Abstract
Background
Aciclovir is effective in herpesvirus infections of the CNS. Aciclovir-induced neuropsychiatric symptoms (AINS) have been reported and are associated with high CSF concentrations of aciclovir metabolite 9-carboxymethoxymethylguanine (CMMG). Risk factors except for renal failure have not been explored, and disruption of the blood–brain barrier (BBB) in acute CNS infection may be of interest.
Objectives
To investigate the impact of risk factors on aciclovir and CMMG concentrations, and to relate the results to AINS.
Methods
We investigated 21 consecutively included, consenting patients treated with aciclovir or valaciclovir for herpesvirus CNS infection. Regression models were constructed to study the impact of risk factors including BBB disruption, as measured with CSF:serum albumin ratio, on CSF aciclovir and CMMG concentrations. Medical records were assessed retrospectively to identify patients with AINS.
Results
Increased CSF:serum albumin ratio, as well as decreased renal function and high aciclovir doses, was associated with increased aciclovir and CMMG concentrations in the CSF. We identified five patients with new neuropsychiatric symptoms; four of those were considered to have AINS and had increased CSF CMMG concentrations. Only one patient without suspicion of AINS had an increased CSF CMMG concentration.
Conclusions
In patients with herpesvirus CNS infections, BBB disruption is associated with increasing aciclovir and CMMG CSF concentrations. We also found an unexpectedly high number of patients with AINS. Evaluation of CSF:serum albumin ratios, renal function and CSF concentrations of aciclovir and CMMG may all contribute to the optimization of aciclovir dosing and avoidance of AINS.
Introduction
Aciclovir is the first-line treatment for patients with infections of the CNS caused by herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and varicella-zoster virus (VZV).1 The efficacy of treatment has only been studied in herpes simplex encephalitis (HSE). Intravenous (iv) aciclovir at 10 mg/kg q8h has in randomized trials been shown to decrease mortality in HSE markedly,2,3 but still the disease is associated with considerable morbidity.4
With the substantial morbidity taken into account, higher dosages than those used in studies on efficacy are sometimes considered in young patients with HSE without renal impairment, although studied retrospectively.5 Furthermore, several national guidelines and international expertise recommend treatment of VZV encephalitis with iv aciclovir at 10–15 mg/kg q8h,1,6 since VZV is less susceptible to aciclovir.7 However, the relationship between aciclovir concentrations, viral in vitro susceptibility and clinical efficacy is uncertain at best. Research on dosing and duration of aciclovir treatment is scarce, and increasing dosages may have negative consequences.
Aciclovir has the well-known and prevalent adverse effect of causing reversible nephrotoxicity, which can be prevented by adequate hydration and reducing the dosage.8 Less noticed is neurotoxicity related to aciclovir, aciclovir-induced neuropsychiatric symptoms (AINS), first reported in the 1980s.9 The AINS incidence in Sweden has been estimated to be at least one AINS case per million inhabitants, when retrospectively studied.10 A multitude of symptoms have been described, including confusion, hallucinations, ataxia, involuntary movements, somnolence and coma, mainly in patients with acute or chronic renal failure.11 However, symptoms occurring in HSE may be difficult to distinguish from AINS, and to date no systematic approach or scoring system to separate them has been published.
The association between AINS and aciclovir concentrations is not fully understood.12 Approximately 90% of aciclovir is excreted unchanged in the urine in patients with normal kidney function. The remaining part is metabolized by alcohol dehydrogenase and aldehyde dehydrogenase to the inactive main metabolite 9-carboxymethoxymethylguanine (CMMG). As renal function declines, metabolism to CMMG makes an increasing contribution to total clearance.13 More recent studies have shown an association between AINS and increased serum and CSF concentrations of CMMG in a more consistent manner compared with aciclovir concentrations,11,13 and as a result CMMG has been proposed as a more reliable marker of AINS than aciclovir.
Besides decreased renal function, there may be other factors influencing CSF concentrations of aciclovir and CMMG. A few studies on CSF concentrations of aciclovir have been published, showing relatively stable aciclovir concentrations in CSF compared with serum, and lower AUC in CSF compared with serum,14–16 suggesting slow diffusion into and active transport out of CSF, substantiating the importance of the integrity of the blood–brain barrier (BBB) for aciclovir penetration to the CSF/CNS compartment. A damaged BBB, such as might occur in acute CNS infection, allows increased passage of molecules, usually accompanied by a decrease in functions of efflux mechanisms.17 Increased drug penetration due to BBB damage in CNS infections has been observed in studies of antiretrovirals18 and antibiotics.19,20 However, studies during aciclovir treatment are lacking.
Our primary aim was to investigate the impact of different risk factors, including disruption of the BBB, on the concentrations of aciclovir and CMMG in patients treated for CNS infections. Our secondary aim was to relate our findings, including CMMG concentrations, to neuropsychiatric symptoms.
Patients and methods
Patients
Patients (≥18 years of age) with suspected or confirmed herpes virus infection of the CNS, treated with iv aciclovir or oral valaciclovir, were consecutively enrolled in the study between 9 September 2013 and 20 January 2016 at the Department of Infectious Diseases at Sahlgrenska University Hospital, Gothenburg, Sweden. The Medical Ethics Committee at Gothenburg University approved the study (Dnr 407-13), and informed consent was obtained.
Sampling and analysis of serum and CSF
Pairs of serum and CSF were obtained on one or multiple occasions from each patient. The samples were obtained on days 1–2, 3–5, 6–9, 10–14, 15–21 and 22–30 after initiation of aciclovir treatment. Lag time between dose administration and sampling was not determined in advance, to enable analysis of the pharmacokinetic profile at group level in a scenario that mimics the clinical setting. CSF and blood samples were coded and stored at −70°C until further analysis.
Estimation of renal function was determined from analysis of serum creatinine and serum cystatin C. CLCR was calculated using the Cockcroft–Gault formula,21 and glomerular filtration rate (GFR) based on cystatin C was calculated using the CAPA (Caucasian, Asian, paediatric and adult) formula.22 Development of acute kidney injury was further classified according to RIFLE (risk, injury, failure, loss of function, end-stage renal disease) criteria.23
Albumin levels in CSF and serum were measured by immunonephelometry on a Beckman image immunochemistry system (Beckman Instruments, Beckman Coulter, Brea, CA, USA). The albumin ratio was calculated [CSF albumin (mg/L)/serum albumin (g/L)] and used as an indicator of the integrity of the BBB,24 with normal upper limits of 6.8 × 10−3 for patients ≤45 years of age and 10.2 × 10−3 for patients >45 years of age,25 similar to the categorization suggested by Reiber and Felgenhauer.26 For ease of interpretation, albumin CSF:serum ratio is presented without adding the magnitude indicator of ×10−3 in the following sections.
The aciclovir and CMMG concentrations were determined at the Department of Clinical Pharmacology, Karolinska University Hospital, Huddinge, Stockholm, Sweden, using solid-phase extraction followed by HPLC with fluorescence detection. The limit of detection and the limit of quantification were 0.15 μmol/L. CMMG concentrations lower than the limit of detection of 0.15 μmol/L were assigned the value of 0.08 μmol/L for visualization purposes.
Assessments of the risk of developing neuropsychiatric symptoms
We retrospectively studied the medical records of all included patients for neuropsychiatric symptoms previously associated with AINS, specifically generalized neuropsychiatric symptoms such as confusion, hallucinations, ataxia, involuntary movements, somnolence and coma.11 The study protocol did not include specific instructions on documentation, but documentation had been made on a daily basis according to standard procedure. Fever, headache and focal CNS symptoms, more typical of viral CNS infection, were considered unlikely to be associated with treatment.27 To further distinguish AINS from symptoms of viral CNS infection, symptoms needed to have developed during aciclovir treatment, and resolved at dose reduction or end of treatment. In addition, concomitant medications or pathologies needed to be ruled out as responsible for such symptoms. The risk assessment also included data on aciclovir doses and changes in serum creatinine, as decreased renal function has been previously associated with AINS. The assessments were performed blinded without knowledge of the aciclovir or CMMG concentrations by three of the authors: an experienced neurologist (J. Lycke) and two clinicians at the Department of Infectious Diseases (M. S., J. Lindström). The patient was considered to have suspected AINS if two or three of the raters were in agreement.
Statistical analysis and subgrouping
Multivariable regression models were constructed to study the impact of relevant independent variables on the studied dependent variables. The SAS procedure mixed was used, considering that some of the patients were sampled at more than one timepoint, using the autoregressive covariance pattern that was found optimal based on the lowest Akaike information criterion. The residual plots were reviewed for assumption of normal distribution. Standard errors and 95% CIs were estimated based on robust sandwich estimators in order to adjust for residuals’ potential deviation from a normal distribution. The mixed models were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Group comparisons were performed with the Mann–Whitney U-test, using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). All tests were two-tailed and conducted at the 0.05 significance level.
Results
During the 29 month period of 2013–16, 21 patients were enrolled in the study. Overall, 34 CSF samples and 32 serum samples were obtained from the 21 patients. Patient data and characteristics are presented in Table 1. As treatment duration and timing of inclusion differed, the number of samplings for each patient varied from one to three. Owing to the study being performed at a referral clinic, all but one patient had their primary diagnostic CSF sampling made before inclusion in the study.
Table 1.
Characteristics of included patients, their treatment and CSF concentrations of aciclovir (ACV) and CMMG at all sampling timepoints
| Patient | Age (years) | Gender | Weight (kg) | Indication for treatment | ACV dose q8h (mg/kg) | Lag time from dose to sampling (h) | Treatment duration (days) | Estimated CLCR (mL/min) | RIFLE | Serum concentration (μmol/L) |
CSF concentration (μmol/L) |
CSF:serum albumin ratio×10-3 | Neuropsychiatric symptoms during treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACV | CMMG | ACV | CMMG | ||||||||||||
| 1a | 70 | M | 56 | herpes encephalitis | 10 | 7.5 | 13 | 80 | R | 5.7 | 0.74 | 8.4 | 0.15 | N/A | |
| 1b | 10 | 9 | 20 | 71 | 2.7 | 0.29 | 7.1 | <0.15 | 15.4 | ||||||
| 1c | 10 | 8.5 | 27 | 57 | 5 | 0.59 | 8.5 | <0.15 | 11.7 | ||||||
| 2 | 79 | F | 52 | herpes encephalitis | 11 | 6.5 | 9 | 61 | R | 70 | 12 | 11 | 0.27 | 7 | |
| 3 | 66 | M | 89 | suspected varicella meningitis | 1000d | 1 | 14 | 84 | F | 29 | 1.7 | 3.1 | <0.15 | 6.9 | |
| 4a | 35 | M | 98 | herpes encephalitis | 15 | 6 | 2 | 30 | F | 110 | 57 | 40 | 3.3 | 29.4 | confusion, improved at dose adjustment |
| 4b | 12 | 7.5 | 9 | 125 | 25 | 2.8 | 7.5 | 0.15 | 15.7 | ||||||
| 4c | 12 | 8 | 16 | 149 | 10 | 1.5 | 10 | 0.19 | 23.8 | ||||||
| 5 | 30 | M | 100 | suspected encephalitis | 10 | 6 | 3 | 109 | 9.6 | 1.3 | 6.7 | <0.15 | 24.3 | ||
| 6a | 64 | F | 60 | herpes encephalitis | 12 | 3.5 | 3 | 40 | R | 50 | 9.7 | 24 | 0.68 | 20.1 | confusion, improved at end of treatment |
| 6b | 12 | 4 | 13 | 65 | 18.6 | 2.7 | 14 | 0.3 | 15.4 | ||||||
| 6c | 12 | 8 | 21 | 70 | 11 | 2.3 | 12 | 0.23 | 11.9 | ||||||
| 7a | 65 | M | 76 | recurrent herpes encephalitis | 15 | 2.5 | 8 | 109 | I | 55 | 18 | 16 | 0.29 | 14.7 | confusion, improved at end of treatment |
| 7b | 15 | 4.5 | 15 | 51 | 120 | 38 | 29 | 1 | 10.5 | ||||||
| 7c | 15 | 21 | 29 | 35 | 11 | 11 | 8.2 | 0.66 | 14.6 | ||||||
| 8 | 55 | M | 77 | herpes encephalitis | 10 | 5.5 | 13 | 122 | 11 | 1 | 6.7 | <0.15 | 7.4 | ||
| 9a | 58 | M | 68 | stroke | 11 | 1 | 5 | 56 | 75 | 10 | 12 | 0.27 | 9.3 | ||
| 9b | 10 | 6.5 | 11 | 52 | 20 | 5 | 11 | 0.26 | 11.7 | ||||||
| 10 | 40 | M | 120 | herpes meningoencephalitis | 10 | 1 | 12 | 184 | 13 | 0.9 | 8.5 | <0.15 | 9.6 | ||
| 11 | 41 | F | 67 | herpes meningitis | 10 | 4.5 | 7 | 117 | 14 | 1.5 | 17 | 0.77 | 26.7 | ||
| 12 | 67 | M | 78 | suspected encephalitis | 9 | 1.5 | 5 | 87 | 31 | 4.9 | 8.9 | 0.17 | 13.8 | ||
| 13 | 26 | F | 50 | suspected encephalitis | 15 | 8 | 3 | 119 | 6.1 | 1.2 | 5.9 | <0.15 | 8.6 | ||
| 14 | 58 | M | 87 | herpes meningitis | 1000d | 1 | 10e | 115 | 13 | 2 | 4.8 | <0.15 | 8.6 | ||
| 15a | 65 | F | 67 | herpes encephalitis | 9 | 6 | 6 | 73 | 10 | 2.3 | 7.7 | 0.16 | 8.5 | ||
| 15b | 9 | 3 | 11 | 79 | 40 | 5.4 | 7.6 | <0.15 | 8.8 | ||||||
| 16 | 39 | F | 77 | herpes encephalitis | 14 | 1 | 13 | 131 | N/A | N/A | 8 | <0.15 | 22.5 | ||
| 17 | 49 | F | 72 | herpes meningitis | 9 | 1.5 | 3 | 129 | 17 | 1.6 | 7.5 | <0.15 | 4.1 | ||
| 18 | 53 | M | 85 | varicella meningitis | 14 | 5.5 | 2 | 123 | N/A | N/A | 14 | 0.24 | 13.3 | ||
| 19a | 75 | F | 57 | varicella encephalitis | 10 | 7 | 7 | 54 | Rf | 15 | 5.2 | 28 | 6.4 | 162.4 | confusion, improved at end of treatment |
| 19b | 10 | 7 | 14 | 68 | 13 | 3.6 | 27 | 3.8 | 108.1 | ||||||
| 20a | 55 | F | 68 | Ramsay Hunt syndrome | 14 | 2.5 | 2 | 131 | F | 31 | 3.8 | 6.5 | <0.15 | 1.7 | anxiety, improved slowly at end of treatment |
| 20b | 5 | 3 | 9 | 60 | 12 | 3 | 3.3 | <0.15 | 1.4 | ||||||
| 21a | 38 | M | 81 | varicella encephalitis | 14 | 4.5 | 1 | 141 | 9.9 | 1.1 | 12 | 0.2 | 28.8 | ||
| 21b | 14 | 2.5 | 3 | 135 | 19 | 1.9 | 14 | 0.24 | 18 | ||||||
ACV dose, mg iv aciclovir per kg body weight in a single dose, administered three times daily, except in patient 20b, where doses were administered twice daily; N/A, not available; M, male; F, female; (RIFLE column) R, risk; I, injury; F, failure; shaded areas indicate patients assessed to have AINS.
First sampling in patient where repeated sampling was performed.
Second sampling.
Third sampling.
Treatment with oral valaciclovir in single dose (mg), three times daily.
Patient had previously received valaciclovir in a lower dose regimen as prophylaxis.
Patient did not fulfil RIFLE criteria based on creatinine, but calculation of GFR according to cystatin C CAPA estimated GFR at 30 mL/min, corresponding to at least risk of acute kidney failure.
Risk factors and CSF concentrations of aciclovir and CMMG
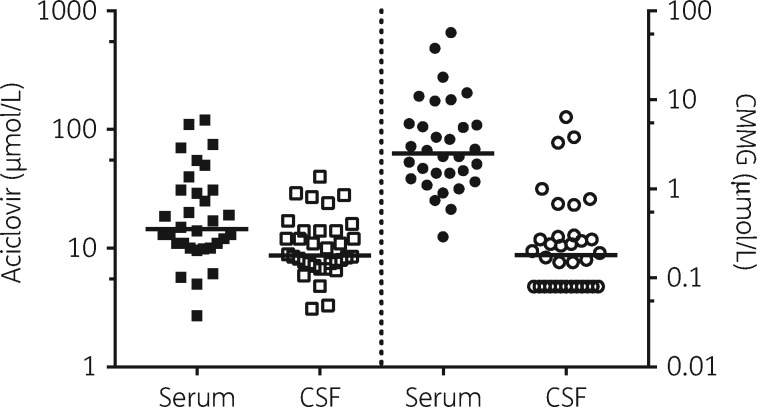
Median aciclovir concentration in serum was 14.5 μmol/L (range 2.7–120) and in CSF 8.7 μmol/L (3.1–40). Median CMMG concentration measured in serum was 2.5 μmol/L (range 0.29–57) and in CSF 0.18 μmol/L (<0.15–6.4) (Figure 1).
Figure 1.
Distribution of aciclovir (left of divider) and CMMG (right of divider) in serum (n=32) and CSF (n=34) samples from patients (n=21) treated with aciclovir for herpesvirus CNS infection. Limit of detection is 0.15; values <0.15 are set to 0.08 for visualization purposes. Bar is set at median.
In our multiple linear regression model, significant associations were made between several independent risk factors and concentrations of aciclovir and CMMG in the CSF (Table 2).
Table 2.
Multiple linear regression models for aciclovir and CMMG CSF concentrations in 21 patients treated with aciclovir for CNS infection (34 samples)
| Independent variable | Comparison | Aciclovir CSF concentration |
CMMG CSF concentration |
||
|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | ||
| Albumin ratio (×10−3) | per 10 unit increase | 1.499 (1.290–1.709) | <0.0001 | 0.388 (0.356–0.421) | <0.0001 |
| Lag time dose to sampling (h) | per 1 unit increase | −0.779 (−1.350 to −0.208) | 0.015 | −0.046 (−0.072 to −0.020) | 0.0038 |
| Creatinine clearance (mL/min) | per 10 unit increase | −1.428 (−2.099 to −0.756) | 0.0015 | −0.161 (−0.245 to −0.077) | 0.0027 |
| Dose (mg/kg) | per 1 unit increase | 1.703 (1.215–2.190) | <0.0001 | 0.093 (0.022–0.163) | 0.017 |
| Weight (kg) | per 10 unit increase | 1.996 (1.000–2.991) | 0.0005 | 0.223 (0.052–0.394) | 0.013 |
| Age (years) | per 10 unit increase | 0.042 (−1.402 to 1.487) | 0.95 | −0.026 (−0.198 to 0.147) | 0.74 |
| Gender | women versus men | 0.774 (−2.276 to 3.824) | 0.60 | 0.292 (−0.179 to 0.762) | 0.21 |
BBB integrity
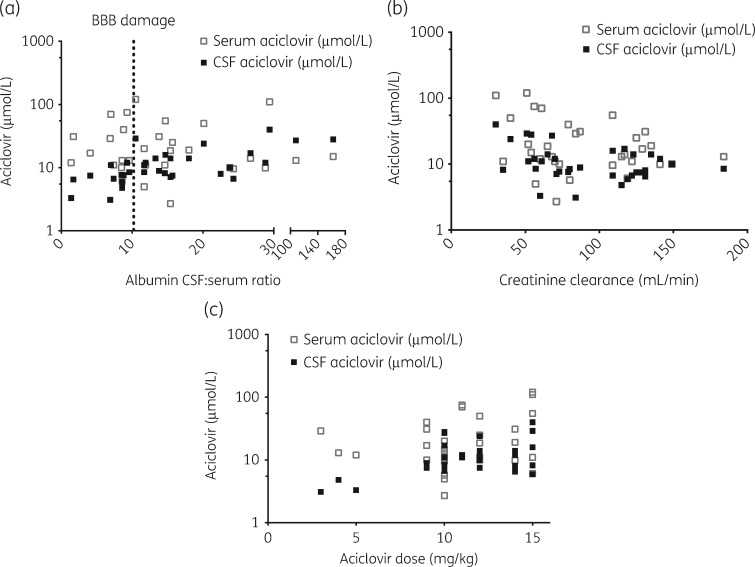
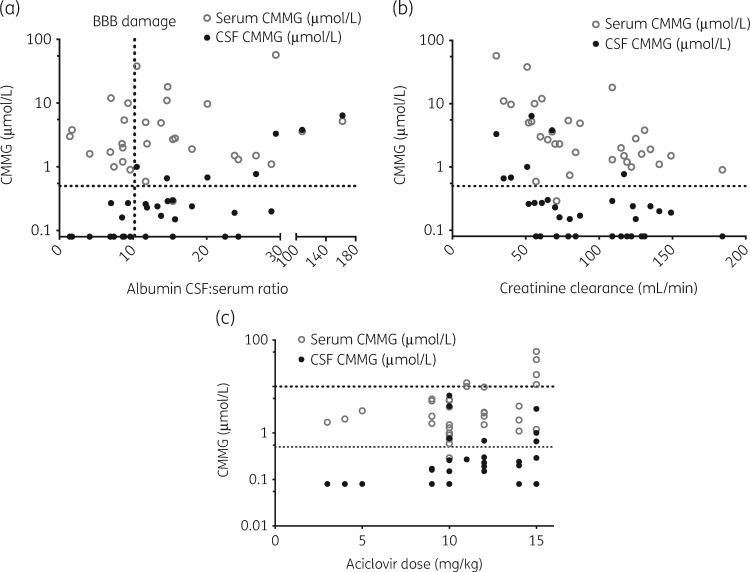
An increase of 10 in CSF:serum albumin ratio was associated with a mean increase in CSF aciclovir concentration of 1.50 μmol/L (95% CI 1.29–1.71, P<0.001) (Figure 2a) and a mean increase in CMMG CSF concentration of 0.39 μmol/L (95% CI 0.36–0.42, P<0.001) (Figure 3a).
Figure 2.
Aciclovir concentrations in serum (n=32) and CSF (n=34) samples from patients (n=21) treated with aciclovir for herpesvirus CNS infection. (a) Increased CSF:serum albumin ratio, interpreted as an indication of BBB damage, is associated with increased aciclovir CSF concentrations. The dotted vertical line represents the upper normal reference for CSF:serum albumin ratio (10.2). (b) Decreased creatinine clearance, calculated using the Cockcroft–Gault formula, is associated with increased aciclovir serum and CSF concentrations. (c) Increasing dose of aciclovir is associated with increased aciclovir serum and CSF concentrations.
Figure 3.
CMMG concentrations in serum (n=32) and CSF (n=34) samples from patients (n=21) treated with aciclovir for herpesvirus CNS infection. (a) Increased CSF:serum albumin ratio, interpreted as an indication of BBB damage, is associated with increased CMMG CSF concentrations. The dotted vertical line represents the upper normal reference for CSF:serum albumin ratio (10.2). The dotted horizontal line represents a CMMG CSF concentration of 0.5 μmol/L, previously associated with AINS. (b) Decreased creatinine clearance, calculated using the Cockcroft–Gault formula, is associated with increased CMMG serum and CSF concentrations. The dotted horizontal line represents a CMMG CSF concentration of 0.5 μmol/L, previously associated with AINS. (c) Increased dosing of aciclovir is associated with increased CMMG serum and CSF concentrations. The upper dotted horizontal line represents a CMMG serum concentration of 10 μmol/L, previously associated with AINS, and the lower dotted horizontal line represents a CMMG CSF concentration of 0.5 μmol/L, previously associated with AINS.
Renal insufficiency
A decrease in CLCR of 10 mL/min was associated with a mean increase in CSF aciclovir concentration of 1.43 μmol/L (95% CI 0.76–2.10, P=0.015) (Figure 2b) and a mean increase in CMMG CSF concentration of 0.16 μmol/L (95% CI 0.08–0.25, P=0.0027) (Figure 3b).
Aciclovir dosing
An increase in aciclovir dose of 1 mg/kg was associated with a mean increase in CSF aciclovir concentration of 1.70 μmol/L (95% CI 1.22–2.19, P<0.001) (Figure 2c) and a mean increase in CMMG CSF concentration of 0.09 μmol/L (95% CI 0.02–0.16, P=0.017) (Figure 3c).
Lag time
CSF aciclovir concentration decreased by a mean of 0.78 μmol/L (95% CI 0.21–1.35, P=0.015) for every hour that passed between dose administration and sampling, and CSF CMMG concentrations decreased by 0.05 μmol/L (95% CI 0.02–0.07, P=0.0038).
Weight
A 10 kg increase in weight was associated with a mean increase in CSF aciclovir concentration of 2.00 μmol/L (95% CI 1.00–2.19, P=0.0005) and a mean increase in CSF CMMG concentration of 0.22 μmol/L (95% CI 0.05–0.39, P=0.013).
Age and gender
Neither age nor gender was significantly associated with changes in CSF aciclovir or CMMG concentrations.
Neuropsychiatric symptoms
Five out of 21 patients were identified with neuropsychiatric symptoms that developed after initiation of aciclovir treatment and subsided after a dose decrease or after the end of treatment (Table 1; see also the short descriptive case series available as Supplementary data at JAC Online). Four of the patients (Patients 4, 6, 7 and 19) deteriorated during treatment, with increased confusion, while one patient (Patient 20) presented with decreased muscle tonus and anxiety, not previously associated with AINS. None of the included patients was suspected to have AINS by the treating clinicians. Looking at expected risk factors, five out of five identified patients had decreased renal function, four out of five were treated with an aciclovir dose >10 mg/kg q8h and four out of five had indication of BBB damage with elevated CSF:serum albumin ratio (Table 1)
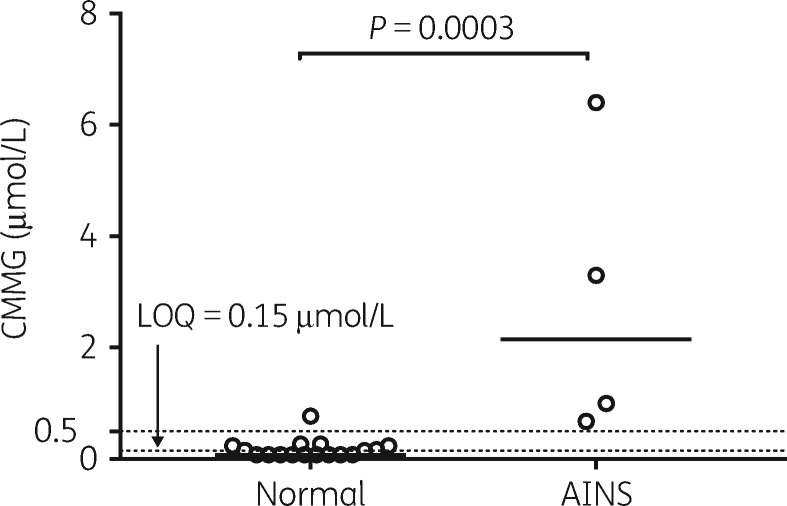
Excluding Patient 20, who did not present a picture similar to earlier published cases, patients assessed with neuropsychiatric symptoms had higher peak CMMG concentrations in any CSF sample (n=4; median 2.15 μmol/L; range 0.68–6.4) compared with patients without typical neuropsychiatric symptoms (n=17; <0.15 μmol/L; <0.15–0.77) (P=0.0003) (Figure 4). Only one patient (Patient 11) had a CMMG concentration in CSF >0.5 μmol/L without recorded symptoms indicating AINS, and although this patient had BBB damage, he had a standard aciclovir dose of 10 mg/kg q8h and normal renal function. Peak serum CMMG concentration was also significantly higher in patients with AINS (n=4; median 23.85 μmol/L; range 5.2–57.0) compared with patients without typical neuropsychiatric symptoms (n=15; 1.7 μmol/L; 0.74–12.0) (P=0.0093).
Figure 4.
Comparison of peak CMMG concentrations in CSF samples from patients (n=21) treated with aciclovir for herpesvirus CNS infection. Comparison is made between patients with (n=4) and without (n=17) suspected AINS, previously associated with CMMG >0.5 μmol/L (upper dotted horizontal line). Bar is set at the median. The lower dotted horizontal line represents the CMMG analysis limit of quantification (LOQ) of 0.15 μmol/L.
Discussion
In this study, we show that damage to the BBB has a significant impact on aciclovir and CMMG pharmacokinetics in a clinical setting. We also reinforce the association between increased concentrations of CMMG in the CSF and neuropsychiatric symptoms, which were surprisingly prevalent.
Looking at the results from our regression models, our findings demonstrate that damage to the BBB in the acute phase of CNS infection is associated with increased penetration of aciclovir and CMMG into the CNS/CSF compartment. The association was proportionally more pronounced when looking at CMMG compared with aciclovir, suggesting a larger impact of BBB damage on CMMG CNS pharmacokinetics, possibly owing to it being more difficult for CMMG to cross the BBB compared with aciclovir, consistent with pharmacokinetic data reported by Smith et al.16 The same study also describes that CMMG concentrations in the CSF do not vary considerably over time when in steady-state. This is not fully supported in our material since there is a change in CMMG concentration in relation to lag time from dose administration to sampling. However, the change is very small whereas the change in the aciclovir counterpart is more dynamic. Both CMMG and aciclovir concentrations were in some cases higher in CSF compared with serum, suggesting accumulation, and slow clearance out of the CNS for both molecules. In addition to BBB damage, and according to expectations, changes in CLCR had an impact on aciclovir and CMMG concentrations, as did increasing aciclovir doses. Notably, increased age was not associated with increased concentrations, possibly as an effect of decreased renal function already being considered in our models. Increased weight, on the other hand, was associated with increased concentrations, suggesting that dosing based on weight may be suboptimal. Patients that weigh more will receive larger doses of aciclovir, while the increase in weight is not necessarily reflected in an increase in renal elimination, resulting in a higher risk of aciclovir and CMMG accumulation. We found concentrations of CMMG in CSF >0.5 μmol/L, previously associated with toxicity,13 in 5/21 patients.
In our retrospective assessment, we were able to identify a surprisingly high number of patients with symptoms consistent with AINS, without taking aciclovir and CMMG concentrations into consideration. Although we identified five patients with neuropsychiatric symptoms, Patient 20 had anxiety as the main finding, a symptom that has not previously been described as typical of AINS. In addition, she did not have increased CMMG in the CSF samples, and we conclude that it is improbable that her anxiety was related to aciclovir treatment. Only in one patient with a CMMG level >0.5 μmol/L (Patient 11) were no neuropsychiatric symptoms found. However, it is possible that the patient may have had symptoms that were not noted in the medical records. Prospective, repeated assessments of neurological and psychiatric symptoms according to a protocol may have decreased the risk of missing such symptoms, but with the risk of impacting therapeutic decisions in this otherwise observational study.
An alternative explanation to impaired neurocognitive recovery could be autoimmune encephalitis. Recovery after cessation of aciclovir treatment, in combination with absence of anti-N-methyl-d-aspartate-receptor IgG antibodies in CSF and serum from the last sampling during admittance (data not shown), makes this highly unlikely. Autoimmune encephalitis is described as a later complication, with autoantibodies detected at 3 months after onset of HSE.28
Considering the invasiveness of CSF sampling, analysis of serum CMMG concentrations could be considered an attractive alternative if proven reliable. Although the patients with AINS had higher median peak serum concentrations of CMMG, there was an overlap between groups, and two out of four patients with suspected AINS had serum CMMG concentrations <10.8 μmol/L, previously associated with AINS.11 However, if CSF sampling is contraindicated, analysis of serum CMMG and aciclovir concentrations may still provide an indication of potentially harmful dosages if concentrations are high.
AINS has in most cases previously been associated with chronic renal failure, which none of our patients with elevated CMMG had in their medical history. However, Patients 4, 6 and 7 could be classified with acute kidney injury according to RIFLE criteria, and Patient 19 had decreased GFR according to calculation based on cystatin C. Only Patient 11 had increased CSF CMMG without any indication of kidney injury, and considering he was not suspected to have AINS, it is possible that renal function may be related to AINS in some respect that is not yet fully understood. While high concentrations of aciclovir in the CSF have been associated with AINS, there are conflicting reports,12 and although CMMG has proven to be more reliable,13 it is unknown how it relates to the pathogenesis.
It is notable that none of the included patients was suspected to have AINS by the treating clinicians, highlighting the diagnostic difficulties and the need for additional diagnostic tools. Even though our assessment was retrospective, the patients were consecutively included, with a lower risk of selection bias when analysing CMMG compared with the previous studies.11,13 Considering the consistency in our study between neuropsychiatric symptoms and increased CMMG concentrations, we can assert that analysis of CMMG in the CSF may be a useful tool when considering aciclovir-induced neuropsychiatric toxicity, especially since a systematic approach is otherwise lacking.
Although pharmacokinetic changes associated with BBB damage may be detrimental with regard to AINS, there may be positive effects associated with increased CNS penetration of aciclovir. Since BBB damage is most pronounced in the early stages of herpesvirus CNS infections, when viral replication is thought to play an important role in the pathogenesis,29 increased concentrations of aciclovir in the CSF might potentially be beneficial, similar to the rationale for consideration of increased aciclovir dosages in selected patients. In the retrospective study on patients with HSE by Stahl et al.5 where dosages of aciclovir were compared, a dosage of 15 mg/kg q8h did not increase the efficacy of treatment over a dosage of 10 mg/kg q8h, measured by looking at poor versus favourable outcome after treatment. Considering the increase in aciclovir concentrations in patients with BBB damage, a possible explanation for the lack of efficacy of increased dosages is that the increased dosages may be excessive. Although there is very little previous data on the penetration of aciclovir into the CNS in regard to BBB damage, a correlation between the albumin CSF:serum ratio and aciclovir CSF concentrations has previously been shown by Pouplin et al.30 in six patients treated with valaciclovir for HSE. In this context, it is proposed that BBB damage may have an impact on the feasibility of oral treatment of HSE, but, as discussed by Bodilsen et al.,31 drawing conclusions on efficacy based on aciclovir CSF concentrations is hazardous.
Although AINS seem to be reversible, they are detrimental to the patient and may obscure the symptomatic progress of the treated disease. Since the morbidity after herpesvirus CNS disease is considerable, it is still possible that some patients may tolerate and benefit from increased doses of aciclovir. However, the recommendation of doses >10 mg/kg q8h in the initial stages of disease is debatable and should perhaps be reconsidered. Instead, it would be safer to consider an increase in dose after a few days of treatment, allowing time for adequate hydration and assurance of stable renal function, as well as factoring in the CSF:serum albumin ratio to identify patients who present with less-pronounced BBB damage, or patients in which the BBB heals over the course of treatment.
In summary, patients with compromised BBB, decreased renal function and high aciclovir doses have an increased risk of accumulating CMMG in the CSF, and increased concentrations of CMMG are associated with AINS. To optimize aciclovir dosing, we suggest that it may be advantageous to reconsider treatment with doses >10 mg/kg q8h, analyse CSF:serum albumin ratios in addition to evaluation of renal function, and, when available, measure CSF concentrations of both aciclovir and CMMG. This approach may be of particular interest when trying to discriminate between AINS and aggravated symptoms due to viral CNS disease.
Supplementary Material
Acknowledgements
We thank our colleagues and staff at the Department of Infectious Diseases at Sahlgrenska University Hospital, Gothenburg, for helpful assistance in including the patients in this study.
Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF-agreement (#ALFBGB-74050).
Transparency declarations
None to declare.
References
- 1. Gilden DH, Mahalingam R, Cohrs RJ. et al. Herpesvirus infections of the nervous system. Nat Clin Pract Neurol 2007; 3: 82–94. [DOI] [PubMed] [Google Scholar]
- 2. Skoldenberg B, Forsgren M, Alestig K. et al. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised Multicentre Study in Consecutive Swedish Patients. Lancet 1984; 2: 707–11. [DOI] [PubMed] [Google Scholar]
- 3. Whitley RJ, Alford CA, Hirsch MS. et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 1986; 314: 144–9. [DOI] [PubMed] [Google Scholar]
- 4. Sili U, Kaya A, Mert A. Herpes simplex encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol 2014; 60: 112–8. [DOI] [PubMed] [Google Scholar]
- 5. Stahl JP, Mailles A, De Broucker T.. Herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect 2012; 140: 372–81. [DOI] [PubMed] [Google Scholar]
- 6. Solomon T, Michael BD, Smith PE. et al. Management of suspected viral encephalitis in adults—Association of British Neurologists and British Infection Association National Guidelines. J Infect 2012; 64: 347–73. [DOI] [PubMed] [Google Scholar]
- 7. Collins P. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J Antimicrob Chemother 1983; 12 Suppl B: 19–27. [DOI] [PubMed] [Google Scholar]
- 8. Richelsen RKB, Jensen SB, Nielsen H.. Incidence and predictors of intravenous acyclovir-induced nephrotoxicity. Eur J Clin Microbiol Infect Dis 2018; 37: 1965–71. [DOI] [PubMed] [Google Scholar]
- 9. Vartian CV, Shlaes DM.. Intravenous acyclovir and neurologic effects. Ann Intern Med 1983; 99: 568.. [DOI] [PubMed] [Google Scholar]
- 10. Helldén A, Odar-Cederlöf I, Bergman U. et al. The incidence of aciclovir-induced neuropsychiatric symptoms in Stockholm. In: Abstracts of the 9th Congress of the European Association for Clinical Pharmacology and Therapeutics, Edinburgh, United Kingdom,2009. Abstract O36, p. 30.
- 11. Hellden A, Odar-Cederlof I, Diener P. et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant 2003; 18: 1135–41. [DOI] [PubMed] [Google Scholar]
- 12. de Knegt R, van der Pijl H, van Es LA.. Acyclovir-associated encephalopathy, lack of relationship between acyclovir levels and symptoms. Nephrol Dial Transplant 1995; 10: 1775–7. [PubMed] [Google Scholar]
- 13. Hellden A, Lycke J, Vander T. et al. The aciclovir metabolite CMMG is detectable in the CSF of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatment. J Antimicrob Chemother 2006; 57: 945–9. [DOI] [PubMed] [Google Scholar]
- 14. Lycke J, Andersen O, Svennerholm B. et al. Acyclovir concentrations in serum and cerebrospinal fluid at steady-state. J Antimicrob Chemother 1989; 24: 947–54. [DOI] [PubMed] [Google Scholar]
- 15. Lycke J, Malmestrom C, Stahle L.. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother 2003; 47: 2438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JP, Weller S, Johnson B. et al. Pharmacokinetics of acyclovir and its metabolites in cerebrospinal fluid and systemic circulation after administration of high-dose valacyclovir in subjects with normal and impaired renal function. Antimicrob Agents Chemother 2010; 54: 1146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelhardt B, Sorokin L.. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009; 31: 497–511. [DOI] [PubMed] [Google Scholar]
- 18. Yilmaz A, Gisslen M, Spudich S. et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One 2009; 4: e6877.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Humbert G, Leroy A, Nair SR. et al. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. J Antimicrob Chemother 1984; 13: 487–94. [DOI] [PubMed] [Google Scholar]
- 20. Karlsson M, Hammers S, Nilsson-Ehle I. et al. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother 1996; 40: 1104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cockcroft DW, Gault MH.. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 22. Grubb A, Horio M, Hansson LO. et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014; 60: 974–86. [DOI] [PubMed] [Google Scholar]
- 23. Bellomo R, Ronco C, Kellum JA. et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tibbling G, Link H, Ohman S.. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 1977; 37: 385–90. [DOI] [PubMed] [Google Scholar]
- 25. Blennow K, Fredman P, Wallin A. et al. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18-88 years of age. Eur Neurol 1993; 33: 129–33. [DOI] [PubMed] [Google Scholar]
- 26. Reiber H, Felgenhauer K.. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta 1987; 163: 319–28. [DOI] [PubMed] [Google Scholar]
- 27. Rashiq S, Briewa L, Mooney M. et al. Distinguishing acyclovir neurotoxicity from encephalomyelitis. J Intern Med 1993; 234: 507–11. [DOI] [PubMed] [Google Scholar]
- 28. Westman G, Studahl M, Ahlm C. et al. N-methyl-d-aspartate receptor autoimmunity affects cognitive performance in herpes simplex encephalitis. Clin Microbiol Infect 2016; 22: 934–40. [DOI] [PubMed] [Google Scholar]
- 29. Esiri MM. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci 1982; 54: 209–26. [DOI] [PubMed] [Google Scholar]
- 30. Pouplin T, Pouplin JN, Van Toi P. et al. Valacyclovir for herpes simplex encephalitis. Antimicrob Agents Chemother 2011; 55: 3624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bodilsen J, Nielsen H, Whitley RJ.. Valaciclovir therapy for herpes encephalitis: caution advised. J Antimicrob Chemother 2019; 74: 1467–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.