Abstract
Background
Earlier age at onset of pubertal events and longer intervals between them (tempo) have been associated with increased breast cancer risk. It is unknown whether the timing and tempo of puberty are associated with adult breast density, which could mediate the increased risk.
Methods
From 1988 to 1997, girls participating in the Dietary Intervention Study in Children (DISC) were clinically assessed annually between ages 8 and 17 years for Tanner stages of breast development (thelarche) and pubic hair (pubarche), and onset of menses (menarche) was self-reported. In 2006–2008, 182 participants then aged 25–29 years had their percent dense breast volume (%DBV) measured by magnetic resonance imaging. Multivariable, linear mixed-effects regression models adjusted for reproductive factors, demographics, and body size were used to evaluate associations of age and tempo of puberty events with %DBV.
Results
The mean (standard deviation) and range of %DBV were 27.6 (20.5) and 0.2–86.1. Age at thelarche was negatively associated with %DBV (p trend = 0.04), while pubertal tempo between thelarche and menarche was positively associated with %DBV (p trend = 0.007). %DBV was 40% higher in women whose thelarche-to-menarche tempo was 2.9 years or longer (geometric mean (95%CI) = 21.8% (18.2–26.2%)) compared to women whose thelarche-to-menarche tempo was less than 1.6 years (geometric mean (95%CI) = 15.6% (13.9–17.5%)).
Conclusions
Our results suggest that a slower pubertal tempo, i.e., greater number of months between thelarche and menarche, is associated with higher percent breast density in young women. Future research should examine whether breast density mediates the association between slower tempo and increased breast cancer risk.
Keywords: Breast cancer, Breast density, Puberty
Background
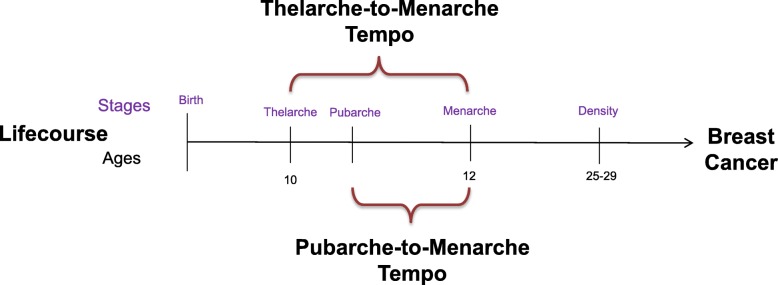
An earlier age at menarche is an established risk factor for breast cancer [1–3]. However, menarche is only one relatively late part of the complex female pubertal transition, which also includes thelarche (the onset of breast development) and pubarche (the onset of pubic hair growth). Thelarche, typically the first sign of puberty, usually occurs 2–4 years before menarche [4]. An earlier recalled age at thelarche is associated with a 20% increased risk of breast cancer [5]. Pubertal tempo, that is the length of time between thelarche and menarche, also was positively associated with increased risk, independent of either age at thelarche or menarche [5]. Possible inaccurate recall of pubertal timing could attenuate the association with breast cancer, highlighting the need to assess both age and tempo of pubertal development prospectively in longitudinal studies. Yet connecting pubertal timing with a breast cancer diagnosis that occurs more than 50 years later is an obstacle for epidemiological studies [6]. One way to address this challenge is to assess intermediate markers that can be measured earlier in life. Breast density is one of the strongest predictors of premenopausal and postmenopausal breast cancer risk [7, 8] and may be a useful intermediate marker between early life factors, such as pubertal development, and breast cancer risk (see Fig. 1).
Fig. 1.
Pubertal and breast density risk factors for breast cancer across the life course. Thelarche and pubarche mark the onset of breast development and pubic hair growth, respectively. Menarche is the onset of menstruation. The age at onset determines the pubertal timing of these events, and the time between these events is known as pubertal tempo
Characterizing the relationship between pubertal development and breast density may help clarify if breast cancer risk is influenced by factors during breast development, when breast tissue might be particularly susceptible to proliferative and carcinogenic stimuli. Given that earlier ages at thelarche and menarche are associated with increased breast cancer risk [5] and that breast density is positively associated with risk [9], we hypothesized that adult women who had an earlier thelarche and slower pubertal development would have denser breasts compared to those who had a later thelarche and faster pubertal development. We tested this hypothesis in the Dietary Intervention Study in Children (DISC) cohort, a prospective study originally designed as a diet intervention in children, who were re-visited in their mid-to-late twenties [10]. The objective of this investigation was to examine the relationship between prospectively assessed pubertal timing (age at thelarche, pubarche, and menarche) and tempo (the interval between thelarche and menarche or pubarche and menarche) with percent dense breast volume (%DBV) measured at ages 25–29 years.
Methods
Population
Between 1988 and 1997, DISC, a multicenter randomized controlled clinical trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI), was conducted to test the safety and efficacy of a dietary intervention to reduce serum low-density lipoprotein cholesterol (LDL-C) in children with elevated LDL-C [10–12]. At baseline, 663 healthy, pre-pubertal, 8–10-year-old children, including 301 girls, with elevated LDL-C were recruited into DISC at six clinical centers and randomized to a behavioral dietary intervention or usual care control group. The intervention continued until the mean age of participants was 16.7 years. In 2006–2008 when participants were 25 to 29 years old, the DISC06 Follow-Up Study was conducted to evaluate the longer-term effects of the diet intervention on breast cancer risk factors in female participants. Prior to randomization, parents/guardians provided informed consent and DISC participants provided assent, while participants provided informed consent prior to the DISC06 follow-up visit. An NHLBI-appointed independent data and safety monitoring committee monitored the original DISC trial. Institutional review boards at all participating clinical centers and the data coordinating center approved both the DISC and the DISC06 Follow-Up Study protocols. The DISC06 Follow-Up Study protocol also was approved by the Fox Chase Cancer Center’s Institutional Review Board.
All female DISC participants were invited to participate in the DISC06 Follow-Up Study, and 260 (86.4%) of the 301 females originally randomized took part. Women who were pregnant or breastfeeding at or within 12 weeks before the study visit (n = 30) and those who had breast implants or breast reduction surgery (n = 16) were not eligible for the current analysis. An additional 32 women were excluded because they had technically unacceptable or missing breast magnetic resonance imaging (MRI) leaving a total of 182 participants for inclusion in analyses.
Data collection
Pubertal staging
Trained and certified clinicians assessed girls for breast and pubic hair Tanner stage (T1–5) at annual visits between the ages of 8 and 17 years until T5 was reached [13]. Ages at thelarche and pubarche were calculated by taking the mid-point of age between the two visits when girls transitioned from T1 to T2+ for the appearance of breast development and pubic hair, respectively. Participants reported whether they had begun menstruating at each visit and if so, their age at menarche in years and months [13]. Age at thelarche was subtracted from age at menarche for each girl to create the thelarche-to-menarche tempo variable, and age at pubarche was subtracted from age at menarche to create the pubarche-to-menarche tempo variable.
Breast density measurement
%DBV was measured using noncontrast MRI on a whole-body 1.5-Tesla or higher field-strength MRI scanner with a dedicated breast-imaging radiofrequency coil as previously described [10, 14]. One investigator (Dr. C. Klifa at the University of California, San Francisco, San Francisco, CA) processed all MRI image data by identifying the chest wall–breast tissue boundary and skin surface and using automated fuzzy C-means to separate breast fibroglandular and fatty tissue [14]. These methods allow for the measurement of total breast volume and absolute dense breast volume (ADBV), which quantifies fibroglandular tissue. We calculated the absolute non-dense breast volume (ANDBV) by subtracting ADBV from total breast volume and %DBV as the ratio of ADBV to total breast volume multiplied by 100. We averaged the density measures of both breasts for analysis. %DBV was the primary outcome and is highly correlated with percent breast density (r = .87) based on mammography [15], which is an established risk factor for breast cancer [16]. ADBV and ANDBV were secondary outcomes.
Covariates
At DISC06 follow-up visits, participants completed several questionnaires on demographic characteristics; medical, reproductive, and menstrual histories; medication use; and health habits. Height, weight, and waist circumference were measured, and body composition was assessed by whole-body dual-energy X-ray absorptiometry (DXA) as previously described [10].
Statistical analysis
We initially explored distributions of breast density measures graphically and by using nonparametric statistics, and prior to modeling, log-transformed measures to improve normality. We calculated geometric means and 95% confidence intervals of %DBV, ADBV, and ANDBV across quartiles of ages at thelarche, pubarche, menarche, and pubertal tempo variables by exponentiating the least square means from multivariable linear mixed-effects regression models with clinic as a random effect and robust standard errors. Model 1 is the crude, unadjusted model. Model 2 adjusted for adult covariates, including parity (nulliparous vs parous), duration of hormone use (continuous), education (some college or less, bachelor’s degree, graduate degree), race (white vs. non-white), smoking status (never vs ever), whole-body percent fat measured by DXA (continuous), and height (continuous). Because childhood BMI is a strong predictor of pubertal timing and was also previously associated with %DBV in DISC06 [17], model 3 adjusted for the same covariates in model 2 as well as BMI at 8–10 years of age, expressed as a z-score relative to CDC 2000 Growth Charts [18]. Missing values of whole-body percent fat (N = 6), age at thelarche (n = 12), and age at pubarche (n = 9) were imputed using values from a prediction model that included adult BMI as an independent variable as well as covariates in model 3; this process was repeated 25 times to create 25 multiply-imputed datasets. Results from each imputed dataset were pooled using Rubin’s rule [19]. Using model 3, we explored the combined effects of pubertal timing (ages at thelarche and menarche) and thelarche-menarche tempo with breast density. Each of the continuous pubertal timing variables was dichotomized at the median and cross-classified with the similarly dichotomized tempo variable creating two dummy variables each with four categories: (1) early menarche/short tempo (reference), early menarche/long tempo, late menarche/short tempo, late menarche/long tempo; and (2) early thelarche/short tempo (reference), early thelarche/long tempo, late thelarche/short tempo, late thelarche/long tempo. We tested for interactions between thelarche-to-menarche tempo and diet intervention assignment as well as between thelarche and menarche by including cross-product terms in the fully adjusted models. In sensitivity analyses, we tested if the observed associations with %DBV held in subsets restricted to white participants, nulliparous participants, women not using hormonal contraceptives, or women whose baseline BMI z-score was < 1.5. All tests were two-sided and considered to be significant if p value < 0.05. All analyses were conducted using STATA 13.0 (College Station, TX).
Results
At baseline, all girls were pre-pubertal, and during the DISC trial, the majority reached thelarche before pubarche. The mean age of 182 women included in the present study was 27.2 years at the DISC06 follow-up visit (Table 1). The majority were white (90%), nulliparous (71%), and ever users of hormonal contraceptives (93%, with 58% current users). Their mean BMI was 25.4 kg/m2. The mean (standard deviation) of %DBV was 27.6 (20.5). Covariates across tempo categories were generally similar (shown in Additional file 1: Table S1). The three related pubertal milestones—menarche, thelarche, and pubarche, were moderately correlated; Pearson correlation coefficients ranged from r 0.42 to 0.46. The correlations between menarche and the tempo variables were higher (thelarche-to-menarche tempo r = 0.68; pubarche-to-menarche tempo r = 0.61).
Table 1.
Characteristics of DISC participants in childhood and as young adults
| Number | Mean | SD | Percentage | |
|---|---|---|---|---|
| Child characteristics | ||||
| Race/ethnicity | ||||
| White | 164 | 90 | ||
| Other | 18 | 10 | ||
| Age at baseline, years | 182 | 9.13 | 0.59 | |
| BMI z-score at baseline | 182 | 0.23 | 0.90 | |
| Age at thelarche, years | 170 | 10.59 | 1.09 | |
| Age at pubarche, years | 173 | 10.97 | 1.19 | |
| Age at menarche, years | 182 | 12.90 | 1.26 | |
| Adult characteristics | ||||
| Age at follow-up, years | 182 | 27.17 | 1.02 | |
| BMI, kg/m2 | 182 | 25.39 | 5.36 | |
| DXA % body fat, % | 176 | 35.41 | 8.82 | |
| Exogenous hormone use | ||||
| Never | 11 | 6 | ||
| Former | 66 | 36 | ||
| Current | 105 | 58 | ||
| Duration of hormone use, years | 171 | 5.6 | 3.5 | |
| Parous (vs nulliparous) | 53/182 | 29 | ||
| Education | ||||
| Graduate degree | 25 | 14 | ||
| Bachelor’s degree | 95 | 52 | ||
| Some college or less | 62 | 34 | ||
| Ever smokers (vs never-smokers) | 82/182 | 45 | ||
| Breast density measures | ||||
| Percent dense breast volume (%) | 182 | 27.6 | 20.5 | |
| Absolute dense breast volume (cm3) | 182 | 104.2 | 70.6 | |
| Absolute non-dense breast volume (cm3) | 182 | 413.3 | 364.3 | |
Table 2 shows associations of pubertal timing and tempo with adult %DBV. Across the three models, the thelarche-to-menarche tempo association was consistently associated with %DBV. Age at thelarche was associated with %DBV after adjustment for child BMI z-score, whereas the association with age at menarche was attenuated. Age at pubarche was not associated with %DBV in any model, and pubarche-to-menarche tempo was not associated with %DBV after adjustment for covariates. In fully adjusted models (model 3), %DBV increased with increasing duration from thelarche- to- menarche tempo (p trend = 0.007). %DBV was 40% higher in women whose thelarche-to-menarche tempo was 2.9 years or longer (geometric mean (95%CI) = 21.8% (18.2–26.2)) compared to women whose thelarche-to-menarche tempo was less than 1.6 years (geometric mean (95%CI) = 15.6% (14.2–20.7)). Girls who were oldest at thelarche (≥ 11.1 years) had an 18% lower %DBV compared to girls who were youngest (8.7 to < 9.9 years; p trend = 0.04).
Table 2.
Geometric mean (95% confidence interval (CI)) from mixed-effect regression models for each pubertal factor in relation to percent dense breast volume (%DBV)
| Pubertal characteristic | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Age at thelarche, years | |||
| 8.7 to < 9.9 | 19.56 (15.62–24.48) | 18.74 (16.93–20.76) | 20.83 (18.42–23.57) |
| 9.9 to < 10.4 | 19.36 (14.15–26.48) | 19.06 (16.24–22.36) | 19.50 (16.36–23.24) |
| 10.4 to < 11.1 | 16.27 (12.01–22.05) | 18.36 (14.54–23.19) | 17.79 (14.15–22.38) |
| 11.1+ | 19.90 (12.41–31.91) | 18.72 (13.55–25.86) | 17.04 (13.28–21.86) |
| p trend | 0.56 | 0.94 | 0.04 |
| Age at pubarche, years | |||
| 8.6 to < 10.3 | 22.08 (18.18–26.81) | 19.04 (16.9–21.45) | 20.84 (18.2–23.86) |
| 10.3 to < 10.9 | 17.31 (12.27–24.42) | 16.88 (11.9–23.94) | 16.97 (12.21–23.57) |
| 10.9 to < 11.5 | 16.20 (13.84–18.98) | 17.51 (14.91–20.56) | 17.73 (14.81–21.23) |
| 11.5+ | 19.72 (13.74–28.32) | 21.66 (18.25–25.71) | 19.47 (17.38–21.83) |
| p trend | 0.87 | 0.39 | 0.64 |
| Age at menarche, years | |||
| 10 to < 12.2 | 16.89 (13.04–21.87) | 15.96 (13.28–19.19) | 17.10 (14.15–20.66) |
| 12.2 to < 12.8 | 14.38 (12.03–17.19) | 15.39 (11.53–20.54) | 16.38 (12.4–21.65) |
| 12.8 to < 13.4 | 19.98 (15.19–26.27) | 22.90 (20.37–25.75) | 21.65 (19.36–24.21) |
| 13.4+ | 25.96 (17.03–39.56) | 21.76 (16.41–28.86) | 20.18 (15.66–25.99) |
| p trend | 0.03 | 0.01 | 0.13 |
| Thelarche-to-menarche tempo, years | |||
| < 1.6 | 13.90 (10.23–18.88) | 15.45 (14.08–16.95) | 15.55 (13.85–17.47) |
| 1.6 to < 2.3 | 19.91 (16.02–24.76) | 18.88 (16.17–22.04) | 19.49 (16.89–22.5) |
| 2.3 to < 2.9 | 18.90 (13.14–27.18) | 18.47 (15.77–21.62) | 18.38 (15.7–21.51) |
| 2.9+ | 24.85 (16.51–37.40) | 22.55 (17.8–28.56) | 21.84 (18.18–26.24) |
| p trend | 0.01 | 0.004 | 0.007 |
| Pubarche-to-menarche tempo, years | |||
| < 1.1 | 14.40 (10.03–20.66) | 15.9 (12.23–20.68) | 16.23 (12.3–21.41) |
| 1.1 to < 1.7 | 17.16 (13.85–21.26) | 18.69 (16.16–21.61) | 18.40 (16.82–20.14) |
| 1.7 to < 2.6 | 16.68 (11.20–24.85) | 17.85 (13.7–23.25) | 17.87 (13.88–23.02) |
| 2.6+ | 29.09 (25.22–33.56) | 22.80 (20.25–25.68) | 22.66 (20.79–24.7) |
| p trend | < 0.001 | 0.06 | 0.06 |
Model 1 is unadjusted
Model 2 adjusts for the following variables as fixed effects: adult covariates, including parity (nulliparous vs parous), duration of hormone use (years, continuous), education (some college or less (reference), bachelor degree, graduate degree), race (white vs. non-white), smoking status (never vs ever), whole-body percent fat measured by DXA (%, continuous), and height (continuous). Clinic included as a random effect
Model 3 adjusts for the same factors in model 2, and in addition, BMI at 8–10 years of age expressed as a z-score relative to CDC 2000 Growth Charts (continuous)
There was a positive multiplicative interaction between thelarche and menarche in association with %DBV (p for interaction < 0.001).We, therefore, explored the combined effects of pubertal timing (age at thelarche and menarche) and thelarche-menarche tempo in cross-classified models and present the %DBV geometric means and 95% confidence intervals from these models in Table 3. Women with early menarche and short tempo had the lowest %DBV compared with women with early menarche and long tempo and women with late menarche and either short or long tempo. Women with early thelarche and short tempo had similar %DBV to women with either later thelarche and/or longer tempo (20.46% vs 19.81–20.4%) (15.73% vs. 18.84–21.33; p ≤ 0.01).
Table 3.
Geometric mean (95% confidence interval (CI)) from mixed-effect regression models, stratified by median of thelarche, menarche, and thelarche-to-menarche tempo in relation to percent dense breast volume (%DBV)
| Number | Mean (95% confidence interval) | p value | |
|---|---|---|---|
| Thelarche effect | |||
| Early menarche/short tempo | 62 | 15.73 (14.3–17.30) | Reference |
| Early menarche/long tempo | 29 | 18.84 (17.05–20.81) | 0.01 |
| Late menarche/short tempo | 29 | 21.33 (18.99–23.97) | < 0.001 |
| Late menarche/long tempo | 62 | 20.88 (17.49–24.92) | < 0.001 |
| Menarche effect | |||
| Early thelarche/short tempo | 29 | 20.47 (16.18–25.91) | Reference |
| Early thelarche/long tempo | 62 | 19.81 (17.49–22.44) | 0.82 |
| Late thelarche/short tempo | 62 | 16.34 (14.64–18.23) | 0.11 |
| Late thelarche/long tempo | 29 | 20.40 (16.52–25.20) | 0.98 |
The means and 95% confidence intervals are generated from stratified models including the same covariates included in model 3 of Table 2. The following variables were adjusted as fixed effects: adult covariates, including parity (nulliparous vs parous), duration of hormone use (years, continuous), education (some college or less (reference), bachelor degree, graduate degree), race (white vs. non-white), smoking status (never vs ever), and whole-body percent fat measured by DXA (%, continuous), height (continuous), and BMI at 8–10 years of age expressed as a z-score relative to CDC 2000 Growth Charts. Clinic was adjusted for as a random effect
There was no interaction between thelarche-to-menarche tempo and diet intervention assignment. In sensitivity analyses, restricting to white women, nulliparous women, women not using hormonal contraceptives, or women with baseline BMI z-score < 1.5 did not change results substantially (data not shown).
Associations of pubertal timing and tempo with ADBV and ANDBV are shown in Additional file 1: Tables S2 and S3. None of the fully adjusted associations were statistically significant.
Discussion
In this prospective study with over 20 years of follow-up, girls with a thelarche-to-menarche tempo of approximately 3 years or more have significantly higher %DBV in their mid-to-late twenties compared to girls with a tempo of ~1.5 years or less. Age at thelarche, but not menarche, also was associated with %DBV in our fully adjusted models.
This prospective study demonstrates an association between clinically assessed pubertal timing and tempo with %DBV in young women. One previous study by Schoemaker et al. evaluated recalled pubertal timing in relation to adult breast density [20]. In that study, which measured breast density from mammograms at age 40–75 years, women with higher absolute dense breast area had a longer thelarche-to-menarche duration independent of the timing of pubertal onset. The association of tempo with percent density followed a similar pattern but it was not statistically significant. The current study prospectively confirms a positive relationship between pubertal tempo with breast density, though not absolute dense breast volume. There were several differences between the previous study and ours. We measured volumetric breast density by MRI, whereas Schoemaker et al. measured areal breast density from mammograms, which could contribute to different results even though MRI and mammographic breast density measures are highly correlated [20]. We ascertained thelarche and pubarche by annual Tanner staging and menarche by self-report to the nearest month during adolescence, whereas in the previous study ages (in years) of thelarche and menarche were recalled decades later. We also measured height and weight in childhood, whereas participants in the study by Schoemaker et al. recalled their body size in relation to peers [20]. Furthermore, we adjusted for adult body fatness in our analysis using percent body fat from DXA as opposed to BMI. Lastly, differences in breast composition across the life course could alter associations with pubertal tempo. In particular, our participants were considerably younger and all were premenopausal at the time of breast density assessment, whereas 80% of participants in the study by Schoemaker et al. were postmenopausal [20]. In one other prospective study by Denholm et al., ages at thelarche and menarche were positively associated with percent breast water (which is positively associated with mammographic percent density) [21]. However, they did not directly asses the association between pubertal tempo and breast density.
The mean (SD) of %DBV in our study was 27.6 (20.5), which is slightly higher than that in another small study (n = 24) of healthy premenopausal Asian women by Chen et al. that found the mean %DBV to be 21.4 (8.4) [22]. In contrast, in a study of young women’s breast tissue composition by Boyd et al. [23], the median percent water was 45%, which is substantially larger than the percent dense breast tissue that we observed. Thicker MRI sections used in the Boyd study were more likely to contain mixtures of water and fat, which may have contributed to higher overall percent water values.
Earlier age at menarche is a long established risk factor for breast cancer [24]. Even though the average age at menarche stabilized around 12 years in the 1960s [25–27], there has been a continual rise in breast cancer incidence in women younger than 50 years old [28]. Several previous studies did not observe an association of age at menarche with breast density [29–31], while others found that later age of menarche is associated with higher density [20, 32–34]. Consistent with Shoemaker et al. [20], we show that the positive association of age at menarche and %DBV is attenuated after adjusting for childhood BMI. Alternatively, there is building evidence of the potential importance of age at breast development for predicting breast cancer risk [5]. Age at thelarche was inversely associated with non-dense breast area in ours and the study by Schoemaker et al. [20]. In another study of girls, Tanner breast stage was positively associated with concurrently measured adolescent breast density, and though attenuated, the association was still present after adjusting for menarcheal status [35]. The continual decline in age at thelarche [27, 36], the corresponding decrease in the correlation between age at menarche and age at thelarche over time [37], and the more recent finding that slow tempo is related to increased risk of breast cancer [5] suggest that the duration between thelarche and menarche may be at least as if not more informative of breast cancer risk than either marker of puberty alone.
Our study has limitations. To be eligible for the original DISC trial, children had to have high LDL-C defined as greater than or equal to the 80th and less than the 98th age- and sex-specific percentiles of the Lipid Research Clinics population, which for girls translates to 117.5–164.5 mg/dL [38]. Additional DISC eligibility criteria that girls be 7.8–10.1 years old and pre-pubertal (Tanner stage 1) may have excluded early as well as late maturers. Thus, our findings may not be generalizable to all healthy children and the resultant truncated distribution of pubertal milestones may have weakened observed associations. Furthermore, because early maturers tend to progress through puberty at a slower tempo [39], associations between tempo and density may have been weakened. While pubertal staging was assessed by a clinician, palpation was not used to distinguish between breast development and lipomastia. Therefore, breast development could have been overestimated in overweight girls. However, even after removing girls with high BMI z-scores (> 1.5), the pattern of associations between tempo and adult %DBV remained, suggesting that the finding is robust to any misclassification of lipomastia for breast development. Our findings suggest associations between age at thelarche and thelarche-to-menarche tempo and %DBV but cannot prove causation or rule out a common cause.
Despite these limitations, our study has several strengths. In particular, we were able to leverage prospectively collected data on pubertal development and breast density measured from the DISC study. Trained and certified personnel following standard protocols collected all data. Clinicians assessed sexual maturation and participants reported onset of menses annually. Finally, breast density was measured at age 25–29 years by MRI, which gives accurate and precise measurement of %DBV.
Conclusions
Our finding that a longer pubertal tempo is associated with increased %DBV may help to explain the current increasing rates of early-onset breast cancer incidence [40]. In the last 50 years, there has been a secular decline in the age of thelarche but not menarche, which translates to pubertal tempo being elongated over time [25]. Now, puberty for the general population has come to look more like those women in our study with longer tempo and associated higher breast density. Longer tempo also has been associated with increased breast cancer risk [5]. Additional studies are needed to determine if breast density mediates the association between pubertal tempo and risk.
Supplementary information
Additional file 1 : Table S1. Characteristics of DISC participants in childhood and as young adults by quartiles of thelarche to menarche tempo duration. Table S2. Geometric mean (95%CI) from mixed-effects regression models for each pubertal factor in relation to Absolute Dense Breast Volume (ADBV). Table S3. Geometric mean (95%CI) from mixed-effects regression models for each pubertal factor in relation to absolute non-dense volume (ANDBV).
Acknowledgements
The authors would like to thank the participants of the DISC06 study.
Abbreviations
- ADBV
Absolute dense breast volume
- ANDBV
Absolute non-dense breast volume
- %DBV
Percent dense breast volume
- LDL-C
Low-density lipoprotein cholesterol
- DISC
Dietary Intervention Study in Children
- T1–T5
Tanner stage
- MRI
Magnetic resonance imaging
- DXA
Dual-energy X-ray absorptiometry
Authors’ contributions
LCH designed, analyzed, interpreted, and drafted the manuscript. JFD designed the study, acquired and interpreted the data, and contributed to multiple revisions of the manuscript. SJ and RT contributed to the data analysis and interpretation. ESLB, LGS, NMH, CK, LVH, KP, and JAS contributed to data acquisition. All authors substantively revised the manuscript and have approved the submitted version.
Funding
The Dietary Intervention Study in Children (DISC) was supported by cooperative agreements U01HL37947, U01HL37948, U01HL37954, U01HL37962, U0137966, U01HL37975, and U01-HL38110 from the National Institutes of Health.
The DISC06 Follow-Up Study was supported by R01CA104670 from the National Institutes of Health.
The first author’s effort on the current manuscript was supported by 5K017CA218166-02 from the National Cancer Institute. The senior author’s effort on the current manuscript was supported by P30CA134274 from the National Institutes of Health and the Maryland Department of Health’s Cigarette Restitution Fund Program.Availability of data and materials
The datasets analyzed for the current study are available from the senior author on reasonable request.
Ethics approval and consent to participate
An NHLBI-appointed independent data and safety monitoring committee monitored the original DISC trial. Institutional review boards at all participating clinical centers and the data coordinating center approved both the DISC and the DISC06 Follow-Up Study protocols. The DISC06 Follow-Up Study protocol also was approved by the Fox Chase Cancer Center’s Institutional Review Board.
Prior to randomization, parents/guardians provided informed consent and DISC participants provided assent, while participants provided informed consent prior to the DISC06 follow-up visit.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13058-019-1209-x.
References
- 1.Sellers TA, Kushi LH, Potter JD, et al. Effect of family history, body-fat distribution, and reproductive factors on the risk of postmenopausal breast cancer. N Engl J Med. 1992;326(20):1323–1329. doi: 10.1056/NEJM199205143262004. [DOI] [PubMed] [Google Scholar]
- 2.Titus-Ernstoff L, Longnecker MP, Newcomb PA, et al. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol Biomark Prev. 1998;7(9):783–789. [PubMed] [Google Scholar]
- 3.Garland M, Hunter DJ, Colditz GA, et al. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol. 1998;147(7):636–643. doi: 10.1093/oxfordjournals.aje.a009504. [DOI] [PubMed] [Google Scholar]
- 4.Biro FM, Lucky AW, Simbartl LA, et al. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J Pediatr. 2003;142(6):643–646. doi: 10.1067/mpd.2003.244. [DOI] [PubMed] [Google Scholar]
- 5.Bodicoat DH, Schoemaker MJ, Jones ME, et al. Timing of pubertal stages and breast cancer risk: the breakthrough generations study. Breast Cancer Res. 2014;16(1):R18. doi: 10.1186/bcr3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahabir S, Aagaard K, Anderson LM, et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer Causes Control. 2012;23(6):983–990. doi: 10.1007/s10552-012-9962-5. [DOI] [PubMed] [Google Scholar]
- 7.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.epi-06-0034. [DOI] [PubMed] [Google Scholar]
- 8.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-attributable risk proportion of clinical risk factors for breast Cancer. JAMA Oncol. 2017. 10.1001/jamaoncol.2016.6326. [DOI] [PMC free article] [PubMed]
- 9.Boyd NF. Mammographic density and risk of breast cancer. Am Soc Clin Oncol Educ B. 2013. 10.1200/EdBook_AM.2013.33.e57. [DOI] [PubMed]
- 10.Dorgan JF, Liu L, Klifa C, et al. Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the dietary intervention study in children follow-up study. Cancer Epidemiol Biomark Prev. 2010;19(6):1545–1556. doi: 10.1158/1055-9965.epi-09-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obarzanek E, Hunsberger SA, Van Horn L, et al. Safety of a fat-reduced diet: the dietary intervention study in children (DISC) Pediatrics. 1997;100(1):51–59. doi: 10.1542/peds.100.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Obarzanek E, Kimm SY, Barton BA, et al. Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the dietary intervention study in children (DISC) Pediatrics. 2001;107(2):256–264. doi: 10.1542/peds.107.2.256. [DOI] [PubMed] [Google Scholar]
- 13.Van Horn L, Obarzanek E, Barton BA, et al. A summary of results of the Dietary Intervention Study in Children (DISC): lessons learned. Prog Cardiovasc Nurs. 2003;18(1):28–41. doi: 10.1111/j.0889-7204.2003.01007.x. [DOI] [PubMed] [Google Scholar]
- 14.Klifa C, Carballido-Gamio J, Wilmes L, et al. Quantification of breast tissue index from MR data using fuzzy clustering. Conf Proc IEEE Eng Med Biol Soc. 2004;3:1667–1670. doi: 10.1109/iembs.2004.1403503. [DOI] [PubMed] [Google Scholar]
- 15.Tagliafico A, Tagliafico G, Astengo D, Airaldi S, Calabrese M, Houssami N. Comparative estimation of percentage breast tissue density for digital mammography, digital breast tomosynthesis, and magnetic resonance imaging. Breast Cancer Res Treat. 2013;138(1):311–317. doi: 10.1007/s10549-013-2419-z. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5). 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed]
- 17.Bertrand KA, Baer HJ, Orav EJ, et al. Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res. 2015;17:95. doi: 10.1186/s13058-015-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000, 2002;(246):1–190 http://www.ncbi.nlm.nih.gov/pubmed/12043359. Accessed 2 Sept 2016. [PubMed]
- 19.Rubin DB. Multiple imputation for nonresponse in surveys: Wiley; 2009. https://books.google.com/books?id=cNvTIOLs_WMC. Accessed 9 Jan 2019.
- 20.Schoemaker MJ, Jones ME, Allen S, et al. Childhood body size and pubertal timing in relation to adult mammographic density phenotype. Breast Cancer Res. 2017;19(1):13. doi: 10.1186/s13058-017-0804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denholm R, De Stavola B, Hipwell JH, et al. Growth trajectories, breast size, and breast-tissue composition in a British prebirth cohort of young women. Am J Epidemiol. 2017. 10.1093/aje/kwx358. [DOI] [PMC free article] [PubMed]
- 22.Chen JH, Chen WP, Chan S, Yeh DC, Su MY, McLaren CE. Correlation of endogenous hormonal levels, fibroglandular tissue volume and percent density measured using 3D MRI during one menstrual cycle. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(9):2329–2335. doi: 10.1093/annonc/mdt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd N, Martin L, Chavez S. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10:569–580. doi: 10.1016/S1470-2045(09)70078-6. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145(3):567–579. doi: 10.1007/s10549-014-2993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplowitz P. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol. 2006;18(5):487–491. doi: 10.1097/01.gco.0000242949.02373.09. [DOI] [PubMed] [Google Scholar]
- 26.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77(3):137–145. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg SM, Newman LA, Partridge AH. Breast cancer in young women: rare disease or public health problem? JAMA Oncol. 2015;1(7):877–878. doi: 10.1001/jamaoncol.2015.2112. [DOI] [PubMed] [Google Scholar]
- 29.Heng D, Gao F, Jong R, et al. Risk factors for breast cancer associated with mammographic features in Singaporean Chinese women. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1751–1758. [PubMed] [Google Scholar]
- 30.Sung J, Song YM, Stone J, Lee K, Lee D. Reproductive factors associated with mammographic density: a Korean co-twin control study. Breast Cancer Res Treat. 2011;128(2):567–572. doi: 10.1007/s10549-011-1469-3. [DOI] [PubMed] [Google Scholar]
- 31.Rice MS, Bertrand KA, Lajous M, et al. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann Epidemiol. 2015;25(11):868–873. doi: 10.1016/j.annepidem.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler LM, Gold EB, Greendale GA, et al. Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN) Breast Cancer Res Treat. 2008;112(1):165–174. doi: 10.1007/s10549-007-9840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dite GS, Gurrin LC, Byrnes GB, et al. Predictors of mammographic density: insights gained from a novel regression analysis of a twin study. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3474–3481. doi: 10.1158/1055-9965.EPI-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lokate M, Kallenberg MG, Karssemeijer N, Van den Bosch MA, Peeters PH, Van Gils CH. Volumetric breast density from full-field digital mammograms and its association with breast cancer risk factors: a comparison with a threshold method. Cancer Epidemiol Biomark Prev. 2010;19(12):3096–3105. doi: 10.1158/1055-9965.epi-10-0703. [DOI] [PubMed] [Google Scholar]
- 35.Novotny R, Daida Y, Morimoto Y, Shepherd J, Maskarinec G. Puberty, body fat, and breast density in girls of several ethnic groups. Am J Hum Biol. 2011;23(3):359–365. doi: 10.1002/ajhb.21145. [DOI] [PubMed] [Google Scholar]
- 36.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126(3):e583–e590. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinics. LR. Population studies data book, I: the prevalence study. Washington, DC: US Public Health Service; 1980. In: lipid research clinics, ed. Washington, DC: US Public Health Service; 1980.
- 39.Pantsiotou S, Papadimitriou A, Douros K, Priftis K, Nicolaidou P, Fretzayas A. Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Paediatr. 2008;97(2):217–220. doi: 10.1111/j.1651-2227.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Table S1. Characteristics of DISC participants in childhood and as young adults by quartiles of thelarche to menarche tempo duration. Table S2. Geometric mean (95%CI) from mixed-effects regression models for each pubertal factor in relation to Absolute Dense Breast Volume (ADBV). Table S3. Geometric mean (95%CI) from mixed-effects regression models for each pubertal factor in relation to absolute non-dense volume (ANDBV).