Abstract
Objective
To describe the management strategies and outcomes of patients with renal medullary carcinoma (RMC) and characterise predictors of overall survival (OS).
Patients and Methods
RMC is a rare and aggressive malignancy that afflicts young patients with sickle cell trait; there are limited data on management to date. This is a study of patients with RMC who were treated in 2000–2015 at eight academic institutions in North America and France. The Kaplan–Meier method was used to estimate OS, measured from initial RMC diagnosis to date of death. Cox regression analysis was used to determine predictors of OS.
Results
In all, 52 patients (37 males) were identified. The median (range) age at diagnosis was 28 (9–48) years and 49 patients (94%) had stage III/IV. The median OS for all patients was 13.0 months and 38 patients (75%) had nephrectomy. Patients who underwent nephrectomy had superior OS compared to patients who were treated with systemic therapy only (median OS 16.4 vs 7.0 months, P < 0.001). In all, 45 patients received chemotherapy and 13 (29%) had an objective response; 28 patients received targeted therapies, with 8-week median therapy duration and no objective responses. Only seven patients (13%) survived for >24 months.
Conclusions
RMC carries a poor prognosis. Chemotherapy provides palliation and remains the mainstay of therapy, but <20% of patients survive for >24 months, underscoring the need to develop more effective therapy for this rare tumour. In this study, nephrectomy was associated with improved OS.
Keywords: kidney cancer, nephrectomy, renal cell carcinoma, renal medullary carcinoma (RMC), #KidneyCancer, #KCSM
Introduction
Renal medullary carcinoma (RMC) is a highly aggressive malignancy arising from the collecting duct epithelium of the kidney. It was first described by Davis et al. [1] in 1995 as the ‘seventh sickle cell nephropathy’, afflicting mostly young patients of African descent with sickle cell trait. However, while sickle cell trait is most commonly carried in those of African descent, it can also occur in Hispanics and Caucasians, particularly those of southern European descent. To date, most reports suggest a median overall survival (OS) of 4–5 months [2].
The pathogenesis of RMC is not completely understood. It has been hypothesised to be related to chronic medullary hypoxia causing up-regulation of the hypoxia-inducible factor pathway, leading to tumour neovascularisation; however, this is not a comprehensive explanation of the pathogenesis [3–5]. RMC typically presents in the second and third decades of life, with male preponderance and primarily right-sided laterality. Most patients present with haematuria or flank pain, and many of these have systemic symptoms such as weight loss [2]. The vast majority of patients present with metastatic disease [6]. RMC generally has a rapidly progressive course. Lymphovascular spread is common with direct invasion to renal sinus veins, regional lymph nodes, and retroperitoneum [7]. Radiographically, tumours arise centrally and demonstrate an infiltrative pattern, distinctly different in appearance from the exophytic peripheral masses of clear-cell RCC [8,9].
Pathologically, these tumours are characterised by large locally advanced renal tumours that frequently show extrarenal extension at the time of presentation. Key pathological features include stromal desmoplasia, prominent nucleoli, and frequent cytoplasmic inclusions resembling rhabdoid tumour cells [10–13]. The diagnosis of RMC is confirmed by demonstrating loss of SMARCB1 (INI1) nuclear staining.
As RMC is extremely rare, there is paucity of data on the optimal management of patients who present with advanced disease. Only small series exist on the treatment of RMC; the largest single-centre data is the report of 34 patients by Davis et al. [1] in 1995. Iacovelli et al. [2] reported on 165 patients pooled from 18 years of literature, but most single-centre treatment data included less than four patients, and individual patient data were not reported. Reported treatment regimens include various cytotoxic chemotherapy and targeted therapy regimens, but the role of nephrectomy to date has been unclear. Topoisomerase overexpression has been evaluated, and based on this rationale, doxorubicin has been used in combination with other chemotherapy agents [14,15]. Platinum-based regimens are also frequently used as they have shown improvement in survival [16]. However, the long-term outcome of patients with RMC remains poor. We report the largest experience to date of 52 patients with RMC treated at eight institutions. Our objectives were to study the clinical presentation, treatment methods, outcomes, and predictors of survival.
Patients and Methods
We conducted this retrospective, multicentre study after Institutional Review Board approval was obtained. A combined de-identified secure database was constructed and all patients with confirmed RMC diagnosed and treated from 2000 to 2015 at eight participating academic institutions in North America and France were included in this study.
Clinical and Pathological Characteristics
Clinical, imaging, and pathological data were available for 52 patients. Clinical information collected at presentation included age, gender, race, symptomatology, presence of haemoglobinopathy, tumour laterality, renal primary tumour size, clinical stage (American Joint Committee on Cancer), Eastern Cooperative Oncology Group performance status (ECOG PS), number of organs involved with metastatic disease, metastatic sites, and baseline laboratory studies. Data on radical or partial nephrectomy and timing of surgery was collected. Information on imaging including full staging evaluation of CT chest, abdomen, and pelvis, as well as bone scan and brain MRI when indicated, was collected. Pathological information included histology from biopsy or nephrectomy material (when nephrectomy was performed), and pathological T and N stage, when available. All available pathology slides were reviewed by genitourinary pathologists at each participating institution. All patients had confirmed RMC histology. Information on systemic therapy was collected including its timing (pre- vs postoperative), duration of therapy, best response, and regimens used. Date of death or last follow-up was collected for survival analysis.
Statistical Methods
Descriptive statistics were used for continuous variables (median, range). OS was measured in months from time of diagnosis until death or last follow-up. Patients who were alive at their last contact were censored on that date. Kaplan–Meier plots were used to estimate OS times [17]. The log-rank test and Cox regression analysis models were used to evaluate the association of co-variables with OS [18,19]. Variables with a P value < 0.1 were included in the multivariable analysis. P values in the multivariable analysis of <0.05 were considered statistically significant.
Results
Patient Characteristics at Presentation
In all, 52 patients (47 Black, 4 White, and 1 Hispanic non-White by self-report) were identified. The median (range) age at diagnosis was 28 (9–48) years and 37 patients (71%) were male. In all, 51 patients had sickle cell trait, and one patient had sickle β thalassaemia. In all, 66% of patients presented with haematuria and/or flank pain and 50% presented with constitutional symptoms, most frequently unintentional weight loss, followed by night sweats; 20% of patients presented with cough, shortness of breath, or abdominal pain related to metastatic disease. In all, 36 patients (69%) had right-sided tumours and the median (range) tumour size at presentation was 6.0 (3.4–11.4) cm. In all, 14 patients (27%) presented with Stage III disease, and 35 patients (67%) presented with Stage IV (distant metastatic) disease. Of those that presented with metastatic disease, one patient was in the ‘good risk’ category by Memorial Sloan-Kettering Cancer Center (MSKCC) risk score, 17 (49%) were in the ‘intermediate risk’ category, and 17 (49%) were in the ‘poor risk’ category. In all, 44 patients (85%) had radiographic lymph node involvement at presentation (>1 cm in the short axis), most commonly in the retroperitoneum. Other common sites of metastatic disease included the lungs (46%), liver (15%), and bone (15%). No patients had brain metastases at presentation. In all, 32 patients (62%) had an ECOG PS of 0–1, and 20 patients (38%) had an ECOG PS of 2–3. Additional details on demographics and presentation are given in Table 1.
Table 1.
The patients’ characteristics.
| Total | Nephrectomy | Systemic therapy only | P | |
|---|---|---|---|---|
| Number of patients | 52 | 38 | 14 | |
| Age at diagnosis, years, median (range) | 28.2 (9.4–48.1) | 28.5 (9.4–48.1) | 27.2 (20–47.2) | 0.61 |
| N (%) | ||||
| Gender | ||||
| Female | 15 (29) | 12 (32) | 3 (21) | 0.73 |
| Male | 37 (71) | 26 (68) | 11 (79) | |
| Race | ||||
| Black | 47 (90) | 34 (89) | 13 (93) | 1.00 |
| Other | 5 (10) | 4 (11) | 1 (7) | |
| Haemoglobinopathy | ||||
| Sickle cell trait | 51 (98) | 38 (100) | 13 (93) | 0.27 |
| Sickle thalassaemia | 1 (2) | 0 | 1 (7) | |
| Tumour Side | ||||
| Right | 36 (69) | 29 (76) | 7 (50) | 0.12 |
| Left | 13 (25) | 7 (19) | 6 (43) | |
| Not available | 3 (6) | 2 (5) | 1 (7) | |
| Tumour size at presentation, cm, median (range) | 6.0 (3.4–11.4) | 6.0 (3.9–11.4) | 6.4 (3.4–9.9) | 0.55 |
| N (%) | ||||
| Clinical stage at presentation | ||||
| I | 3 (6) | 3 (8) | 0 | 0.07 |
| II | 0 | 0 | 0 | |
| III | 14 (27) | 13 (34) | 1 (7) | |
| IV | 35 (67) | 22 (58) | 13 (93) | |
| ECOG PS at presentation | ||||
| 0 | 9 (17) | 9 (24) | 0 | 0.06 |
| 1 | 23 (44) | 17 (45) | 6 (43) | |
| 2 | 11 (21) | 8 (21) | 3 (21) | |
| 3 | 9 (17) | 4 (10) | 5 (36) | |
| Lymph nodes at presentation | 44 (85) | 30 (79) | 14 (100) | 0.09 |
| Metastatic sites at diagnosis | ||||
| Lymph Nodes | 44 (85) | |||
| Lungs | 24 (46) | |||
| Liver | 8 (15) | |||
| Bone | 8 (15) | |||
| Pleura | 3 (6) | |||
| Adrenal gland | 2 (4) | |||
| Psoas muscle | 2 (4) | |||
| Ovary | 1 (2) | |||
| Skin | 1(2) | |||
| Pancreas | 1 (2) | |||
| Thyroid | 1 (2) | |||
| Brain | 0 (0) | |||
| No. of organs with metastatic disease (at any time) | ||||
| 1–2 | 14 (27) | |||
| >2 | 38 (73) | |||
| Pathological staging | 38 (nephrectomy) | |||
| T stage | ||||
| T1 | 7 (19) | |||
| T2 | 2 (5) | |||
| T3 | 27 (71) | |||
| T4 | 2 (5) | |||
| N stage | ||||
| N0 | 4 (11) | |||
| N1 | 24 (63) | |||
| Nx | 10 (26) |
Pathology and Surgery
In all, 25 patients (48%) had renal mass biopsy as part of their initial evaluation. All patients had pathological confirmation of RMC on either biopsy or nephrectomy specimen and 38 patients (73%) underwent nephrectomy during their treatment course (37 radical nephrectomies and one partial nephrectomy). Eight of the patients who underwent nephrectomy received preoperative systemic chemotherapy, whereas 30 patients underwent upfront nephrectomy before any systemic therapy. Seven patients had pT1 disease, 29 patients had pT3 or pT4 disease, and 24 patients had pathological confirmation of disease in lymph nodes. Additional pathology details are given in Table 1.
Survival
The median follow-up time was 34.4 months (95% CI: 27.5–N/A) for the entire cohort, and the median OS was 13.0 months (95% CI: 9.0–17.9; range 0.2–45.2). The 12-month OS rate was 53% (95% CI: 40–69), and the 24-month OS rate was 17% (95% CI: 9–33). The log-rank test did not show a significant difference in OS by subgroup variables of age, gender, presence of hypertension, MSKCC risk score, clinical T stage, or body mass index (Table 2). For the three patients who had organ-confined disease at presentation and underwent upfront nephrectomy, the median time from nephrectomy to metastatic disease was only 3 months, indicating rapid postoperative recurrence.
Table 2.
OS analysis.
| Variable | Median OS, months (95% CI) | Univariable analysis P | Multivariable analysis |
|
|---|---|---|---|---|
| HR (95% CI) | P | |||
| Gender | ||||
| Female | 12.3 (9.0, N/A) | 0.24 | ||
| Male | 13.0 (8.4, 17.9) | |||
| Hypertension at baseline | ||||
| No | 13.0 (7.8, 19.9) | 0.63 | ||
| Yes | 14.3 (7.0, N/A) | |||
| Initial AJCC stage | ||||
| I | 33.3 (7.2, N/A) | 0.47 | ||
| III | 14.3 (12.3, N/A) | |||
| IV | 11.1 (8.4, 16.4) | |||
| Initial T stage | ||||
| 1 | 33.3 (8.9, N/A) | 0.17 | ||
| 2 | 12.0 (10.4, N/A) | |||
| 3 | 13.0 (9.0, 18.1) | |||
| 4 | 8.4 (7.5, N/A) | |||
| Initial LN involvement | ||||
| Yes | 11.1 (8.7, 15.0) | 0.01 | 3.3 (1.0, 11.1) | 0.06 |
| No | 33.3 (19.9, N/A) | |||
| MSKCC risk score | ||||
| 0 | 13.7 (N/A, N/A) | 0.20 | ||
| 1–2 | 13.0 (9.0, 20.0) | |||
| ≥3 | 8.9 (7.2, 15.3) | |||
| ECOG PS | ||||
| 2–3 | 8.9 (4.3, 15.3) | 0.008 | 1.6 (0.8, 3.2) | 0.22 |
| 0–1 | 16.4 (11.0, 22.5) | |||
| Nephrectomy | ||||
| Yes | 16.4 (13.0, 22.5) | <0.001 | 0.22 (0.09, 0.5) | <0.001 |
| No | 7.0 (3.5, N/A) | |||
| Response to first-line therapy | ||||
| CR/PR | 12.3 (8.5, N/A) | 0.11 | ||
| SD | 17.9 (13.0, N/A) | |||
| PD | 8.9 (7.5, 22.5) | |||
| Body mass index, kg/m2 | ||||
| <30 | 13.0 (7.8, 19.9) | 0.42 | ||
| >30 | 18.4 (8.7, N/A) | |||
AJCC, American Joint Committee on Cancer.
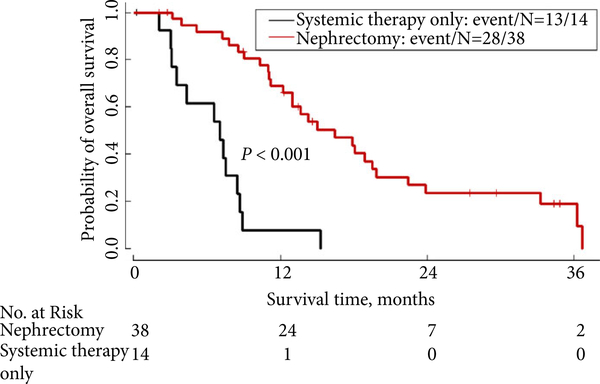
By multivariable analysis (Table 2), patients without clinical enlargement of lymph nodes at presentation had a median OS of 33.3 months, compared with 11.1 months for those with enlarged lymph nodes at presentation [P = 0.056; hazard ratio (HR) 3.28, 95% CI: 0.97–11.14]. Patients with an ECOG PS of 0–1 at baseline had a median OS of 16.4 months, while those with an ECOG PS of 2–3 at baseline had a median OS of 8.9 months (P = 0.22; HR 1.57, 95% CI: 0.77–3.19). Patients who underwent nephrectomy had a lower risk of death compared with patients who received systemic therapy only (median OS 16.4 vs 7.0 months, respectively, P < 0.001; HR 0.22, 95% CI: 0.09–0.51) after adjusting for ECOG PS and initial LN involvement (Fig. 1).
Fig. 1.
OS: comparison of nephrectomy plus systemic therapy vs systemic therapy only (N = 52 patients).
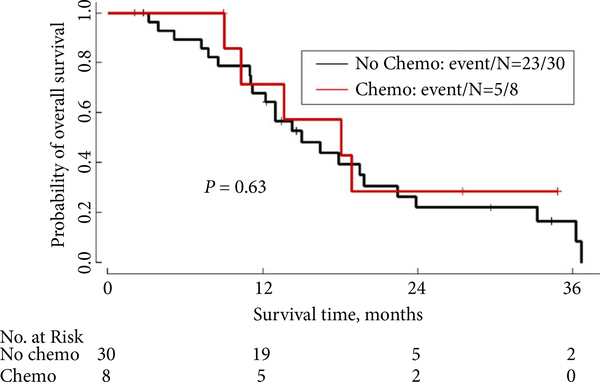
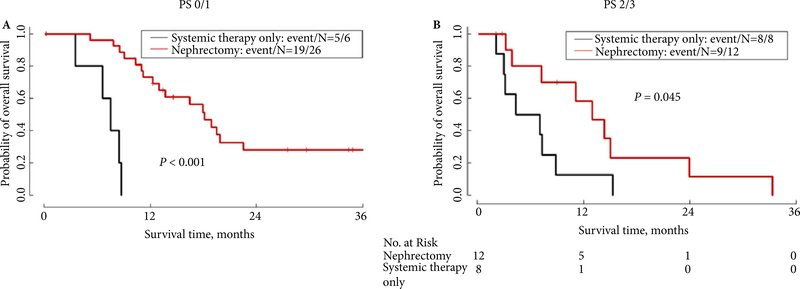
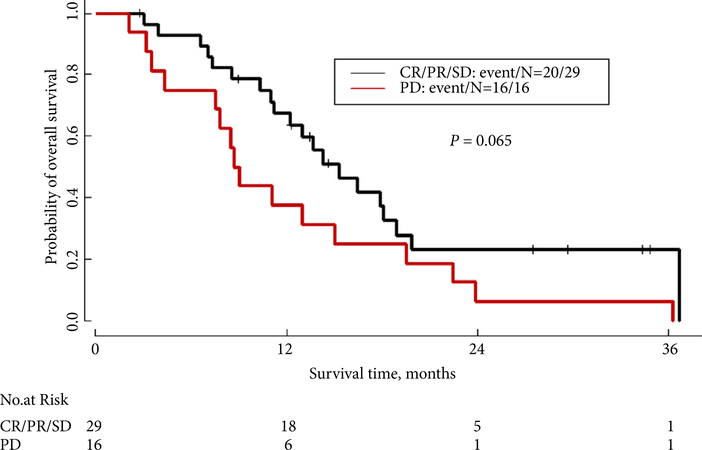
As shown in Table 3, in the subgroup of 38 patients who had nephrectomy, initial staging and performance status did not significantly affect survival. The median OS for Stage I–III patients was 18.9 months, whereas the median OS for Stage IV patients was 15.0 months (P = 0.89). In this cohort of patients who underwent nephrectomy, timing of first-line chemotherapy, stratified as pre- vs postoperative, did not affect survival. As shown in Fig. 2 and Table 4, the median OS for patients who received preoperative first-line chemotherapy was 18.1 months, compared with 15.0 months for patients who received postoperative first-line systemic therapy (P = 0.63). Interestingly, as shown in Fig. 3B, even in the cohort of patients with an ECOG PS of 2–3 at presentation, patients who ultimately underwent nephrectomy after receiving chemotherapy had longer survival than those who did not undergo nephrectomy (median OS 13.0 vs 5.7 months, P = 0.045). The difference in survival between the cohort of patients who had nephrectomy vs the cohort who did not was even larger for patients with an ECOG PS of 0–1 (18.1 months for nephrectomy patients vs 7.5 months for non-nephrectomy patients, P < 0.001; Fig. 3A). Additionally, as shown in Fig. 4, patients who achieved a complete radiographic response (CR), a partial response (PR) or had stable disease (SD) on first-line treatment had a median OS of 15.3 months, compared with median OS of 8.9 months for patients who had progressive disease (PD) on first-line treatment (P = 0.065).
Table 3.
Subgroup analysis of nephrectomy patients (N = 38).
| Variable | Median OS, months | OS rate at 12 months, % | P |
|---|---|---|---|
| Stage at presentation | |||
| I–III | 18.9 | 74 | 0.89 |
| IV | 15.0 | 65 | |
| ECOG PS | |||
| 0–1 | 18.1 | 73 | 0.09 |
| 2–3 | 13.0 | 58 | |
| Preoperative therapy | |||
| Yes | 18.1 | 71 | 0.63 |
| No | 15.0 | 68 | |
Fig. 2.
OS of nephrectomy patients: comparison of preoperative systemic therapy vs upfront surgery followed by systemic therapy (N = 38).
Table 4.
First-line chemotherapy data.
| Variable | Value |
|---|---|
| Pre-operative chemotherapy, n | |
| Yes | 8 |
| No | 30 |
| Duration of preoperative therapy, weeks, median (range) | 16.9 (15.9–38.9) |
| N: | |
| Best response to preoperative chemotherapy by RECIST (n = 8) | |
| CR | 1 |
| PR | 4 |
| SD | 3 |
| PD | 0 |
| Regimens used for preoperative therapy | |
| Paclitaxel/carboplatin | 4 |
| Paclitaxel/carboplatin/bevacizumab | 1 |
| Paclitaxel/carboplatin/gemcitabine | 1 |
| Gemcitabine/cisplatin/bevacizumab | 1 |
| Gemcitabine/doxorubicin | 1 |
| Best response to first-line chemotherapy by RECIST in upfront nephrectomy patients | |
| CR | 1 |
| PR | 4 |
| SD | 12 |
| PD | 9 |
| Best response to first-line chemotherapy in non-nephrectomy patients | |
| CR | 0 |
| PR | 4 |
| SD | 2 |
| PD | 8 |
| Number of lines of systemic therapy throughout course | |
| 0 | 0 |
| 1 | 20 |
| 2 | 9 |
| 3 | 11 |
| ≥4 | 12 |
RECIST, Response Evaluation Criteria in Solid Tumors.
Fig. 3.
OS by nephrectomy and performance status. (A) ECOG PS 0–1; (B) ECOG PS 2–3.
Fig. 4.
OS by best response to first-line therapy (CR/PR/SD vs PD).
Systemic Therapy and Response
All 52 patients received at least one line of systemic therapy during their treatment course. For the eight patients who received preoperative systemic therapy, the median (range) duration of first-line chemotherapy was 16.9 (15.9–38.9) weeks. One patient had a CR, four had a PR, and three had SD. Carboplatin/paclitaxel (±bevacizumab) was the most common regimen, used in five of the eight patients. For the patients who underwent upfront nephrectomy and then received postoperative chemotherapy, 65% achieved CR/PR/SD with first-line chemotherapy, while 35% of patients had PD. In all, 14 patients did not undergo nephrectomy, and 8/14 had PD with first-line chemotherapy. Details on first-line systemic therapy are listed in Table 4.
In all, 28 (54%) of patients received targeted therapies (sunitinib, bevacizumab, bortezomib, pazopanib, sorafenib, everolimus, imatinib) during their treatment course. The median duration of single-agent targeted therapy was 8 weeks. No objective (CR or PR) responses were seen with single-agent targeted therapy. Data on targeted therapies and best responses are presented in Table 5.
Table 5.
Targeted therapies used and responses achieved.
| Agent | Patient no. | Line of therapy, n | Duration of therapy, weeks | Best response |
|---|---|---|---|---|
| Sunitinib | 6 | 1 | 12 | PD |
| 9 | 1 | 27 | SD | |
| 10 | 1 | 5 | PD | |
| 11 | 1 | 4 | PD | |
| 13 | 2 | 5 | PD | |
| 15 | 1 | 25 | SD | |
| 17 | 1 | 4 | PD | |
| 44 | 2 | 13 | PD | |
| Bevacizumab | 2 | 1 (with gemcitabine/cisplatin) | 16 | PR |
| 3 | 6 (with erlotinib) | 20 | PD | |
| 5 | 1 (with gemcitabine/cisplatin) | 7 | SD | |
| 9 | 2 | 8 | PD | |
| 11 | 2 (with gemcitabine/doxorubicin) | 21 | PD | |
| 12 | 2 (with carboplatin/paclitaxel) | 10 | SD | |
| 13 | 1 (with erlotinib) | 49 | SD | |
| 14 | 2 (with erlotinib) | 23 | SD | |
| 17 | 2 (with gemcitabine/capecitabine) | 10 | SD | |
| 18 | 2 (with carboplatin/paclitaxel) | 10 | SD | |
| 19 | 2 (with carboplatin/paclitaxel) | 17 | PR | |
| 21 | 2 (with carboplatin/paclitaxel) | 6 | SD | |
| 24 | 1 (with carboplatin/paclitaxel) | 18 | SD | |
| 25 | 1 (with gemcitabine/cisplatin) | 10 | PD | |
| 26 | 3 (with carboplatin/paclitaxel) | 43 | SD | |
| 31 | 2 | 30 | SD | |
| 36 | 1 (with carboplatin/paclitaxel) | 26 | PR | |
| 41 | 2 | 12 | SD | |
| 46 | 1 (with gemcitabine/cisplatin) | 6 | PR | |
| Sorafenib | 12 | 5 (with everolimus) | 5 | PD |
| 48 | 3 | 6 | PD | |
| Imatinib | 3 | 2 (with gemcitabine/cisplatin) | 6 | PD |
| 4 | 1 | 3 | PD | |
| 37 | 3 (with ipilimumab) | 7 | PD | |
| 48 | 1 | 8 | PD | |
| Bortezomib | 9 | 7 | 8 | SD |
| 28 | 1 | 7 | PD | |
| 48 | 4 | 6 | PD | |
| Everolimus | 9 | 4 | 11 | PD |
| Pazopanib | 20 | 2 | 9 | PD |
The most frequently used chemotherapy regimens were carboplatin/paclitaxel (±bevacizumab), gemcitabine/cisplatin (±bevacizumab), gemcitabine/doxorubicin (±bevacizumab), and dose-dense MVAC (methotrexate, vinblastine, doxorubicin, cisplatin). A 29% objective response rate was seen in patients receiving cytotoxic chemotherapy. Further data regarding cytotoxic chemotherapy regimens used and responses achieved are presented in Table 6.
Table 6.
Chemotherapy-based regimens used and responses achieved.
| Agent | Patient no. | Line of treatment, n | Duration of therapy, weeks | Best response |
|---|---|---|---|---|
| Carboplatin/paclitaxel (±bevacizumab) | 2 | 3 | 15 | SD |
| 12 | 2 | 10 | SD | |
| 14 | 1 | 16 | PD | |
| 18 | 1 | 29 | SD | |
| 19 | 1 | 33 | PR | |
| 20 | 1 | 15 | CR | |
| 21 | 1 | 14 | SD | |
| 24 | 1 | 31 | SD | |
| 26 | 2 | 48 | SD | |
| 27 | 2 | 49 | SD | |
| 29 | 1 | 14 | SD | |
| 30 | 1 | 19 | PR | |
| 31 | 3 | 23 | SD | |
| 32 | 1 | 39 | PR | |
| 33 | 1 | 28 | SD | |
| 35 | 1 | 15 | PR | |
| 36 | 1 | 26 | PR | |
| 37 | 1 | 9 | PD | |
| 38 | 1 | 16 | SD | |
| 40 | 1 | 8 | SD | |
| 43 | 2 | 28 | CR | |
| 45 | 2 | 18 | SD | |
| Gemcitabine/cisplatin (±bevacizumab) | 1 | 1 | 16 | PR |
| 2 | 1 | 16 | PR | |
| 3 | 2 | 6 | PD | |
| 5 | 1 | 7 | SD | |
| 9 | 3 | 15 | PD | |
| 12 | 1 | 13 | PD | |
| 19 | 3 | 20 | SD | |
| 23 | 1 | 35 | SD | |
| 25 | 1 | 10 | PD | |
| 42 | 1 | 14 | PR | |
| 46 | 1 | 12 | PR | |
| 47 | 1 | 16 | PD | |
| Gemcitabine/doxorubicin (±paclitaxel or bevacizumab) | 2 | 2 | 11 | PD |
| 3 | 1 | 6 | SD | |
| 8 | 2 | 7 | PD | |
| 9 | 5 | 25 | SD | |
| 11 | 1 | 21 | SD | |
| 18 | 2 | 11 | SD | |
| 19 | 2 | 24 | PR | |
| 20 | 3 | 16 | SD | |
| 30 | 3 | 10 | PD | |
| 33 | 2 | 12 | PR | |
| 52 | 1 | 27 | PR | |
| MVAC | 18 | 4 | 6 | SD |
| 26 | 1 | 7 | PD | |
| 30 | 2 | 13 | PD | |
| 31 | 1 | 14 | SD | |
| 38 | 2 | 9 | PD | |
| 41 | 1 | 20 | SD | |
| 44 | 1 | 8 | SD | |
| 45 | 1 | 24 | SD | |
| 48 | 2 | 24 | PR | |
| 49 | 1 | 12 | PR | |
| Gemcitabine/cisplatin/ifosfamide | 8 | 1 | 11 | SD |
| 16 | 1 | 2 | PD | |
| 26 | 3 | 17 | PD | |
| Gemcitabine/capecitabine (±bevacizumab) | 13 | 3 | 6 | SD |
| 14 | 3 | 10 | PD | |
| 17 | 2 | 10 | SD | |
| 21 | 2 | 8 | PD | |
| Gemcitabine/carboplatin/paclitaxel | 50 | 1 | 16 | SD |
| 51 | 1 | 22 | PR | |
| Gemcitabine/cisplatin/paclitaxel | 43 | 1 | 26 | CR |
Long-term Responders
Seven patients survived for >24 months; two of them are alive without evidence of disease at >36 months from diagnosis. One of these patients had a clinical CR with preoperative chemotherapy (carboplatin/paclitaxel). At nephrectomy, the surgical specimen revealed no evidence of tumour in the resected lymph nodes and only a small focus of residual tumour in the resected kidney. The patient developed metastases to the lungs 8 weeks after nephrectomy, and was treated with multiple lines of systemic therapy (pazopanib, gemcitabine/doxorubicin, and carboplatin/ paclitaxel). He had PD on pazopanib, but achieved repeat CR with carboplatin/paclitaxel. He remains off chemotherapy and free of disease 45 months from diagnosis. The other surviving patient was aged 9 years at diagnosis, and had lymph node and lung metastasis at presentation. He underwent upfront nephrectomy, followed by two lines of systemic therapy (gemcitabine/cisplatin/paclitaxel and carboplatin/paclitaxel). He is now 42 months from nephrectomy and 30 months from completion of chemotherapy, and remains with no evidence of disease to date.
Discussion
RMC is a rare and an aggressive subtype of RCC. The vast majority of patients have sickle cell trait and present with advanced disease, but even the few who present with local disease develop tumour recurrence within a few months after nephrectomy. In the present largest-reported cohort to date, the median OS of 13 months compares favourably with the historical median OS of 5 months, but the prognosis remains poor, as <20% of patients survive for >24 months.
We found that clinical lymph node involvement, lack of nephrectomy, and an ECOG PS of 2–3 at presentation, were associated with worse survival. Patients who underwent nephrectomy at some point during their course of treatment had a lower risk of death, even after adjusting for effects of lymph node involvement and ECOG PS. A limitation of the present retrospective study is that it is unclear whether the benefit of nephrectomy is due to a therapeutic effect, a confounder from more favourable tumour biology, or due to patient selection bias. While a selection bias is inherent in a retrospective study, we recommend that patients should be considered for nephrectomy when clinically appropriate. The MSKCC risk score did not appear to be an appropriate metric for selecting patients for surgery; most patients in the present study had intermediate- and high-risk disease, but over half of the poor-risk group were able to undergo nephrectomy at some point during their course of treatment. Patients who present with a high tumour burden with extensive visceral or bone metastasis or rapidly progressive metastatic disease should not undergo upfront nephrectomy, as these patients invariably will have rapid deterioration after surgery and will not be able to receive systemic therapy. However, in well-selected patients, who respond to initial chemotherapy, or who have low-volume metastasis, nephrectomy can potentially play an important role, even in the metastatic setting, with radical nephrectomy and resection of all visible retroperitoneal disease being a goal of surgery. Of note, there is no role for distant metastasectomy in this disease, in contrast to RCC. Iacovelli et al. [2] also showed a trend towards benefit of nephrectomy, although this was not statistically significant and included only small numbers of patients from each treatment centre. Our present data suggest a therapeutic benefit from nephrectomy in selected patients whether it is performed upfront or after response to chemotherapy. However, the decision to perform nephrectomy is influenced by many patient-specific variables, so a thorough patient evaluation and a multidisciplinary deliberation are necessary before a surgical treatment plan is formulated.
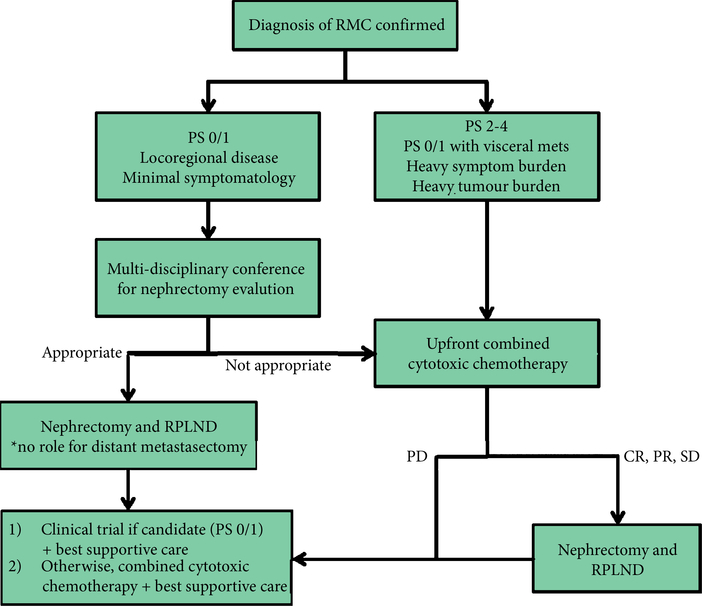
In our present study, response to first-line chemotherapy was associated with a trend toward improved OS. A trend for worse outcome was noted in patients who had PD in response to first-line chemotherapy, compared with those who had SD or achieved an objective response. Based on our present results, we recommend the use of combined cytotoxic chemotherapy over single agents or targeted therapies, particularly for palliation of symptoms. Most patients in our present study received carboplatin/paclitaxel (±bevacizumab), or gemcitabine/cisplatin (±bevacizumab) in the first-line setting. Based on our present data, targeted agents do not appear to produce any meaningful response as single agents. Our suggested treatment algorithm for RMC is shown in Fig. 5, and focuses on the multidisciplinary management of this disease.
Fig. 5.
Suggested treatment algorithm. RPLND. retroperitoneal lymph node dissection.
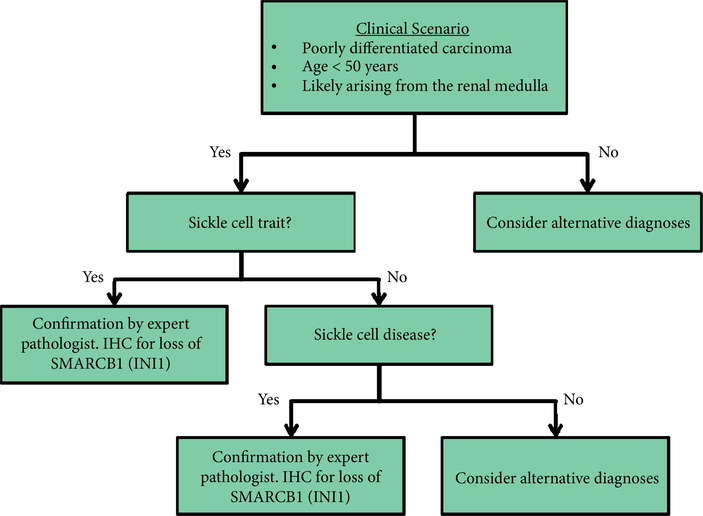
The next frontier in RMC research is a rigorous molecular characterisation to identify biologically relevant targets that can be exploited for the development of rational and more effective therapies. Molecular profiling of RMC has shown a signature more similar to urothelial carcinoma or TCC of the renal pelvis than to RCC [20]. Recent comprehensive genomic profiling has shown loss of SMARCB1, a tumour suppressor gene on chromosome 22 [21,22]. The loss of SMARCB1 protein is becoming increasingly important in the confirmation of RMC, as shown in the recommended diagnostic algorithm in Fig. 6. In addition, balanced translocations disrupting SMARCB1 have been reported as the hallmark of genetic alterations in RMC [23]. SMARCB1 acts as a chromatin remodelling regulator, which is a repressor of cyclin D1 transcription; therefore, loss of SMARCB1 leads to increased cyclin D1 transcription in RMC [24]. This has downstream effects on overexpression of EZH2 (a histone methyltransferase), providing the rationale for using EZH2 inhibitors, such as tazemetostat, as therapeutic options [23,25]. Phosphatase and tensin homologue (PTEN) loss and use of mammalian target of rapamycin (mTOR) inhibitors has also been under recent investigation, although results have been limited [26]. As we gain insights into the biology of this aggressive tumour, we hope to develop novel and more effective therapies that will lead to improved patient outcomes.
Fig. 6.
Suggested diagnostic algorithm. IHC, immunohistochemistry.
Conclusions
RMC is an aggressive disease, with limited treatment options and universally poor outcomes. In the present retrospective study, we did not observe any responses to targeted agents when used as monotherapy; however, cytotoxic chemotherapy provided palliative effects. Patients who underwent nephrectomy during the course of their disease had a longer survival than those who were treated with systemic therapy only. Timing of administration of chemotherapy (pre- vs postoperatively) did not affect survival, but a trend towards a longer survival was noted in patients who achieved an objective response or SD to first-line chemotherapy. Studies are urgently needed to identify molecular alterations that could provide the foundation for development of novel, rational, and more effective therapy for RMC.
Acknowledgments
Conflicts of interest
Dr Malouf reports grants from Pfizer, grants from Novartis, outside the submitted work; Dr Heng reports grants and consultancy fees from Pfizer, grants and consultancy fees from Novartis, outside the submitted work; Dr Guancial reports other from Genentech, outside the submitted work; Dr Fung reports personal fees from Janseen Scientific Affairs, personal fees from Dendreon, personal fees from Bayer, personal fees from Novartis, outside the submitted work; Dr Wood reports grants from Pfizer, grants from Ono Pharma, grants from Boehringer Ingelheim, personal fees from Novartis, during the conduct of the study; Dr Tannir reports grants, personal fees and non-financial support from BMS, grants, personal fees and non-financial support from Exelixis, personal fees and nonfinancial support from Nektar, grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Argos, personal fees and non-financial support from Calithera, grants from Miranti, grants from Epizyme, outside the submitted work; Dr Shah, Dr Karam, Dr Rao, Lianchun Xiao, Dr Jonasch, Dr Vaishampayan, Dr Lowas, Dr Tamboli, Dr Sircar and Dr Matin have nothing to disclose.
Abbreviations
- CR
complete response
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HR
hazard ratio
- MSKCC
Memorial Sloan-Kettering Cancer Center
- MVAC
methotrexate, vinblastine, doxorubicin, cisplatin
- OS
overall survival
- PD
progressive disease
- PR
partial response
- RMC
renal medullary carcinoma
- SD
stable disease
References
- 1.Davis CJ Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma: the seventh sickle cell nephropathy. Am J Surg Pathol 1995; 19: 1–11 [DOI] [PubMed] [Google Scholar]
- 2.Iacovelli R, Modica D, Palazzo A, Trenta P, Piesco G, Cortesi E. Clinical outcome and prognostic factors in renal medullary carcinoma: a pooled analysis from 18 years of medical literature. Can Urol Assoc J 2015; 9: E172–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Dor Y, Herbert JM et al. Role of HIF-1a in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature 1998; 394: 485–90 [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol 2000; 63: 205–11 [DOI] [PubMed] [Google Scholar]
- 5.Pham PT, Pham PC, Wilkinson AH, Lew SQ. Renal abnormalities in sickle cell disease. Kidney Int 2000; 57: 1–8 [DOI] [PubMed] [Google Scholar]
- 6.Warren KE, Gidvani-Diaz V, Duval-Arnould B. Renal medullary carcinoma in an adolescent with sickle cell trait. Pediatrics 1999; 103: E22. [DOI] [PubMed] [Google Scholar]
- 7.Figenshau RS, Basler JW, Ritter JH, Siegel CL, Simon JA, Dierks SM. Renal medullary carcinoma. J Urol 1998; 159: 711–3 [PubMed] [Google Scholar]
- 8.Davidson AJ, Choyke PL, Hartman DS, Davis CJ Jr. Renal medullary carcinoma associated with sickle cell trait: radiologic findings. Radiology 1995; 195: 83–5 [DOI] [PubMed] [Google Scholar]
- 9.Blitman NM, Berkenblit RG, Rozenblit AM, Levin TL. Renal Medullary Carcinoma: CT and MRI Features. AJR Am J Roentgenol 2005; 185: 268–72 [DOI] [PubMed] [Google Scholar]
- 10.Swartz MA, Karth J, Schneider DT, Rodriguez R, Beckwith JB, Perlman EJ. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology 2002; 60: 1083–9 [DOI] [PubMed] [Google Scholar]
- 11.Assad L, Resetkova E, Oliveira VL et al. Cytologic features of renal medullary carcinoma. Cancer 2005; 105: 28–34 [DOI] [PubMed] [Google Scholar]
- 12.Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol 2008; 21: 647–52 [DOI] [PubMed] [Google Scholar]
- 13.Rao P, Tannir NM, Tamboli P. Expression of OCT 3/4 in renal medullary carcinoma represents a potential diagnostic pitfall. Am J Surg Pathol 2012; 36: 583–8 [DOI] [PubMed] [Google Scholar]
- 14.Albadine R, Wang W, Brownlee NA et al. Topoisomerase II alpha status in renal medullary carcinoma: immuno-expression and gene copy alterations of a potential target of therapy. J Urol 2009; 182: 735–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaeffer EM, Guzzo TJ, Furge KA et al. Renal medullary carcinoma: molecular, pathological and clinical evidence for treatment with topoisomerase-inhibiting therapy. BJU Int 2010; 106: 62–5 [DOI] [PubMed] [Google Scholar]
- 16.Strouse JJ, Spevak M, Mack AK, Arceci RJ, Small D, Loeb DM. Significant responses to platinum-based chemotherapy in renal medullary carcinoma. Pediatr Blood Cancer 2005; 44: 407–11 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J R Stat Soc Series B Stat Methodol 1958; 53: 457–81 [Google Scholar]
- 18.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–70 [PubMed] [Google Scholar]
- 19.Cox D Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol 1972; 34: 187–220 [Google Scholar]
- 20.Yang XJ, Sugimura J, Tretiakova MS et al. Gene expression profiling of renal medullary carcinoma: potential clinical relevance. Cancer 2004; 100: 976–85 [DOI] [PubMed] [Google Scholar]
- 21.Pal SK, Choueiri TK, Wang K et al. Characterization of clinical cases of collecting duct carcinoma of the kidney assessed by comprehensive genomic profiling. Eur Urol 2016; 70: 516–21 [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Smith SC, Agaimy A et al. Collecting duct carcinoma versus renal medullary carcinoma: an appeal for nosologic and biological clarity. Am J Surg Pathol 2014; 38: 871–4 [DOI] [PubMed] [Google Scholar]
- 23.Calderaro J, Masliah-Planchon J, Richer W et al. Balanced Translocations Disrupting SMARCB1 are Hallmark Recurrent Genetic Alterations in Renal Medullary Carcinomas. Eur Urol 2016; 69: 1055–61 [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Beltran A, Cheng L, Raspollini MR, Montironi R. SMARCB1/INI1 genetic alterations in renal medullary carcinoma. Eur Urol 2016; 69: 1062–4 [DOI] [PubMed] [Google Scholar]
- 25.US National Institutes of Health, Clinical Trials. gov. A Phase II, Multicenter Study of the EZH2 Inhibitor Tazemetostat in Adult Subjects with INI1-Negative Tumors or Relapsed/Refractory Synovial Sarcoma, 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02601950. Accessed November 2016
- 26.Lipkin JS, Rizvi SM, Gatalica Z et al. Therapeutic approach guided by genetic alteration: use of MTOR inhibitor in renal medullary carcinoma with loss of PTEN expression. Cancer Biol Ther 2015; 16: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]