Abstract
Type 2 diabetes is characterised by a progressive decline in insulin secretion, and sooner or later patients require insulin therapy. However, physicians are reluctant to initiate insulin therapy because of perceived inadequacy in managing insulin therapy, cost and lack of benefits. Experts from across the country met at a workshop during 12th National Insulin Summit which was held in September at Hyderabad and came up with key recommendations to build capacity and confidence in general practitioners for insulin usage. Barriers can be overcome through self-education and training; effective patient education; imparting coping skill training to patients; and bridging gaps to improve adherence. Moreover, optimum insulinization requires knowledge about the available options for initiation and intensification of insulin therapy; various insulin regimens; dosing and titration; and choosing effective and simple insulin therapy as per patient characteristics. Hence, the objective of this review article is to help build capacity and confidence among general practitioners on optimising insulin therapy.
Keywords: Diabetes, general practitioners, insulin initiation, insulin intensification, insulin therapy
Introduction
Need for insulin
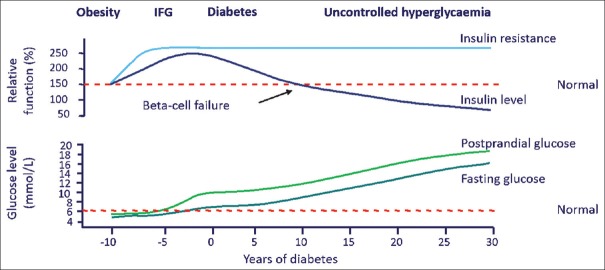
The pathophysiology of type 2 diabetes mellitus (T2DM) is characterised by insulin resistance and a progressive defect in insulin secretion. The United Kingdom Prospective Diabetes Study (UKPDS) highlighted that 40%–50% of beta-cell function is already lost at the time of diagnosis with a further decrease in beta-cell function as diabetes progresses.[1] Reduction in mean volume of beta cells by approximately 50% relative to normal subjects has also been seen.[2] In the early stages, beta-cells compensate for insulin resistance by secreting more insulin, but with time, their function is reduced [Figure 1] ultimately leading to a state of insulin deficiency. Consequently, exogenous insulin supplement is required to control hyperglycaemia.[3]
Figure 1.
Natural history of type 2 diabetes
Benefits of insulin
Insulin maintains normal blood glucose levels by facilitating cellular glucose uptake, and regulating carbohydrate, lipid and protein metabolism.[4] In addition, it has other benefits as mentioned in Table 1.[5] Several studies have shown that by preserving beta-cell function, early intensive insulin therapy helps in modifying the natural history of diabetes in people with newly diagnosed T2DM.[6]
Table 1.
| Vasodilatory effect |
| Anti-oxidant effect |
| Anti-platelet effect |
| Anti-thrombotic effect |
| Anti-inflammatory effect |
| Anti-apoptotic effect |
| Cardioprotective effect |
| Neuroprotective effect |
Long-term study like UKPDS has shown that with good glycemic control, the risk of micro- and macro-vascular complications are reduced.[8] Three large trials, namely, the Action in Diabetes and Vascular Disease—Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE, n = 11,140); Action to Control Cardiovascular Risk in Diabetes (ACCORD, n = 10,251); and Veterans Affairs Diabetes Trial (VADT, n = 1,791) assessed the beneficial effect of intensive glycaemic control in T2DM patients with cardiovascular risk profiles or prior events. The percentages of participants with known major cardiovascular disease (CVD) were 32%, 35%, and 40% in the three trials, respectively. These studies demonstrated a significant benefit of intensive glycaemic control on CVD in patients with shorter duration of diabetes, lower glycosylated hemoglobin (HbA1c) at entry, and/or absence of known CVD.[9] It should be noted that among all the available options, insulin has the maximum efficacy in achieving good glycaemic control.[8] With the help of newer insulin analogues, the risk of complications can be reduced and the benefits can be maximized.
Current state of insulin usage and delay in initiation
Results of DiabCare India study suggested that 93.2% of patients with diabetes in India are on oral antidiabetic drug (OAD) while 35.2% are on insulin (with or without oral antidiabetic drugs). Among insulin users, 71.1% were taking human insulin and 32% patients were on insulin analogues. Premix insulin was used by majority of patients followed by meal time insulin (39.4%) and basal-meal time insulin (19.4%). Only 19.7% of patients with type 2 diabetes had good glycaemic control. The median duration of insulin treatment was 2 years; mean dose of insulin was 32.1 ± 17 units/day.[10]
The International Diabetes Federation (IDF) global guidelines for diabetes management recommend that insulin therapy must be initiated based on individual patient characteristics; their glycaemic profile; the risk of hypoglycaemia; presence of comorbidities; and after failure to achieve glycaemic targets with single- dual-, or triple-oral therapy. The Diabetes Attitudes, Wishes and Needs (DAWN) survey indicated that Indian physicians delay insulin initiation longer than physicians from 13 other countries included in the survey. Physicians delay initiation and intensification of insulin due to cost, fear of side effects and sub-optimal knowledge about insulin therapy. Healthcare professionals delay the initiation of insulin in patients with type 2 diabetes until their HbA1c exceeds 10%. Patients are started insulin therapy after a median period of 6.2 (1-30) years after diagnosis of T2DM. As a result, patients are exposed to a significant glycaemic burden.[11]
Methods
Meeting of experts from across India was conducted as a workshop during the 12th annual National Insulin Summit in September 2018 at Hyderabad. The experts evaluated inputs from various guidelines, approved pack inserts and published scientific literature. The information was debated and discussed within the group to arrive at key recommendations which are highlighted in this article. [Figure 2].
Figure 2.
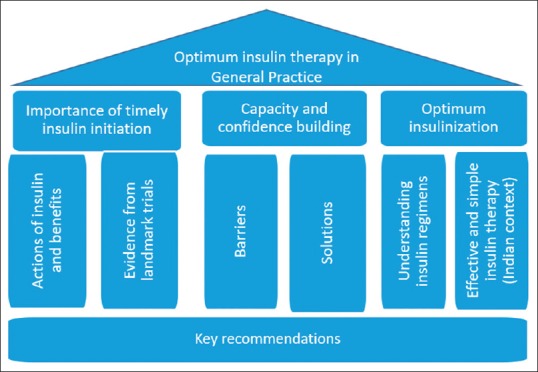
Framework for developing key recommendations
This article highlights stepwise tools for primary care physicians to understand the barriers and the various abilities which are required towards self-management education to patients with diabetes. It covers the efforts required to bridge the barriers through education and training, by overcoming time constraint, taking diabetes peer-support intervention and patient-centered approach including for both emotional and practical aspects. As a result, it will help in building capacity and confidence in the general practitioners for optimum utilisation of insulin during the management of diabetes in their patients.
Physician-related barriers to initiate insulin therapy
Physicians experience multiple barriers while trying to initiate appropriate insulin therapy in a timely manner. To overcome these barriers, physicians must acknowledge and understand the same [Table 2].
Table 2.
Physician and provider related barriers and bridges[12]
| Barrier | Subcategory | Bridges |
|---|---|---|
| Perceived inadequacy | Inadequate communication/motivation skills | Soft skills training |
| Inability to initiate, optimise, intensify insulin | CME on insulin use | |
| Perceived high cost | 3 T (time taken to teach) | Create and utilise paramedical support |
| Loss of Clientele | Create awareness of financial assistance programmes | |
| Psychological – Compassion fatigue | Coping skills training | |
| Biomedical | CME on appropriate insulin regimen, preparations and techniques | |
| Perceived lack of benefit | Low priorities of diabetes care | CME on epidemiology and its impact |
| Effects of uncontrolled diabetes | CME on diabetes complication and care |
CME: Continuing medical education
Perceived inadequacy
Many physicians feel they are ill equipped to initiate, optimise or intensify insulin therapy. Physician-related barriers due to perceived inadequacy are attributed to the physician's inability to manage patients, lack of skill, and lack of time.[12]
Inability to manage patients
In a qualitative analysis of systemic barriers to diabetes management in primary care, nearly 62% of the physicians reported inability to provide adequate self-management education (SME). Although physicians recognised the critical need for SME, they did not have reliable access to SME providers. They lacked a clear communication path to arrange SME in a timely fashion. To overcome these barriers, physicians attempted to provide SME in-clinic, which however was not successful. They perceived that their time-consuming efforts were insufficient because of limited time and knowledge, particularly with respect to nutrition counselling.[13]
Lack of skills and time
In a web-based survey of 600 physicians across six countries, nearly 40% of primary care physicians and 30% of specialists viewed administration of insulin therapy as a difficult task. They desired more support staff and resources to assist them. About 49% of doctors acknowledged that they lacked experience with available types of insulin. They also perceived that educating patients about progression of disease would take too much time. They cited lack of time to educate patients as the main barrier to insulin intensification.[14]
As of 2017, nearly 73 million people were affected by diabetes in India and the population with diabetes is expected to touch 134 million by 2045.[15] However, many healthcare professionals are unable to offer adequate counselling to their patients because of time constraints.[10]
Perceived high cost
Physicians attributed perceived high cost as another barrier because occurrence of hypoglycaemia, weight gain and local site reactions continue to be the obstacles for physicians to initiate and intensify insulin therapy.[12]
A multinational Global Attitudes of Patients and Physicians (GAPP) in Insulin Therapy study assessed patient and physician beliefs regarding insulin therapy and adherence to their insulin regimens. The study included 1250 physicians (600 specialists and 650 primary care physicians). Majority of physicians (72%) and specialists (79%) opined that initiation of aggressive treatment was limited by treatment-related hypoglycaemia, as it was difficult to efficiently manage both efficacy (reducing hyperglycaemia) and safety (reducing risk of hypoglycaemia) simultaneously.[16] Another study which assessed physician's attitudes about the initiation of insulin in patients with T2DM found that nearly one-fourth of the physicians were reluctant to initiate insulin because of its side-effect of weight gain.[17]
Although local skin reactions are transient and reduced with improved insulin formulations and devices, physicians are reluctant to initiate insulin therapy due to local allergic reactions to insulin and local site reactions to insulin injection such as lipohypertrophy, lipoatrophy, abscess formation and oedema.[18]
Perceived lack of benefits
Lack of awareness about diabetes management, short-term and long-term benefits of insulins and low prioritisation of diabetes compared to other diseases also contribute as barriers for initiating insulin therapy.[12]
A cross-sectional survey of physicians assessed the knowledge, attitude and practices towards diabetes. The study included 767 physicians (756 males and 11 females, average age of 42.18 years) with a mean clinical practice duration of 13.41 years. A total of 681 physicians practicing in urban areas and 86 in rural areas participated in the study. Less than 50% of physicians answered questions correctly with respect to diagnostic blood values of glucose; treating children with diabetes; managing gestational diabetes; monitoring people with diabetes; and insulin injection techniques.[19]
Physicians did not perceive diabetes as a management priority when compared with other diseases and disorders that they encounter in daily practice.[10] Diabetes care is neither a part of the roles and responsibilities of health personnel in the rural healthcare set-up nor in pre- and in-service training of personnel.[20]
Solutions to overcome physician-related barriers
Physician-related barriers can be overcome by providing education and training to them. Moreover, by implementing effective patient education, it is possible to improve patient adherence to insulin therapy.
Education and training
General physicians and family physicians are the first point of care for diabetes and associated morbidities for most patients. They must take initiatives to attend conferences, meetings and workshops to keep themselves updated about the growing prevalence of diabetes and the recent guidelines on treatment of diabetes.
They should also be updated about sequential therapy in diabetes management and know when to refer their patients to endocrinologists and other specialists.[20] Practical case-based continuous medical education (CME) programmes on insulin therapy help to build capacity and confidence in identifying the right patient for insulin therapy during their day-to-day clinical practice. CME also helps in overcoming clinical inertia. Hands-on training programmes on proper administration of insulin helps to build capacity and confidence to teach patients the right insulin injection technique.[15,20] CMEs must address issues such as lack of knowledge; lack of familiarity and concerns about the risk of hypoglycaemia, and weight gain. Such CMEs must be conducted by the diabetologists/endocrinologists with emphasis on benefits of timely insulin therapy, its indications in patients with T2DM, different insulin regimens; dosing and titration algorithms, and usage in special populations.[21]
Diabetes peer support intervention
Peer support is a promising strategy for improving and sustaining diabetes self-management behaviours especially in areas with shortage of professionals and economic resources. A recent meta-analysis concluded that peer support interventions for diabetes help to achieve a statistically significant improvement in HbA1c levels.[22]
Patient-centred approach
To change the attitude of patients and their caretakers, it is necessary to counsel and educate patients. Patient-centred approach involves providing diabetes care that is respectful of and responsive to individual patient needs, preferences and thereby ensuring that patient values guide all clinical decisions. To provide patient-centred care, diabetes care provider needs to have the following five attributes (CARES):[23,24]
C - Confident competence
A - Authentic accessibility
R - Reciprocal respect
E - Expressive empathy
S - Straightforward simplicity
Five steps to self-directed goal setting for behaviour change
IDF provides five-step recommendations to self-directed goal setting for behaviour change and to strengthen the doctor–patient conversations. According to the recommendations, the physician must
Identify the problem
Explore feelings
Set goals
Make a plan
Evaluate the results.
These five steps help the patients change their behaviour towards diabetes management.[25]
5As approach to improve patient self-management
The ‘5As’ model of counselling is appropriate to facilitate insulin initiation, patient self-management and behaviour change, and is possible to apply in primary care.[26] The 5As include:
Assessing patient level of behaviour, beliefs and motivation
Advising the patient based upon personal health risks
Agreeing with the patient on a realistic set of goals
Assisting to anticipate barriers and develop a specific action plan
Arranging follow-up support.
Handling emotions relating to insulin use
Discussing insulin therapy with patients evokes emotions such as denial, distress or anxiety, helplessness, and anger or hatred, which act as obstacles for insulin initiation. These emotions can be handled niftily using approaches given in [Table 3]. In this way, insulin therapy can be initiated at the right time.
Table 3.
Mnemonics to help in handling emotions related to insulin use[27]
| Dealing with denial: OPEN OUT |
| O open-ended conversation |
| P points of concern |
| E equipoise in information |
| N non-judgmental suggestion |
| O offer time to reflect |
| U understand other’s opinion |
| T therapeutic patient education |
| Dealing with hopelessness: SHAKTI |
| S support with a smile |
| H holding hands, helpfully |
| A assess limitations/strengths |
| K ‘kreate’ (create) bridges over barriers |
| T therapeutic patient education |
| I identify sources of support |
| Dealing with anxiety and distress: ASHA/HOPE |
| A acknowledgement of fears and concerns |
| S strength mapping-self, society, system (healthcare) |
| H help build resources |
| A anticipate challenges, plan ahead |
| H help acknowledge fears and concern |
| O offer optimism and strength |
| P prepare a pragmatic plan of action |
| E explore available resources and expand upon them |
| Dealing with anger: SHANTI/SHALOM (peace) |
| S stop for a second |
| H hear out the person |
| A appreciate other’s viewpoint |
| N note fears and concerns |
| T ‘translate’/paraphrase core issues |
| I intervention to be planned |
| S stop for a second |
| H hear out the person |
| A appreciate other’s viewpoint |
| L list fears and concerns |
| O optimism for a resolution |
| M monitoring and motivation |
| Take-home message: LISTEN |
| L list patient’s concerns and fears |
| I information equipoise |
| S share sources of support |
| T therapeutic patient education/teamwork |
| E empathic understanding/expression |
| N neutral non-judgmental communication |
Patient motivation for insulin/injectable therapy
Often most of the patients are reluctant to accept insulin initiation or intensification. Physicians must motivate such patients to accept insulin repeatedly. The WATER approach [Table 4] is a useful method for motivating people with diabetes.
Table 4.
The WATER approach for the motivation of patients with diabetes[28]
| W: welcome with warmth |
| A: ask and assess complaints, medical status |
| T: tell the truth, while counselling |
| E: explain, with empathy, the need for insulin |
| R: reassure and ensure return |
Coping skills training
Diabetes is a stressor and people with diabetes need to find ways to cope with diabetes. Diabetes patients can benefit significantly by learning certain coping strategies to deal with diabetes. This approach is often called ‘coping skills training’ or ‘problem-solving skills training’. The AEIOU approach helps physicians to train the patients [Table 5] to cope with diabetes.
Table 5.
AEIOU approach for coping skill training[29]
| A - Assess coping skills |
| E - Explain and Eliminate the negative strategies |
| I – Introduce and Internalise positive skills |
| O- Observe the changes regularly |
| U - Upgrade the patient’s health-related behaviour |
Educational facilities for improving diabetes care in clinics
Diabetes educational materials tailored to the literacy levels and learning styles would positively impact patient's knowledge about their condition. Supplementation of ready reckoners or videos on various aspects of diabetes management will improve self-management.[30] Patients must be provided information on the following aspects:
Lifestyle modification
Diet
Advice on complications
Advice on exercise
Injection technique
Monitoring
Foot care.
Psychological management
Psychosocial treatment is an integral part of diabetes management. Psychological assessment and treatment should be included in routine care rather than waiting to identify a specific problem or deterioration of psychological status. Practice of yoga and meditation by patients with diabetes has shown to control its overall symptoms and complications. Yogic breathing programme has been shown to improve the physical, psychological, and social domains and total quality of life in people with diabetes taking oral anti-diabetic drugs (OADs) and is therefore a recommended effective complementary or integrative therapy programme for diabetes management.[31]
Bridging gaps to improve adherence
The benefit of medications can be realised only if patients adhere to prescribed treatment regimens. However, non-adherence among patients with chronic disease is common. Treatment non-adherence is caused by several factors [Table 6].[32] Strategies to improve adherence are given in [Table 7] in order to help bridge the gaps.[33] Prescribing once daily injections provide better adherence and simplicity to diabetes therapy. Physicians must check patient's recall and comprehension of treatment regimens by asking patients to restate dosing instructions and recommended treatment changes.[34] Physicians must explain the benefits of treatments prescribed to patients. Physicians must also enquire patients, if they are having any cost-related adherence issues and try to minimise the cost of treatment by choosing an appropriate regimen.
Table 6.
Factors responsible for non-adherence[32]
| Social and economic factors |
| Socioeconomic variables |
| Cost of treatment |
| Health system-related factors |
| Characteristics of the healthcare provision |
| Patient and prescriber interaction |
| Prescribers follow-up |
| Multiple providers |
| Condition-related factors |
| Characteristics of disease |
| Severity |
| Chronic or acute |
| Patient-related factors |
| Patient’s own view of required therapy |
| Cognitive functioning |
| Health literacy |
| Motivation for self-care |
| Social support |
| Therapy-related factors |
| Multiple medications |
| Complexity of therapy |
| Adverse drug reactions |
| Duration of therapy |
Table 7.
Strategies to improve adherence[33]
| Strategies | Specific Interventions |
|---|---|
| Simplifying regimen characteristics | Adjusting the timing, frequency, amount, and dosage |
| Matching to patient’s activities of daily living | |
| Using adherence aids, such as medication boxes and alarms | |
| Imparting knowledge | Discussion with patient, nurse, or pharmacist |
| Distribution of written information or pamphlets | |
| Accessing health-education information on the web | |
| Modifying patient beliefs | Assessing perceived susceptibility, severity, benefit, and barriers |
| Rewarding, tailoring, and contingency contracting | |
| Patient and family communication | Active listening and providing clear, direct messages |
| Including patients in decisions | |
| Sending reminders via mail, email, or telephone | |
| The convenience of care, scheduled appointment | |
| Home visits, family support, and counselling | |
| Leaving the bias | Tailoring the education to patient’s level of understanding |
| Evaluating adherence | Self-reports (most commonly used) |
| Pill counting, measuring serum or urine drug levels |
Optimum insulinisation
Options for initiation of insulin therapy
According to the American Diabetes Association Standards of Medical Care in Diabetes - 2019, insulin must be initiated if there is evidence of ongoing catabolism (weight loss), if symptoms of hyperglycaemia are present, if HbA1c level is >10% or when blood glucose levels are very high (≥300 mg/dL).[35] Insulin should also be initiated if patients are not on target after usage of 2-3 OADs. Following insulin regimens are available for initiation of insulin therapy:[36]
Basal insulin regimen
Once-daily premix insulin regimen
Twice-daily premix insulin regimen
Basal-bolus regimen.
Each regimen has its own advantages and limitations and hence different patient factors must be considered when starting insulin therapy in order to choose the best regimen possible for initiating insulin therapy.[36]
Basal insulin regimen
Basal insulin regimen predominantly controls fasting plasma glucose (FPG) levels. There are three types of basal insulin: intermediate-acting (isophane insulin, i.e. NPH), long-acting (insulin detemir, insulin glargine U100) and ultra-long acting insulin (insulin degludec, insulin glargine U300). Newer basal insulin analogues provide coverage throughout the 24-hour period with a ‘peakless’ action. Basal insulin regimen suppresses lipolysis, proteolysis, and endogenous hepatic glucose production but is not effective in lowering post-prandial blood glucose directly.[36,37,38]
Dosing of once daily basal insulin: Basal insulins must be started at bedtime as 10 units once-daily. Titration of basal insulin should be performed using FPG level of 80–130 mg/dL as the target. Dose modification can be done every fourth day while using NPH, Levemir or glargine and dose modification may also be done once weekly, while using degludec, based on the lowest/mean value of the three most recent FPG values.[37]
Advantages: A once-daily basal insulin regimen is an effective and safe option in patients with T2DM and a baseline HbA1c >8.5%. It is simple and easy for early initiation to insulin therapy. It is associated with potentially less weight gain and less risk of hypoglycaemia. Basal insulin regimen is useful for symptomatic relief if tight control is not a major issue.[38]
Limitations: Basal insulin regimen is not useful to achieve glycaemic targets particularly if the PPG level is high or if the diet of the patient includes high carbohydrates as it does not offer optimum control of post-prandial excursions. Some individuals may require a more intensified insulin regimen.[38]
Once-daily premix insulin
Premix insulins have prandial and basal insulin components in 30:70, 25:75 or 50:50 ratios. Examples of premix insulin include Human premix insulin 30/70 and Human premix insulin 50/50, Biphasic aspart 30/70, Biphasic aspart 50/50, Biphasic lispro 25/75, Biphasic lispro 50/50. Unlike other premix insulin analogues, IDegAsp is an insulin co-formulation and has distinct bolus and basal components in 30/70 ratio. These regimens effectively control both FPG and PPG levels in many patients.[38]
Dosing of once daily premix insulin: Premix insulin/co-formulation should be started as 10 units once-daily as pre-breakfast or pre-dinner dose. The timing of administration must be decided according to the size of the meal, its carbohydrate content or post-prandial glucose excursions. Titration of once-daily premix insulin should be performed once in a week to achieve FPG level of 80-130 mg/dL if given pre-dinner and titration should be based on the lowest/mean value of the three most recent blood glucose levels.[38]
Advantages: Administration of once-daily premix insulin/co-formulation is relatively easy to teach and simple for the patient to understand. It offers better post-prandial glucose control and is more effective in lowering HbA1c than basal insulin alone. Premix insulin regimen is associated with improved compliance since the same device provides control for both PPG and FPG.[38]
Limitations: Once-daily premix insulin/co-formulation requires a fixed daily routine with regards to lifestyle, carbohydrate content and meal timing. A time gap of 30 minutes is required between injection administration and meal consumption while using human premix insulin while analogue premix insulin can be given immediately before the meal. Once-daily dosing offers PPG control only for one meal.[38]
Twice-daily premix insulins
In this regimen, premix insulins are administered twice daily and help control PPG levels after two meals in addition to controlling FPG.
Dosing of twice-daily premix insulins: When twice-daily premix analogue insulin is necessary, it must be started as 6U BID or 0.1-0.2 U/kg body weight, divided into two equal doses as pre-breakfast and pre-dinner subcutaneous injections. However, human premix insulin is given as two-third dose in the morning and one-third dose in the evening. Premix analogues achieve lower PPG levels than human premix insulins. Dose titration should be based on the pre-meal value of 80–130 mg/dL. The dose should be reduced by 10%–20% for patients experiencing hypoglycaemia (<70 mg/dL).[37]
Advantages: Twice-daily premix insulin regimen is easy to learn and implement while initiating insulin. It offers better PPG control for two meals even during the initiation of insulin therapy. It is more effective in lowering HbA1c than basal insulin alone because of additional PPG control.[38]
Limitations: Unlike basal insulin regimen, twice-daily premix insulins are associated with one extra injection. Patients are unable to adjust the bolus or basal components independently. These regimens require a fixed daily routine with regards to lifestyle, carbohydrate content and meal timing. A time gap of 30 minutes is required between conventional mixture, that is, biphasic human insulin administration and meal intake.[38]
Basal-bolus regimen
The basal-bolus regimen can be used as an insulin initiation option in case of severe hyperglycaemia. Patients take one basal insulin injection along with 2–3 rapid-acting insulins. It is considered the most effective insulin regimen and controls FPG and PPG levels post all the meals before which rapid acting analogues are taken.[38] It immediately helps lower the high blood glucose values in case of severe hyperglycaemia.
Options for intensification of insulin therapy
As T2DM is associated with progressive beta-cell decline, once-daily insulin may eventually fail to maintain glycaemic control in many patients with type 2 diabetes. When the recommended A1C level of <7.0% is not reached or when aggressive titration is limited by hypoglycaemia, treatment should be intensified by adding insulin injections. The available options for intensifying insulin therapy include:[38,39]
Basal-plus regimen
Basal-bolus regimen
Twice-daily premix insulin regimen
Thrice-daily premix insulin regimen.
Adding an extra insulin injection depends on the initial insulin therapy and glycemic profile of the patient [Figure 3].
Figure 3.
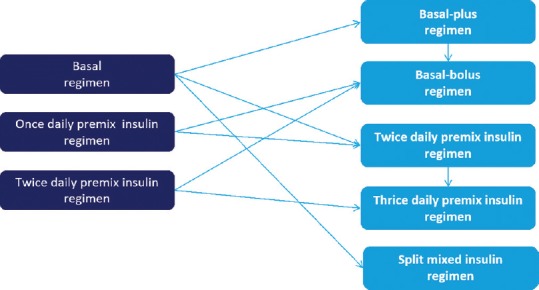
Options for intensification of insulin therapy
Basal-plus regimen
In a basal-plus regimen, a rapid-acting insulin injection is given just before the main meal along with a basal insulin injection which is usually taken at bedtime. For example, basal insulin once daily (insulin glargine or insulin degludec), rapid-acting insulin once daily (insulin aspart, insulin lispro, insulin glulisine), or short-acting insulin once daily (regular human insulin given half an hour before a meal). Basal-plus regimen helps to control FPG level along with PPG level post one meal. It is used as a part of a step-wise approach for intensifying insulin therapy.[38]
Dosing of basal-plus regimen: When basal-plus regimen is necessary, one bolus injection is given just before the largest meal of the day as chosen by the patient and the basal insulin is continued. Starting dose of bolus insulin is 4U or 10% of the basal dose. The dose of bolus insulin should be titrated twice weekly based on 2 hour post-meal value of 180 mg/dL. Basal insulin dose is titrated once in a week based on FPG levels. The dose should be reduced by 10%-20% for patients suffering from hypoglycaemia (<70 mg/dL).[37]
Advantages: The basal-plus regimen produces greater HbA1c reductions compared to basal-only insulin regimen. It offers a step-wise approach to insulin intensification thereby offering increased patient acceptability. Moreover, it is associated with a low risk of hypoglycaemia compared to a basal-bolus regimen.[38]
Limitations: Basal-plus regimen offers PPG control only at one meal. Patients may eventually need a second or third prandial injection to achieve adequate glycaemic control. Delivery of two different insulins require two separate pens and two separate injections. Also, there are chances of confusion between pens and difficulty in distinguishing the effect of two separate insulins in case of hypoglycaemia.[38]
Basal-bolus regimen
In the basal-bolus regimen, patients need to take one basal insulin injection along with 2–3 bolus insulins. It is considered as the most effective and gold-standard insulin regimen to control FPG and PPG levels.[38]
Dosing of the basal-bolus regimen: Total insulin dose is calculated as 0.3-0.5 U/kg body weight. Out of the total dose, 40% of the total dose is given as basal insulin at bedtime and 60% of the total dose is given as prandial insulin divided into three doses.[37]
Advantages: Basal-bolus regimen offers optimum flexibility in terms of diet and activity. It closely mimics normal physiology and has the potential for better metabolic control if used optimally.[38]
Limitations: Basal-bolus regimen requires multiple insulin injections. It is more complicated to support or teach and necessitates carbohydrate counting. It is associated with a risk of hypoglycaemia and weight gain. The basal-bolus regimen needs maximum patient motivation and requires regular monitoring.[38]
Twice-daily premix insulin
Twice-daily premix insulin can be given when basal insulin or once-daily premix insulin are insufficient to achieve glycaemic control. It helps to control FPG and PPG levels post two meals before which it is injected.[38] Newer insulin co-formulation such as IDegAsp can be used twice daily with two main meals as an intensification option because it offers glycaemic control with convenience.[40]
Dosing of twice-daily premix insulin: When shifted from other insulins to twice-daily premix insulins, total basal-insulin dose should be considered and administered as two equal doses before breakfast and before dinner. Dose titration should be done using a pre-meal value of 80-130 mg/dL as a target. Pre-breakfast dose, if titrated, should be based on pre-dinner values and vice versa. The dose should be reduced by 10%–20% for patients suffering from hypoglycaemia (<70 mg/dL).[37]
Advantages: Twice-daily premix insulin is easy to learn and implements while intensifying insulin therapy. It has the potential for better PPG control. Twice-daily premix insulin is more effective in lowering HbA1c than basal-plus insulin regimen because of PPG control after two separate meals.[38]
Limitations: Twice-daily premix insulin regimen is less flexible (i.e., the patient is unable to adjust the bolus or basal component of insulin independently). It requires a fixed daily routine with respect to lifestyle, carbohydrate content and meal timing. Conventional mixture requires time delay of injection with need to inject 30 minutes before a meal.[38]
High-mix insulins and Hetero-mix insulin regimen
High-mix insulins are mixtures of insulins that contain 50% or more of bolus insulin component. Such high-mix insulins are used to take care of high PPG levels in patients having relatively controlled FPG values. Such insulins include both biphasic human insulin and analogue premixes: Biphasic aspart 50/50 (BI Asp50), lispro mix 50/50 (LM50), BIAsp 70/30 (BI Asp70), and LM 75/25 (LM75).
The hetero-mix regimen includes use of two different types of premix insulin preparations. High-mix insulin prescribed before breakfast/lunch helps achieve adequate post-prandial control while low-mix insulin administered before dinner helps achieves glycaemic control with minimal risk of nocturnal hypoglycaemia. Various regimens are listed in [Table 8]. Reverse hetero-mix regimen can be considered in patients fasting during Ramadan. For example, Low-mix BIAsp 30/70 before morning meal (Suhur) and high-mix BIAsp 50/50 before evening meal (Iftar). This allows for good glycemic control, with minimal risk of hypoglycaemia during the daytime fasting period.[41]
Table 8.
High-mix, Hetero-mix and reverse hetero-mix insulin regimens[41]
| High-mix regimen: Regimen using a single high-mix insulin |
| Once daily |
| Twice daily |
| Thrice daily |
| Hetero-mix regimen: Regimen using more than one insulin preparation |
| Twice daily: e.g., high-mix 50:50 with breakfast, premix 30:70 with dinner |
| Thrice daily: e.g., high-mix 50:50 with breakfast and lunch, premix 30:70 with dinner |
| Reverse mix regimen: Regimen using low mix during day time, and high-mix at night |
| Twice daily: e.g., premix 30:70 with breakfast, high-mix 50:50 with dinner |
Thrice-daily premix insulins
Thrice-daily premix analogue insulins help control FPG apart from PPG levels post three meals. Thrice-daily premix insulins should be used for intensification of insulin therapy and has been shown to provide control similar to basal-bolus regimen.[38]
Dosing of thrice-daily premix insulins: In patients on BID premix insulins, 2–6 U or 10% of total daily premix dose is added before the lunch. Down-titration of morning dose (by 2–4 U) may be needed after adding the lunch dose. Titration is performed using pre-meal glucose levels of 80–130 mg/dL as target and may be done once in a week based on pre-dinner or pre-breakfast values. Modification of dose is based on the lowest/mean value of the three most recent pre-meal values.[37]
Advantages: Thrice-daily premix insulins offer PPG control for each meal. It is close to physiological insulin secretion. Thrice daily premix insulins offer similar glycaemic control, risk of hypoglycaemia and weight gain as a basal-bolus regimen with an advantage of one less dose/prick.[38]
Limitations: Thrice-daily premix insulins require multiple doses/pricks. Additional adjustment for a bolus component is not possible. This regimen requires frequent monitoring and is associated with increased risk of hypoglycaemia.[38]
Effective and simple insulin therapy
Starting of insulin therapy and choosing the appropriate regimen depends on several factors, including the patient's acceptance and willingness to adhere to the therapy. Co-existing physiologic and medical conditions such as pregnancy and chronic renal failure and drugs such as glucocorticoids may alter insulin requirements.
Factors affecting the choice of insulin regimen
Factors affecting the choice of insulin regimen include phase of life, diet, clinical situation, psychological state and the glucophenotype.
Phase of life: Selection of insulin regimen also depends on the phase of life of patients such as frail elderly patients (≥60 years) with limited daily activities; planning a pregnancy or antenatal; and children and adolescents. Analogue insulins are safe for usage in an elderly population. Latest product labels should always be referred for using them in special populations.
Diet: Indian diet is composed of high amount of carbohydrate. Indians have less frequent heavy meals (two major meals a day), and meal timing is also not fixed. In addition, patients often request for meal time flexibility to take insulin injections. These things should be considered while selecting an insulin regimen.[41,42] Post-prandial glucose control is crucial in patients with high carbohydrate diet. Analogue premix insulins and newer co-formulation fulfil both meal-related and basal insulin requirements and are thus simplified and convenient options. Bolus insulins can be used for customised titration and control but will require need for a separate injection. Also, analogue premix insulins offer increased flexibility while closely mimicking physiological insulin response compared to conventional human insulins.[43,44]
Clinical situation: There is a misconception among patients that if they are sick enough or when they do not eat or do vomit, they should hold off on taking their insulin. Patients who are ill should be instructed to continue their insulin therapy, maintain fluid intake, eat smaller meals as tolerated, test their glucose levels every 1–4 hours and titrate their insulin doses accordingly. Hyperglycaemia frequently occurs with acute medical illness. Even patients who are normoglycaemic can develop hyperglycaemia in response to acute metabolic stress. Acute conditions demand immediate control of blood glucose levels. Individualised, patient-centred approach based on the acute concomitant condition is essential to ensure optimal outcomes and safety of insulin therapy.[45,46]
Psychosocial factor: Social factors have an important role in the acceptance, adherence to treatment and overall outcome of diabetes management. Economic class, financial expenditure, regional disparities, family structure, affordability to medical care, educational profile, ethnicity, customs, food habits, fasting conditions, and community beliefs should be considered while selecting the insulin regimen.[31]
Glucophenotype: Glucophenotype is defined as the clinical and biochemical attributes, which allow characterisation of the glycaemic status, understanding of the etiopathogenesis of dysglycaemia, and planning of therapeutic strategies in an individual. Glucophenotype may be predominant insulin deficiency or insulin resistance; non-obese or obese; predominant fasting; post-prandial or combined hyperglycaemia; and with or without acute/modifiable precipitating factors.[47] A study suggested that Indian population have higher post-prandial glucose excursions.[44] Insulins that reduce post-prandial excursion like premix insulins/co-formulation are more relevant in such a scenario.
Evolution of premix insulins from human insulins to new generation co-formulation
Premix insulins were developed to overcome the disadvantage of manually mixing basal and prandial insulin in the syringe. Premix analogues can be used as once-daily, twice-daily and thrice-daily regimens; for initiation as well as intensification, respectively [Table 9].
Table 9.
Summary of recommendations from guidelines for insulin initiation and intensification
| Guideline | Initiation | Intensification |
|---|---|---|
| RSSDI 2017[36] | Basal OD | Add GLP-1 RA |
| Premixed OD/BID | Basal-plus or basal-bolus | |
| Co-formulation | Premix BID or TID | |
| OD | Co-formulation BID | |
| High-mix insulins | ||
| Diabetes Australia 2016[48] | Basal OD Premix OD |
Basal-plus or basal−bolus |
| Premixed BID or TID Add GLP-1 RA | ||
| Canadian Diabetes Association 2018[49] | Basal OD Premix | Basal-plus or basal-bolus Premix |
| IDF[50] | Basal OD Premix OD/BID |
Basal-plus, basal-bolus or premix |
| NICE 2015[51] | Basal OD/BID Premix | Basal-plus or basal-bolus Premix |
| AACE 2019[52] | Basal | Add GLP-1 RA or prandial insulin (Premix among other options) |
| ADA 2019[35] ADA/EASD position statement 2018[53] | Basal | Basal-plus or basal-bolus Premix BID or TID Add GLP-1 RA |
ADA, American Diabetes Association; AACE, American Association of Clinical Endocrinologists; EASD, European Association for the Study of Diabetes; BID, twice daily; GLP-1 RA, glucagon-like peptide-1 receptor agonist; IDF, International Diabetes Federation; NICE, National Institute for Health and Care Excellence; OD, once daily; RSSDI, Research Society of the study of diabetes in India; TID, three-times daily; T2D, type 2 diabetes
The Consensus on initiation and intensification of premix insulin for T2DM recommended the following:[37]
Once-daily premix insulin (for initiation): Premix insulin analogues/co-formulation could be preferred over basal insulins for insulin initiation to achieve recommended glucose targets (10 U pre-breakfast or pre-dinner).
-
Twice-daily premix insulin (for initiation): It is recommended to initiate twice-daily premix analogues over once daily basal insulins to achieve desired glucose targets
(6 U pre-breakfast and 6 U pre-dinner).
Twice-daily premix insulin (for intensification): It is recommended to switch patients uncontrolled on OD basal insulin or OD premix insulin to twice-daily premix analogues to achieve desired glucose targets.
Thrice-daily premix insulin (for intensification): Premix insulins analogue TID or insulin co-formulation BID are comparable with basal-bolus regimen and offer more convenience as an option for intensive insulin therapy.
Injection techniques
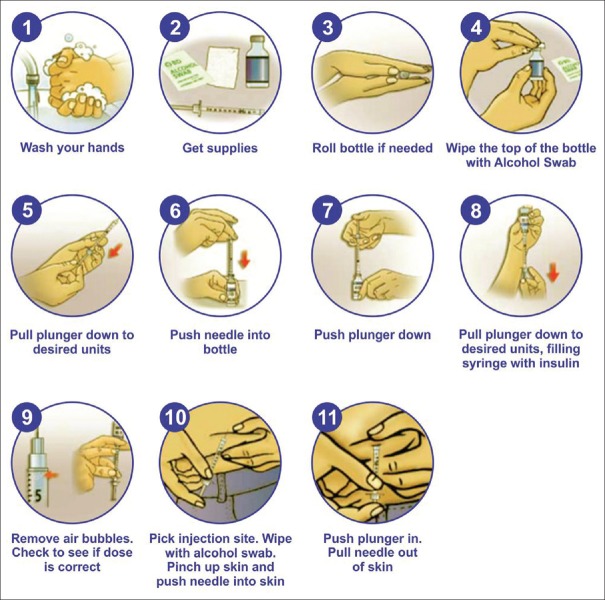
Proper injection technique is of utmost importance for optimal glycaemic control.[54] Insulin injections through vials and injections is cumbersome and involves multiple steps [Figure 4]. This drawback can be overcome by using modern insulin pens which are more user friendly and involve fewer steps [Figure 5]. Insulin pens are recommended over vial and syringes for convenience, better accuracy, reduced fear of injections, lesser pain, greater social acceptance, and improved treatment adherence.
Figure 4.
Steps for drawing, mixing and injecting insulins through vials and syringes
Figure 5.
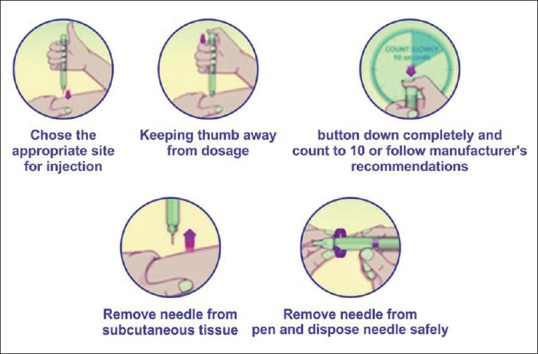
Steps for insulin injection through pen devices
Summary
As T2DM is progressive in nature, exogenous supplementation of insulin often becomes necessary overtime. Timely insulin initiation helps to achieve glycaemic targets and prevent complications. However, patients and physicians are reluctant to initiate insulin therapy. Physician-related barriers may be because of inadequacy of skill or time, perceived cost due to complications, inadequate understanding of benefits of insulin therapy or personal lack of prioritisation for diabetes management compared to other diseases. These barriers can be overcome through education and training and thereby building individual capacity and confidence.
Proper insulin dosing and timely titration are essential for better outcomes and lesser side-effects including hypoglycaemia. As the duration of diabetes increases, insulin therapy needs to be monitored and intensified when required to achieve the glycaemic target. Each insulin regimen has its own advantages and limitations and hence patient lifestyle, food habits and convenience along with its safety and efficacy needs to be considered so as to choose best regimen possible for initiating and intensifying insulin therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Novo Nordisk India Pvt Ltd for supporting the conduct of the workshop wherein the recommendations were arrived at in consensus. The authors acknowledge medical writing assistance by JSS Medical Research India Pvt. Ltd.
References
- 1.Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-Cell function: Review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH, Kim JW, Shin JA, Shin J, Yoon KH. β-cell mass in people with type 2 diabetes. J Diabetes Investig. 2011;2:6–17. doi: 10.1111/j.2040-1124.2010.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BW, Kim JH, Ko SH, Hur KY, Kim NH, Rhee SY, et al. Insulin therapy for adult patients with type 2 diabetes mellitus: A position statement of the Korean Diabetes Association, 2017. Diabetes Metab J. 2017;41:367–73. doi: 10.4093/dmj.2017.41.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93:S52–9. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol. 2009;53:S14–20. doi: 10.1016/j.jacc.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:28–34. doi: 10.1016/S2213-8587(13)70006-8. [DOI] [PubMed] [Google Scholar]
- 7.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: A guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569–76. doi: 10.7326/M17-0939. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RK, Cobble ME, Reid TS, Shomali ME. Distinguishing among incretin-based therapies. Pathophysiology of type 2 diabetes mellitus: Potential role of incretin-based therapies. J Fam Pract. 2010;59:S5–9. [PubMed] [Google Scholar]
- 9.Dailey G, Wang E. A review of cardiovascular outcomes in the treatment of people with type 2 diabetes. Diabetes Ther. 2014;5:385–402. doi: 10.1007/s13300-014-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V, Shah SN, Joshi SR, Seshiah V, Sahay BK, Banerjee S, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: Results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18:370–8. doi: 10.4103/2230-8210.129715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zografou I, Strachan MW, McKnight J. Delay in starting insulin after failure of other treatments in patients with type 2 diabetes mellitus. Hippokratia. 2014;18:306–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Kalra S, Ghosal S, Shah P. Consensus on bridges for barriers to insulin therapy. J Assoc Physicians India. 2017;65:23–30. [PubMed] [Google Scholar]
- 13.Elliott DJ, Robinson EJ, Sanford M, Herrman JW, Riesenberg LA. Systemic barriers to diabetes management in primary care: A qualitative analysis of Delaware physicians. Am J Med Qual. 2011;26:284–90. doi: 10.1177/1062860610383332. [DOI] [PubMed] [Google Scholar]
- 14.Cuddihy RM, Philis-Tsimikas A, Nazeri A. Type 2 diabetes care and insulin intensification: Is a more multidisciplinary approach needed? Results from the MODIFY survey. Diabetes Educ. 2011;37:111–23. doi: 10.1177/0145721710388426. [DOI] [PubMed] [Google Scholar]
- 15.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–90. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes RP1, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62:860–8. doi: 10.1111/j.1742-1241.2008.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile S, Guarino G, Giancaterini A, Guida P, Strollo F on behalf of the AMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. SpringerPlus. 2016;5:563. doi: 10.1186/s40064-016-1978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shera AS, Jawad F, Basit A. Diabetes-related knowledge, attitude and practices of family physicians in Pakistan. J Pak Med Assoc. 2002;52:465–70. [PubMed] [Google Scholar]
- 20.Venkataraman K, Kannan AT, Mohan V. Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Ctries. 2009;29:103–9. doi: 10.4103/0973-3930.54286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunaert P, Willems S, Feyen L, Bastiaens H, De Maeseneer J, Jenkins L, et al. Engaging GPs in insulin therapy initiation: A qualitative study evaluating a support program in the Belgian context. BMC Fam Pract. 2014;15:144. doi: 10.1186/1471-2296-15-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil SJ, Ruppar T, Koopman RJ, Lindbloom EJ, Elliott SG, Mehr DR, et al. Peer support interventions for adults with diabetes: A meta-analysis of hemoglobin A1c outcomes. Ann Fam Med. 2016;14:540–51. doi: 10.1370/afm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalra S, Baruah MP, Unnikrishnan AG. Responsible patient-centered care. Indian J Endocrinol Metab. 2017;21:365–6. doi: 10.4103/ijem.IJEM_543_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalra S, Kalra B. Communication in diabetes care. J Pak Med Assoc. 2017;67:1625–27. [PubMed] [Google Scholar]
- 25.International Diabetes Federation. International Curriculum for Diabetes Health Professional Education. Brussels: International Diabetes Federation; 2008. [Google Scholar]
- 26.Glasgow RE, Emont S, Miller DC. Assessing delivery of the five ‘As’ for patient-centeredcounseling. Health Promot Int. 2006;21:245–55. doi: 10.1093/heapro/dal017. [DOI] [PubMed] [Google Scholar]
- 27.Kalra S, Verma K. Handling insulin-related emotions. Diabetes Ther. 2018;9:1415–9. doi: 10.1007/s13300-018-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra S, Kalra B, Batra P. Patient motivation for insulin/injectable therapy: The Karnal model. Int J Clin Cases Investig. 2010;1:11–5. [Google Scholar]
- 29.Kalra S, Kalra B, Sharma A, Sirka M. Coping skills training: The AEIOU approach. Endocr J. 2010;57:S391. [Google Scholar]
- 30.Koonce TY, Giuse NB, Kusnoor SV, Hurley S, Ye F. A personalized approach to deliver health care information to diabetic patients in community care clinics. J Med Libr Assoc. 2015;103:123–30. doi: 10.3163/1536-5050.103.3.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalra S, Sridhar GR, Balhara YS, Sahay RK, Bantwal G, Baruah MP, et al. National recommendations: Psychosocial management of diabetes in India. Indian J Endocrinol Metab. 2013;17:376–95. doi: 10.4103/2230-8210.111608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa E, Giardini A, Savin M, Menditto E, Lehane E5, Laosa O, et al. Interventional tools to improve medication adherence: Review of literature. Patient Prefer Adherence. 2015;9:1303–14. doi: 10.2147/PPA.S87551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atreja A, Bellam N, Levy SR. Strategies to enhance patient adherence: Making it simple. MedGenMed. 2005;7:4. [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118:27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42:S1–193. [Google Scholar]
- 36.Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38:1–115. doi: 10.1007/s13410-018-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovil R, Chawla M, Rajput R, Singh AK, Sinha B, Ghosal S, et al. Consensus on insulin dose and titration algorithms in ambulatory care of type 2 diabetes in India. J Assoc Physicians India. 2017;65:17–30. [PubMed] [Google Scholar]
- 38.Strachan MJ, Frier BM. London, England: Springer-Verlag; 2013. Insulin Therapy: A Pocket Guide. [Google Scholar]
- 39.Mohan V, Kalra S, Kesavadev J, Singh AK, Kumar A, Unnikrishnan AG, et al. Consensus on initiation and intensification of premix insulin in type 2 diabetes management. J Assoc Physicians India. 2017;65:59–73. [PubMed] [Google Scholar]
- 40.Rodbard HW, Cariou B, Pieber TR, Endahl LA, Zacho J, Cooper JG. Treatment intensification with an insulin degludec (IDeg)/insulin aspart (IAsp) co-formulation twice daily compared with basal IDeg and prandial IAsp in type 2 diabetes: A randomized, controlled phase III trial. Diabetes Obes Metab. 2016;18:274–80. doi: 10.1111/dom.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalra S, Farooqi MH, El-Houni AE. High-mix insulins. Indian J Endocrinol Metab. 2015;19:686–90. doi: 10.4103/2230-8210.163214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elizarova S, Galstyan GR, Wolffenbuttel BH. Role of premixed insulin analogues in the treatment of patients with type 2 diabetes mellitus: A narrative review. J Diabetes. 2014;6:100–10. doi: 10.1111/1753-0407.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59) Br J Nutr. 2009;102:1498–506. doi: 10.1017/S0007114509990468. [DOI] [PubMed] [Google Scholar]
- 44.Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, et al. IMPROVE Study Group Expert Panel. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: Safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. Int J Clin Pract. 2009;63:522–31. doi: 10.1111/j.1742-1241.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 45.Donner T. Insulin – Pharmacology, therapeutic regimens and principles of intensive insulin therapy. In: De Groot LJ, Chrousos G, Dungan K, editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc.; 2000. [Updated 2015 Oct 12]. [PubMed] [Google Scholar]
- 46.McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinol Metab Clin North Am. 2012;41:175–201. doi: 10.1016/j.ecl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalra S, Gupta Y. The gluco-phenotype. J Pak Med Assoc. 2016;66:118–9. [PubMed] [Google Scholar]
- 48.The Royal Australian College of General Practitioners, Diabetes Australia. General Practice Management of Type 2 Diabetes-2016-18. The Royal Australian College of General Practitioners, Diabetes Australia. 2016 [Google Scholar]
- 49.Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018. Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42:S1–S325. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 50.International Diabetes Federation 2012 Clinical Guidelines Task Force. Global guideline for type 2 diabetes. [Last accessed 2019 Jun 09]. Available from: https://www.idf.org/component/attachments/attachments.html?id=725 and task=download .
- 51.National Institute for Health and Care Excellence (NICE). NG28: Type 2 diabetes in adults: management National Institute for Health and Care Excellence. 2015 [PubMed] [Google Scholar]
- 52.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25:69–100. doi: 10.4158/CS-2018-0535. [DOI] [PubMed] [Google Scholar]
- 53.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tandon N, Kalra S, Balhara YPS, Baruah MP, Chadha M, Chandalia HB, et al. Forum for injection technique and therapy expert recommendations, India: The Indian recommendations for best practice in insulin injection technique, 2017. Indian J Endocrinol Metab. 2017;21:600–17. doi: 10.4103/ijem.IJEM_97_17. [DOI] [PMC free article] [PubMed] [Google Scholar]