Abstract
Purpose:
Percutaneous radio-frequency ablation is a minimally invasive treatment option for osteoid osteomas. The ablation process is straightforward in the more common locations like the femur/tibia. Surgery has historically been the gold standard, but is currently used in lesions, that may not be effectively and safely ablated, i.e. close to skin/nerve. Radio-frequency ablation can still be used in such cases along with additional techniques/strategies to protect the sensitive structures and hence improve the outcomes. The authors describe their experience with four challenging osteoid osteoma ablation cases.
Methods:
We retrospectively reviewed radio-frequency ablations of four osteoid osteomas in rather atypical locations, the protective techniques/strategies employed, the adequacy and safety of the radio-frequency ablation with the use of these techniques.
Results:
All patients had complete resolution of pain with no recurrence in the follow-up period. No complications were reported.
Conclusion:
RFA has been proven to be an effective and safe option for treatment of OOs in the common locations. It is generally recommended to have a 1 cm safety margin between the RF probe and any critical structures in the vicinity. However, with OOs in atypical locations this may not be always possible and hence additional techniques may be needed to ensure protection of the surrounding sensitive structures and also allow for effective ablation.
Keywords: Atypical locations, nerve damage, osteoid osteoma, protective techniques, radio-frequency ablation
Introduction
An osteoid osteoma (OO) is a painful benign bony lesion, with a male predominance, and usually occurs between the ages of 10–30.[1] It often presents with night-pain which responds to salicylates or non-steroidal anti-inflammatory drugs (NSAIDs).[2] In over 50% of the cases, they are located in the diaphysis of the femur or tibia with the proximal femur being the most frequently affected.[3] Radio-frequency ablation (RFA) of OOs in these locations has been extensively described. There is scant literature for RFA of OOs in uncommon locations. The authors describe their experience with RFA of OOs in four rather atypical locations and the use of techniques to protect adjacent sensitive structures.
Methods
This was an Institutional Review Board approved retrospective chart review study. We reviewed RFAs of four OOs in uncommon locations, with techniques to protect critical structures, particularly, skin, nerves and cartilage adjacent to the ablation zone. These patients were followed up to assess relief from their symptoms, and possible recurrence or complications [Table 1].
Table 1.
Patient Demographics, lesion distribution, nidus size, response to treatment, biopsy results and procedure time
| Patient | Age (years) | Sex | Site of OO | Nidus size (mm) | Pre-operative Pain Score | Post-operative Pain Score | Biopsy Results | Procedure time |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | M | Femoral head | 5 | 8/10 | 0/10 | OO | 53 min |
| 2 | 20 | M | 2nd Metacarpal | 2 | 4/10 | 0/10 | Not obtained | 71 min |
| 3 | 10 | M | Glenoid | 6 | 8/10 | 0/10 | OO | 30 min |
| 4 | 18 | M | Posterior distal femoral cortex | 7 | 7/10 | 0/10 | OO | 43 min |
Pre-procedure
All patients were discussed in the multidisciplinary tumor board and then referred to the interventional radiology (IR) clinic. The severity of pain was assessed subjectively using the visual analogue scale (VAS). Routine lab workup included a complete blood count, comprehensive metabolic panel, and coagulation profile.
Procedural technique
All RFA procedures were performed under general anesthesia in a Computed tomography (CT) suite by a single interventional radiologist (IR) with seven years of experience in performing these procedures. Antibiotic prophylaxis was performed in all patients just prior to the procedure. CT guidance was used to localize the lesion, monitor needle advancement and confirm final positioning of the RF probe. The On-control drill (Teleflex Morrisville, NC) with its proprietary 11 Gauge (G) needle was used to create a tract to the center of the nidus. A biopsy was performed with a 13 G needle, in three of the four cases [Table 1]. The 16-guage Cool-tip RF ablation probe (Medtronic, Minneapolis, Minnesota) was advanced through the 11 G needle and positioned with its tip at the far margin of the nidus. Once the RF probe was in place within the nidus, the outer needle was withdrawn by 1 cm to prevent heat conduction along the shaft of the cannula. Then, using a radio-frequency generator, the nidus was ablated at 90°C for six minutes, based on the protocol used in prior studies.[4] RF probes with capabilities to generate different ablation sizes were used, as determined by the size of the nidus, taking care to match or slightly oversize the ablation zone in relation to the nidus.
Case 1
20-year-old male with a 10-month history of insidious right-hand pain and numbness, worse at night. Radiographs and MRI demonstrated periosteal thickening [Figure 1A and 1B] along the ulnar aspect of the 2nd metacarpal. CT showed a subtle 2 mm lucency within the inner aspect of the cortex [Figure 1C] suggestive of the nidus.
Figure 1 (A-E).
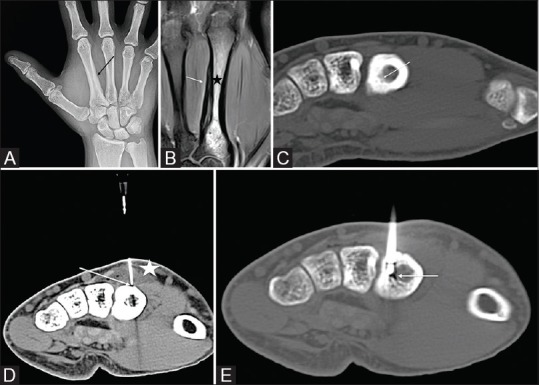
(A) AP radiograph showing periosteal reaction on the medial side of the 2nd metacarpal. (B) Coronal PD fat saturated MRI of the hand showing periosteal reaction on the ulnar side of the 2nd metacarpal (white arrow) with marrow edema (black star). (C) Axial non contrast CT image of the hand demonstrating the nidus (white arrow). (D) Axial CT of the hand with a 20 G needle (white arrow), placed on the dorsal surface of the metacarpal for infusion of D5W while the ablation is in progress to prevent skin burns. The infused fluid is seen creating a buffer between the skin and the bone (white star). (E) Axial CT demonstrating the RF probe in the OO nidus (white arrow)
The ablation was performed with the right hand in the prone position. A 20 G needle was advanced under CT guidance to the dorsal aspect of the metacarpal. The surrounding tissue was then infiltrated with 30 mL of 5% Dextrose in water (D5W) [Figure 1D]. Following this, RFA was performed using the technique and protocol mentioned above [Figure 1E]. A cold pack was placed on the skin to prevent thermal injury.
Case 2
10-year-old male with right shoulder pain. CT showed a 6 mm OO in the glenoid [Figure 2A].
Figure 2 (A-C).
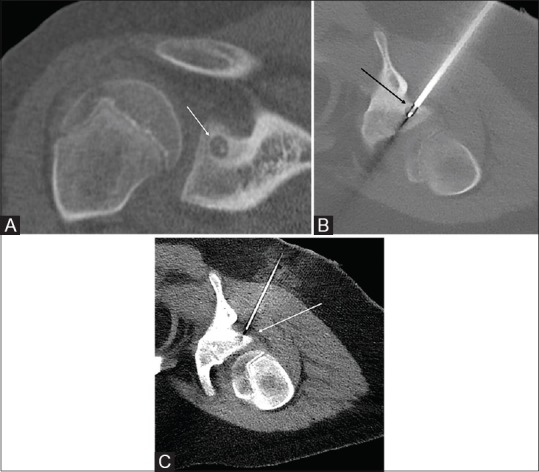
(A) CT coronal reconstruction shows a right glenoid osteoid osteoma (white arrow). (B) Intra-procedural CT showing the ablation probe with tip within the nidus (black arrow). (C) Intraprocedural CT with a 20 G spinal needle placed in the spino-glenoid notch (white arrow) to facilitate D5W injection during the ablation for protection of the suprascapular nerve
RFA was performed in the prone position with a posterior approach [Figure 2B] through the infraspinatus muscle. A 20 G spinal needle was placed parallel to the ablation probe, and D5W was infused during the ablation to protect the suprascapular nerve [Figure 2C].
Case 3
23-year-old male who developed persistent left hip pain after falling off a chair. MRI and Tc-99m bone scan demonstrated a 7 mm subchondral lesion suggestive of an OO, in the left femoral head [Figure 3A and B].
Figure 3 (A-C).
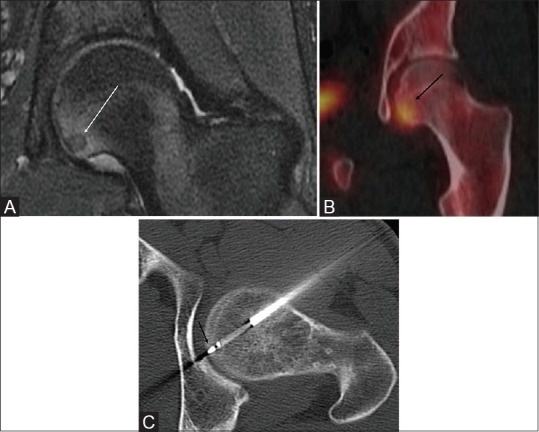
(A) Coronal fat-sat PD MR of the left hip shows a hypointense subchondral OO with adjacent marrow edema (white arrow). (B) SPECT/CT shows focal uptake within the lesion (black arrow). (C) Axial procedural CT showing the lateral approach for the RFA avoiding the femoral head articular cartilage and neurovascular bundle. RF probe in the nidus (black arrow)
RFA was performed in the supine position. An anterior/lateral subchondral approach through the tensor fascia lata and the rectus femoris muscles was used to avoid neurovascular or cartilage injury [Figure 3C].
Case 4
18-year-old male with pain behind the knee for 1-2 years. CT and MRI demonstrated a lesion highly suggestive of an OO in the posterior cortex of the distal femur [Figure 4A and B]. This was ablated via a posterior approach with the patient in the prone position [Figure 4C]. A 20 G needle was placed medial to the RFA probe abutting the posterior cortex of the femur and used to infuse D5W throughout the ablation to protect the tibial and common peroneal nerves [Figure 4D].
Figure 4 (A-D).
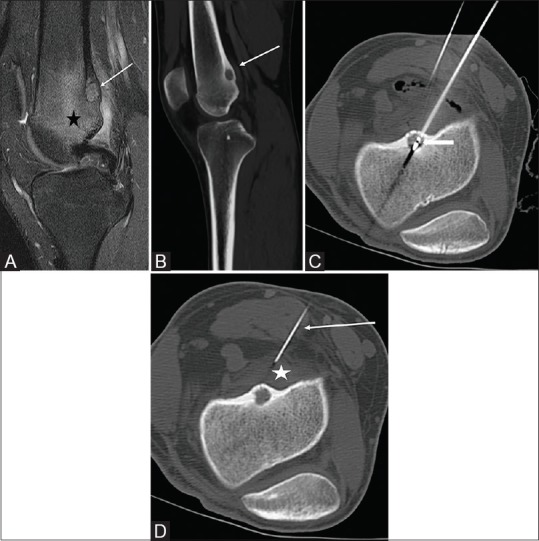
(A) Fat suppressed sagittal MRI of the knee with edema in the distal femur (black star). There is a hyperintense lesion in the posterior cortex of the distal femur (white arrow) suggestive of an OO nidus. (B) The margins of the nidus are better seen on the CT (white arrow). (C) Ablation probe in the nidus (white arrow). (D) A 20 G needle (white arrow) is placed in the popliteal fossa adjacent to the nidus and used to infuse D5W (white star) to protect the adjacent nerves from thermal damage
Research ethics standards compliance
This original article was completed under an institutional review board approved protocol. The IRB number was 2004777. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
The RFA was technically and clinically successful in 100% of cases with resolution of symptoms in all patients (4/4). The mean procedure time was 49 minutes. Furthermore, no complications, such as injury to the skin, nerves, or cartilage were encountered. None of the cases recurred. Biopsies were obtained in 3 of the 4 patients and was conclusive of OO. The minimum clinical follow-up was 12 months (mean, 27.8 months; range, 12-37 months).
Discussion
OO, first described by Jaffe in 1935, is a benign osteoblastic lesion characterized by a nidus of osteoid tissue. It constitutes 10% of all benign bone tumors.[5] CT is the initial cross-sectional imaging modality employed. The classic radiological finding is a lucent area (nidus), with dense and solid periosteal reaction.[4] CT is more effective than MRI for localization of tumors.[6] MRI better demonstrates OOs in cancellous bones, and the associated soft tissue/intramedullary edema.
Treatment options for OOs include conservative management, surgical excision, and percutaneous ablation techniques.[7]
Aspirin or other NSAIDs frequently provide effective pain control, however, long-term therapy may be unacceptable due to gastrointestinal complications. Articular or periarticular OOs are particularly resistant to conservative therapy, and more aggressive intervention is often necessary.[4]
In the past, conventional surgical excision and more recently minimally invasive surgical techniques were the treatment of choice for OOs refractory to conservative treatment.[8,9,10,11,12] Traditional surgical approach involves curetting the nidus and allografting. However, this may be associated with difficulty in intraoperative localization, prolonged hospital stay, weakening of the bone during post-operative remodeling, and longer downtime following treatment.[13] RFA of OOs requires a small osseous access just enough to allow insertion of the probe. Therefore, loss of bone substance is minimal and the ablation does not cause significant structural weakening.[4]
RFA utilizes a generator as a source of alternating current and a probe to transmit the current into the target lesion/tissue. The shaft of the probe is insulated, while its tip is not. When high frequency alternating current from the generator passes through the probe and into the tissue to be ablated, it produces oscillation of ions in the vicinity. This results in frictional heating of surrounding tissues and ultimately coagulation necrosis.[14]
Rosenthal et al.[15] reported the efficacy of CT-guided thermal ablation of OOs. Since then, there has been an increasing shift towards ablation. RFA has been the most commonly used ablation modality,[7] however, interstitial laser ablation,[16] cryotherapy,[17] and microwave ablation[18] have also been used.
RFA of OOs has been extensively reported in the past[19,20,21,22] with success rates approaching 100%. The key to successful treatment of OOs is complete removal or destruction of the tumor nidus. Recurrence rates for OOs after surgical resection is 9-28%,[23] as opposed to 7-16% recurrence rates after RFA.[24,25] However, the likelihood for recurrence or incomplete removal is increased for OOs located in anatomic areas that are technically difficult to access, such as the acetabulum, femoral head and neck.[21] Additionally, the presence of sensitive structures adjacent to the ablation zone may prevent the application of maximum power needed to accomplish complete destruction of the nidus.
Very few papers have reported RFA of OOs in unusual locations. Moreover, none of them have used techniques to protect sensitive structures adjacent to the ablation zone. However, Akhlaghpoor et al. reported limited skin burns in a case of a phalangeal OO ablation.[26] This was anticipated in our case of metacarpal OO ablation and adequate skin protection was ensured by a D5W fluid buffer in the subcutaneous tissues and placing a cold bag of D5W on the skin.
Garge et al.,[27] reported radial nerve paralysis in their series of OO ablations. We anticipated this complication while ablating the OOs in the posterior femur and glenoid and minimized the risk of nerve injury by infusing fluid through the 20 G needles placed adjacent to the ablation zones in both cases. Most patients with OO are of the younger age group and any deficit from nerve injury could limit the child's future potential. Therefore, this should be of primary focus of the operator performing the ablation.
The femoral head OO is unusual, as only a few cases have been reported.[28] Articular cartilage injury is possible with this location and care should be taken not to traverse the articular cartilage to reach the OO. The antero-lateral approach was therefore used in the femoral head OO, to stay away from the articular cartilage and neurovascular bundle.
Since RFA uses alternating current to induce tissue necrosis, continuous infusion of ionic solutions, like normal saline, with an intent to protect critical structures may actually extend the electrical conductivity in these tissues, thus potentially exposing them to thermal injury.[29] Hence, we used D5W infusion (which is nonionic) to protect critical structures adjacent to the ablation zone.
The study is limited by its retrospective design, small sample size and absence of control subjects who did not get the protective techniques utilized in the study subjects.
Conclusion
RFA has been proven to be an effective and safe option for treatment of OOs in the common locations. It is generally recommended to have a 1 cm safety margin between the RF probe and any critical structures in the vicinity. However, with OOs in atypical locations, this may not be always possible and hence additional techniques may be needed to ensure protection of the surrounding sensitive structures and also allow for effective ablation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge Joanne Cassani, our Director of Research, and Salwa Ali for their assistance in coordinating this publication.
References
- 1.Kransdorf MJ, Stull MA, Gilkey FW, Moser RP., Jr Osteoid osteoma. Radiographics: A review publication of the Radiological Society of North America, Inc. 1991;11:671–96. doi: 10.1148/radiographics.11.4.1887121. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan A. Benign bone-forming lesions: Osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993;22:485–500. doi: 10.1007/BF00209095. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MD, Harrington TM, Ginsburg WW. Osteoid osteoma: 95 cases and a review of the literature. Semin Arthritis Rheum. 1983;12:265–81. doi: 10.1016/0049-0172(83)90010-0. [DOI] [PubMed] [Google Scholar]
- 4.Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, et al. Thermal ablation of osteoid osteoma: Overview and step-by-step guide. Radiographics: A review publication of the Radiological Society of North America, Inc. 2009;29:2127–41. doi: 10.1148/rg.297095081. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe HL. “Osteoid-osteoma”: A benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg. 1935;31:709–28. [Google Scholar]
- 6.Yip PS, Lam YL, Chan MK, Shu JS, Lai KC, So YC. Computed tomography-guided percutaneous radiofrequency ablation of osteoid osteoma: Local experience. Hong Kong Med J. 2006;12:305–9. [PubMed] [Google Scholar]
- 7.Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–5. doi: 10.1148/radiol.2291021053. [DOI] [PubMed] [Google Scholar]
- 8.Fenichel I, Garniack A, Morag B, Palti R, Salai M. Percutaneous CT-guided curettage of osteoid osteoma with histological confirmation: A retrospective study and review of the literature. Int Orthop. 2006;30:139–42. doi: 10.1007/s00264-005-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcove RC, Heelan RT, Huvos AG, Healey J, Lindeque BG. Osteoid osteoma.Diagnosis, localization, and treatment. Clin Orthop Relat Res. 1991:197–201. [PubMed] [Google Scholar]
- 10.Ozaki T, Liljenqvist U, Hillmann A, Halm H, Lindner N, Gosheger G, et al. Osteoid osteoma and osteoblastoma of the spine: Experiences with 22 patients. Clin Orthop Relat Res. 2002:394–402. doi: 10.1097/00003086-200204000-00046. [DOI] [PubMed] [Google Scholar]
- 11.Parlier-Cuau C, Champsaur P, Nizard R, Hamze B, Laredo JD. Percutaneous removal of osteoid osteoma. Radiol Clin North Am. 1998;36:559–66. doi: 10.1016/s0033-8389(05)70044-0. [DOI] [PubMed] [Google Scholar]
- 12.Sans N, Galy-Fourcade D, Assoun J, Jarlaud T, Chiavassa H, Bonnevialle P, et al. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212:687–92. doi: 10.1148/radiology.212.3.r99se06687. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop Relat Res. 1999:186–91. doi: 10.1097/00003086-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: Tricks of the trade. AJR Am J Roentgenol. 2002;179:1633–42. doi: 10.2214/ajr.179.6.1791633. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: A new procedure. Radiology. 1992;183:29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 16.Gangi A, Dietemann JL, Gasser B, Mortazavi R, Brunner P, Mourou MY, et al. Interstitial laser photocoagulation of osteoid osteomas with use of CT guidance. Radiology. 1997;203:843–8. doi: 10.1148/radiology.203.3.9169714. [DOI] [PubMed] [Google Scholar]
- 17.Skjeldal S, Lilleas F, Folleras G, Stenwig AE, Samset E, Tillung T, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii--A case report. Acta Orthop Scand. 2000;71:637–8. doi: 10.1080/000164700317362307. [DOI] [PubMed] [Google Scholar]
- 18.Kostrzewa M, Diezler P, Michaely H, Rathmann N, Attenberger UI, Schoenberg SO, et al. Microwave ablation of osteoid osteomas using dynamic MR imaging for early treatment assessment: Preliminary experience. J Vasc Interv Radiol. 2014;25:106–11. doi: 10.1016/j.jvir.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni SS, Shetty NS, Polnaya AM, Janu A, Kumar S, Puri A, et al. CT-guided radiofrequency ablation in osteoid osteoma: Result from a tertiary cancer centre in India. Indian J Radiol Imaging. 2017;27:318–23. doi: 10.4103/ijri.IJRI_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankharia B, Burute N. Percutaneous radiofrequency ablation for osteoid osteoma: How we do it. Indian J Radiol Imaging. 2009;19:36–42. doi: 10.4103/0971-3026.44523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wortler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg British. 2001;83:391–6. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- 22.Rehnitz C, Sprengel SD, Lehner B, Ludwig K, Omlor G, Merle C, et al. CT-guided radiofrequency ablation of osteoid osteoma: Correlation of clinical outcome and imaging features. Diagn Interv Radiol. 2013;19:330–9. doi: 10.5152/dir.2013.096. [DOI] [PubMed] [Google Scholar]
- 23.Healey JH, Ghelman B. Osteoid osteoma and osteoblastoma. Current concepts and recent advances. Clin Orthop Relat Res. 1986:76–85. [PubMed] [Google Scholar]
- 24.Flanagin BA, Lindskog DM. Intraoperative radiofrequency ablation for osteoid osteoma. Am J Orthop. 2015;44:127–30. [PubMed] [Google Scholar]
- 25.Shields DW, Sohrabi S, Crane EO, Nicholas C, Mahendra A. Radiofrequency ablation for osteoid osteoma–recurrence rates and predictive factors. Surgeon. 2018;16:156–62. doi: 10.1016/j.surge.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Akhlaghpoor S, Aziz Ahari A, Arjmand Shabestari A, Alinaghizadeh MR. Radiofrequency ablation of osteoid osteoma in atypical locations: A case series. Clin Orthop Relat Res. 2010;468:1963–70. doi: 10.1007/s11999-010-1265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garge S, Keshava SN, Moses V, Koshy G, Ahmed M, Mammen S, et al. Radiofrequency ablation of osteoid osteoma in common and technically challenging locations in pediatric population. Indian J Med Paediatr Oncol. 2017;38:302–5. doi: 10.4103/ijmpo.ijmpo_61_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahata KM, Keshava SK, Jacob KM. Osteoid osteoma of the femoral head treated by radiofrequency ablation: A case report. J Med Case Reports. 2011;5:115. doi: 10.1186/1752-1947-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa T, Kubota T, Horigome R, Kimura N, Honda H, Iwanaga A, et al. Radiofrequency ablation during continuous saline infusion can extend ablation margins. World J Gastroenterol. 2013;19:1278–82. doi: 10.3748/wjg.v19.i8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]


