Summary
Vaccination against meningococcal serogroup B is recommended for patients with a complement deficiency; however, although immunogenicity in this patient group has been shown, efficacy has not yet been established. In this study, we collected serum from children with a complement deficiency in the alternative pathway or in late terminal pathway before and after vaccination with multi‐component meningococcal serogroup B (MenB)‐4C. MenB‐4C is a multi‐component, protein‐based vaccine against MenB consisting of factor H‐binding protein, Neisserial heparin‐binding protein, Neisserial adhesion A and outer membrane vesicles containing Porin A. We assessed the vaccine immunogenicity and vaccine‐mediated protection by a whole cell enzyme‐linked immunosorbent assay with Neisseria meningitidis serogroup B strains H44/76, 5/99 and NZ98/254, which shows that vaccination induced antibody titers against meningococcus. We show that the classical serum bactericidal activity assay with exogenous serum indicates the presence of vaccine‐induced antibodies and capacity to activate complement‐mediated pathogen lysis. However, in children with a late terminal pathway deficiency, no complement‐mediated pathogen lysis was observed when autologous serum was applied in the serum bactericidal activity assay, demonstrating a lack of serum bactericidal activity in children with complement deficiencies. However, MenB‐4C vaccination still induced effective complement‐dependent opsonophagocytic killing against N. meningitidis serogroup B in reconstituted whole blood with autologous serum from children with an alternative pathway or late terminal pathway deficiency. These findings support the recommendation to vaccinate all complement‐deficient children against MenB.
Keywords: children, complement‐deficient, MenB‐4C, Neisseria meningitidis, vaccine
Patients with a complement deficiency are extensively vaccinated against Meningococcal infection, including with the 4C‐MenB vaccine against N. meningitidis serogroup B. We show that vaccination induces functional antibodies, but does not result in bacterial killing via the complement system in patients with a late terminal pathway deficiency. Despite the complement deficiency, killing can occur via opsonophagocytosis.
Introduction
Upon infection in the human host, the complement system is one of the first systems to respond and can kill bacteria directly via pore formation, label them for phagocytosis and stimulate the immune system by the release of anaphylatoxins. Activation of the complement system occurs through three different pathways. The classical pathway (CP) is mainly activated by antibody complexes that recognize bacterial epitopes, the lectin pathway (LP) recognizes specific sugar moieties on the bacterial surface and the alternative pathway (AP) mainly functions as amplification loop of the other two pathways, but can also be activated spontaneously by hydrolysis of C3 1. Activation of either of these three pathways leads to the formation of C3 convertase and deposition of C3b on the bacterial surface. Eventually, C3b deposition will cause formation of the C5 convertase leading to formation of the membrane attack complex (MAC) 2. The MAC is a pore that is formed on the bacterial surface and inserts itself into the bacterial membrane, and thereby lyses Gram‐negative bacteria 1. This process from the cleavage of C5 to inserting the pore in the membrane is also called the ‘terminal pathway’ and the process after the cleavage of C5 is often called the ‘late terminal pathway’ (LTP).
That the complement system is important for protection against microbial infections can be seen in patients with a complement deficiency. Depending on the complement deficiency, the type of bacterial infection can differ. The most frequently encountered pathogens are Streptococcus pneumoniae, Haemophilus influenza and Neisseria meningitidis 3. In particular, a complement deficiency in the terminal pathway or the AP can have a 250–10 000‐fold increased risk of infection with meningococci 3, 4. Prevention of meningococcal infection in these patients is indicated with antibiotic prophylaxis and vaccination with the tetravalent vaccine against meningococcal serogroups A, C, Y and W and a vaccine against serogroup B 4.
Meningococcal serogroup B vaccine is protective in the general population, as assessed following the introduction of multi‐component meningococcal serogroup B (MenB)‐4C vaccine (Bexsero) in the national immunization program in the United Kingdom 5. MenB‐4C is a multi‐component, protein‐based vaccine consisting of factor H‐binding protein, Neisserial heparin‐binding protein, Neisserial adhesion A and outer membrane vesicles containing Porin A. Children and adults with complement deficiencies have an increased risk of meningococcal disease and several guidelines recommend meningococcal serogroup B vaccine for inherited or chronic complement deficiencies, including C3, C5–C9, properdin, factor D and factor H deficiencies 6. However, these patients may have a deficiency of complement opsonization, complement anaphylatoxin activation or serum bactericidal activity (SBA), which are important mechanisms for vaccine‐induced meningococcal killing. Meningococcal serogroup B vaccination is obligated for patients suffering from a complement dysregulation, such as paroxysmal nocturnal hemoglobinuria or atypical hemolytic uremic syndrome, who are treated with the C5 inhibitor eculizumab 6, 7, 8. Previous studies in patients with primary terminal complement deficiencies have demonstrated that vaccine‐induced anti‐capsular antibodies against serogroups A, C, Y and W confer protection against meningococcal disease by increasing the opsonophagocytic activity despite inability to kill bacteria by SBA 9, 10. Whether this is also the case for the meningococcal serogroup B MenB‐4C multi‐component, protein‐based vaccine is not known.
Recent assessment of MenB‐4C vaccine in the context of eculizumab treatment caused reason for alarm. Eculizumab is a monoclonal antibody directed at C5 in order to prevent the cleavage of C5 into C5a and C5b, and thereby further activation of the LTP. Blocking the cleavage of C5 by eculizumab essentially creates a complement deficiency in these patients, as C5 can no longer be cleaved to activate the LTP and other pathways. This led to a number of patients who were treated with eculizumab and, despite vaccination, acquired a meningococcal infection 11.
In this study, we demonstrate effective opsonophagocytic killing of meningococcal serogroup B by serum from MenB‐4C‐vaccinated children with complement deficiencies.
Methods
Subjects
Our patient group consisted of two children with an AP deficiency and three children with a LTP deficiency. Of these children, the activity of the AP (AP50) and classic complement pathway (CP) (CH50) were measured via enzyme‐linked immunosorbent assay (ELISA) adapted from Seelen et al. 12 performed by our hospital’s diagnostic laboratory. Some children were on antibiotic prophylaxis. Patient characteristics are described in more detail in Table 1.
Table 1.
Patient and control characteristics
| Child | Age (years) | Gender | Complement deficiency | Complement pathway | Symbol | Prophylaxis (daily dose) | Inflammatory history | AP50 (normal range 67–133%)* | CH50 (normal range 67–149%)* | Deficient complement protein level | Associated mutation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 7 | Male | C6 deficiency | LTP | Amoxicillin (14·4 mg/kg) | Septic arthritic knee | 12% | 31% | 2·1 µg/ml (normal level: 45 ± 16 µg/ml) | Cys867Arg and rs76202909 | 13 | |
| B | 6 | Male | Factor I deficiency | AP | – | Meningococcal meningitis (serotype B) | 51% | > 85% | n.d. | Asp519Asn | – | |
| C | 9 | Female | C8 deficiency | LTP | Feneticillin (< 12·5 mg/kg) | Meningococcal sepsis (serotype Z and C) | < 20% | < 10% | n.d. | Gln91Stop | – | |
| D | 15 | Female | C8 deficiency | LTP | Feneticillin (< 12·5 mg/kg) | – | < 20% | < 10% | n.d. | Gln91Stop | – | |
| E | 16 | Male | Factor D deficiency | AP | – | Meningococcal sepsis (serotype B) | < 5% | – | < 0·03 mg/l (normal level: 1·0–2·0 mg/l) | Cys214Arg and Val213Gly# | 14 | |
| Control 1 | 39 | Male | ||||||||||
| Control 2 | 25 | Male | ||||||||||
| Control 3 | 36 | Female |
AP50 and CP50 values below normal range shown in bold type.
Both homozygous mutations were found, but contributions of either mutation could not be distinguished.
AP = alternative complement pathway; CP = classic complement pathway; LTP = late terminal pathway; n.d. = not determined.
All children received MenB‐4C vaccine in concordance with current guidelines. Assessment of children’s vaccine responses was offered as part of routine clinical care in the immunodeficiency clinic. As assessment of MenB‐4C vaccine responses was implemented in our pediatric immunodeficiency clinic as a test in routine clinical care, no research protocol to assess vaccine responses was submitted to the institutional ethics committee for approval. Patients and/or their parents provided oral informed consent for blood sample collection in accordance with standard good clinical practice. Healthy adult controls were vaccinated due to laboratory exposure to N. meningitidis serogroup B. A protocol for blood collection from healthy volunteers was approved by the institutional ethical committee and all samples from laboratory workers were obtained following written informed consent. Both children and controls received two vaccination with MenB‐4C with a 1–2‐month interval. One unvaccinated healthy control was included as a serum source for the exogenous serum bactericidal assay. All experiments were carried out in accordance with local guidelines and regulations and comply with the Declaration of Helsinki and the Good Clinical Practice guidelines.
Bacterial growth conditions
The N. meningitidis serogroup B strain 5/99 and NZ98/254, kindly provided by Public Health England (PHE) (Manchester Laboratory, UK), and strain H44/76 were grown overnight at 37°C with 5% CO2 on a GC‐agar plate with Isovitalex, followed by resuspension in tryptic soy broth (TSB) and grown to an optical density of 0·23 [approximately 2·3 × 108 colony‐forming units (CFU)] at 620 nm. These strains were used to establish the individual contributions of the factor H‐binding protein (H44/76), neisserial adhesin A (5/99) and NZ outer membrane vesicle components (NZ98/254) 15, 16.
Serum collection
Blood was collected before vaccination and 1 month after the second vaccination into a clot activator tube (BD Diagnostics, Wokingham, UK) on ice and coagulated for 1 h on ice, after which the serum was aliquoted and frozen. To neutralize any present β‐lactam antibiotics due to antibiotic prophylaxis used by these children, all sera (including control) were pretreated in all experiments with 20 µg/ml β‐lactamase for 10 min.
Whole cell ELISA
The N. meningitidis serogroup B strain H44/76, 5/99 or NZ98/254 in phosphate‐buffered saline (PBS) were coated in a 96‐well plate and dried overnight at 37°C. The next day, the plate was incubated for 1 h at 37°C with a threefold serum dilution series, starting with a 50‐fold serum dilution. Next, the samples were incubated with mouse anti‐human immunoglobulin (Ig)G in PBS with 0·1% Tween‐80 and 0·5% milk powder for 1 h at 37°C. As a second antibody, horseradish peroxidase (HRP)‐conjugated anti‐mouse IgG in PBS with 0·1% Tween‐80 and 0·5% milk powder was added for 1 h at 37°C. Between each step, the plate was washed three times with PBS with 0·05% Tween‐80. HRP activity was detected by the addition of tetramethylbenzidine containing substrate solution for 10 min, after which the reaction was stopped with 2 M H2SO4 and subsequently read by an ELISA microplate reader 17. In Fig. 1, only the results of the 4050‐fold serum dilution are depicted.
Figure 1.
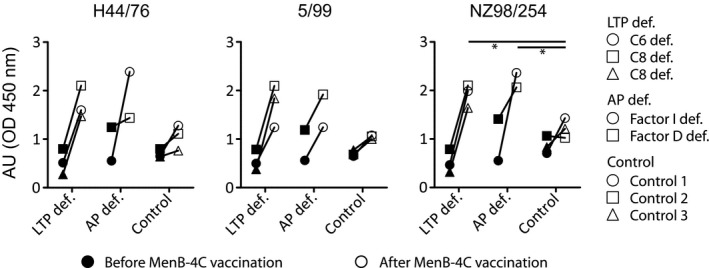
Multi‐component meningococcal serogroup B (MenB)‐4C vaccine responses of sera from complement‐deficient patients or controls assessed by whole cell total immunoglobulin (Ig)G enzyme‐linked immunosorbent assay (ELISA). Sera were collected from three patients with a late terminal complement pathway (LTP def.); C6 deficiency (○) or C8 deficiency (Δ and □) or two patients with alternative pathway deficiency (AP def.); factor I deficiency (○) or factor D deficiency (□) before (filled symbols) and after (open symbols) vaccination with MenB‐4C. The sera were assessed for the presence of antibodies against Neisseria meningitides serotype B strain H44/76, 5/99 and NZ98/254 by whole cell total IgG ELISA. Each of these strains express at least one of the antigens in the MenB‐4C vaccine. Results with a P‐value of < 0·05 were considered significant and are marked with an asterisk. Pre‐vaccination titers were compared between groups. The same was performed for post‐vaccination titers.
Exogenous human complement source SBA assay
The classical serum bactericidal activity with donor serum as exogenous human complement (eSBA) was determined. In short, the TSB with optical density (OD) = 0·23 was diluted 10 000 times and 10 µl was mixed with 20 µl of the heat‐inactivated serum, which was diluted in a twofold series. Lastly, 10 µl of active serum was added and the samples were incubated for 1 h at 37°C and plated on a GC‐agar plate with Isovitalex. eSBA titers were based on the initial serum dilution that showed ≥ 90% killing of colonies, compared to colonies surviving in serum from a healthy unvaccinated individual with low bactericidal activity. A stringent threshold for killing (90%) was chosen due to the small number of children included in the study. eSBA titers of ≥ 4 are considered protective 18, 19. Exogenous human complement is used to reduce interindividual variation in the assay.
Autologous human complement source SBA assay
As eSBA does not reflect real‐life SBA in complement‐deficient patients, we also performed the classical serum bactericidal activity with active autologous serum as human complement source (aSBA). In short, the TSB with OD = 0·23 was diluted 10 000 times and 10 µl was mixed with 20 µl of the active autologous serum that was diluted in a twofold series, and the samples were incubated for 1 h at 37°C and plated on a GC‐agar plate with Isovitalex. aSBA titers were based on the initial serum dilution that showed ≥ 90% killing of colonies compared to colonies surviving in serum from a healthy unvaccinated individual with low bactericidal activity. A stringent threshold for killing (90%) was chosen due to the small number of children included in the study. aSBA titers of ≥ 4 are considered protective 18, 19.
Whole blood killing assay
The whole blood killing assay was performed as described previously, with some minor adjustments 20. Blood was collected in an ethylenediamine tetraacetic acid (EDTA) tube from one healthy volunteer, washed with PBS, and the cells were suspended in PBS to the initial blood volume. One hundred microliters of cells were transferred to each well of a 96‐well plate and mixed with 12·5‐µl active serum with β‐lactamase. The bacterial culture with OD = 0·23 was diluted 50 times and 15 µl was mixed with the cells with serum. The samples were incubated in the 96 well plate in a shaking incubator (Edmund Buhler TH 10 Swip, Bodelshausen, Germany) at 37℃C for 45 min while shaking at 250 rpm. Samples were plated before and after incubation, in which one 10‐fold dilution was made, and subsequently a twofold dilution series of the samples was plated on GC‐agar plate with Isovitalex. After incubation the colonies were counted and the survival between t = 0 min and t = 45 min was calculated.
Statistical analysis
Statistical analysis was performed with Graphpad Prism version 5.03. Due to the small sample size and the presence of the same values in the groups, a one‐sided analysis of variance (anova) was chosen with a post‐hoc Dunnet’s multiple comparison test. The significance of the LTP or AP deficiency samples compared to the control samples in either the pre‐ or post‐vaccination condition was determined for each strain. A P‐value of < 0·05 was considered statistically significant.
Results
Patient characteristics
Children who participated in this study had an average age of 10·6 years (range = 6–16 years). All were seen by a pediatric infectious disease specialist who determined the complement deficiency in each child. To locate the pathway in which the complement deficiency resided, CH50 and AP50 were performed for most children (Table 1).
MenB‐4C vaccine induces adequate MenB IgG antibody titers in children with primary AP or LTP complement deficiencies
Production of vaccine‐induced N. meningitidis serogroup B IgG was detected in post‐vaccination serum samples of children and controls for all strains, with the exception of the NZ98/254 strain in one of the controls (Fig. 1). MenB‐4C vaccine‐specific IgG levels tended to be higher for children with complement deficiencies compared to healthy controls, which was significant for the NZ98/254 strain (one‐way anova, Dunnet’s multiple comparison test, P < 0·05).
Using an exogenous human complement serum source, the classical bactericidal antibody assay was used to confirm the effect of anti‐meningococcal antibodies on serum bactericidal activity. A titer is the serum dilution where 90% or more killing of N. meningitidis is observed. A protective titer was found in most MenB‐4C‐vaccinated children and controls (Fig. 2a). One child (E) with an AP deficiency showed a protective pre‐vaccination eSBA titer for all strains and one control showed a protective pre‐vaccination eSBA titer to strain NZ98/254. Immunization boosted eSBA titers in all but one child with AP deficiency (child E), which had an eSBA titer of 4 for strain NZ98/254. In all vaccinated children and controls, a lower eSBA titer was observed for strain NZ98/254 compared to strains H44/76 or 5/99.
Figure 2.
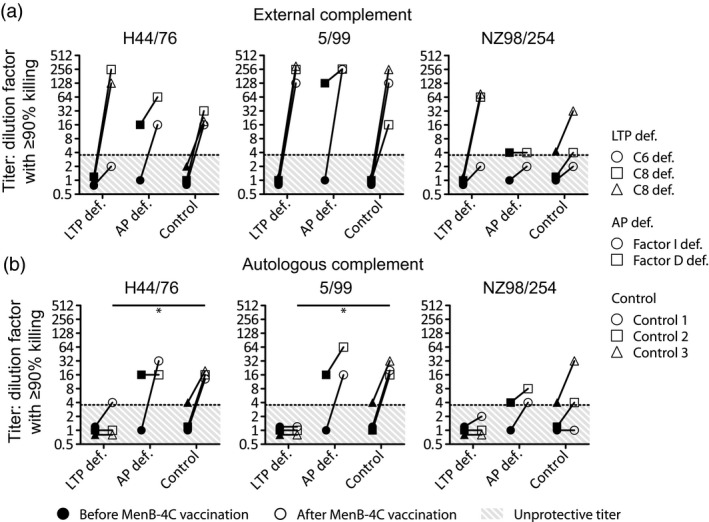
Exogenous human complement source serum bactericidal activity assay with sera from complement‐deficient patients or controls. Sera were collected from three patients with a late terminal complement pathway (LTP def.); C6 deficiency (○) or C8 deficiency (Δ and □) or two patients with alternative pathway deficiency (AP def.); factor I deficiency (○) or factor D deficiency (□) before (filled symbols) and after (open symbols) vaccination with multi‐component meningococcal serogroup B (MenB)‐4C. Classical serum bactericidal activity assay with exogenous human serum was determined (a). Serum bactericidal activity assay with autologous human serum was determined (b). Titers were based on the initial serum dilution that showed 90% or more killing. Results with a P‐value of < 0·05 were considered significant and are marked with an asterisk. Pre‐vaccination titers were compared between groups. The same was performed for post‐vaccination titers.
MenB‐4C vaccine fails to induce effective meningococcal killing in autologous‐SBA assay in children with LTP deficiencies
Children with a LTP deficiency showed no protective titer in an autologous human complement source serum bactericidal activity assay, except for a single child for strain H44/76 (Fig. 2b). Children with AP deficiencies showed effective meningococcal killing comparable to controls (Fig. 2b). Meningococcal killing for controls was similar applying aSBA and eSBA.
MenB‐4C vaccine induces effective opsonophagocytic killing of MenB by vaccinated children with AP and LTP complement deficiencies
MenB‐4C induced opsonophagocytic killing for all strains in children with AP and LTP deficiencies in the whole blood killing assay, which was equivalent to the controls (Fig. 3a). Opsonophagocytic killing was largely dependent on complement activity, as heat‐inactivated post‐vaccination autologous serum failed to induce effective opsonophagocytic killing (Fig. 3b).
Figure 3.
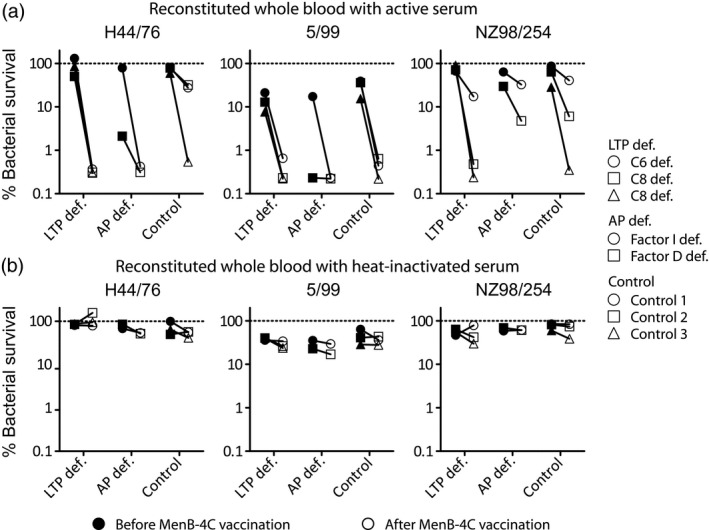
Bacterial survival in reconstituted whole blood with sera from complement‐deficient patients or controls. Sera were collected from three patients with a late terminal complement pathway (LTP def.); C6 deficiency (○) or C8 deficiency (Δ and □) or two patients with alternative pathway deficiency (AP def.); factor I deficiency (○) or factor D deficiency (□) before (filled symbols) and after (open symbols) vaccination with multi‐component meningococcal serogroup B (MenB)‐4C. Clearance of the bacterium in whole blood reconstituted with patient sera (a) or heat‐inactivated patient sera (b) was determined. Results with a P‐value of < 0·05 were considered significant and are marked with an asterisk. Pre‐vaccination titers were compared between groups. The same was performed for post‐vaccination titers.
Discussion
Our main findings are that we show that MenB‐4C vaccination induces an increase in anti‐meningococcal antibodies in children with AP and LTP deficiencies, and we demonstrate that these antibodies can induces effective opsonophagocytic killing of meningococci. MenB‐4C vaccination‐induced antibody titers tended to be lower for the healthy controls compared to the children with complement deficiencies, which might be related to the differences in age between the controls (mean age = 33 years) and children with complement deficiencies (mean age = 10·6 years).
As expected, by the nature of the complement defect, serum bactericidal antibody activity of autologous post‐immunization serum failed to induce meningococcal killing when serum from LTP‐deficient children was employed. Post‐immunization serum from children with AP defects demonstrated effective killing of meningococci comparable to controls.
One child with an AP deficiency had a protective pre‐titer (Fig. 2a,b). This is explained by an episode of pre‐vaccination meningococcal serogroup B sepsis, as documented in the patient’s history. Although this child had a protective titer before vaccination, the titer still tended to increase in most cases after vaccination (Fig. 2a,b), suggesting that vaccination induces additional protection. To date, the children did not sustain another infection with MenB after vaccination. One child with a C6 deficiency showed limited killing of H44/76 and NZ98/254 (Fig. 2b), which can be explained by the compound heterozygous mutations in the C6 gene resulting in C6 levels of 5% compared to normal. Despite these mutations, MAC formation is still present, causing a CH50 of 31% 13.
Recently, a large study was published in which 239 participants were vaccinated with MenB‐4C. Of these 239 participants, only 31 had a congenital complement deficiency and nine participants were treated with eculizumab. One hundred and twelve participants had an asplenia or splenic dysfunction and 87 participants were in the control category. In this study, Martinon‐Torres et al. performed an eSBA and an aSBA on serum from children with a complement deficiency and showed results that were similar to ours 21. In addition to their results, we performed a whole blood killing, showing that opsonophagocytosis is involved in the killing of meningococci in children with a complement deficiency.
It is important to note that in the classical eSBA, an external complement source is used and bypasses individual complement deficiencies (Fig. 4). Thus, an eSBA only confirms induction of effective anti‐meningococcal antibodies, but does not assess real protective potential in individuals with complement deficiencies. However, the eSBA has been shown to correlate with protection and therefore was used for licensing. Although the aSBA, which mimics the complement‐mediated killing capacity in these patients more effectively, the aSBA has not been validated to be a correlate of protection.
Figure 4.
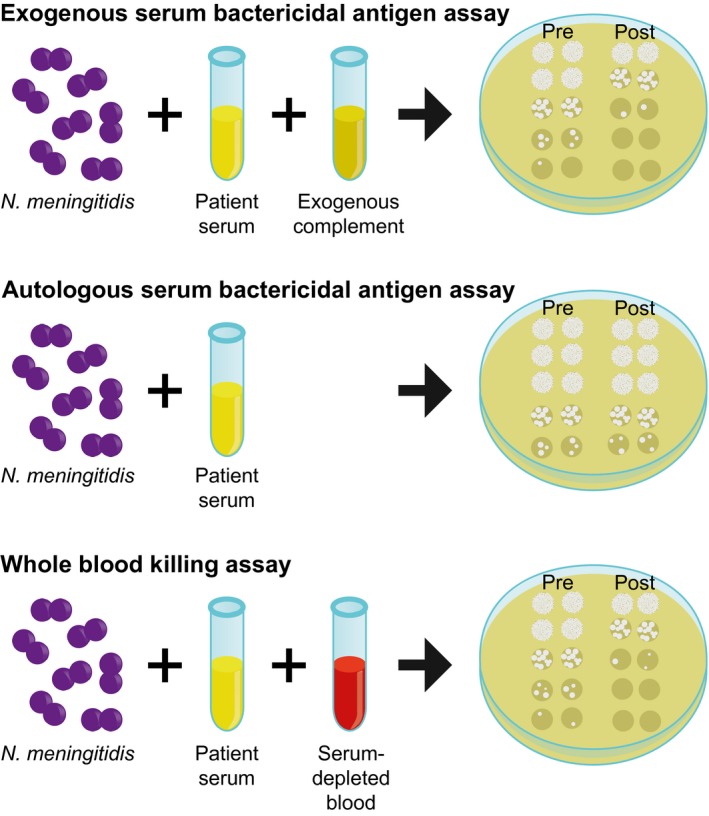
Schematic presentation of the differences between the assays used.
Recently, assessment of MenB‐4C vaccine in the context of eculizumab treatment revealed that immunization failed to induce effective opsonophagocytic killing of meningococci in the presence of eculizumab in a whole blood killing assay 11. Konar and Granoff elegantly show that blocking C5a production by eculizumab, thus blocking anaphylatoxin‐mediated activation of neutrophils via the C5a‐receptor, hampers effective vaccine‐induced opsonophagocytosis in eculizumab treatment. Additionally, they show that blocking factor D does not necessarily lead to outgrowth of the meningococci. When sufficient antibodies are present, this can cause bacteriolysis without the need for AP amplification 11. However, another study showed that, depending on the vaccine epitope, AP amplification might be necessary and could be dependent on CP activation 22. Our data with C6‐ and C8‐deficient children show effective killing in the whole blood killing assay. In contrast to eculizumab treatment, these C6‐ and C8‐deficient children still have normal C5 cleavage and release of C5a. Based on these data and the paper by Konar and Granoff, we hypothesize that C5a production is important for neutrophil activation and effective opsonophagocytosis in the absence of MAC formation. Despite the inability of LTP‐deficient children to kill meningococci via MAC formation in an aSBA assay, whole blood killing shows that, in the presence of immune cells, C3b deposition and production of anaphylatoxin C3a and C5a, killing occurs and is comparable to that of the control group. The AP‐deficient children and control group can kill meningococci via both MAC formation and opsonophagocytosis, but the contribution of each separate pathway to the killing of the meningococci could not be determined in this whole blood killing assay. Further studies in patients with specific primary complement deficiencies, including patients with C5 and C3 deficiency, may help to further confirm this hypothesis and help to predict in which primary complement deficiencies MenB‐4C and other vaccines may fail to offer protection. This information is important to assess the indication for other preventive management such as antibiotic prophylaxis.
Clearly, in‐vitro opsonophagocytic killing (Fig. 4) remains a surrogate marker, and cannot replace true clinical efficacy evaluation 10. At present, it is unclear if vaccination is equally effective as antibiotic prophylaxis to prevent meningococcal infection in complement‐deficient children.
In conclusion, this is the first report, to our knowledge, that assesses the effectiveness of the MenB‐4C vaccination in children with primary AP and LTP deficiencies. These findings support the recommendation to administer group B meningococcal vaccine to patients with primary complement deficiency. Patients with defects in C5a generation (C3 and C5 deficiency) may be deficient in immunization‐induced opsonophagocytic killing and require further study to provide more effective preventive treatment.
Disclosures
The authors declare that they have no conflicts of interest.
Author contributions
B. v.d. B. and B. K. performed the experiments. B. v.d. B., C. A. C. M. v. Els, B. K., J. L. and M. v.d. F. designed the study and analysed the data. B. v.d. B., J. L. and M. v.d. F. wrote the manuscript. B. v.d. B., C. A. C. M. v. Els, B. K., K. v. A., S. H., R. d. G., M. I. d. J., J. L. and M. v.d. F. critically reviewed the article. K. v. A., S. H. and M. v.d. F. treated the patients and collected the samples.
Acknowledgements
We thank Professor Dr Ray Borrow from PHE for providing strains and Dr Diana Wouters for critical reading of the manuscript.
Contributor Information
J. D. Langereis, Email: jeroen.langereis@radboudumc.nl.
M. van der Flier, Email: michiel.vanderflier@radboudumc.nl.
References
- 1. Hovingh ES, van den Broek B, Jongerius I. Hijacking complement regulatory proteins for bacterial immune evasion. Front Microbiol 2016; 7:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merle NS, Church SE, Fremeaux‐Bacchi V, Roumenina LT. Complement system part I – molecular mechanisms of activation and regulation. Front Immunol 2015; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol Immunol 2011; 48:1643–55. [DOI] [PubMed] [Google Scholar]
- 4. Rosain J, Hong E, Fieschi C, Martins PV, El Sissy C, Deghmane AE. Strains responsible for invasive meningococcal disease in patients with terminal complement pathway deficiencies. J Infect Dis 2017; 215:1331–8. [DOI] [PubMed] [Google Scholar]
- 5. Parikh SR, Andrews NJ, Beebeejaun K et al Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet 2016; 388:2775–82. [DOI] [PubMed] [Google Scholar]
- 6. Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR, Centers for Disease Control . Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:608–12. [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Ani F, Chin‐Yee I, Lazo‐Langner A. Eculizumab in the management of paroxysmal nocturnal hemoglobinuria: patient selection and special considerations. Ther Clin Risk Manag 2016; 12:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cody EM, Dixon BP. Hemolytic uremic syndrome. Pediatr Clin North Am 2019; 66:235–46. [DOI] [PubMed] [Google Scholar]
- 9. Granoff DM. Relative importance of complement‐mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine 2009; 27(Suppl 2):B117–B125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Kayhty H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 2003; 21:4437–47. [DOI] [PubMed] [Google Scholar]
- 11. Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood 2017; 130:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seelen MA, Roos A, Wieslander J et al Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods 2005; 296:187–98. [DOI] [PubMed] [Google Scholar]
- 13. Westra D, Kurvers RA, van den Heuvel LP et al Compound heterozygous mutations in the C6 gene of a child with recurrent infections. Mol Immunol 2014; 58:201–5. [DOI] [PubMed] [Google Scholar]
- 14. Sprong T, Roos D, Weemaes C et al Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood 2006; 107:4865–70. [DOI] [PubMed] [Google Scholar]
- 15. Giuliani MM, Biolchi A, Serruto D et al Measuring antigen‐specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine 2010; 28:5023–30. [DOI] [PubMed] [Google Scholar]
- 16. Santolaya ME, O'Ryan ML, Valenzuela MT et al Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer‐blind, placebo‐controlled study. Lancet 2012; 379:617–24. [DOI] [PubMed] [Google Scholar]
- 17. Kuipers B, Dobbelsteen Gvd, Wedege E, Alphen Lv, Pollard AJ, Maiden MCJ. Serological characterization Meningococcal Disease: Methods and Protocols: Humana Press; 2001. [Google Scholar]
- 18. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med 1969; 129:1327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection – serum bactericidal antibody activity. Vaccine 2005; 23:2222–7. [DOI] [PubMed] [Google Scholar]
- 20. van der Maten E, de Jonge MI, de Groot R, van der Flier M, Langereis JD. A versatile assay to determine bacterial and host factors contributing to opsonophagocytotic killing in hirudin‐anticoagulated whole blood. Sci Rep 2017; 7:42137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinon‐Torres F, Bernatowska E, Shcherbina A et al Meningococcal B vaccine immunogenicity in children with defects in complement and splenic function. Pediatrics 2018; 142. [DOI] [PubMed] [Google Scholar]
- 22. Giuntini S, Reason DC, Granoff DM. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor h binding protein. Infect Immun 2012; 80:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


