Abstract
We present a case of a 59-year-old woman with a malignant tumor arising within presacral teratoma associated with Currarino syndrome (CS). A characteristic crescent-shaped sacrum was detected on preoperative image examination and the presacral mass was pathologically diagnosed as a malignant tumor associated with CS. To our knowledge, this is the first case report of presacral teratoma associated with CS coexisting with both adenocarcinoma and a neuroendocrine tumor.
Keywords: Adenocarcinoma, Currarino syndrome, neuroendocrine tumor, teratoma
Introduction
Currarino syndrome (CS) is a hereditary disorder characterized by the classical triad of sacral agenesis, anorectal malformation, and a presacral mass. It was first described by Currarino et al. and considered the result of abnormal endoectodermal adhesions and notochordal defects in early fetal life.[1] The phenotypic expression of CS is variable.[2,3,4] Some patients are asymptomatic and incidentally diagnosed late in life, whereas the illness is fatal in infancy in other cases because of severe complications such as bacterial meningitis and urinary tract infections. Malignancy in CS is rare, with a reported frequency of approximately 1%.[2,5] Concerning malignancy in adults with CS, only six cases have been described to date [Table 1].[6,7,8,9,10,11] This case report is the first documented instance of presacral teratoma associated with CS coexisting with both adenocarcinoma and a neuroendocrine tumor.
Table 1.
Malignancy in adults with Currarino syndrome
| Author, year | Age, sex | CS diagnosis | Malignant tumor | Treatment |
|---|---|---|---|---|
| Ashcraft et al., 1974 | 32, female | Clinical | Malignant presacral teratoma | Not described |
| Yates et al., 1983 | 54, male | Clinical | Malignant presacral teratoma | Surgery |
| Norum et al., 1991 | 59, female | Clinical | Presacral leiomyosarcoma | Surgery, radiotherapy |
| Urioste et al., 2004 | 22, male | HLXB9 mutation | Neuroendocrine tumor from presacral teratoma | Surgery, chemotherapy |
| Pendlimari et al., 2010 | 22, female | Clinical | Neuroendocrine tumor from presacral teratoma | Surgery |
| Ciotti et al., 2011 | 44, female | HLXB9 mutation | Neuroendocrine tumor from presacral teratoma | Surgery, chemotherapy |
| Present case | 59, female | Clinical | Adenocarcinoma and neuroendocrine tumor from presacral teratoma | Surgery, radiotherapy |
Case Report
A 59-year-old Japanese woman with hypertension and diabetes mellitus was admitted to our hospital to undergo pelvic surgery. She had a presacral mass that was incidentally detected on CT before cholecystectomy for cholelithiasis, and adenocarcinoma was detected in the mass via endoscopic ultrasound-guided fine needle aspiration through the rectum in a prior hospital. Complete blood count, liver function, basic metabolic panel, and urinalysis were unremarkable. CEA, CA19-9, and CA125 were within their normal ranges.
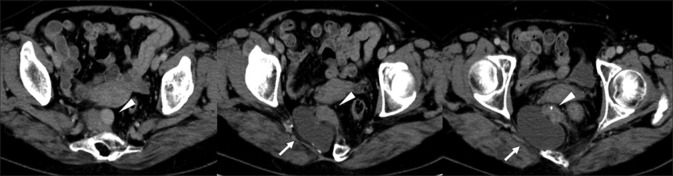
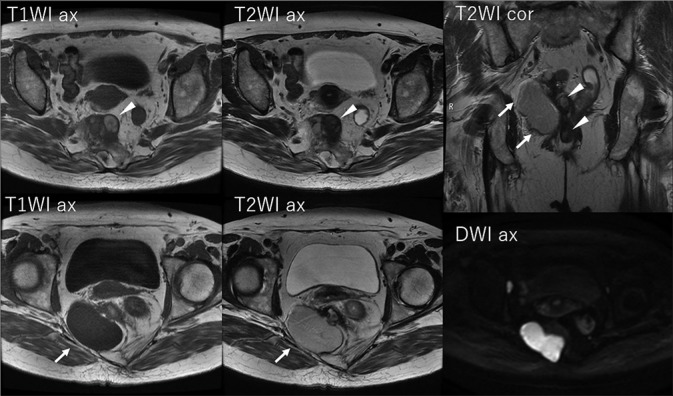
A pelvic radiograph revealed a scimitar sacrum with a right-sided defect [Figure 1]. Contrast-enhanced CT revealed two presacral masses in the pelvis. The upper mass had cystic and solid components containing subtle calcification, and the second mass was a simple cyst through an enlarged neural foramen [Figure 2]. Pelvic MRI illustrated the upper mass with cystic and solid components with various intensities on T1- and T2-weighted images (T2WI). The other presacral cystic lesion displayed high intensity on T2WI, and it had no mural nodule, which was suggestive of an anterior meningocele [Figure 3]. The patient was diagnosed with a malignant tumor associated with CS preoperatively, and she underwent surgical resection of the presacral mass in the pelvis. The tumor was completely resected, although the lower cystic lesion was accidentally ruptured because of adhesion.
Figure 1.
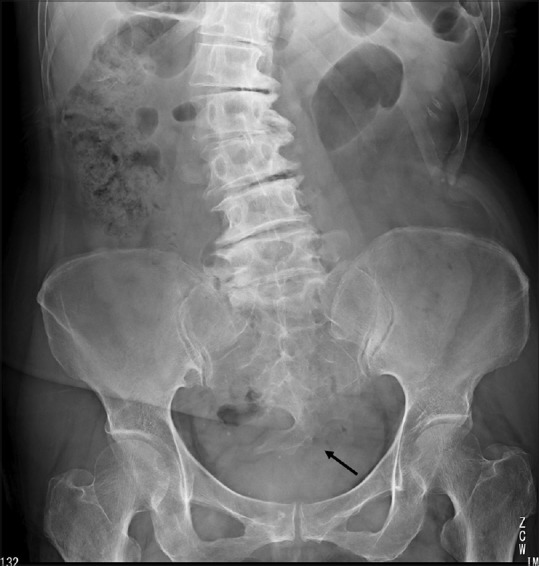
Pelvic X-ray revealed a scimitar sacrum with a right-sided defect
Figure 2.
Contrast-enhanced CT revealed two presacral masses in the pelvis. One mass had cystic and solid components containing subtle calcification (arrowhead). The other mass was a cystic lesion through an enlarged neural foramen (arrow)
Figure 3.
Pelvic MRI revealed cystic and solid components with various intensities on T1- and T2-weighted images (arrowheads). The other presacral cystic lesion exhibited high intensity on T2-weighted images, and it featured no mural nodule (arrows)
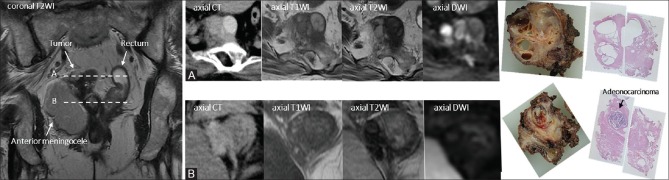
Pathologic examination confirmed that the cystic and solid tumor contained adenocarcinoma and neuroendocrine tumor (NET) components (G2) [Figure 4]. The resected tumor included many cysts covered by stratified columnar epithelium, smooth muscle tissues, and a glomus tumor. Subtle fat tissue was present in the resected tumor, although it was not detected on preoperative MRI. Histologically, the tumor contained several different tissue types, suggesting a teratomatous nature. Most cysts inside the tumor that exhibited varied intensity on MRI included necrotic tissues. Adenocarcinoma was found in the cyst-like structure, and it was not distinguishable from other cysts on preoperative image examinations [Figure 5]. The adenocarcinoma component exhibited ductal and papillary structures, and a diagnosis of moderately differentiated adenocarcinoma was rendered. In the NET component, which was close to the adenocarcinoma lesion, immunohistochemical staining was positive for synaptophysin and chromogranin A. Residual NET tissue was observed along the surgical margins, and postoperative adjuvant radiotherapy (50.4 Gy/28 fr) was administered without adjuvant chemotherapy. After 8 months of follow-up, contrast-enhanced CT and pelvic MRI revealed no tumor recurrence, but genetic testing has not been performed in line with the patient's preference.
Figure 4 (A and B).
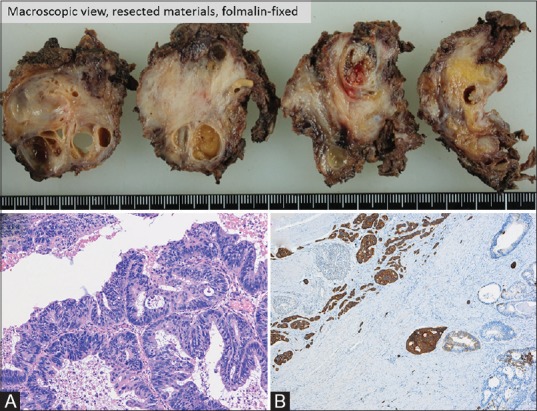
(A) Moderately differentiated adenocarcinoma, H and E stain. (B) Neuroendocrine tumor, synaptophysin stain. The histological presence of a neuroendocrine tumor, smooth muscle tissue, cysts covered by stratified squamous epithelium, and glomus tumor suggested a teratomatous nature (not shown)
Figure 5 (A and B).
(A and B) The presacral tumor near the rectum contained cysts with varied intensity on MRI. One of these cysts included adenocarcinoma
Discussion
CS is a rare autosomal dominant disorder, and mutations in the coding sequence of HLXB9 located at 7q36 have been identified in nearly all cases of familial CS, as well as approximately 30% of patients with sporadic CS.[2] Sacral defects in CS vary from lateral deviation of the coccygeal bone to a hemisacrum or the absence of the lower sacral segments.[2,3,4] Presacral masses contain anterior myelomeningocele, malignant or benign teratoma, dermoid or epidermoid cysts, leiomyoma, leiomyosarcoma, lipoma, or combinations of these lesions. More than 80% of patients with the classic triad are diagnosed within the first decade of life because of the symptoms of anorectal malformation.[12] However, one-third of patients are asymptomatic at the time of diagnosis.[2] The combination of all three defects is not necessary for a diagnosis of CS because the clinical presentation of the syndrome is variable. Although genetic tests have not been performed, the present case featured a hemisacrum and a presacral mass with anterior meningocele, and no anorectal malformation was present, suggesting the mild type of CS in the classification by Martucciello et al.[4] Therefore, the present case was diagnosed incidentally at older age than typical cases.
Malignancy in CS is rare. Only a small number of patients of CS with malignancy have been reported. The risk of malignant transformation of presacral teratomas was estimated in previous reports at around 1% of CS cases.[2,5] On top of that, as for malignancy in adults with CS, only six cases have been described until now [Table 1].[6,7,8,9,10,11] The median age of diagnosis for CS with malignancy is 38 years (range, 22–59). Among the previously reported cases, HLXB9 mutations were detected in two cases, and the remaining cases were diagnosed as CS clinically. NETs from presacral teratoma were reported in three cases, and malignant tumors were associated with presacral teratoma in all but one case of leiomyosarcoma. Surgical resection of a presacral mass carries risks of severe complications related to adhesion of the anterior meningocele such as bacterial meningitis and cerebrospinal fluid leakage.[13] In our case, although the anterior meningocele was accidentally ruptured during surgery, no severe postoperative complication occurred.
In general, somatic malignant transformation of mature cystic teratoma is uncommon, and the risk of transformation is estimated at 1%–3%.[14,15] Although any tissue component of a teratoma can potentially undergo malignant transformation, squamous cell carcinoma is the most commonly associated malignancy. Other reported malignant tumors arising within a mature cystic teratoma include adenocarcinoma, basal cell carcinoma, adenosquamous carcinoma, thyroid carcinoma, sebaceous carcinoma, malignant melanoma, sarcoma, carcinoid tumor (NET), and neuroectodermal tumor.[16,17] A synchronous primary neuroendocrine tumor and primary adenocarcinoma arising within mature cystic teratoma is extremely rate, and only five cases have been reported [Table 2].[18,19,20,21,22] To the best of our knowledge, no prior report described a mature cystic teratoma in CS coexisting with both adenocarcinoma and NET.
Table 2.
Clinical characteristics of adenocarcinoma and neuroendocrine tumor arising from mature cystic teratoma
| Author, year | Age, sex | Symptoms | Lesion site | Treatment | Follow-up |
|---|---|---|---|---|---|
| Kim et al., 2003 | 62, female | Lower abdominal pain | Ovary | Hysterectomy with BSO, chemotherapy, radiotherapy | Alive without evidence of disease at 13 months after surgery |
| Armah et al., 2009 | 50, female | Low back and right hip pain | Horseshoe kidney | Partial nephrectomy | Alive without evidence of disease at 6 months after surgery |
| Hinshaw et al., 2012 | 74, female | Incidental finding on CT | Ovary | Hysterectomy with BSO | Not described |
| Agarwal et al., 2015 | 24, female | Chest pain | Mediastinum | Surgical resection | Alive without evidence of disease at 1 month after surgery |
| Higgins et al., 2017 | 66, male | Epigastric pain | Horseshoe kidney | Partial nephrectomy, chemotherapy | Died 7 months after surgery from metastatic disease |
| Present case | 59, female | Incidental finding on CT | Presacral teratoma associated with CS | Surgical resection, radiotherapy | Alive without evidence of disease at 6 months after surgery |
BSO=Bilateral salpingo-oophorectomy; CS=Currarino syndrome
Conclusion
CS is a rare congenital disease with variable phenotypic expression, thereby producing a range of symptoms. The occurrence of synchronous primary adenocarcinoma and NET arising within a teratoma is rare. The present patient was diagnosed with CS incidentally in middle age, and this is the first reported case to our knowledge of a mature cystic teratoma coexisting with adenocarcinoma and NET in a patient with CS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Currarino G, Coln D, Votteler T. Triad of anorectal, sacral, and presacral anomalies. Am J Radiol. 1981;137:395–8. doi: 10.2214/ajr.137.2.395. [DOI] [PubMed] [Google Scholar]
- 2.Lynch SA, Wang Y, Strachan T, Burn J, Lindsay S. Autosomal dominant sacral agenesis: Surrarino syndrome. J Med Genet. 2000;37:561–6. doi: 10.1136/jmg.37.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emans PJ, Kootstra G, Marcelis CL, Beuls EA, van Heurn LW. The currarino triad: The variable expression. J Pediatr Surg. 2005;40:1238–42. doi: 10.1016/j.jpedsurg.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Martucciello G, Torre M, Belloni E, Lerone M, Pini Prato A, Cama A, et al. Currarino syndrome: Proposal of diagnostic and therapeutic protocol. J Pediatr Surg. 2004;39:1305–11. doi: 10.1016/j.jpedsurg.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Dirix M, van Becelaere T, Berkenbosch L, van Baren R, Wijnen RM, Wijnen MH, et al. Malignant transformation in sacrococcygeal teratoma and in presacral teratoma associated with currarino syndrome: A comparative study. J Pediatr Surg. 2015;50:462–4. doi: 10.1016/j.jpedsurg.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Ashcraft KW, Holder TM. Hereditary presacral teratoma. J Pediatr Surg. 1974;9:691–7. doi: 10.1016/0022-3468(74)90107-9. [DOI] [PubMed] [Google Scholar]
- 7.Yates VD, Wilroy RS, Whitington GL, Simmons JC. Anterior sacral defects: An autosomal dominantly inherited condition. J Pediatr. 1983;102:239–42. doi: 10.1016/s0022-3476(83)80528-9. [DOI] [PubMed] [Google Scholar]
- 8.Norum J, Wist E, Bostad L. Incomplete currarino syndrome with a presacral leiomyosarcoma. Acta Oncol. 1991;30:987–8. doi: 10.3109/02841869109088256. [DOI] [PubMed] [Google Scholar]
- 9.Urioste M, Garicai-Andrade M, del Carmen, Valle L, Robledo M, González-Palacios F. Malignant degeneration of presacral teratoma in the Currarion anomaly. Am J Med Genet Part A. 2004;128:299–304. doi: 10.1002/ajmg.a.30028. [DOI] [PubMed] [Google Scholar]
- 10.Pendlimari R, Leonard D, Dozois EJ. Rare malignant neuroendocrine transformation of presacral teratoma in patient with Currarino syndrome. Int J Colorectal Dis. 2010;24:1383–4. doi: 10.1007/s00384-010-0953-2. [DOI] [PubMed] [Google Scholar]
- 11.Ciotti P, Mandich P, Bellone E, Ceppa P, Bovio M, Ameri P, et al. Currarino syndrome with pelvic neuroendocrine tumor diagnosed by post-mortem genetic analysis of tissue specimens. Am J Med Genet Part A. 2011;155:2750–3. doi: 10.1002/ajmg.a.34031. [DOI] [PubMed] [Google Scholar]
- 12.Baltogiannis N, Mavridis G, Soutis M, Keramidas D. Currarino triad associated with Hirschsprung's disease. J Pediatr Surg. 2003;38:1086–9. doi: 10.1016/s0022-3468(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 13.AbouZeid AA, Mohammad SA, Abolfotoh M, Radwan AB, Ismail MME, Hassan TA. The currarino triad: What pediatric surgeons need to know. J Pediatr Surg. 2017;52:1260–8. doi: 10.1016/j.jpedsurg.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths D, Wass J, Look K, Sutton G. Malignant degeneration of a mature cystic teratoma five decades after discovery. Gynecol Oncol. 1995;59:427–9. doi: 10.1006/gyno.1995.9948. [DOI] [PubMed] [Google Scholar]
- 15.Rose PG, Tak WK, Reale FR. Squamous cell carcinoma arising in a mature cystic teratoma with metastasis to the paraaortic nodes. Gynecol Oncol. 1993;50:131–3. doi: 10.1006/gyno.1993.1178. [DOI] [PubMed] [Google Scholar]
- 16.Curling OM, Potsides PN, Hundson CN. Malignant change in benign cystic teratoma of the ovary. Br J Obstet Gynecol. 1979;86:399–402. doi: 10.1111/j.1471-0528.1979.tb10619.x. [DOI] [PubMed] [Google Scholar]
- 17.Chandha S, Schaberg A. Malignant transformation in benign cystic teratomas: Dermoids of the ovary. Eur J Obstet Gynecol Repro Biol. 1988;29:329–38. doi: 10.1016/0028-2243(88)90074-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim SM, Choi, HS, Byun JS, Kim YH, Kim KS, Rim SY, et al. Mucinous adenocarcinoma and stromal carcinoid tumor arising in one mature cystic teratoma of the ovary with synchronous cervical cancer. J Obstet Gynaecol Res. 2003;29:28–32. doi: 10.1046/j.1341-8076.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 19.Armah HB, Parwani AV, Perepletchikov AM. Synchronous primary carcinoid tumor and primary adenocarcinoma arising within mature cystic teratoma of horseshoe kidney: A unique case report and review of the literature. Diagn Pathol. 2009;4:17. doi: 10.1186/1746-1596-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw HD, Smith AL, Desouki MM, Olawaiye AB. Malignant transformation of a mature cystic ovarian teratoma into thyroid carcinoma, mucinous adenocarcinoma and stromal carcinoid: A case report and literature review. Case Rep Obstet Gynecol. 2012;2012:269489. doi: 10.1155/2012/269489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal S, Mullick S, Gupta K, Dewan RK. Mediastinal teratoma with coexisting adenocarcinoma and carcinoid tumor (somatic-type malignancy): A case report with a review of the literature. Turk Thorac J. 2015;16:101–4. doi: 10.5152/ttd.2014.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins A, Eisa W, Walton J, Baber J, Zhu S, Williams H. Metastatic mucinous adenocarcinoma and carcinoid tumor arising from a mature cystic teratoma of a horseshoe kidney. Urol Case Rep. 2017;11:39–41. doi: 10.1016/j.eucr.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]