Abstract
Objective:
The present study aimed to extract apparent diffusion coefficient (ADC) values from the diffusion-weighted imaging (DWI) sequence of endometrial lesions and compare them with tissue specimen results in order to determine the precision of ADC values in grading of malignant endometrial lesions.
Methods:
The present prospective study was conducted on 22 patients complaining of abnormal vaginal bleeding or evidence for endometrial thickening or masses detected using the ultrasound. Sampling was performed for pathological examination. MRI T2W+DWI+T1W+Post Contrast T1W were performed for patients. The statistical analysis was performed in SPSS 20 and MedCalc.
Results:
In this study, although the mean ADC value was lower in patients with endometrial cancer than those with benign endometrial lesions, the difference was not significant (0.86 ± 0.2 mm2/sec versus 1.33 ± 0.53 mm2/sec; P = 0.13). Using the cutoff point of 0.53, the sensitivity and specificity of ADC value for differentiating benign and malignant lesions, respectively, equaled 90.91 and 9.09, with an equal positive and negative predictive value of 50%. In patients with endometrioid adenocarcinoma, mean ADC value was 0.93 ± 0.15 in FIGO Grade I, and 0.76 ± 0.165 in FIGO Grade II. Based on the statistical test, no significant difference existed between the two groups in terms of ADC values.
Conclusion:
Results indicate that the use of a DWI sequence (ADC values) can prevent invasive measures in the diagnosis of benign endometrial lesions and the identification of malignant lesions with a high precision in many patients having accompanying diseases or other cases for which invasive measures cannot be used. Also, there is no significant difference in the mean ADC values between G1 and G2 of endometrioid carcinoma.
Keywords: Apparent diffusion coefficient, diffusion wheighted imaging, endometrial cancer
Introduction
Endometrial cancer is the most common malignancy of the female reproductive system, which often involve menopausal women.[1] Over half of the patients above 50 years of age and approximately 15% of cases below 40 years of age. This disease is invasive in about 20% of cases, and no reliable imaging method is available for differentiating benign and malignant endometrial lesions.[2] Transvaginal ultrasound is recommended as the first diagnostic method for evaluating postmenopausal bleeding. However, ultrasound findings, including the heterogeneity and irregular thickening of the endometrium are not reliable for differentiating benign proliferation, hyperplasia, polyps, and endometrial cancer. For a definitive diagnosis, endometrial biopsy or dilation and curettage (D and C) are required. The disadvantages of biopsy include the high risk of obtaining insufficient specimen and a sensitivity of less than 50%.[1,2] Although the false negative cases of D and C are low (<10%), it is considered an invasive method. Modern imaging provides the possibility of preoperational evaluation of endometrial cancer for the optimal planning of treatment and surgery.[3] Magnetic resonance imaging (MRI) is the imaging method of choice for the preoperational evaluation of endometrial cancer due to its high tissue resolution contrast and replicability. Still, there is no consensus as to the usefulness of MRI, and the focus does not always clearly show endometrial cancer.[2,4] Based on results of a meta-analysis, dynamic T1-weighted (T1W) postcontrast sequences were superior to ultrasound, computed tomography (CT), and MRI without contrast.[3] Dynamic MRI postcontrast sequences have a higher precision compared with T2-weighted (T2W) sequences in identifying tumors and evaluating invasion. Still, due to the risk of nephrogenic systemic fibrosis progress and, therefore, renal failure following MRI, the necessity of imaging without contrast has increased in examining endometrial pathologies and myometrium invasion in patients with endometrial cancer. Nevertheless, regular MRI sequences cannot differentiate cancer, hyperplasia, and benign endometrial polyps.[1] Diffusion-weighted imaging (DWI) is a functional MRI (fMRI) providing a tissue contrast different from that observed using regular T1W and T2W.[5] In many imaging centers, DWI is routinely performed in addition to regular MRI for preoperational grading of endometrial cancer.[6] In general, malignant lesions have a higher cellularity than benign ones. Thus, the use of apparent diffusion coefficient (ADC) values helps the differentiation of benign and malignant lesions.[7,8] The usefulness of ADC values has been proved in making diagnoses in the breasts, liver, kidneys, prostate, bladder, and cervix.[7,9] It has been reported that the ADC map resulting from the DWI sequence can assist the differentiation of benign and malignant endometrial lesions. However, the diagnostic value of DWI and the quantitative analysis of ADC is controversial, especially in examining the depth of myometrial invasion and tumor grade.[10] As endometrial cancer occurs in postmenopausal women with accompanying diseases, including diabetes, hypertension, and obesity, the effective selection of patients at risk of relapse following radical surgery and auxiliary therapy is of utmost importance. In addition, tumor grade, depth of myometrial invasion, and pelvic lymph node involvement are important for selecting the method of surgery. It has been reported that DWI can assist the identification of myometrium invasion depth and tumor grade. Few researchers agree with previous reports on the capability of ADC values in differentiating tumor grades. In other words, contradictory findings have been reported in this regard.[1,11]
The present study aimed to extract ADC values from the DWI sequence of endometrial lesions and compare them with tissue specimen results in order to determine the precision of ADC values in the identification of benign and malignant endometrial lesions while also determining the appropriate cutoff for these lesions and determination of mean ADC values and its significance in different grades of endometrial cancer. In this way, invasive measures for the diagnosis of benign and malignant endometrial lesions can be prevented, especially in the case of patients with accompanying diseases. Also, this study contributes to the development of an appropriate strategy for the treatment of patients with different grades of cancer.
Materials and Methods
The present epidemiological prospective study—which was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences—was conducted in a 1-year period on eligible 22 patients visiting the Gynecology Clinic of Ahvaz Imam Khomeini Hospital, Iran, complaining of abnormal vaginal bleeding with evidence for endometrial thickening or masses in ultrasound. All patients gave written informed consent.
Exclusion criteria were having any MRI contraindication, claustrophobia, or inability to undergo MRI, any contraindication for receiving the contrast T1W medium, being treated in other hospitals, having endometrial cancer for which no following surgery was going to be performed, having received chemotherapy or radiotherapy before the surgery, and undergoing MRI and surgery over 30 days apart. A questionnaire was administered to collect demographic information, including age, menopausal status, BMI, and risk factors for endometrial cancer. Sampling was performed for pathological examination in terms of benignity or malignancy, type of benign lesions, cancer subtype, and histological grade of cancer. In the next step, MRI T2W+DWI+T1W+Post Contrast T1W were performed for patients in Golestan Hospital, Ahvaz, with settings presented in Table 1. All patients fasted for 6 h and emptied their bladder about 1 h before MRI. Right before the MRI, to decrease the artifact caused by bowel movement, 20 mg of butylscopolamine was intramuscularly injected. For all patients, MRI1.5 T (Symphony: Siemense, Erlangen, Germany) of the pelvis (and abdomen, for malignant cases) including axial and sagittal sequences of turbo spin-echo T2W; axial DWI with the b-value of 0, 500, and 1000; axial Post Contrast T1W gradient echo dynamic with fat saturation at 0 sec and 120 sec; and sagittal Post Contrast T1W gradient echo dynamic with fat saturation at 180 sec following contrast injection were performed. MRI sequences were reported in separate files by two experienced radiologist in a blind manner, including endometrial thickness, longest tumor diameter, and lesion or endometrial signal in T2W images. Moreover, lesions were examined in terms of extension to myometrium and cervical stroma as well as pelvic or lumboaortic lymph node involvement. An ADC map was prepared from DWI isotropic images using the Syngo software (Siemens, Erlangen, Germany). Regions of interest (ROI) was manually drawn for involving more parts of the lesion or endometrium in order to obtain ADC values. Necrotic regions, tumor–air interface, and blood circulation were removed from the ROI. In cases when the suspicious region was not clear in ADC images, the noted region was identified using T2W images, and the ROI was drawn in its corresponding region in ADC images. In patients with malignant lesions based on the initial sampling and suspicious imaging evidence, full abdominal hysterectomy with bilateral salpingo-oophorectomy, pelvic lymphadenectomy, and omental sampling were performed for treatment and staging. In patients with nonmalignant lesions, the required treatment including simple hysterectomy with bilateral oophorectomy or D and C with pharmacotherapy and follow-up with resampling were performed based on their clinical conditions, menopausal status, or need for fertility preservation. Patients with indications for surgery underwent surgery in 3–30 days following the MRI. The surgical sample was sent to an experienced pathologist for histological examination in terms of cancer subtype, cancer histological grade, and level of myometrium, cervical stroma, and lymph node involvement.
Table 1.
MRI T2W+DWI+T1W+Post Contrast T1W for patients in Golestan Hospital, Ahvaz
| Sequences | Sagittal t2w | Axial t2w | Axial DWI | Axial t1 + GAD flash - 2D + fat suppressed T=0 s | Axial t1 + GAD flash - 2D + fat suppressed T=120 s | Axial t1 + GAD flash - 2D + fat suppressed T=180 s | Axial t2 TSE fat suppressed | Axial t2 TSE | Axial t1 flash-2D fat standard | Axial t1 + GAD flash-2D fat standard |
|---|---|---|---|---|---|---|---|---|---|---|
| Range | pelvic | pelvic | pelvic | pelvic | pelvic | pelvic | Abdomen | Abdomen | Abdomen | Abdomen |
| sections | 19 | 19 | 20 | 19 | 19 | 19 | 26 | 26 | 25 | 25 |
| TR | 3570 ms | 3754 ms | 4400ms | 236 ms | 236 ms | 236 ms | 4000 ms | 4000 ms | 140 ms | 140 ms |
| TE | 90 ms | 90 ms | 117 ms | 2.89 ms | 2.89 ms | 2.89 ms | 86 ms | 86 ms | 2.89 ms | 2.89 ms |
| Slice thickness (mm) | 3mm | 4mm | 4mm | 4mm | 4mm | 4mm | 6mm | 6 mm | 6mm | 6mm |
| Gap (mm) | 1.5 mm | 1.5 mm | 0.4 mm | 1.5 mm | 1.5 mm | 1.5 mm | 1.2 mm | 1.2 mm | 1.2 mm | 1.2 mm |
| FOV (mm) | 280 | 320 | 250 | 280 | 280 | 280 | 300 | 300 | 300 | 300 |
| B | b=0 b=500 b=1000 |
- | - | - | - | |||||
| Matrix | 280*120 | 320*320 | 250 *160 | 280*256 | 280*256 | 280*256 | 380*256 | 380*256 | 380*256 | 380*256 |
| Acquisition time (sec) | 119 s | 125s | 138 s | 22 s | 22 s | 22 s | 32 s | 32 s | 40 s | 40 s |
Statistical analysis
In this study, data were entered into the SPSS 20 software and analyzed. Quantitative data were described using mean and standard deviation and qualitative data were described using by frequency and percentage. In this study, the mean of quantitative data between the two groups was analyzed by Independent sample T-test and Chi-square test was used to compare the qualitative data. All results of statistical tests were considered less than 0.05. Also, sensitivity and specificity analysis was used to check and compare the diagnostic accuracy and ROC curve test was used to determine the desired cutting point.
Results
From May 2017 to July 2018, 22 patients (mean age of 50.52 ± 9.12 years) complaining of abnormal vaginal bleeding or evidence for endometrial thickening or mass were recruited. Of these patients, 11 (50%) had benign lesions based on D and C sampling or pathologic results (three cases with endometrial polyps, three with endometrial hyperplasia, and five with normal proliferative endometrium) and 11 patients (50%) had endometrial cancer based on the pathological reports (nine cases, i.e., 81.82%, with low-grade endometrioid tumor, and two cases, i.e., 18.18%, with high-grade nonendometrioid tumor). Nonendometrioid cases included one case of carcinosarcoma and one case of serous adenocarcinoma. Endometrioid cases included five cases (55.56%) of FIGO Grade I and four cases (FIGO %) of FIGO Grade II, and no patient was FIGO Grade III [Tables 2 and 3].
Table 2.
Diagnostic accuracy of myometrial invasion, cervical stromal infiltration, pelvic or lumboaortic lymphadenopathy, FIGO staging (2009) on T2W+DWI correlated with pathologic findings
| Radiological findings | Pathological findings | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Myometrial invasion | < 50% | ≥50% | |||||||
| < 50% | 4 | 1 | 5 | ||||||
| ≥50% | 3 | 3 | 6 | ||||||
| Total | 7 | 4 | 11 | ||||||
| Cervical stromal infiltration | No | Yes | Total | ||||||
| No | 6 | 3 | 9 | ||||||
| Yes | 1 | 1 | 2 | ||||||
| Total | 7 | 4 | 11 | ||||||
| Pelvic or Lumboaortic metastatic lymphadenopathy | No | Yes | Total | ||||||
| No | 7 | 0 | 7 | ||||||
| Yes | 3 | 1 | 4 | ||||||
| Total | 10 | 1 | 11 | ||||||
| FIGO Staging | IA | IB | II | IIIA | IIIB | IIIC | IVA | IVB | Total |
| IA | 3 | - | - | - | - | - | - | 3 | |
| IB | 1 | - | 2 | - | - | - | - | - | 3 |
| II | - | - | - | - | - | - | - | - | - |
| IIIA | - | - | - | - | - | - | - | - | - |
| IIIB | - | - | - | - | - | - | - | - | - |
| IIIC | - | - | - | - | 1 | 1 | - | - | 4 |
| IVA | 2 | - | - | - | - | - | - | 1 | 1 |
| IVB | - | - | - | - | - | - | - | - | - |
| Total | 6 | 2 | - | 1 | 1 | - | 1 | 11 | |
Note: Accuracy, 63.81% in depth of myometrial invasion, 63.81% in cervical stromal infiltration, 72.72% in detection of metastatic lymphadenopathy.
Table 3.
Tumor characteristics among patients
| Characters | Patients (Mean, Range) |
|---|---|
| Age | 52.5±12.08 |
| MR presentation of malignant lesions on T2W | |
| Mass | 8 (36.4%) |
| Endometrial thickness | 8 (36.4%) |
| Normal endometrium | 6 (27.3%) |
Imaging data
Eight cases (72.73%) from malignant lesions in the form of a mass, two cases (18.18%) in the form of endometrial thickening, and one case (9.09%) with normal endometrial thickness were manifested by MRI T2W. Mean endometrial thickness was 19.46 ± 14.56 mm in the ultrasound of patients with endometrial cancer, and 18.79 ± 9.33 mm in those with benign lesions. Thus, no significant difference was found between the two groups in terms of mean endometrial thickness based on ultrasound (P = 0.90). Mean endometrial thickness was 13.27 ± 14.56 mm in the T2W MRI of patients with benign lesions, and 25.09 ± 14.34 mm in those with endometrial cancer. Based on the statistical analysis, the mean endometrial thickness reported by T2W MRI was significantly more in patients with endometrial cancer than those with benign endometrial lesions (P = 0.03) [Table 4].
Table 4.
Comparison mean of endometrial thickness via ultrasonography or T2W MRI
| Imaging findings | Benign lesions | Malignant lesions | P |
|---|---|---|---|
| Mean endometrial thickness on ultrasound | 18.79±9.34 | 19.45±14.56 | 0.90 |
| Mean endometrial thickness on T2W | 13.27±6.93 | 25.09±14.34 | 0.03 |
In six cases (54.55%) of patients with benign lesions, and one case (9.09%) with endometrial cancer, adenomyosis was found as a coexisting finding in T2W MRI. All patients with endometrial cancer has a high signal in T2W MRI images, but clear restriction in the ADC map was observed in only eight cases qualitatively [Tables 3 and 4].
In this study, although the mean ADC value was lower in patients with endometrial cancer [Figures 1 and 2] than those with benign endometrial lesions, the difference was not significant (0.86 ± 0.2 mm2/sec versus 1.33 ± 0.53 mm2/sec; P = 0.13). Using the cutoff point of 0.53, the sensitivity and specificity of ADC value for differentiating benign and malignant lesions, respectively, equaled 90.91 and 9.09, with an equal positive and negative predictive value of 50%. Based on the ROC curve, ADC value has a high diagnostic power for differentiating benign and malignant endometrial lesions [Table 3].
Figure 1.
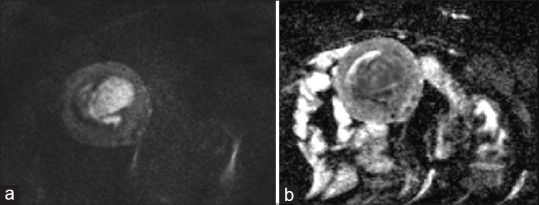
MR images of a 70-year-old woman with histopathologically proven uterine endometrial carcinosarcoma. (a) the mass show high signal intensity in DWI (b value = 1000),(b) with diffusion restriction on ADC map, ADC Values = 0.92 × 10-3 mm2/s
Figure 2.
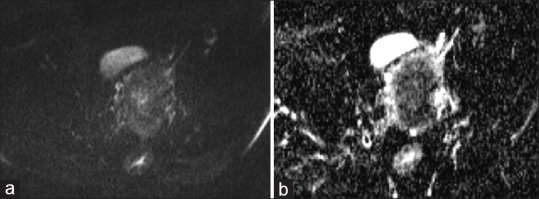
MR images of a 56-year-old woman with histopathologically proven endometrial endometrioed carcinoma. (a) show intermediate signal intensity in DWI (b value = 1000), (b) with diffusion restriction on ADC map, ADC Values = 0.65 × 10-3 mm2/s
In patients with endometrioid adenocarcinoma, mean ADC value was 0.93 ± 0.15 in FIGO Grade I [Figure 3], and 0.76 ± 0.165 in FIGO Grade II. Based on the statistical test, no significant difference existed between the two groups in terms of ADC values (P = 0.18) [Table 5].
Figure 3.
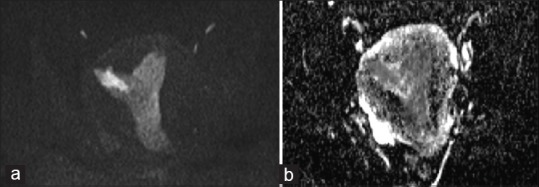
MR images of a 46-year-old woman with histopathologically proven endometrial well-differentiated endometrioed carcinoma (FIGO grade 1) (a) show high signal intensity in DWI (b value = 1000), (b) with diffusion restriction on ADC map, ADC Values = 0.88 × 10-3 mm2/s
Table 5.
Tumor ADC value in relation to histological characteristics among study patients
| Study variables | n | ADC value | P |
|---|---|---|---|
| Histology | |||
| Benign | 11 | 1.33±0.53 | 0.013 |
| Malignant | 11 | 0.87±0.21 | |
| Histological subtypes of endometrial carcinoma | |||
| Endometrioid | 9 | 0.85±0.16 | 0.73 |
| Nonendometrioid | 2 | 0.89±0.16 | |
| Histological grade of endometrioid type | EE | 0.18 | |
| G1 | 5 | 0.93±0.15 | |
| G2 | 4 | 0.76±0.165 |
The level of myometrium invasion was estimated by T2W + DWI images at below 50% in five patients (45.45%) and equal to or above 50% in six patients (54.55%). Nevertheless, in pathological results, it was reported at below 50% in seven patients (63.64%) and equal to or above 50% in four patients (36.36%). In three cases, the level of myometrium invasion was estimated to be higher, and in one case, it was estimated to be lower. Based on these points, the precision, sensitivity, and specificity of T2W+DWI, respectively, were 63.81%, 75%, and 57% in estimating the level of myometrial invasion compared with pathological results. Also, the positive and negative predictive value of T2W+DWI were, respectively, 50% and 80% compared with pathological results [Table 2].
In two cases (18.18%) of patients, cervical stroma infiltration was observed in T2W + DWI images, but stroma infiltration was observed in four patients (36.36%) based on pathological results. In one case, the MRI estimated involvement lower than the value reported by pathological results. Based on these results, the precision, sensitivity, and specificity of T2W+DWI, respectively, were 63.81%, 25%, and 85.71% in identifying cervical stroma infiltration compared with pathological results. Also, the positive and negative predictive value of T2W+DWI were, respectively, 50% and 33.33% in this case [Table 2].
The metastatic involvement of lumboaortic and pelvic lymph nodes was seen in four patients (36.36%) based on T2W+MRI images, but only one patient (9.09%) showed an actual metastatic involvement of the noted regions based on pathological findings. Based on these results, the precision, sensitivity, and specificity of T2W+DWI, respectively, were 72.72%, 100%, and 70% in identifying the metastatic involvement of lumbaraortic and pelvic lymph nodes compared with pathological results. Also, the positive and negative predictive value of T2W+DWI were, respectively, 25% and 100% in this case [Table 2].
FIGO stage was IA in three patients (27.27%), IB in three patients (27.27%), IIIC in four (36.36%), and IVB in one (9.09%) based on the imaging view. However, based on pathologic results, it was IA in six (54.55%), II in two (18.18%), IIIA in one (9.09%), IIIB in one (9.09%), and IVB in one (9.09%) based on pathologic results [Table 2].
Discussion
The present study aimed to extract ADC values from endometrial lesions and compare them with tissue specimen results in order to determine the precision of ADC values in the identification of benign and malignant endometrial lesions while also determining the appropriate cutoff for these lesions and assessment of mean ADC values in grades of endometrial carcinoma. Results revealed that the mean endometrial thickness reported by T2W MRI was significantly more in patients with endometrial cancer than those with benign endometrial lesions. Moreover, all patients with endometrial cancer had a high signal in T2W MRI images, but a clear restriction in the ADC map was observed in only 72.72% of them. Although the mean ADC value was lower in patients with endometrial cancer than those with benign endometrial lesions, the difference was not significant. There was no significant difference in the mean ADC values between G1 and G2 of endometrioid carcinoma. Furthermore, results indicated that the ADC value has a high diagnostic power for differentiating benign and malignant endometrial lesions. In the prospective study by Shen et al. on 32 patients suspicious of endometrial cancer using transvaginal ultrasound, postcontrast T1W and MRI (DWI) was requested for further evaluation. Mean ADC value was 0.864 × 10-3 mm2/sec in patients with endometrial cancer, and 1.277 × 10-3 mm2/sec in those with benign lesions, showing a significant difference (P = 0.0058). The diagnostic precision in evaluating myometrial invasion was 61.9% for DWI, and 71.4% for T1W postcontrast. In five cases, DWI provided information on tumor expansion and showing tumor focus, thereby changing the preoperational staging.[2] Similar to the mentioned study, in the present study, mean ADC values significantly differed in patients with benign lesions compared to those with endometrial cancer. In the prospective study by Tamai et al., in the DWI sequence of 18 cases of endometrial cancer and 12 cases of normal endometrium (in patients with cervical cancer), all cases had a high signal. Mean ADC values were 0.88 ± 0.10 × 10-3 mm2/sec in endometrial cancer, and 1.53 ± 0.10 × 10-3 mm2/sec in normal endometrium, which was significant (P < 0.01). Moreover, mean ADC values were significantly less in G3 than G1 (P < 0.05).[10] Fujii et al. in a retrospective study, performed DWI + T2W sequences for 25 patients with endometrial lesions. ADC values significantly differed across benign and malignant endometrial lesions (P < 0.01). Also, the ADC values’ cutoff point of 1.15 × 10-3 mm2/sec mm was defined with the highest precision (sensitivity of 84.6%, specificity of 100%, and precision of 92%) for malignant lesions.[12] In the present study, using the cutoff point of 0.53, the sensitivity and specificity of ADC value for differentiating benign and malignant lesions, respectively, equaled 90.91 and 9.09, with an equal positive and negative predictive value of 50%, showing a significant difference similar to the study by Fujii et al. In line with the result of the present study, in the study by Kilikesmez et al., ADC values significantly differed across benign and malignant endometrial lesions (P < 0.01). For malignant lesions, the ADC value cutoff point of 1.05 × 10-3 mm2/sec with the sensitivity of 95.83%, specificity of 94.55%, and precision of 94.94% was defined.[13] In the study by Reichichi et al., mean ADC values of endometrial cancer were significantly lower than those of normal endometrium and myometrium with no overlap (P < 0.0001), but no significant difference existed between grades of endometrial cancer (G1, G2, G3), deep and superficial myometrium involvement, or presence or absence of metastatic lymphadenopathy.[14] Results of the present study show that the precision, sensitivity, and specificity of T2W+DWI, respectively, are 63.81%, 75%, and 57% in estimating the level of myometrial invasion compared with pathological results. Also, the positive and negative predictive value of T2W+DWI are, respectively, 50% and 80% compared with pathological results. In the study by Seo et al., of 52 patients with endometrial cancer, DWI, postcontrast T1W, and T2W sequences were prepared. Mean ADC values in endometrial cancer were significantly below those in normal myometrium (P < 0.001) and lower in Grades 2 and 3 than Grade 1 of tumor (P < 0.01). In predicting myometrial invasion, the specificity, precision, and area-under-the-curve in T2W+DWI reported by both radiologists interpreted similar postcontrast T1W images (P < 0.05).[15] Moreover, in the study by Woo et al. on 33 patients with endometrial cancer, in all ADC slices showing the tumor, ROIs including necrotic regions were prepared to draw the ADC volume-based histogram. The SD, quartile, and 75, 90, and 95 percentiles for ADC values showed a significant difference among various cancer grades (P < 0.03) and between high and low grades (P < 0.024). No significant difference existed between other parameters, and they had ROC curve analysis, sensitivity, and specificity of 75%, 96%, 62.5%, 92%, 100%, 52%, 100%, 72%, 100%, and 88% for SD, quartile, and 75, 90, and 95 percentile in the identification of high-grade lesions.[11] In the study by Bonatti et al., MRI (T2W + DWI + Post Contrast T1W) was performed on 52 patients with endometrial cancer. T2W+DWI sequences had a higher diagnostic power compared with T2W+Post-Contrast T1W sequences in identifying the infiltration depth of the myometrium (precision, sensitivity, and specificity of 89%, 89%, and 89% versus 86%, 84%, and 86%) (P < 0.05). However, both had similar diagnostic power in examining cervical stroma infiltration (precision, sensitivity, and specificity of 95%, 98%, and 80%) (P < 0.05).[4] On the other hand, results of the present study show that, in one case, MRI estimated involvement lower than the value reported by pathological results, and the precision, sensitivity, and specificity of T2W+DWI, respectively, equaled 63.81%, 25%, and 85.71% in identifying cervical stroma infiltration, with the positive and negative predictive value of 50% and 33.33% in this case. Abkenari et al. requested T2W+DWI+T1W Post Contrast sequences in a prospective study on 30 patients with endometrial cancer. The level of convergence between radiological and pathological results in identifying the cancer stage was approximately 0.8 (P < 0.001). The diagnostic accuracy of dynamic and diffusion-based techniques was 0.83. In differentiating metastatic and nonmetastatic lymph nodes, a complete agreement existed between radiologic and pathological findings.[16] In the present study, in three cases, the level of myometrium invasion was estimated to be higher, and in one case, it was estimated to be lower. Based on these points, the precision, sensitivity, and specificity of T2W+DWI, respectively, are 63.81%, 75%, and 57% in estimating the level of myometrial invasion compared with pathological results. Also, the positive and negative predictive values of T2W+DWI are, respectively, 50% and 80% compared with pathological results. Research shows that the incidence of endometrial cancer is increasing as a result of prolonged life and higher obesity.[3] The major manifestation of endometrial cancer is abnormal uterine bleeding, which is often characterized by endometrial thickening or masses. Some benign endometrial lesions, including polyps and hyperplasia, may also lead to uterine bleeding, endometrial thickening, or masses.[1] Awareness of tumor extension and the accurate examination of myometrium invasion depth and cervical stroma involvement before the surgery are key factors for choosing the treatment method, which includes radical hysterectomy and lymphadenectomy.[6] Prognosis of endometrial cancer depends on the stage of the disease, depth of myometrial invasion, involvement of lymph nodes, tumor grade, cellular type, and the patients’ age.[17] Endometrial cancer is staged based on surgical and pathological findings and the International Federation of Gynecology and Obstetrics (FIGO) system.[3] It is difficult to perform biopsy and D and G in patients with vaginal and cervical stenosis[2] or may be impossible.
To perform or require general anesthesia in menopausal women due to endometrial atrophy or endometrial adhesions.[1] On the other hand, noninvasive techniques are more appropriate than transvaginal ones in those with no experience of sexual intercourse.[2] It has also been reported that low-risk patients do not necessarily benefit from lymphadenectomy, which has complications, especially in those with accompanying diseases.[6,18] The classic MRI protocol for preoperational staging in endometrial cancer includes postcontrast dynamic T2W and T1W sequences.[4] Endometrial cancer is characterized by a thick endometrium in the T2W sequence. Because it may differ from a high to a low signal (which is usually not differentiable from normal endometrium or normal myometrium), MRI does not always clearly show the focus on endometrial cancer.[19] To overcome the morphologic limitations of DWI images, a combination of T2W and DWI sequences with a high b-factor has been used for screening for malignant endometrial tumors in T1.5 MRI.[20] In DWI images, the tumor has a low signal and clearly restricted diffusion, which facilitates the differentiation of tumor from normal tissue. Consequently, the precision of MRI increases upon combination with the T2W sequence in identifying myometrial invasion.[21]
Based on the results of the present study, it can be concluded that the use of ADC values (DWI) has an important role in preventing invasive measures in the diagnosis of benign endometrial lesions and the identification of malignant lesions with a high precision in many patients having accompanying diseases or other cases for which invasive measures cannot be used. There is no significant difference in the mean ADC values between G1 and G2 of endometrioid carcinoma, however, similar studies are recommended with more patients.
Conclusion
Results indicate that the use of a DWI sequence (ADC values) can prevent invasive measures in the diagnosis of benign endometrial lesions and the identification of malignant lesions with a high precision in many patients having accompanying diseases or other cases for which invasive measures cannot be used. Also, there is no significant difference in the mean ADC values between G1 and G2 of endometrioid carcinoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Çavuşoǧlu M, Ciliz DS, Ozsoy A, Duran S, Elverici E, Atalay C, et al. Diffusion-weighted MRI of postmenopausal women with vaginal bleeding and endometrial thickening: Differentiation of benign and malignant lesions. J Belg Soc Radiol. 2016;100:70. doi: 10.5334/jbr-btr.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen S-H, Chiou Y-Y, Wang J-H, Yen M-S, Lee R-C, Lai C-R, et al. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. Am J Roentgenol. 2008;190:481–8. doi: 10.2214/AJR.07.2155. [DOI] [PubMed] [Google Scholar]
- 3.Barwick T, Rockall A, Barton D, Sohaib S. Imaging of endometrial adenocarcinoma. Clin Radiol. 2006;61:545–55. doi: 10.1016/j.crad.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti M, Stuefer J, Oberhofer N, Negri G, Tagliaferri T, Schifferle G, et al. MRI for local staging of endometrial carcinoma: Is endovenous contrast medium administration still needed? Eur J Radiol. 2015;84:208–14. doi: 10.1016/j.ejrad.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Inada Y, Matsuki M, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, et al. Body diffusion-weighted MR imaging of uterine endometrial cancer: Is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol. 2009;70:122–7. doi: 10.1016/j.ejrad.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Husby J, Salvesen Ø, Magnussen I, Trovik J, Bjørge L, Salvesen H, et al. Tumour apparent diffusion coefficient is associated with depth of myometrial invasion and is negatively correlated to tumour volume in endometrial carcinomas. Clin Radiol. 2015;70:487–94. doi: 10.1016/j.crad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–8. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 8.Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: A clinical prospective study. J Magn Reson Imaging. 2006;24:319–24. doi: 10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 9.Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: Comparison with the normal uterine cervix. Eur Radiol. 2005;15:71–8. doi: 10.1007/s00330-004-2529-4. [DOI] [PubMed] [Google Scholar]
- 10.Tamai K, Koyama T, Saga T, Umeoka S, Mikami Y, Fujii S, et al. Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging. 2007;26:682–7. doi: 10.1002/jmri.20997. [DOI] [PubMed] [Google Scholar]
- 11.Woo S, Cho JY, Kim SY, Kim SH. Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: A preliminary correlation study with histological grade. Acta Radiol. 2014;55:1270–7. doi: 10.1177/0284185113514967. [DOI] [PubMed] [Google Scholar]
- 12.Fujii S, Matsusue E, Kigawa J, Sato S, Kanasaki Y, Nakanishi J, et al. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: Initial results. Eur Radiol. 2008;18:384–9. doi: 10.1007/s00330-007-0769-9. [DOI] [PubMed] [Google Scholar]
- 13.Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A. Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol. 2009;50:340–7. doi: 10.1080/02841850902735858. [DOI] [PubMed] [Google Scholar]
- 14.Rechichi G, Galimberti S, Signorelli M, Franzesi CT, Perego P, Valsecchi MG, et al. Endometrial cancer: Correlation of apparent diffusion coefficient with tumor grade, depth of myometrial invasion, and presence of lymph node metastases. Am J Roentgenol. 2011;197:256–62. doi: 10.2214/AJR.10.5584. [DOI] [PubMed] [Google Scholar]
- 15.Seo JM, Kim CK, Choi D, Kwan Park B. Endometrial cancer: Utility of diffusion-weighted magnetic resonance imaging with background body signal suppression at 3T. J Magn Reson Imaging. 2013;37:1151–9. doi: 10.1002/jmri.23900. [DOI] [PubMed] [Google Scholar]
- 16.Seyed Abkenari S, Faeghi F, Arian A. Diagnostic accuracy of diffusion weighted imaging and dynamic imaging techniques in endometrial and lymph nodes cancer staging. Horizon Med Sci. 2016;22:253–60. [Google Scholar]
- 17.Berman ML, Ballon SC, Lagasse LD, Watring WG. Prognosis and treatment of endometrial cancer. Am J Obstet Gynecol. 1980;136:679–88. doi: 10.1016/0002-9378(80)91024-8. [DOI] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: Management of endometrial cancer. Obstet Gynecol. 2005;106:413–25. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 19.Manfredi R, Gui B, Maresca G, Fanfani F, Bonomo L. Endometrial cancer: Magnetic resonance imaging. Abdom Imaging. 2005;30:626–36. doi: 10.1007/s00261-004-0298-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsushima Y, Takano A, Taketomi-Takahashi A, Endo K. Body diffusion-weighted MR imaging using high b-value for malignant tumor screening: Usefulness and necessity of referring to T2-weighted images and creating fusion images. Acad Radiol. 2007;14:643–50. doi: 10.1016/j.acra.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lin G, Ng KK, Chang CJ, Wang JJ, Ho KC, Yen TC, et al. Myometrial invasion in endometrial cancer: Diagnostic accuracy of diffusion-weighted 3.0-T MR imaging—initial experience 1. Radiology. 2009;250:784–92. doi: 10.1148/radiol.2503080874. [DOI] [PubMed] [Google Scholar]


