Carmeli S, Kloog Y. Novel LIMK2 inhibitor blocks Panc-1 tumor growth in a mouse xenograft model. Oncoscience. 2014;1(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morioka M 3-Cyano-6-(5-methyl-3-pyrazoloamino) pyridines (Part 2): a dual inhibitor of Aurora kinase and tubulin polymerization. Bioorgan Med Chem Lett. 2016;26(24):5860–5862. [DOI] [PubMed] [Google Scholar]
- 184.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. [DOI] [PubMed] [Google Scholar]
- 185.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5(1):1–10. [DOI] [PubMed] [Google Scholar]
- 186.Lin Y-S, Su L-J, Yu C-TR, et al. Gene expression profiles of the Aurora family kinases. Gene Expression. 2006;13(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pollard JR, Mortimore M. Discovery and development of Aurora kinase inhibitors as anticancer agents. J Med Chem. 2009;52(9):2629–2651. [DOI] [PubMed] [Google Scholar]
- 188.Bavetsias V, Linardopoulos S. Aurora kinase inhibitors: current status and outlook. Front Oncol. 2015;5(278). 10.3389/fonc.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lok W, Klein RQ, Saif MW. Aurora kinase inhibitors as anti-cancer therapy. Anti-Cancer Drugs. 2010;21(4):339–350. [DOI] [PubMed] [Google Scholar]
- 190.Lin H-Y, Han H-W, Sun W-X, et al. Design and characterization of α-lipoic acyl shikonin ester twin drugs as tubulin and PDK1 dual inhibitors. Eur J Med Chem. 2018;144:137–150. [DOI] [PubMed] [Google Scholar]
- 191.Bohnacker T, Prota AE, Beaufils F, et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat Commun. 2017;8:14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leung YY, Yao Hui LL, Kraus VB. Colchicine--update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. 2013;4:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Müller P, Martin K, Theurich S, von Bergwelt-Baildon M, Zippelius A. Cancer chemotherapy agents target intratumoral dendritic cells to potentiate antitumor immunity. Oncoimmunology. 2014;3(8):e954460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Muller P, Martin K, Theurich S, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2(8):741–755. [DOI] [PubMed] [Google Scholar]
- 196.Martin K, Müller P, Schreiner J, et al. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol Immunother. 2014;63(9):925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wen CC, Chen HM, Chen SS, et al. Specific microtubule-depolymerizing agents augment efficacy of dendritic cell-based cancer vaccines. J Biomed Sci. 2011;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Din FVN, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59(12):1670–1679. [DOI] [PubMed] [Google Scholar]
- 199.Allison M, Garland C, Chlebowski R, et al. The association between aspirin use and the incidence of colorectal cancer in women. Am J Epidemiol. 2006;164(6):567–575. [DOI] [PubMed] [Google Scholar]
- 200.Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13(9):11071–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cytokines Klampfer L., inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11(4):451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fife CM, McCarroll JA, Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharmacol. 2014;171(24):5507–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis. 2010;25(2):135–140. [DOI] [PubMed] [Google Scholar]
- 204.Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol. 2007;25(29):4542–4549. [DOI] [PubMed] [Google Scholar]
- 205.Chopra A, Anderson A, Giardina C. Novel piperazine-based compounds inhibit microtubule dynamics and sensitize colon cancer cells to tumor necrosis factor-induced apoptosis. J Biol Chem. 2014;289(5):2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chopra AS, Kuratnik A, Scocchera EW, Wright DL, Giardina C. Identification of novel compounds that enhance colon cancer cell sensitivity to inflammatory apoptotic ligands. Cancer Biol Ther. 2013;14(5):436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Demidowich AP, Davis AI, Dedhia N, Yanovski JA. Colchicine to decrease NLRP3-activated inflammation and improve obesity-related metabolic dysregulation. Med Hypotheses. 2016;92:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guarda G, Zenger M, Yazdi AS, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186(4):2529–2534. [DOI] [PubMed] [Google Scholar]
- 209.Misawa T, Takahama M, Kozaki T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14(5):454–460. [DOI] [PubMed] [Google Scholar]
- 210.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. [DOI] [PubMed] [Google Scholar]
- 211.Tovar C, Higgins B, Deo D, et al. Small-molecule inducer of cancer cell polyploidy promotes apoptosis or senescence: Implications for therapy. Cell Cycle. 2010;9(16):3364–3375. [DOI] [PubMed] [Google Scholar]
- 212.Senovilla L, Vitale I, Martins I, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337(6102):1678–1684. [DOI] [PubMed] [Google Scholar]
- 213.Cheng B, Crasta K. Consequences of mitotic slippage for antimicrotubule drug therapy. Endocr Relat Cancer. 2017;24(9):T97–T106. [DOI] [PubMed] [Google Scholar]
- 214.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8(4):244–250. [DOI] [PubMed] [Google Scholar]
- 215.Field JJ, Kanakkanthara A, Miller JH. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg Med Chem. 2014;22(18):5050–5059. [DOI] [PubMed] [Google Scholar]
- 216.Mitchison TJ, Pineda J, Shi J, Florian S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017;7(11):170182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54(2):289–296. [DOI] [PubMed] [Google Scholar]
- 219.Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]