Abstract
Background and Aims:
Irritable bowel syndrome (IBS) is a chronic relapsing disorder characterized by abdominal pain-discomfort and altered bowel habits. The IBS-diarrhoea predominant subtype (IBS-D) is defined as >25% of bowel movements representing type 6 or 7 of the Bristol Stool Form Scale. Management of IBS-D is mainly symptomatic, including lifestyle modification. Due to absence of standard treatment, multiple drugs are used. A controlled release (CR) form of mebeverine, recommended for spasmodic gastrointestinal disorders (including IBS) has recently been introduced in Indian market. We have conducted a placebo-controlled double blind randomized controlled trial [CTRI/2018/03/012897] to evaluate the effectiveness and safety of this product.
Methods:
40 patients of IBS-D were recruited from medicine out-patient department (OPD) of a tertiary care hospital and randomized to two parallel groups. One received mebeverine 200 mg CR tablets twice daily for 8 weeks, while other received matching placebo. Outcome parameters were number of bowel movements per day over past 7 days (NoBM7d), severity of abdominal cramps and IBS quality of life (IBSQoL) score. Medication adherence record and treatment emergent adverse events were captured.
Results:
Mebeverine group showed modest but statistically significant improvement in NoBM7d, cramps and IBSQoL from baseline to 4 and 8 weeks. The changes within the placebo group were not statistically significant. Also, the intergroup differences at both 4 and 8 weeks were not statistically significant. Adherence was better in mebeverine group and both interventions were well tolerated.
Conclusions:
Mebeverine 200 mg CR twice daily has modest effect in IBS-D and therefore will not be a good choice for patients with severe symptoms.
Keywords: Diarrhoea, irritable bowel syndrome, mebeverine, randomized controlled trial
Introduction
Irritable bowel syndrome (IBS) is a long-lasting, relapsing disorder characterized by abdominal pain-discomfort and altered bowel habits. Intestinal motility impairment and visceral hypersensitivity are the key factors among its multifactorial pathogenesis.[1] The IBS classification comprises 3 subtypes based on the predominant bowel disorder and includes IBS with diarrhoea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed symptoms of constipation and diarrhoea (IBS-M).[2] The IBS-D subtype is defined as follows: more than 25% of bowel movements using the Bristol Stool Form Score (BSFS) are type 6 or 7, and less than 25% of bowel movements are type 1 or 2.[3] Alternatively, for epidemiologic studies and in clinical practice, if the patient reports abnormal bowel movements that are usually diarrhoea, the patient can be considered to have IBS-D. Experiencing bowel movement patterns with at least 3 different types of stool in a week also supports a diagnosis of IBS-D.
At present, there is no standard treatment algorithm for IBS-D. Therapeutic options focus on alleviating symptoms, often encompassing lifestyle and dietary modifications for initial and adjunctive treatment, over-the-counter medications, prescription medications, and psychological therapies.
Mebeverine is an antispasmodic drug that has been used in the management of bowel spasms for many years. It is a musculotropic agent that has antispasmodic activity and regulatory effects on the bowel function. During oral administration at doses of 135-270 mg thrice daily, it shows minimum typical anticholinergic adverse effects.[4,5] There is no indication that the incidence of adverse effects experienced on mebeverine is higher than that with placebo.[6] Recently, the 200 mg controlled release (CR) preparation of mebeverine has been launched in the Indian market. This formulation is expected to improve patient adherence to treatment since the frequency of drug dosing is reduced to twice daily from the thrice daily regimen of the previously available formulations.
Mebeverine became treatment of interest for IBS in the 1960s. In an early study by Connell,[7] the drug decreased all sigmoid colonic motility, especially in hyperactive subjects, and had less or no effect in hypoactive subjects. In a subsequent part of the study, mebeverine was superior to placebo at each time point over 12 weeks of treatment in IBS patients in terms of symptom improvement and general well-being. Using prolonged ambulant manometry in 12 IBS patients and 6 healthy controls, compared to a placebo period, mebeverine had no significant effects on inter-digestive small bowel motor parameters in controls. In contrast, a higher phase 2 motility index was observed in both IBS-D and IBS-C patients, and phase 3 motility was also affected. These alterations in small bowel activity by mebeverine suggest possible spasmolytic and prokinetic effects in IBS patients. Regarding symptom control in IBS, non-placebo-controlled studies have shown positive results. Significant improvement was observed after 6 weeks treatment with both the plain and sustained-release forms of mebeverine, with a minimal number of adverse events. However, when the effects of mebeverine have been compared to placebo and not compared to another drug or measured by self-control, the results have been controversial.
In 2010, a meta-analysis[8] was published on the efficacy and tolerability of mebeverine in IBS in its usual dosages. 8 randomized trials recruiting 555 patients of all IBS subtypes, randomized to receive either mebeverine or placebo, met the meta-analysis criteria. The pooled relative risk (RR) for clinical improvement with mebeverine was 1.13 (P = 0.7) and 1.33 (P = 0.12) for relief of abdominal pain. The efficacy of mebeverine 200 mg compared to mebeverine 135 mg indicated RRs of 1.12 (P = 0.168) for clinical or global improvement and 1.08 (P = 0.463) for relief of abdominal pain.[9] Therefore, although mebeverine was shown to be well-tolerated, its efficacy in global improvement of IBS did not reach statistical significance. Similarly, no positive effects of mebeverine over placebo were seen in an exploratory study performed in 135 IBS patients, fulfilling the Rome III criteria and recruited from general practice, when mebeverine, methylcellulose and placebo were compared, with or without the combination of a cognitive behavioural therapy-based self-management website (with or without additional telephone and e-mail support) over 6 weeks. Disappointingly, the use of the website also did not improve IBS symptom severity scores or quality of life scores significantly over the ‘no website’ group; nevertheless, there was a trend towards continued improvement in the self-management group (particularly those with telephone support) throughout the study, while the ‘no website’ group and the medication groups seemed to lose their therapeutic gains from weeks 6 to 12.
The objective of the present study was to carry out a head-to-head comparison of mebeverine 200 mg twice daily in CR formulation with matching multivitamin placebo formulation in the management of IBS-D subtype with respect to both effectiveness and safety.
Methods
The study was designed as a parallel group, double blind, placebo controlled, randomized, controlled clinical trial and duly registered with Clinical Trial Registry of India [CTRI/2018/03/012897]. The study protocol was abided by the Declaration of Helsinki and the Indian Council of Medical Research (ICMR) ethical guidelines for biomedical research with human subjects, and received approval of the Institutional Ethics Committee (IEC). Written informed consent was obtained from all subjects prior to screening.
Patients of either sex were screened at the out-patient clinic (OPD) of the Department of General Medicine and recruited if they were at least 18 years of age and presenting with recurrent abdominal pain or discomfort for atleast 3 days per month in the last 3 months, along with history of loose or watery stools more than three times per day (IBS as per ROME IV criteria). Patients having infrequent bowel movements (less than 3 per week), hard or lumpy stools or straining regularly during defecation were excluded. Other exclusions were women who were pregnant or breast-feeding, history of debilitating chronic diseases including uncontrolled diabetes, immunocompromising disorders such as HIV/AIDS, alcoholism or substance abuse, psychiatric disorders liable to render patients unreliable study subjects and lack of adequate caregiver support at home to supervise daily drug administration. Use of systemic antimicrobials, corticosteroids or any other medication known to influence IBS symptoms currently or within last 4 weeks was not permitted. Subject recruitment was expected to be completed within the first 12 months.
Subjects were randomized to receive any one of the formulations in a double-blind manner using computer generated random number list. Randomization was done in fixed blocks of 16. Allocation concealment was done through the serially numbered, opaque, sealed envelope technique. The test drug was controlled release mebeverine hydrochloride, equivalent to 200 mg mebeverine, in capsule form. Treatment was continued for 8 weeks with an interim follow-up visit at 4 weeks. Control subjects received a marketed multivitamin preparation, also in capsule form, as placebo, for 8 weeks, and were then followed-up at the same time intervals. The use of placebo was justified on the basis that the role of pharmacotherapy in IBS is oriented towards symptom control and no treatment has been shown to be unequivocally superior. To ensure double blinding, the medication was removed from original blister packaging and repacked in small plastic jars with airtight screw caps. These were then labelled appropriately. Medication was dispensed twice to each subject, with each lot sufficient for 4 weeks use. Subjects were instructed to take one capsule twice daily before meals.
Outcome measures were the number of bowel movements per day over 7 days prior to a visit, subjective assessment of severity of abdominal cramps on a 3-point grading system (1 = absent/mild; 2 = moderate; 3 = severe) and IBS quality of life (IBS QoL) score.[10] Safety parameters were treatment emergent adverse signs or symptoms or abnormal values detected through laboratory tests done on suspicion. Adherence to medication was assessed by the traditional pill count method. It was deemed to be excellent if not more than 10% of scheduled doses were missed, good if not more than 20% were missed, fair if not more than 30% were missed and poor for any situation worse than fair.
For the purpose of sample size calculation, reduction in the number of average daily bowel movements was considered as the primary outcome measure. It was estimated that 35 subjects would be required per group in order to detect a difference of 2 in this number with 80% power and 5% probability of Type 1 error. This calculation assumed a standard deviation of 3 for the average number of daily bowel movements and two-sided testing. Assuming a drop-out rate of 20% we proposed to recruit 44 subjects in each group. The sample size was calculated using nMaster version 2.0 (Department of Biostatistics, Christian Medical College, Vellore; 2011) software.
Data were captured from source documents on a structured Case Report Form (CRF). The data were first transcribed to Microsoft Excel spreadsheet and then analysed by Statistica version 6 [Tulsa, Oklahoma: StatSoft Inc., 2001] and GraphPad Prism version 5 [San Diego, California: GraphPad Software Inc., 2007] software. Data have been summarized by routine descriptive statistics, namely mean and standard deviation for numerical variables that are normally distributed, median and interquartile range (IQR) for skewed numerical variables and counts and percentages for categorical variables. Fisher's exact test or Pearson's Chi-square test was employed for intergroup comparison of categorical variables. Numerical variables were compared between groups by Student's independent samples t test, if normally distributed or by Mann-Whitney U test, if otherwise. Within group comparisons were done by pair-wise application of Tukey's honestly significant difference test following repeated measures analysis of variance (ANOVA) or by Dunn's test following Friedman's ANOVA as applicable. Analyses were two-tailed, and the level of statistical significance was set at P < 0.05 for all comparisons.
Results
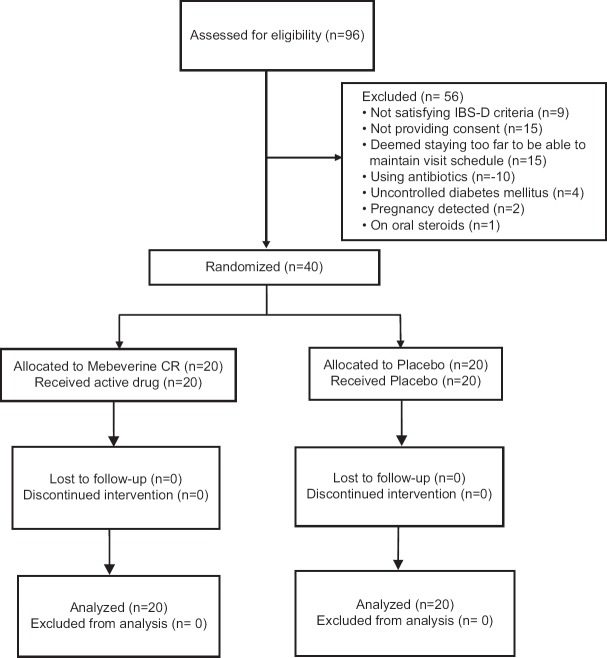
We screened 96 subjects but excluded 56 owing to various reasons as depicted in the study flow diagram in Figure 1. Average age of subjects was 37.6 years in placebo group and 33.7 years in the mebeverine group. There was female preponderance in the placebo group (female: male 18:2) in contrast to the mebeverine group (8:12). Median duration of IBS-D symptoms was also comparable at 32 months in mebeverine arm versus 35 months in placebo arm. Table 1 depicts a summary of the baseline demographics and clinical characteristics.
Figure 1.
Flow of participants in the study
Table 1.
Baseline profile of the study subjects
| Parameter | Mebeverine group (n=20) | Placebo group (n=20) | p |
|---|---|---|---|
| Age (years) | 0.236 | ||
| Range | 16 - 53 | 21 - 60 | |
| Mean±SD | 33.65±9.60 | 37.6±11.09 | |
| Gender | 0.065 | ||
| Male: Female | 8:12 | 2:18 | |
| Residence | 0.523 | ||
| Rural : Urban | 13 : 7 | 10 : 10 | |
| Duration of IBS (months) | 0.675 | ||
| Range | 6.0 - 60.0 | 6.0 - 60.0 | |
| Mean±SD | 19.7±13.16 | 21.7±19.01 | |
| Median (IQR) | 32.0 (38.5 - 29.2) | 35.0 (44.8 - 32.0) |
Abbreviations: IBS=Irritable bowel syndrome, IQR=Interquartile range; SD=Standard deviation. p value in the last column is from Student’s unpaired t test for age, from Mann-Whitney U test for symptom duration and Fisher’s exact test for gender and residence distribution
The median number of bowel movements per day in the past 7 days in the mebeverine group decreased from 5 at the screening visit to 4 and 3.5 at the end of 4th and 8th week of treatment, respectively. The corresponding figures in the placebo group were 5.5, 4 and 4. When we compared the average number of bowel movements on screening and at 4th and 8th weeks of treatment, there was no statistically significant difference between the two groups. However, within the mebeverine group this parameter showed a statistically significant decline from screening to 4th week and 8th week. In the placebo group, a similar pattern was observed. The decline from 4th week to 8th week was not significant in either group [Table 2].
Table 2.
Changes in number of daily bowel movements in past seven days over 8 weeks study period
| Visit time | Mebeverine group (n=20) | Placebo group (n=20) | p | |
|---|---|---|---|---|
| Screening | Range | 4.0 - 12.00 | 4.0 - 10.00 | 0.617 |
| Mean±SD | 5.6±2.06 | 5.6±1.53 | ||
| Median (IQR) | 5.0 (4.0 - 6.0) | 5.0 (4.0 - 6.0) | ||
| End of 4 weeks | Range | 2.0 - 12.0 | 2.0 - 8.0 | 0.766 |
| Mean±SD | 4.3±2.18 | 4.2±1.30 | ||
| Median (IQR) | 4.0 (3.0 - 5.0) | 4.0 (3.5 - 4.0) | ||
| End of 8 weeks | Range | 2.0 - 12.0 | 2.0 - 8.0 | 0.756 |
| Mean±SD | 3.9±2.34 | 3.8±1.51 | ||
| Median (IQR) | 3.5 (2.0 - 4.5) | 4.0 (3.0 - 4.0) | ||
| Within group P | < 0.001 | < 0.001 |
Abbreviations: IQR=interquartile range; SD=standard deviation. p value in the last column is for intergroup comparison by Mann-Whitney t test. The P value for within group comparison is from Friedman’s analysis of variance
The median abdominal pain severity was matching in the two arms at all the visits [Table 3]. The within group decline was significant in the mebeverine arm but not within the placebo arm. In the assessment of IBS QoL score, we found statistically significant difference at screening visit between these two groups, but at further visits there was no significant difference. The within group change mirrored the situation with abdominal pain severity, that is being significant in the mebeverine group but not in the placebo group. Figure 2 presents these comparisons graphically.
Table 3.
Changes in abdominal pain severity over 8 weeks study period
| Visit time | Mebeverine group (n=20) | Placebo group (n=20) | p | |
|---|---|---|---|---|
| Screening | Range | 1.0 - 3.0 | 1.0 - 3.0 | 0.914 |
| Mean±SD | 1.8±0.69 | 1.8±0.83 | ||
| Median (IQR) | 2.0 (1.0 - 2.0) | 2.0 (1.0 - 2.5) | ||
| End of 4 weeks | Range | 1.0 - 3.0 | 1.0 - 3.0 | 0.482 |
| Mean±SD | 1.4±0.59 | 1.6±0.75 | ||
| Median (IQR) | 1.0 (1.0 - 2.0) | 1.0 (1.0 - 2.0) | ||
| End of 8 weeks | Range | 1.0 - 3.0 | 1.0 - 3.0 | 0.615 |
| Mean±SD | 1.3±0.57 | 1.5±0.75 | ||
| Median (IQR) | 1.0 (1.0 - 1.5) | 1.0 (1.0 - 2.0) | ||
| Within group P | < 0.001 | 0.339 |
Abbreviations: IQR=interquartile range; SD=standard deviation p value in the last column is for intergroup comparison by Mann-Whitney t test. The P value for within group comparison is from Friedman’s analysis of variance
Figure 2.
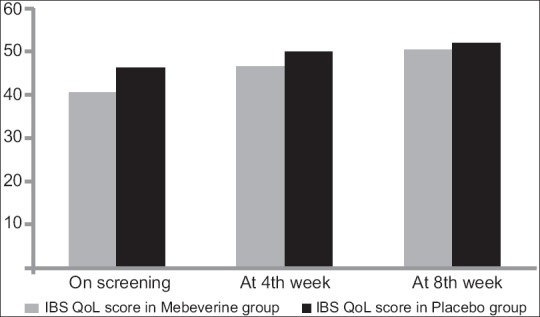
Bar chart showing changes in irritable bowel syndrome quality of life (IBS-QoL) score in the two study groups. Note that the modest increase in IBS-QoL was statistically significant in mebeverine arm but not in placebo arm
There were no serious adverse events or hospitalizations during the study. We observed one probable adverse drug reaction in mebeverine group where one patient developed heartburn sensation within a couple of days of initiation of mebeverine and this was managed by increasing her regular proton pump inhibitor (rabeprazole) dosage. No other adverse events were captured.
Adherence to study medication was comparable between the two groups. Notably, the adherence was poor to fair in a relatively large number of subjects which probably points to lack of remarkable symptom ameliorating effects of study medication.
Discussion
With the availability of the 200 mg CR formulation of mebeverine, there is increasing tendency to use this formulation rather than the conventional 135 mg tablet. In a multicentric, randomized, double dummy, double-blind study designed to demonstrate the equivalence of two forms of mebeverine hydrochloride, the 200 mg twice daily CR capsule and 135 mg thrice daily tablet, in IBS, this aim was achieved with no identifiable safety concerns.[11] Another study concluded that the twice-daily dosage regimen of the 200 mg CR capsule was a well-tolerated alternative to the three times daily regimen of the 135 mg plain tablet, in terms of pharmacokinetics, and likely to improve compliance.[12]
Mebeverine in sustained release formulation was expected to provide beneficial results in IBS-D subtype patients in our study. However, when we compared mebeverine and placebo over 8 weeks study period with respect to outcome parameters like changes in number of daily bowel movements in 7 days prior to a visit, severity of abdominal pain/cramps and IBS-QoL score, we did not find remarkable differences. These findings contradict the view taken in a position paper on mebeverine,[13] but conform to results found in the meta-analysis done by Darvish-Damavandi et al.[8] The possible reasons for the variation might be due to difference in clinical profile of study population, the duration of the study or pharmaceutical differences in the mebeverine formulations used. However, unlike in the placebo group, we did find a modest but statistically significant within group improvement in the mebeverine arm.
From a safety point of view, the active drug mebeverine was well tolerated in the dose of 200 mg twice daily without producing any serious adverse events. However, adherence to treatment remains an issue even with the controlled release formulation. The lack of marked clinical benefit may be the possible explanation for the less than satisfactory adherence in several study subjects.
Our study has limitations; the major one being our inability to reach the target sample size of 35 evaluable patients in each group. The slow pace of recruitment was due to the inability to find patients readily satisfying both the inclusion and exclusion criteria, particularly the criterion of patients not being on medication known to influence disease symptoms currently or within last 4 weeks since many patients were already on various anticholinergic or prokinetic medication. Although we did not have any dropouts from among recruited subjects, the less than satisfactory adherence was another limitation.
Overall, we can conclude that mebeverine 200 mg controlled release preparation on twice daily basis has modest effect in IBS diarrhoea predominant subtype and therefore will not be an appropriate choice for patients with severe symptoms. However, it can be given a fair trial in subjects dissatisfied with other types of medication or experiencing anticholinergic side effects.
Conclusion
Primary care physicians play a pivotal role in our society providing initial as well as emergency treatment to community individuals. Most patients suffering from irritable bowel syndrome visit them initially for symptom relief.[14] So any new information about use of controlled release preparation of mebeverine should be helpful for those physicians. Unfortunately, despite the promise with which it has been introduced, our study indicates that the 200 mg controlled release formulation of mebeverine will not relieve severe symptoms. Thus the cost of treatment will not be justified in such patients, despite the reduced pill burden.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Clinical trial registry number- CTRI/2018/03/012897
References
- 1.Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150:1393–407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 3.Şimşek İ. Irritable bowel syndrome and other functional gastrointestinal disorders. J Clin Gastroenterol. 2011;45:86–8. doi: 10.1097/MCG.0b013e31821fbd6f. [DOI] [PubMed] [Google Scholar]
- 4.Lindner A, Selzer H, Claassen V, Gans P, Offringa OR, Zwagemakers JM. Pharmacological properties of mebeverine, a smooth-muscle relaxant. Arch Int Pharmacodyn Ther. 1963;145:378–95. [PubMed] [Google Scholar]
- 5.Lu CL, Chen CY, Chang FY, Chang SS, Kang LJ, Lu RH, et al. Effect of a calcium channel blocker and antispasmodic in diarrhoea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2000;15:925–30. doi: 10.1046/j.1440-1746.2000.02230.x. [DOI] [PubMed] [Google Scholar]
- 6.Kruis W, Weinzierl M, Schüssler P, Holl J. Comparison of the therapeutic effect of wheat bran, mebeverine and placebo in patients with the irritable bowel syndrome. Digestion. 1986;34:196–201. doi: 10.1159/000199329. [DOI] [PubMed] [Google Scholar]
- 7.Connell AM. Physiological and clinical assessment of the effect of the musculotropic agent mebeverine on the human colon. Br Med J. 1965;2:848–51. doi: 10.1136/bmj.2.5466.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darvish-Damavandi M, Nikfar S, Abdollahi M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J Gastroenterol. 2010;16:547–53. doi: 10.3748/wjg.v16.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maneerattanaporn M, Chang L, Chey WD. Emerging pharmacological therapies for the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:223–43. doi: 10.1016/j.gtc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: Development of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 11.Gilbody JS, Fletcher CP, Hughes IW, Kidman SP. Comparison of two different formulations of mebeverine hydrochloride in irritable bowel syndrome. Int J Clin Pract. 2000;54:461–4. [PubMed] [Google Scholar]
- 12.Winsemius A, Meuwsen IM, Boon C, van der Laan A, Brekle A, de Vries M. A pharmacokinetic comparison of the modified release capsule and a plain tablet formulation of mebeverine. Int J Clin Pract. 2002;56:659–62. [PubMed] [Google Scholar]
- 13.Dumitrascu DL, Chira A, Bataga S, Diculescu M, Drug V, Gheorgh C, et al. The use of mebeverine in irritable bowel syndrome. A position paper of the Romanian society of neurogastroenterology based on evidence. J Gastrointestin Liver Dis. 2014;23:431–5. doi: 10.15403/jgld.2014.1121.234.mibs. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome: The view from general practice. Eur J Gastroenterol Hepatol. 1997;9:689–92. doi: 10.1097/00042737-199707000-00008. [DOI] [PubMed] [Google Scholar]