Abstract
Background:
To compare the efficacy and safety of intravenous levetiracetam and phenytoin in status epilepticus.
Methodology:
A prospective, randomized controlled, nonblinded study was conducted in children 1 month to 12 years of age with active seizure and with status epilepticus. A total of 104 children were randomly allocated to either group 1 (levetiracetam) or group 2 (phenytoin) on the basis of computer-generated random number table. Children already on antiepileptic drugs, very sick children with shock, impending respiratory failure, or head injury, and children hypersensitive to phenytoin or levetiracetam were excluded. Data analysis was done by IBM SPSS statistics.
Results:
The mean age was 4.09 years with a male preponderance with the most common type of seizure being generalized type (74%). The seizures were controlled in all 104 patients initially within 40 min. Seizure control for 24 h was significantly better in group 1 (96%) when compared with group 2 (59.6%) (P = 0.0001). Minibolus of drug was given in 28.8% in group 1 and 46.2% in group 2 (P = 0.068). The seizure recurrence in groups 1 and 2 in the first hour was 1.9% and 5.8%, respectively (P = 0.61), whereas the recurrence between 1 and 24 h was significantly more in group 1 (34.6%) when compared with group 2 (3.8%) (P = 0.0001). The mean time to control seizure was comparable between both the groups (P = 0.71). There was no significant adverse effect in both the groups.
Conclusion:
Levetiracetam is more effective than phenytoin for seizure control for 24 h in children with status epilepticus, and it is safe and effective as a second-line therapy.
Keywords: Efficacy, levetiracetam, phenytoin, status epilepticus
Introduction
Status epilepticus is most common in children younger than 5 years, with incidence between 10 and 60 per 100,000 population.[1] Convulsive status for 30 min leads to irreversible neuronal injury.[2] Established Status Epilepticus Treatment Trial proposed that 35%–45% of patients with convulsive status epilepticus did not respond to benzidizapine; levetiracetam can be given in them.[3] There are retrospective studies on the use of intravenous (IV) levetiracetam in acute seizures in children, in view of paucity of studies of levetiracetam and its comparison with phenytoin in status epilepticus in children; this study was done to compare their efficacy and safety.
Methodology
It is a prospective, randomized study conducted on 104 children in the age group of 1 month to 12 years presenting with status epilepticus. The approval was taken from the Institutional Ethics Committee and a written informed consent was taken from their parents or guardians (Ethics committee approval was obtained dated 29/06/2017). Children already on antiepileptic medication, having head injury, very sick children with shock or impending respiratory failure and renal failure, and children hypersensitive to phenytoin or levetiracetam were excluded as the underlying etiology of seizures makes them more resistant to anticonvulsant response. The patients were randomly allocated to one of the group by computer-generated random number table. Group allocation was concealed in opaque envelops. After enrolment, an envelope was opened and the patient was assigned to that particular group. After taking care of airway and breathing, an IV access was established, and blood samples were obtained for complete blood count, blood sugar, serum electrolytes, renal function, and liver function.
Children who came with active seizures were given midazolam at a dose of 0.1 mg/kg slowly followed by IV levetiracetam and phenytoin depending on group allotment. Children with a history of status epilepticus and presently not in active seizure were given only levetiracetam or phenytoin. The subjects were randomized to receive either IV levetiracetam (group 1) or IV phenytoin (group 2). Children in group 1 were given levetiracitam at a dose of 40 mg/kg diluted in 50 mL of normal saline over 10 min followed by a maintenance dose of 20 mg/kg/day to be given in two divided doses 12 h after initial dose. Children in the control group or group 2 were given IV phenytoin as 20 mg/kg diluted in normal saline over 20 min. For both the groups, if seizures recurred after the first loading of the drug, a further additional dose of 10 mg/kg of the same drug was given. If seizures still recurred, the patients were loaded with valproate with a dose of 20 mg/kg dissolved in 50 mL of normal saline over 10 min and further a maintenance dose of 20 mg/kg in two divided dose was given.
The parameters involving level of consciousness (Glasgow coma scale), heart rate, respiratory rate, blood pressure, and oxygen saturation were recorded at the time of presentation and then again at 30 min, 1 h, 6 h, 12 h, and 24 h. Detailed neurological examination of all children was done. The children were monitored to see whether there was any recurrence of seizure in the subsequent 24 h. Seizure control was defined as the absence of seizure for 24 h after initial loading of the drug. All patients were monitored for adverse effects such as hypotension, bradyarrhythmia, vomiting, agitation, and any behavioral changes. Neuroimaging, electroencephalogram, lumbar puncture, and other investigations for etiological diagnosis were carried out as and when required.
We estimated the sample size for randomized controlled trial comparing levetiracetam to phenytoin for control of acute seizures. From previous literature, we hypothesized that the proportion of children responding to levetiracetam was 88% and those to phenytoin was 68%. Keeping an alpha level of 5% (probability of making type 1 error) and power of 80%, the sample size in each group was kept as 52 with a total sample size of 104 subjects. Discrete categorical data were represented in the form of either a number or percentage (%). The normality of quantitative data was checked by measures of Kolmogrov–Smirnov tests of normality. Skewed data were compared using Mann–Whitney test. The means of normality distributed data for two groups were compared using Student's t-test. For time-related variables, repeated measures analysis of variance or Friedman's test followed by Wilcoxon signed rank test were applied. The proportions were compared using Chi-square or Fisher's exact test. All the statistical tests were two-sided and were performed at a significance level of 0.05. The analysis was conducted using IBM SPSS statistics (version 22.0).
Results
In this study, 104 children in the age group of 1 month–12 years were randomized into group 1 and group 2 by computer-generated random number table to receive levetiracetam and phenytoin, respectively. In group 1, the number of children with age less than 1 year, between 1 and 5 years, and more than 5 years was 18 (34.6%), 23 (44.2%), and 11 (21.2%), respectively. In group 2, it was 15 (28.8%), 16 (30.8%), and 21 (40.4%), respectively. The two groups were comparable with regard to age distribution (P = 0.20). The mean age in group 1 and group 2 was 3.39 ± 3.32 and 4.80 ± 4.11, respectively. Group 1 had 32 (61.5%) males and 20 (38.5%) females, and group 2 had 34 (65.4%) males and 18 (34.6%) females. The sex ratio was statistically insignificant (P = 0.65). The most common type of seizure [Graph 1] was generalized seizures, followed by simple partial seizure and then complex partial seizure. The distribution of cases in view of seizures was comparable between the two groups (P = 0.21). Out of 104 patients, 58.1% presented with one episode of seizures, followed by 24% with two episodes and 21% with three or more episodes of seizures [Graph 2].
Graph 1.
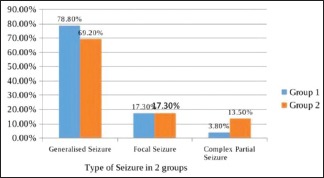
Type of seizure in both the groups
Graph 2.
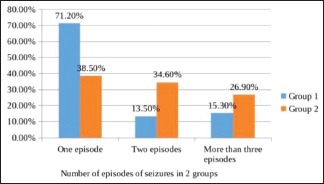
Number of episodes of seizure in both the groups
On comparing the history of seizures and fever, we found that 18 (17.3%) patients had seizures in the past, of which 8 (15.4%) were from group 1 and 10 (19.2%) were from group 2 which was not statistically significant (P = 0.60). Overall 53 (51%) had fever associated with seizure, of which 29 (55.8%) were from group 1 and 24 (46.2%) from group 2, and it was not statistically significant (P = 0.33). About 17 (16.3%) patients had developmental delay, of which 8 (15.4%) were from group 1 and 9 (17.3%) were from group 2. It was not statistically significant (P = 0.79).
When we compared the time taken to control seizures in both the groups [Graph 3], we found that in group 1 seizure was controlled in 65.4%, 32.7%, and 1.9% of cases within 5 min, 5–20 min, and 20–40 min, respectively of levetiracetam administration. In group 2, they were controlled in 69.2%, 28.8%, and 1.9%, respectively, of phenytoin administration. Initial seizure control with respect to time was comparable between the two groups (P = 0.91). Seizures were initially controlled in all patients of both groups within 40 min. The mean time to control seizures in groups 1 and 2 was 6.02 and 5.65 min, respectively. There was no significant difference between the two groups with regard to time to control seizure (P = 0.71).
Graph 3.
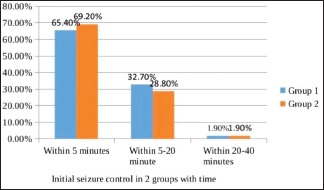
Initial seizure control in both the groups with time
In the levetiracetam group, 13 (25%) patients needed minibolus of 10 mg/kg after a loading dose of 40 mg/kg for initial control of seizure, while in the phenytoin group, 7 (13.5%) needed minibolus. This difference was also statistically insignificant (P = 0.135). Seizures were totally controlled for 24 h [Graph 4] in 50 (96.2%) and 31 (59.6%) in groups 1 and 2, respectively. Seizure control for 24 h was significantly better in the levetiracetam group (P = 0.0001) than the phenytoin group although more patients (13) in the levetiracetam group needed minibolus after the loading dose when compared with patients (7) in the phenytoin group. Seizure recurrence in the first hour was seen in 1 (1.9%) and 3 (5.8%) of patients in groups 1 and 2, respectively. There was no significant difference (P = 0.61). Seizure recurrence between 1 and 24 h [Graph 5] was seen in 2 (3.8%) and 18 (34.6%) of patients in groups 1 and 2, respectively. There was significantly more seizure recurrence in the phenytoin group (P = 0.0001).
Graph 4.
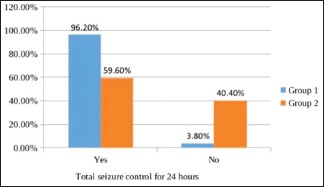
Total seizure control for 24 h in both the groups
Graph 5.
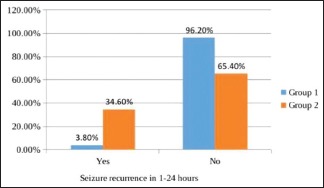
Seizure recurrence in 1–24 h in both the groups
Only 3 (2.9%) patients in group 1 and one patient in group 2 required additional antiepileptic. The remaining 101 patients did not require any additional antiepileptic to control seizure. It was statistically not significant (P = 0.56). All the enrolled cases were reviewed for adverse effect and there was no adverse effect in any subject in the two groups. Out of 104 patients, 98 (94.2%) improved, 51 in group 1 and 47 in group 2. The difference between both groups with regard to the outcome was statistically not significant (P = 0.227). The most common cause of seizure in our study was febrile seizure followed by seizure disorder, neurocysticercosis, meningoencephalitis, and then hypocalcemic seizures being least common [Graph 6].
Graph 6.
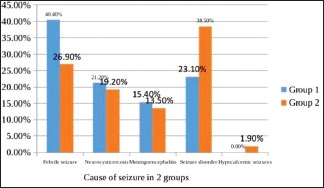
Cause of seizure in both the groups
Discussion
In this prospective, randomized controlled study, we compared the efficacy and safety of levetiracetam and phenytoin in the treatment of status epilepticus in children. The age range was 1 month to 12 years and the mean age was 4.09 years, with a slight male preponderance. The retrospective studies by Altunbasak et al.[4] and Unalp et al.[5] also had male preponderance and the mean age was 69.39 and 41.3 months, respectively. Nicholas et al.[6] found the mean age to be 60 months.
In this study, the most common type of seizure was generalized type followed by simple partial seizure followed by complex partial seizure. Unalp et al.[5] and Altunbasak et al.[4] found generalized seizure in 82% and 18% and focal seizure in 65.7% and 34.3%, respectively.
We observed that seizure control for 24 h was significantly better in the levetiracetam group when compared with the phenytoin group (P = 0.0001). Unalp et al.[5] noted that 104 (78%) out of 133 children who were given levetiracetam showed total seizure control and did not recur during the follow-up period. Singh et al.[7] conducted a study on 44 patients and concluded that phenytoin achieved seizure control in 68.2% and levetiracetam in 59.1%. Both the groups showed comparable result (P = 0.34). In a prospective study by Satishchandra et al.,[8] seizure control in phenytoin, valproate, and levetiracetam subgroups was 68%, 68%, and 78%, respectively. Altunbasak et al.[4] used levetiracetam as a first-line drug for status epilepticus in patients with respiratory depression, cardiac arrhythmia, hepatic failure, and thrombocytopenia and found that it was effective in controlling seizure in 73.1% of patients [Table 1].
Table 1.
Outcome parameters of various studies
| Study | Type of study | Age group | Study groups and number | Efficacy for seizure control |
|---|---|---|---|---|
| Nicholas et al. (2009) | Retrospective study | 1-14 years | Levetiracetam group (n=10) | Levetiracetam (73%) |
| Unalp et al. (2014) | Retrospective study | 1 month-18 years | Levetiracetam group (n=133) | Levetiracetam (78%) |
| Satishchandra et al. (2015) | Prospective, randomized controlled | Not available | Levetiracetam, phenytoin, valproate (n=150) | Levetiracetam (78%) Phenytoin (68%) Valproate (68%) |
| Visudtibhan et al. (2015) | Retrospective study | Less than 18 years | Levetiracetam group (n=50) | Levetiracetam (60%) |
| Singh et al. (2015) | Randomized controlled trial | Not available | Levetiracetam and phenytoin groups (n=144) | Levetiracetam (59.1%) Phenytoin (68.2%) |
| Altunbasak et al. (2016) | Retrospective study | 1-192 months | Levetiracetam group (n=108) | Levetiracetam (73.1%) |
| Isgueder et al. (2016) | Retrospective study | Not available | Levetiracetam group (n=133) | Levetiracetam (78.2%) |
| This study (2017) | Randomized controlled trial | 1 month-12 years | Levetiracetam and phenytoin groups (n=104) | Levetiracetam (96.2%) Phenytoin (59.6%) |
In our study, 75% of patients achieved seizure control after initial loading dose (40 mg/kg) of levetiracetam and the remaining 25% needed an additional minibolus (10 mg/kg) to achieve seizure control. After seizure control, we used a maintenance dose of 20 mg/kg/day at 12 hourly interval. In a study by Altunbasak et al.,[4] the mean IV loading dose of levetiracetam was 28.33 (10–40 mg/kg) and a mean maintenance dose was 33.7 mg/kg/dose (20–40 mg/kg) was found to be effective in treating 73.1% of patients. In a study by Satishchandra et al.,[8] IV loading dose of levetiracetam was 25 mg/kg which was found to be effective in controlling seizures in 78% of patients. In a study by Singh et al.,[7] IV levetiracetam was given at a loading dose of 20 mg/kg and there was successful termination of seizure within 30 min in 59.1% of patients. Unalp et al.[5] used a mean loading dose of 18.2 mg/kg/dose and a mean maintenance dose of 35.3 mg/kg/day and seizures were controlled in 78% of patients.
In this study, 2 (3.8%) patients in the levetiracetam group and 18 (34.6%) in the phenytoin group had seizure recurrence within 1–24 h. In a study by Nicholas et al.,[6] 1 out of 10 children had recurrence of seizure within 24 h and needed an additional minibolus of drug after 1.5 h of initial loading dose.
Levetiracetam was found to have no significant side effects on cardiorespiratory parameters as reported in previous studies.[9,10,11] In our study also there were no significant adverse effects in either of the group which was similar to other studies.[6,12] On the contrary, Unalp et al.[5] noted side effects in 2.2% of patients which were resolved after dose reduction. Altunbasak et al.[4] also noted side effects in the form of agitation and mild erythematous rash after IV levetiracetam.
Among the etiological factor, febrile seizure was found to be the most common followed by seizure disorder, neurocysticercosis, and meningoencephalitis, and the least common was hypocalcemic seizures. On the contrary in study by Unalp et al.,[5] the three most common causes of seizure were hypoxic ischemic encephalopathy, meningitis and neurometabolic disorder. In a study by Altunbasak et al.,[4] cerebral palsy and hypoxic ischemic encephalopathy were common followed by meningitis and cranial involvement in hematological malignancy.
Hence, to conclude, levetiracetam was found to be more efficacious when compared with phenytoin in controlling seizures with no side effects and it can be used as an alternative second-line antiepileptic drug in children with status epilepticus.
The strength of this study was its randomized design using standard protocol, strict allocation of children in two groups as per computer-generated randomized table which resulted in good match for reported symptom, sign on examination, and baseline parameters and laboratory findings at presentation. Two arm treatment groups had strengthened the comparison to infer the results and tried to remove bias.
The limitation of this study was its nonblinded study design, which would have introduced some bias during patient assessment. Second, drug level of levetiracetam and phenytoin was not done due to financial constraints, and the dose given in our study was similar to other studies in which similar dosage maintained the drug level. Still in our study population, there may be some unknown factors that may alter the drug pharmacokinetics and pharmacodynamics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mikati MA, Hani AJ, Kliegman Robert M, Behrman RE, Jenson HB, Stanton FB. Nelson Textbook of Pediatrics. 20th ed. Philadelphia: Saunders; 2016. Status epilepticus; pp. 2854–56. [Google Scholar]
- 2.Fountain NB. Status epilepticus: Risk factors and complications. Epilepsia. 2000;41:23–30. doi: 10.1111/j.1528-1157.2000.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleck T, Cock H, Chamberlain J, Cloyd J, Connor J, Elm J, et al. The established status epilepticus trial 2013. Epilepsia. 2013;54:89–92. doi: 10.1111/epi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altunbasak S, Incecik F, Horoz OO, Herguner OM, Yildizdas D, Besen S, et al. Intravenous levetiracetam in critically ill children for treatment of acute repetitive seizures or status epilepticus. Ann Indian Acad Neurol. 2016;19:79–82. doi: 10.4103/0972-2327.167702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unalp A, Guzel O, Agin H, Yilmaz U, Akarcan SE, Celik T, et al. Efficacy and safety of intravenous levetiracetam in children with acute repetitive seizures. J Pediatr Neurol. 2014;51:688–95. doi: 10.1016/j.pediatrneurol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas SA, Heather MM, Daniel JL, Dennis JD. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatric Crit Care Med. 2009;10:505–10. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P, Chakravarthi S, Goyal MK, Manish Modi M, Bhalla A. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci. 2015;22:959–63. doi: 10.1016/j.jocn.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Satishchandra P, Mundlamuri RC, Sinha S, Subbakrishna DK, Prathyusha PV, Nagappa M, et al. Management of generalized convulsive status epilepticus: A prospective randomized controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam. J Epilepsy Res. 2015;114:52–8. doi: 10.1016/j.eplepsyres.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Wheless JW, Clarke D, Hovinga CA, Ellis M, Durmeier M, McGregor A, et al. Rapid infusion of a loading dose of intravenous levetiracetam with minimal dilution: A safety study. J Child Neurol. 2009;13:946–51. doi: 10.1177/0883073808331351. [DOI] [PubMed] [Google Scholar]
- 10.Sasidaran K, Singhi S, Singhi P. Management of acute seizure and status epilepticus in pediatric emergency. Indian J Pediatr. 2012;13:510–7. doi: 10.1007/s12098-011-0604-9. [DOI] [PubMed] [Google Scholar]
- 11.Weinstock A, Ruiz M, Gerard D, Toublanc N, Stockis A, Farooq O, et al. Prospective open-label, single-arm, multicenter, safety, tolerability, and pharmacokinetic studies of intravenous levetiracetam in children with epilepsy. J Child Neurol. 2013;13:1423–9. doi: 10.1177/0883073813480241. [DOI] [PubMed] [Google Scholar]
- 12.Visudtibhan A, Khongkhatithum C, Thamprantankul L, Wiwattanadittakul N. Intravenous levetiracetam in Thai children and adolescents with status epilepticus and acute repetitive seizures. Eur J Pediatr Neurol. 2015;19:429–34. doi: 10.1016/j.ejpn.2015.02.010. [DOI] [PubMed] [Google Scholar]


