Abstract
Freshwater systems are faced with a myriad of stressors including geomorphological alterations, nutrient overloading and pollution. Previous studies in marine fish showed polyaromatic hydrocarbons (PAHs) to be cardiotoxic. However, the cardiotoxicity of anthropogenic pollutants in freshwater fishes is unclear and has not been examined across multiple levels of cardiac organization. Here we investigated the effect of phenanthrene (Phe), a pervasive anthropogenic pollutant on a sentinel freshwater species, the brown trout (Salmo trutta). We first examined the electrical activity of the whole heart and found prolongation (∼8.6%) of the QT interval (time between ventricular depolarization and repolarization) of the electrocardiogram (ECG) and prolongation (∼13.2%) of the monophasic action potential duration (MAPD) following ascending doses of Phe. At the tissue level, Phe significantly reduced trabecular force generation by ∼24% at concentration 15 μM and above, suggesting Phe reduces cellular calcium cycling. This finding was supported by florescent microscopy showing a reduction (∼39%) in the intracellular calcium transient amplitude following Phe exposure in isolated brown trout ventricular myocytes. Single-cell electrophysiology was used to reveal the mechanism underlying contractile and electrical dysfunction following Phe exposure. A Phe-dependent reduction (∼38%) in the L-type Ca2+ current accounts, at least in part, for the lowered Ca2+ transient and force production. Prolongation of the MAPD and QT interval was explained by a reduction (∼70%) in the repolarising delayed rectifier K+ current following Phe exposure. Taken together, our study shows a direct impact of Phe across multiple levels of cardiac organization in a key freshwater salmonid.
Keywords: Poly aromatic hydrocarbons, PAHs, brown trout, Cardiac action potential, AP, ECG, Electrophysiology, Toxicology, Fish
Highlights
-
•
Phenathrene reduced cardiac muscle contractility.
-
•
Phenathrene disrupted electrical activity of the whole heart and cardiomyocytes by inducing QT interval and action potential aprolongation.
-
•
Phenathrene disrupts electrical activity by inducing QT and action potential prolongation.
1. Introduction
The aquatic environment, particularly coastal and inland waterbodies, are extremely vulnerable to pollution. Rising population, industrial development and demand for energy has resulted in an increase in the transport of oil by water (Thompson et al., 2017). Increase in global demand means increased production and transport of oil and oil-based products which has led to disasters within the aquatic environment. Major oil spills, such as the 1989 Exxon Valdez and the 2010 Deepwater Horizon disasters, released large quantities of hydrocarbons including large amounts of polyaromatic hydrocarbons (PAHs) into the aquatic ecosystem. Aside from spills, PAHs are an ever increasing anthropogenic pollutant in the aquatic environment constantly receiving input through atmospheric deposition of exhaust particles, soot from vehicular emissions, industrial emissions and forest fires (Lima et al., 2003; National Research Council (US) Committee on Oil in the Sea, 2003; Van Metre and Mahler, 2003; Brette et al., 2017; Thompson et al., 2017). There are also allochthonous input routes for PAH pollution through urban runoff and seepage from oil transport pipes (Incardona et al., 2004; Mahler et al., 2005; Brette et al., 2017). Most worrisome is the fact that PAHs are persistent organic pollutants (Behera et al., 2018) and are known to cause a myriad of effects in a wide range of organisms including carcinogenicity (Baars, 2002; Laffon et al., 2006; Moorthy et al., 2015), disruption of the endocrine system (Gentes et al., 2007; Zhang et al., 2016), DNA damage and adduct formation (Hazilawati et al., 2017), developmental toxicity (Incardona et al., 2006; Carls et al., 2008; Li et al., 2011; Sorhus et al., 2016) and cardiotoxicity (Incardona et al., 2009, 2015; Zhang et al., 2013a, 2013b; Brette et al., 2014, 2017; Edmunds et al., 2015).
Developing embryos of fish exposed to petrogenic PAHs showed low survival, increased mortality, delayed hatching, impacted swimming (Le Bihanic et al., 2014), pericardial and yolk-sac oedema, jaw malformations, skeletal defects, lordosis, scoliosis, bradycardia, cardiac arrhythmia, and tachycardia (Incardona et al., 2004, 2005, 2009; Lucas et al., 2014; Adeyemo et al., 2015; Sorhus et al., 2016). The mechanisms underlying the developmental toxicity and cardiotoxicity of PAHs have been summarised in the recent review by Incardona (2017) where the focus is on embryonic developmental stages and cellular function in marine fishes. Fewer studies have investigated the toxicity of PAHs at the whole heart level. Nelson et al. (2016), showed that the cardiovascular system of mahi-mahi (Coryphaena hippurus) was compromised when it was exposed to high energy water accommodated fraction (HEWAF) of crude oil containing 9.6 ± 2.7 μg/l ΣPAH for 24 h. The results revealed a significant reduction in stroke volume (by 44%), cardiac output (by 39%), stroke work (by 52%), ventricular contractility (by 28%), and contractility index (by 24%) of the whole heart. Whole heart function is controlled by excitation-contraction coupling in the cardiomyocytes that make up the heart. PAHs have been found to impair cardiac excitation-contraction coupling in a number of marine fish species (Brette et al., 2014), by disrupting Ca2+ dynamics through inhibition of intracellular Ca2+ flux and decreases in extracellular Ca2+ influx via the L-type Ca2+ current ICaL. Cellular electrical activity was also disrupted following PAH exposure via the delayed rectifier potassium current (IKr) inhibition and action potential (AP) prolongation which is the basis for cardiac arrhythmogenesis. Subsequently, these effects were found to be due to Phe, a 3-ringed PAH, which is a significant component in crude oil (Brette et al., 2017).
To date, the majority of studies on cardiotoxicity focused on the marine environment. However, freshwater ecosystems are biologically diverse containing over 60% of all fish species (Convention on Biological Diversity, 2015). Freshwater systems are under extreme threat due to geomorphological alterations, land use changes, water abstraction, invasive species, nutrient overloading and pollution (Dudgeon et al., 2005) and reports suggest that inland waters receive smaller but more frequent oil spills than its marine counterpart (Michel and Ploen, 2017). Recently, a rail way freight incident released 230,000 gallons of crude oil into Rock River, Iowa (Elyachar, 2018). An estimated 819,000 gallons of crude oil was released into the Kalamazoo River from a ruptured Enbridge Lakehead Line 6B pipeline impacting an estimated 1,600 acres of river, stream, floodplains and uplands (USFWS, 2015). The M/T Westchester oil spill released estimated 554,400 gallons of Nigeria crude oil into the Mississippi River in 2000 wherein the lower molecular weight PAHs accounted for 75% of the total PAH content. These PAHs exhibited significant acute toxicity to aquatic species (Michel et al., 2002). Consequently, there has been a ∼80% decline in freshwater species diversity over the past half-decade (Darwall et al., 2018). Therefore, understanding the impact of PAHs on biota in this fragile ecosystem is imperative.
Here we investigate the impact of Phe on the whole heart electrical activity, cardiac tissue contractile activity and ventricular cardiomyocyte ion flux in the brown trout, a key freshwater indicator species (Edwards et al., 1990). We show changes in whole heart electrical activity consistent with arrhythmogenesis due to prolongation of the monophasic action potential duration (MAPD) and interval between ventricular depolarization and repolarization (QT interval) as shown in the electrocardiogram (ECG). This corresponds to APD prolongation and Ikr (erg channel) inhibition in isolated ventricular cardiomyocytes. Phe also disrupted cellular Ca2+ dynamics resulting in reduced intracellular Ca2+ transients and inhibition of L-type Ca2+ channels which explains, at least in part, the reduction in tissue level contractile force following Phe exposure. As such, we show the mechanisms underlying the pro-arrhythmogenic and negative ionotropic effects of Phe on the brown trout heart. We concluded that, similar to the marine environment, PAHs in freshwater can have profound effects on fish cardiac health. The plight of salmonids and the role of the cardiac system in powering arduous migrations has received considerable attention in relation to warming, hypoxia, and altered flow regimes (Farrell, 2009). We suggest that PAH pollutants represent another cardiac stressor for freshwater fish and that future work in this area should go beyond the heart and address the impact of Phe at the organismal level.
2. Materials and methods
2.1. Animal husbandry
Brown trout (Salmo trutta) were obtained from Dunsop Trout Farm Ltd Clitheroe, UK and transported to the University of Manchester's aquarium facility in cold dechlorinated aerated water. The fish were held in 800 l aerated re-circulatory outdoor tanks at 10 °C for a minimum of 2 weeks prior to experimentation. Fish experienced a natural photoperiod and were fed ad libitum with commercial trout pellets three times a week. Water quality was maintained by large biological filters and 25% water changes 3 times a week, with regular testing for nitrogen compounds.
2.2. Assessing electrical activity of the whole heart
Brown trout (mean mass 332.4 ± 29.6 g; n = 10) were stunned by a sharp blow to the head, the spine cut and the brain destroyed. The heart was quickly excised and placed in ice-cooled physiological saline (see composition below). The excised heart (mean mass 0.61 ± 0.05 g; n = 10) was mounted in a Langendorff perfusion system where it was retrograde perfused with 10 °C physiological saline (see composition below) containing 5 μM adrenaline to maintain muscle tone (Graham and Farrell, 1989) and supplemented with blebbistatin (10 μM) to minimise contraction and thus movement artefacts during recording of electrical activities. The atrium was dissected to remove pacemaker tissue to allow external control of heart rate. A stimulating electrode was placed at the atrioventricular junction and the heart paced at 0.5 Hz while monophasic action potential (MAP) and electrocardiogram (ECG) electrodes where placed on the surface of the ventricle. The electrodes were connected to a bio amp (ADInstruments), then to a power lab 4/35 (ADInstruments) and data was recorded via LabChat software 8.0. Once steady, ECG and MAP recordings were achieved at 0.5 Hz, 0.6 Hz and 0.8 Hz. These frequencies were chosen to reflect in vivo heart rate range of trout at 10 °C (Wood et al., 1979). After control recordings, Phe was added to the saline perfusing the heart in cumulative doses to reach 5, 15 and 25 μM concentrations. At each concentration, MAPs and ECGs were recorded at 0.5, 0.6 and 0.8 Hz. From these recording, the QT duration was extracted from the ECG data and subsequently corrected for heart rates; QTc = QT/[√ (60/HR)]. Monophasic action potential duration was assessed at 90% repolarization (APD90), and triangulation was calculated as APD90-APD30. Saline samples entering the heart and flowing out of the heart were collected to assess Phe absorption into heart tissues (Jenway 6305 UV-VIS spectrophotometer, 251 nm).
2.3. Assessing force development of cardiac tissues
Brown trout (body mass 195 ± 8.7 g, n = 13) were killed and the heart (mean mass 0.29 ± 0.01 g, n = 13) excised as described above. The ventricle and atrium were separated and bisected to expose the lumen. Four thin ventricular and atrial strips were cut lengthwise and positioned between a fixed post of a force transducer and lowered into water-jacketed myobath containing 15 ml of oxygenated physiological saline at 10 °C. The ventricle strips had a mean mass of 5.8 ± 0.4 mg (n = 58) and length of 4.7 ± 0.2 mm (n = 58) while the atria strips had a mean mass of 4.1 ± 0.3 (n = 46) and length 3.2 ± 0.3 mm (n = 50).
The strips were lengthened to remove slack and left for 1 h after which 5 nM adrenaline was added to maintain muscle tone (Shiels and Farrell, 1997). The strips were stimulated to contract using an SD9 square pulse stimulator (USA Grass) at 70 V with10 ms pulse duration. Output from the force transducers was coupled to a 4 channel power lab 4/35 (ADInstruments) where it was digitized and stored using Labchart software (7.0). The muscle length at which active tension is maximized (Lmax) was established and the length was adjusted to L98 (length at 98% of Lmax) and allowed to equilibrate at this length for 30 min under basal stimulation (0.2 Hz), before being subjected to the experimental protocols. Each protocol consisted of 3 force-frequency trials performed sequentially on the same muscle strip: a control, a Phe exposed, and an adrenaline (1 μM) exposed. In each force–frequency trial, stimulation frequency was increased from 0.2 to 1.0 Hz in 0.2 Hz increments. After each change in stimulation frequency, the muscles were allowed to stabilize (∼5 min) before new force measurements were made. Muscles were allowed to recover at 0.2 Hz for 30 min between force-frequency trials. After the control force-frequency trial, a muscle strip was exposed to fresh saline containing either a DMSO control or one of three concentrations of Phe (5 μM, 15 μM or 25 μM) for 30 min. During each saline change, 5 nM adrenaline was added to each organ bath before the force–frequency trial was repeated to maintain muscle tone (Graham and Farrell, 1989). The saline changed ensured there was a control value of force for each exposure and also a control experiment where strips were only exposed to DMSO. Deterioration of the preparation over the duration of the experiment was monitored using the control strip.
At each stimulation frequency and under each experimental condition, force, time to peak force, time to 63% relaxation (tau) and the rates (df/dt) of contraction and relaxation were measured. Before removing the muscle strips from the bath, the length was measured (to 0.1 mm) using a Vernier calliper and the region of the tissue that contributed to force development was weighed (to the nearest 0.01 mg). Mean cross-sectional area was calculated using muscle mass, muscle strip length and an assumed muscle density of 1.06 g cm−3(Layland et al., 1995; Shiels et al., 1999) and used to standardised force, expressed as mN mm−2.
2.4. Assessing mechanisms of cardiotoxicity at the cellular level
Myocyte isolation: Myocyte were isolated according to (Shiels et al., 2000, 2006). Briefly, brown trout (body mass 347.2 ± 39.7 g, n = 6) were killed and the heart (mean mass 0.43 ± 0.05 g, N = 8) was isolated and cannulated through the bulbus arteriosus and perfused from a height of 30 cm with a Ca2+ free isolation solution (Shiels et al., 2006, Shiels et al., 2000) for 8–10mins followed by enzymatic digestion (5 mg Trypsin type III, 7.5 mg Collagenase Type 1A and 7.5 mg bovine serum albumin in 10 ml isolation solution) for 18–20mins. The ventricle was isolated and cut into small pieces and gently titrated via Pasteur pipette to liberate single cells. These were held at 4 °C in isolation solution for up to 8 h.
Intracellular Ca2+transients: Intracellular Ca2+ transients were recorded using the Ca2+ indicator, Fluo 4-AM. A 500 μl suspension of myocytes was incubated with 1.4 μM of Fluo 4-AM for 10 min at 11 °C. This temperature and time combination was determined with a set of preliminary studies where dye loading was optimised. Following the protocol of Brette et al. (2017), the dye-loaded cell suspensions were then diluted by adding 2.5 ml ringer solution containing different concentrations of Phe and allowed to de-esterify whilst being exposed to Phe for 1 h. Control cells were treated with DMSO. Using a Nikon Eclipse TE2000-U epifluorescence microscope, the dye was excited at 488 nm and emission was measured at 500 ± 40 nm while the cell was stimulated at 0.2 Hz. Signal was acquired using a PMT coupled with an OptOscanmonochromator(CAIRN research, UK) set to a gain of 9.20 and 878 V. This was connected to a Digidata 1440A (Axon instrument CA) and signal was acquired using Clampex 11 (Axon Instruments CA) and analysed offline using a Clampfit11.0.3 (Axon Instruments CA).
Whole-cell patch clamp: Electrophysiological data was recorded as previously described (Brette et al., 2017). Briefly, isolated myocytes were allowed to settle for ∼10 min in the recording chamber at the beginning of each trial and then perfused with external solution (see composition below). Membrane potentials and currents were recorded from each myocyte in whole-cell mode under baseline (control) conditions and in the presence of Phe in the extracellular solution. The wave-forms used to elicit ion currents and APs are provided in the figure legends.
APs were evoked using 10 ms sub-threshold current steps at a frequency of 0.5 Hz. The L-type Ca2+ channel current (ICaL) was elicited by a pulse to 0 mV (the approximate peak of the current-voltage relationship) after a pre-pulse to −40 mV to inactivate Na+ current. ICaL was measured as the difference between peak and the end of pulse current. IKr was activated by a pre-pulse to +40 mV (to fully activate K+ channels as determined in preliminary experiments) and measured as the tail current at −40 mV. Data was recorded via a Digidata 1322A A/D converter (Axon Instruments, CA) controlled by an Axopatch 200B (Axon Instruments, CA) amplifier running pClamp 10.3 software (Axon Instruments, CA). Signals were filtered at 1–10 kHz using an 8-pole Bessel low pass filter before digitization at 10–20 kHz and storage. Patch pipette resistance was typically 2–3 MΩ when filled with intracellular solution. Cell membrane capacitance (mean ± S.E.M = 31.85 pF ± 4.8, n = 34 myocytes from 8 fishes) was measured using the “membrane test module”.
2.5. Statistical analysis
Data are reported as mean ± S.E.M. Data from whole heart and tissues data were subjected to a two-way ANOVA and the statistical significance was tested using a Fisher's LSD posthoc test at p < 0.05. Data from myocyte exposure were analysed using a one-way ANOVA followed by a Fisher's LSD post-hoc test at p < 0.05. The data for force of contraction were reported as a percentage of the control but were transformed using formula Y = Log(Y) for statistical analysis. All data were analysed using the GraphPad Prism 7.0 software. Data for Ca2+ transient was extracted from Clampfit into Graphpad and the change in the amplitude of the florescent signal was normalized to its respective base line (ΔF/F0). Tau (τ) was fit using the experimental standard equation;
Electrophysiological data were analysed using Clampfit and GraphPad Prism software. All AP parameters were stable over the time of recording (<10 min) in controls. Currents are expressed as current density (pApF−1).
2.6. Physiological solutions and chemicals
The physiological saline is same as previously described (Shiels and Farrell, 1997). The Ringer solution contained (in mM) 150 NaCl, 3.5 KCl, 1.5 MgSO4, 0.4 NaH2PO4, 2 CaCl2, 10 glucose and 10 HEPES, with pH set to 7.7 with NaOH. This Ringer solution was used for the whole heart and tissue studies. Whole-cell patch clamp recording external solution contained (in mM) 150 NaCl, 3.5 KCl, 1.5 MgCl2, 3.2 CaCl2, 10 glucose and 10 HEPES, with pH set to 7.7 with NaOH. For AP recording, pipette solutions contained (in mM): 10 NaCl, 140 KCl, 5 MgATP, 0.025 EGTA, 1 MgCl2, and 10 HEPES, pH adjusted to 7.2 with KOH. For ICa measurement, the pipette solution contained (in mM) 130 CsCl, 15 TEA-Cl, 5 MgATP, 1 MgCl2, 5 Na2-phosphocreatine, 5 EGTA, 10 HEPES, and 0.03 Na2GTP, pH adjusted to 7.2 with CsOH. CsCl and TEA-Cl were included to inhibit activities of K+ channels. For IKr measurements the pipette solution contained (in mM): 10 NaCl, 140 KCl, 5 MgATP, 5 EGTA, 1 MgCl2, and 10 HEPES, pH adjusted to 7.2 with KOH. For IKr recordings, tetrodotoxin (TTX, 0.5 μM), nifedipine (10 μM), and glibenclamide (10 μM) were included in the external solution to inhibit Na+, Ca2+, and ATP-sensitive K+ channels. For ICaL recordings, tetrodotoxin (TTX, 0.5 μM), E-4031 (2 μM), and glibenclamide (10 μM) were included in the external solution to inhibit Na+, IKr and ATP-sensitive K+ channels, respectively.
All reagents including Phe (analytic grade), were acquired from Sigma Aldrich except blebbistatin (MedChemExpress, MCE USA), Fluo 4-AM (Molecular Probes, Oregon USA) and TTX (Tocris, UK). All stock solutions were made using Milli-Q supplied water (Millipore, USA). Phe stock (25 mM) was prepared by dissolving 22 mg of Phe into 5 ml DMSO (tissue culture grade, Sigma). A 10 mM stock of blebbistatin was made by dissolving 25 mg in 1 ml DMSO. From these stocks, working solutions of 5, 15 and 25 μM of Phe and 10 μM blebbistatin were achieved. In all experiments, maximal DMSO was maintained below 1:1000.
The range of concentrations of Phe used in this study reflect those previously employed to understand the mechanism of cardiotoxicity in whole embryos (28–56 μM, Incardona et al. (2004)) and isolated cardiomyocytes from marine fish species (5–25 μM, Brette et al. (2017)). However, these ranges are higher than those routinely reported for the freshwater habitat. Moeckel et al. (2013), reported total and freely dissolved PAH in a typical upland stream in the UK to range from 2.71 to 18.9 ng L−1 and 2.61–16.8 ng L−1, respectively. However, concentrations up to 7420 μg L−1 have been reported in stream and rivers in Ogoniland, Nigeria (Lindén and Pålsson, 2013).
3. Results
3.1. Phenanthrene disrupts electrical properties of trout heart
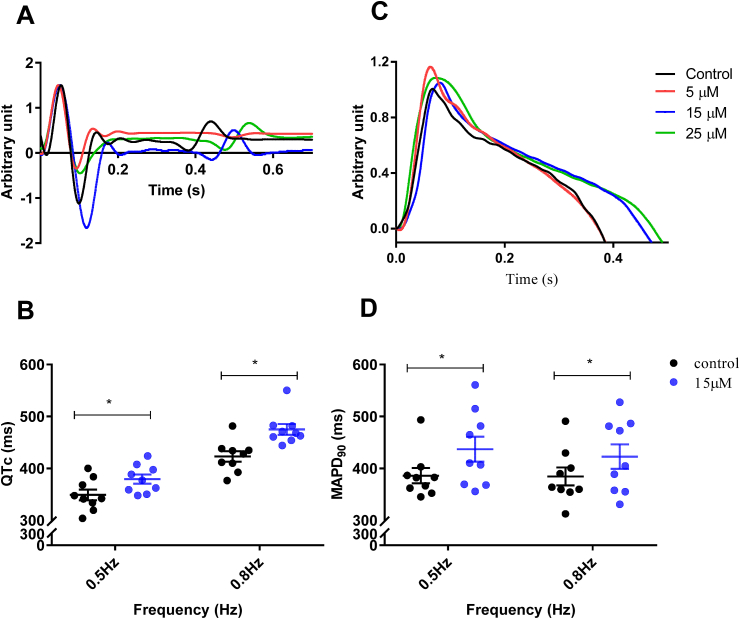
ECG recordings provide information on the electrical activity sweeping across the heart with each beat. The QT interval in the ECG reflects the time between ventricular depolarization and repolarization and it is used clinically to assess the electrical health of the heart. A change in this interval is indicative of arrhythmia risk. Fig. 1 shows the effect of Phe on the ECG of the brown trout heart. The raw ECG recording (Fig. 1A) show the prolongation of the QT interval following Phe exposure. Mean data where the QT interval is corrected for heart rate (QTc; Fig. 1B) shows that Phe caused significant prolongation of QTc interval on exposure to 15 and 25 μM concentrations, across all frequencies (also see Table S1). At the highest cardiac pacing frequency (0.8 Hz), 5 μM Phe caused significant elongation of QTc indicating the potential for Phe to be cardiotoxic even at low concentrations. A representative MAP trace from an isolated heart (Fig. 1C) shows the effects of Phe on the MAP which reveals the time course (but not voltage change, presented in arbitrary units) of an action potential taken from the surface of the heart. Phe caused significant MAP prolongation at concentrations ≥15 μM, which explains the prolongation of depolarization observed in the QTc. MAP prolongation at 90% repolarization (MAPD90) was significant at pacing frequencies of 0.5, 0.6 and 0.8 Hz at ≥ 15 μM Phe (Fig. 1D, Table S1). Phe also effected MAP triangulation (calculated as MAPD90 – MAPD30) across frequencies of 0.5, 0.6 and 0.8 Hz which is a measure of electrical dispersion (Table S1).
Fig. 1.
The impact of Phenanthrene (Phe) on the electrical properties of the brown trout heart. (A) Representative ECG traces from a heart contracting at 0.5 Hz showing QT interval prolongation in presence of increasing Phe concentration. (B) The corrected QT interval (QTc, see methods) under control conditions and upon exposure to 15 μM of Phe at 0.5 Hz, and 0.8 Hz . (C) Representative MAP traces from an isolated heart at 0.5 Hz showing QT interval prolongation in presence of Phe concentration at 15 μM and above. (D) Mean data showing MAP duration at 90% repolarization (MAPD90) in control conditions and following exposure to 15 μM Phe. Individual hearts are shown by circles with bar showing mean ± S.E.M (n = 7–9 hearts). *p < 0.05, two-way ANOVA. MAP and QTc data for all exposure concentrations and all frequencies are shown in Table S1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Phenanthrene reduced cardiac contractility
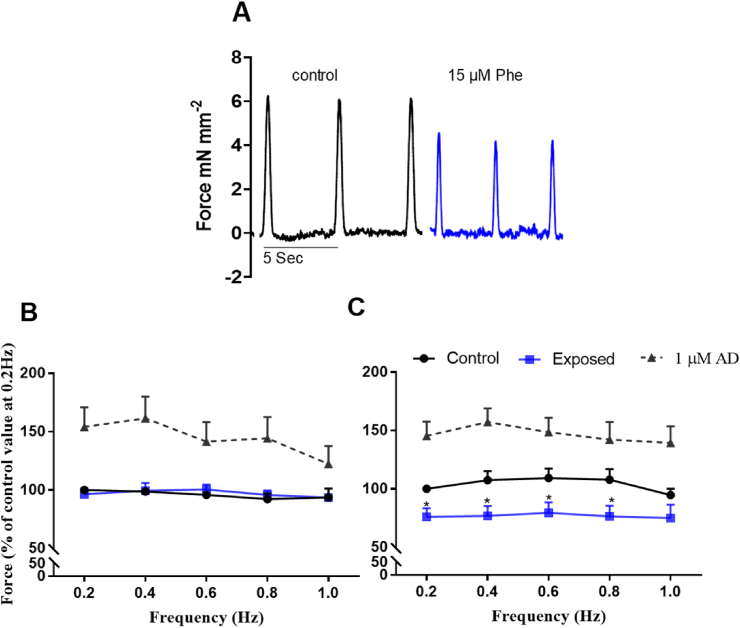
Alterations in cardiac electrical activity are known to affect cardiac contractility (i.e. the ability of the heart to pump blood against a resistance). Thus, we next examined whether Phe affected cardiac force using an isolated isometric cardiac muscle preparation. The raw ventricular force traces (Fig. 2A) show that Phe exposure (at 15 μM) depresses force. This reduction occurred across pacing frequencies between 0.2 and 0.8 Hz in the presence of Phe (Fig. 2C) but not the DMSO vehicle (Fig. 2B). When frequency responses were combined, Phe resulted in a 27 ± 2.3 and 19 ± 1.9% reduction in force at 15 μM and 25 μM exposure, respectively. The effect was attenuated at frequencies ≥1.0 Hz, probably due to the negative force-frequency relationship (Shiels et al., 2002). Similar results were observed in atrial tissue (Fig. S1). Phe at 5, 15 and 25 μM caused 12 ± 3.5, 24 ± 0.5 and 25 ± 2.3% reduction, respectively in atrial force when averaged across frequencies between 0.2 and 0.8 Hz (Table S2, Fig. S1). The rate of contraction (Table S3) and relaxation (Table S4) of both atrial and ventricular force were reduced but not significantly by Phe whereas resting tension, the time to peak contraction and the decay constant of relaxation (tau) were unchanged in both tissues (not shown). Interesting, in both cardiac tissues, the positive inotropic agonist adrenaline (1 μM) was able to ameliorate the negative effect of Phe on peak force (Fig. 2 and Fig. S1) and on the rate of contraction (Table S3) and relaxation (Table S3).
Fig. 2.
The impact of Phenanthrene (Phe) on the contractile properties of the brown trout heart. Force of contraction was significantly reduced when ventricular strips were exposed to 15 μM Phe and stimulated at varying frequency (0.2–1.0 Hz). (A) Representative trace from a ventricular strip contracting at 0.2 Hz showing peak height which was then converted to force. Mean ± S.E.M force expressed as percentage, normalised to control force contracting at 0.2 Hz in (B) DMSO control exposed group and (C) 15 μM exposed group. The solid black line represents the basal force of contraction prior to exposure of the strips to either DMSO or 15 μM Phe (blue line) whereas the dotted black line shows adrenaline (1 μM) exposure increasing contractility, attenuating the effects of Phe. (n = 8–12) *p < 0.05, two-way ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Phenanthrene disrupts the intracellular Ca2+ transient
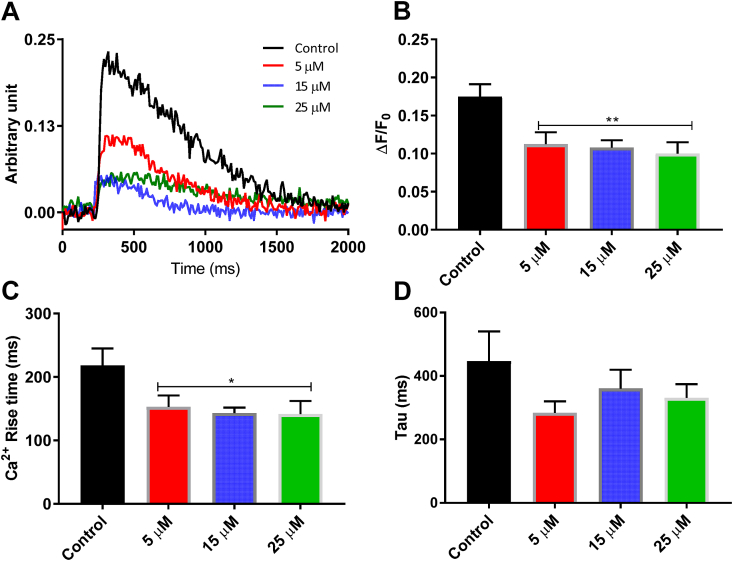
The rate and magnitude of cardiac contractile force is underpinned by the rate and magnitude of Ca2+ cycling in the myocytes of the working myocardium (Yue, 1987). Thus, reduced tissue contractility following Phe exposure may be due to reductions in the intracellular Ca2+ transient. To assess this, isolated ventricular cardiomyocytes from brown trout were exposed to Phe and the Ca2+-sensitive dye, Fluo-4, was used to record Ca2+ transients during stimulation at 0.2 Hz. The Ca2+ transient signal recorded from a single myocyte contracting at 0.2 Hz was significantly reduced when exposed to Phe (Fig. 3A). Phe significantly decreased Ca2+ transient amplitude (∼39%) (Fig. 3 B), which accounts for the shorter Ca2+ transient rise time (Fig. 3C) but did not slow the time course of transient relaxation (τ) (Fig. 3D). The impact of Phe on the Ca2+transient showed no clear concentration-dependence.
Fig. 3.
Intracellular Ca2+ recording from ventricular myocytes of brown trout exposed to 5, 15 and 25 μM Phe incubation 1 h prior to recording. Phe significantly reduced intracellular Ca2+ transients. (A) Ca2+ transient signals recorded from myocytes contracting at 0.2 Hz. Black trace represent control condition (exposed to ≤ 0.1% DMSO ) while the red, blue and green line represent traces from cells exposed to 5, 15 and 25 μM Phe respectively. (B) Ca2+ transient mean amplitude (ΔF/F0) in control and increasing Phe concentrations (red bar, 5 μM; blue bar 15 μM; red bar, 25 μM) (** p < 0.01, one-way ANOVA). (C) Bar graph showing significant effect by Phe on Ca2+ rise time (* p < 0.05, one-way ANOVA). (D) Bar graph showing non-significant reduction of decay of Ca2+ transient calculated as tau (ms). Values are mean ± S.E.M (N = 6, n = 69, control; 54, 5 μM; 67, 15 μM; and 62, 25 μM). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Phenanthrene inhibits ion flux in isolated cardiomyocytes
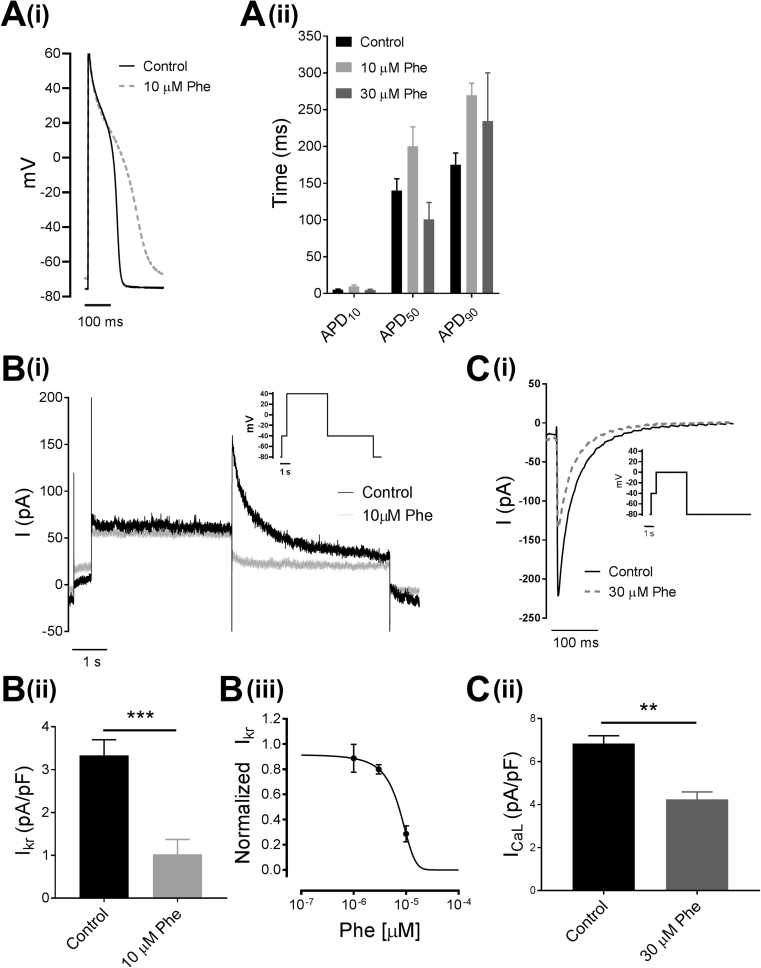
The effect of Phe on the QTc of the ECG and on MAPD, indicate that Phe is prolonging depolarization or slowing repolarization of the heart. The AP of a cardiomyocyte is characterised by the synchronous activity of various transmembrane ionic currents such as INa, ICa, IK, which are regulated by various ion channels. The effect of Phe on cellular AP and the underlying ion channels was assessed using whole-cell patch-clamp on isolated ventricular cardiomyocytes at 0.5 Hz frequency. Phe caused significant prolongation of AP duration at 90% (APD90) at 10 μM (Control - 175 ± 16 ms; Phe - 270 ± 17 ms) (Fig. 4Ai, Aii). Interestingly, at higher concentration of 30 μM, Phe caused significant reduction in APD at 50% (APD50) (Control - 140 ± 16 ms; Phe - 101 ± 23 ms) whilst prolonging APD90(Phe - 234 ± 66 ms)(Fig. 4Aii).
Fig. 4.
Phenanthrene (Phe) disrupts cardiac ion flux in isolated brown trout ventricular cardiomyocytes. (A) Effect of Phe on action potential duration (APD). (Ai) Representative traces of action potential elicited at 0.5 Hz in absence (Control) and presence of 10 μM Phe and (Aii) Mean action potential duration at 10% (APD10), 50% (APD50) and 90% (APD90) in absence (Control) and presence of 10 μM and 30 μM Phe (n = 5 for each data set from min. two fish). (B) Effect of Phe on Ikr currents. (Bi) Representative traces of Ikr currents in absence (Control) and presence of 10 μM Phe; inset – voltage protocol used to elicit Ikr currents. (Bii) Bar graph of mean Ikr current density in absence (Control) and presence of 10 μM Phe (*** p=0.0002; n = 6 from min. two fish). (Biii) concentration-response curve yielded an the IC50 value of 7.2 ± 0.6 μM (n = 6 for each data set from min. two fish). (C) Effect of Phe on ICaL currents. (Ci) Representative trace of ICaL currents in absence (Control) and presence of 30 μM Phe; inset – voltage protocol used to elicit ICaL currents. (Cii) Mean ICaL current density in absence (Control) and presence of 30 μM Phe (** p=0.001; n = 4 from two fish). Values are mean ± S.E.M (total n = 34 from total of 8 fishes).
The fast repolarization (i.e. phase 3, seen at APD90) of the cardiomyocyte AP is mainly driven by outward-rectifying ether-a-go-go (erg) channel (referred to as IKr current) while phase 2 (APD50) of the AP is mainly driven by L-type calcium channels (referred to as ICaL current). To understand Phe's effect on APD90 and APD50, the corresponding IKr and ICaL currents were investigated using whole-cell voltage-clamp of single ventricular myocytes. Brown trout ventricular myocytes exhibited robust IKr current with an average current density of 3.3 ± 0.4 pApF−1 (Fig. 4Bii). This was inhibited up to 70% to 1 ± 0.3 pApF−1 in the presence of 10 μM Phe (Fig. 4 Bi and ii) in-line with the observed effect of APD90 prolongation. Concentration-response curve revealed an IC50 of approximately 7.2 ± 0.6 μM (Fig. 4Biii). ICaL currents were also significantly inhibited (by ∼30%; Control - 6.8 ± 0.4 pApF−1; Phe - 4.2 ± 0.3 pApF−1) by Phe, but only at the higher dose of 30 μM Phe (Fig. 4C), which is in-line with the shortening of APD50 at this concentration but not at 10 μM Phe (Fig. 4 Ai and ii).
4. Discussion
The growth of anthropogenic stressors impacting the freshwater environment has led to grave concern for the fish living within them as exemplified by a recent study highlighting the susceptibility of salmonids to climate change (Jarić et al., 2019). Here we investigated the impact of a prevalent anthropogenic PAH pollutant, Phe, on cardiac function in a sentinel fresh water salmonid, the brown trout. Studies following the 1989 Exxon Valdez and the 2010 Deepwater Horizon disasters emphasized the developmental toxicity of various PAHs on fish early life stages and the developing cardiovascular system (Incardona et al., 2009, 2013). However, the direct negative effects of PAH exposure on cardiac parameters in adult life stages are becoming increasingly evident (Brette et al., 2014, 2017; Nelson et al., 2016). Our study identified three key findings. (1) Phe alters the electrical activity of the heart causing QT prolongation of ECG and MAPD, which is pro-arrhythmiogenic. (2) Phe depresses cardiac contractility due to a reduction in cellular Ca2+ flux in the cardiomyocytes. (3) The electrical and contractile dysfunction of the heart are due to the inhibition of depolarizing Ca2+currents and repolarising K+ currents in the cardiomyocytes. Taken together, our findings suggest that Phe pollution has the capacity to cause serious disruption to the trout heart. As the heart is the organ which drives oxygenated blood around the body whilst supplying nutrients and removing waste, failure in function can be catastrophic. Concurrently, there are host of studies (Pörtner and Farrell, 2008; Clark et al., 2011; Eliason et al., 2011; Eliason and Farrell, 2016) which indicate that it is collapse of the salmonid cardiovascular system which underlies the death of individual fish and the collapse of populations in hostile environments. Our study underscores the impact of anthropogenic pollutants on fish cardiac function and cautions that pollutants need to be considered in parallel with abiotic factors driven by climate change such as temperature and hypoxia if salmonid populations are to survive in this era of multiple environmental stressors.
4.1. The cardiotoxic mechanisms of phenanthrene
Our study is the first to examine the influence of Phe on any fish heart across multiples levels of biological organization. We are thus uniquely placed to attribute mechanisms at the myocyte to functional changes observed at the tissue and whole heart level. Indeed, our cellular work supports the mechanisms established by Brette et al. (2017) and reviewed by (Incardona, 2017), but additionally reveals the impact these cellular disturbances have on whole heart contractile and electrical function. Our findings also support previous work which shows direct cardiotoxicity independent of the activation of the aryl hydrocarbon receptor (AHR) (Incardona, 2017).
Electrical Excitability: Our data confirms that Phe is acutely and directly cardiotoxic to fish (Brette et al., 2017) and is the first to demonstrate this in a salmonid. Phe impairs membrane excitability by disrupting the ion channels responsible for the plateau (phase 2) and repolarization (phase 3) of the AP; the ICaL and IKr currents, respectively. This cellular inhibition is responsible for prolongation of the MAP and QT elongation at the level of the whole heart, meaning that Phe is able to induce long QT syndrome in salmonids. This pro-arrhythmic effect suggests that Phe could trigger irregular heartbeats leading to fibrillation (abnormal firing of electrical stimuli) and even death (Fermini and Fossa, 2003; Brette et al., 2017). Previous studies have reported arrhythmia caused by crude oil derived PAHs (Incardona et al., 2004, 2009). Phe (56 μM) induced bradycardia and arrhythmia in zebrafish (Danio rerio) embryos which progressed to atrioventricular (AV) block (Incardona et al., 2004). Similarly, Zhang et al. (2013b) reported onset of arrhythmia in zebrafish embryos due to disruption in calcium handling by SERCA2a (sarcoplasmic reticulum calcium ATPase). Following the onset of arrhythmia, Zhang et al. (2013a) revealed that Phe caused significant reduction in ventricular end diastolic volume (EDV) and end systolic volume (ESV) leading to significant reduction in stroke volume and cardiac output in developing zebrafish heart. They concluded that the effect observed was due to the ability of Phe to induce the activity of matrix metalloproteinase-9 (MMP-9); an enzyme involved in cardiac remodelling. Evidence of the ability of Phe to induce hypertrophy in a mammalian heart by inhibiting miR-133a expression and induced DNA methyltransferases (DNMTs) in rat cardiomyocytes and H9C2 cardiomyoblast cells have also been reported (Huang et al., 2016). In humans, long QT syndrome can lead to temporary loss of consciousness and sudden death in young adults as a result of altered ionic flux in the cardiomyocytes (Etheridge et al., 2019). Because of fairly conserved excitation-contraction coupling processes across vertebrates, our findings for fish suggest similar dysfunction may occur in humans exposed to PAH via air pollution (Kim et al., 2015).
Altered cellular Ca2+cycling: Phe also significantly impaired the Ca2+dynamics of the cells which inhibit the Ca2+ transient and culminated in the reduced contractility observed in the cardiac tissues. The percentage reduction in tissue contractility observed from this study is consistent with the reduction in cardiac output reported by Nelson et al. (2016) following exposure of mahi-mahi (Coryphaena hippurus) to crude oil containing a measured total PAH of 9.6 ± 2.7 μg l−1 with phenanthrene/anthracene ratio making up to 14%. The reduction in the Ca2+ transient following exposure to Phe reported in this study will undoubtably be due, at least in part, to the inhibtion of ICa. However, the reduction in Ca2+transient amplitude was seen at doses lower than those where ICa inhibtion was extensive. This may be explained by the lesser role of the sarcoplasmic reticulum in salmonid heart function compared with pelagic fish (Shiels and Galli, 2014). However, there are many other Ca2+ cycling pumps and transporters which may be influenced by Phe in cardiomyocytes and future studies should investigate the role of the sarcoplasmic reticulum and the Na+-Ca2+ exchanger in a range of vertebrates.
An interesting aspect of our study was the amelioration of reduced contractility following Phe exposure with treatment of adrenaline (1 μM). Adrenaline causes a robust increase in contractility in trout heart (Mc Donald and Milligan, 1992; Shiels et al., 1998; Mercier et al., 2002) because it is able to stimulate the β-adrenergic receptors leading to a positive inotropic signalling cascade which culminates in the activation of protein kinase A(PKA). PKA phosphorylates the L-type calcium channels, increasing their open probability and ICaL (Shiels et al., 1998; Gordan et al., 2015). The effect of AD was only tested in cardiac tissues but future work should look at the effect of this endogenous hormone at multiple levels as it may be cardioprotective.
4.2. Environmental relevance
Fish are an important source of protein and wild fish are a major source of commercially available fish (Asagbra et al., 2015). The diversity and numbers of commercial fish species is impaired due to climate change and pollution. For example, pollution through anthropogenic activities leads to consistent release of harmful substances such as hydrocarbons into aquatic communities. Zenetos et al. (2004), attributed the reduction in benthic species richness and diversity to hydrocarbons in water and sediments in the Gulf of Southern Evoikos (Greece). In regions of frequent spills, high surface water concentrations have been reported. PAHs contamination of rivers, creeks and groundwater in Ogoni land (Nigeria) revealed levels reaching 7,420 μg L−1 in surface waters, and 42 ,200 μg L−1 in drinking waters (Lindén and Pålsson, 2013). PAHs accumulate in sediments thereby constituting a major continuous and persistent source within the water column (Reddy et al., 2002; Peterson et al., 2003; Short et al., 2004; Incardona et al., 2005). The amount of PAHs present in sediments varies but Vane et al. (2007), reported Σ PAHs in the Mersey estuary (UK) sediments to range between 0.6 and 3.8 μg g−1 dry weight (dw) with the concentrations of Phe ranging between 0.05 and 0.25 μg g−1 dw. Much higher values were reported for sediments from the Haihe River in Tiajin (China) with Phe concentrations up to 74.4 μg g−1 (dw) accounting for approximately 25% of the overall amount of PAHs present (Jiang et al. (2007). A study from the Klang River (Malaysia) reported Σ PAH concentration in sediments in the range of 3.8–7.44 μg g−1 dry weight and a dominance of 3 ringed PAHs (Phe family) representing on average 38% of the overall amount of PAHs present (1.4–2.8 μg g−1 dry weight). These data suggest that benthic and pelagic organisms are routinely exposedto (Keshavarzifard et al., 2015). Asagbra et al. (2015) analysed PAHs in 3 economically important fish species from the Warri River (Nigeria) and reported concentration values as high as 1.1 μg g−1 in the fish tissues, with the sediments in the same river having concentrations of 4.6 μg g−1 dw, although the values in the water column was 0.03 μg ml−1. Although the freshwater environment did not receive the disastrous point spills which have plagued the marine environment, the ubiquity of PAHs and rising loads of hydrocarbon emission, depositions, coastal flooding, freshwater-marine intrusion and urban runoff suggest that the fresh water environment receives a significant quantity of these pollutants. It is therefore important to understand the response of fresh water organisms to PAH pollutants.
In this study, Phe at doses 15 and 25 μM are clearly cardiotoxic to the salmonid heart providing new insight into the integrated cardiac response to PAH pollutants. Exposing larval zebrafish to higher concentrations (56 μM) gave similar cardiotoxic effects by inducing bradycardia and arrhythmia (Incardona et al., 2004). Therefore, ecological tools relevant in biodiversity assessment and management/conservation strategies should consider the cardiotoxicity of pollutants alongside other parameters such as temperature and climate change. Accounting for multiple environmental stressors is paramount for an in-depth assessment of peril for fish in this era of rapid climate change.
5. Conclusion
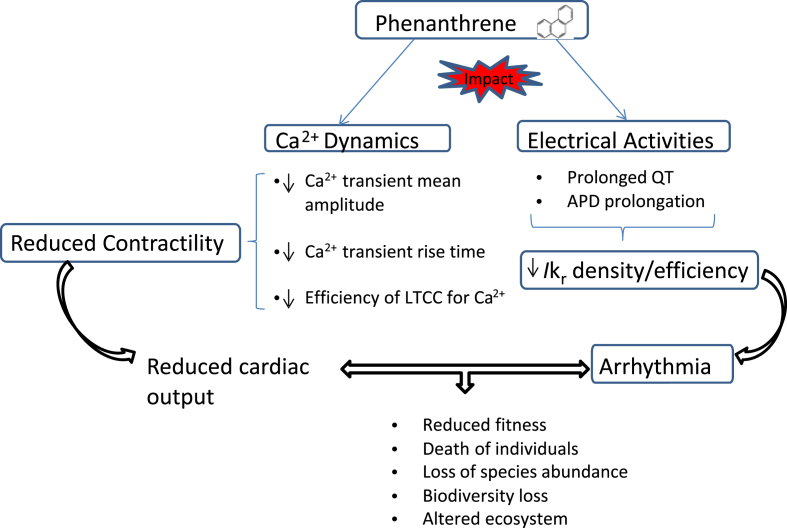
This study has determined the impact of a key PAH, Phe, on the heart of a freshwater indicator species, the brown trout, across layers of biological organization. Our findings support earlier investigations into PAH cardiotoxicity in cardiomyocytes of marine species but adds to this literature by providing evidence of reduced contractile performance and whole heart electrical disruption. We summarise the importance of our findings in Fig. 5, where we show how Phe pollution, through impairment of cardiac function, can impact fish survival. Importantly, the schema presented in Fig. 5 will rarely exist in isolation. Fishes must accommodate additional anthropogenic pollution load with warmer temperatures and lower levels of dissolved oxygen. Each of these stressors alone has a large and deleterious effect on fish cardiac function. Hence, consistent monitoring and remediation of environmental matrices containing this cardiotoxic pollutant is strongly recommended, particularly in systems already under stress from other anthropogenic inputs.
Fig. 5.
Summary of the effects of phenanthrene on fish cardiovascular system and the potential implications of exposure on fish species population.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We thank the Nigerian Petroleum Technology Development Fund (PTDF) for support to Ainerua Martins, the British Heart Foundation (PG/17/77/33125) for support for Shiva Kompella and the University of Manchester for support of Jake Tinwell. We also thank James Merchant for help in fish transport and the members of the Shiels lab that were involved in fish care.
Handling Editor: Jim Lazorchak
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2019.124608.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adeyemo O.K., Kroll K.J., Denslow N.D. Developmental abnormalities and differential expression of genes induced in oil and dispersant exposed Menidia beryllina embryos. Aquat. Toxicol. 2015;168:60–71. doi: 10.1016/j.aquatox.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Asagbra M.C., Adebayo A.S., Anumudu C.I., Ugwumba O.A., Ugwumba A.A.A. Polycyclic aromatic hydrocarbons in water, sediment and fish from the Warri River at Ubeji, Niger Delta, Nigeria. Afr. J. Aquat. Sci. 2015;40:193–199. [Google Scholar]
- Baars B.J. The wreckage of the oil tanker ‘Erika’—human health risk assessment of beach cleaning, sunbathing and swimming. Toxicol. Lett. 2002;128:55–68. doi: 10.1016/s0378-4274(01)00533-1. [DOI] [PubMed] [Google Scholar]
- Behera B.K., Das A., Sarkar D.J., Weerathunge P., Parida P.K., Das B.K., Thavamani P., Ramanathan R., Bansal V. Polycyclic Aromatic Hydrocarbons (PAHs) in inland aquatic ecosystems: perils and remedies through biosensors and bioremediation. Environ. Pollut. 2018;241:212–233. doi: 10.1016/j.envpol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Brette F., Machado B., Cros C., Incardona J.P., Scholz N.L., Block B.A. Crude oil impairs cardiac excitation-contraction coupling in fish. Science. 2014;343:772–776. doi: 10.1126/science.1242747. [DOI] [PubMed] [Google Scholar]
- Brette F., Shiels H.A., Galli G.L.J., Cros C., Incardona J.P., Scholz N.L., Block B.A. A novel cardiotoxic mechanism for a pervasive global pollutant. Sci. Rep. 2017;7:41476. doi: 10.1038/srep41476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carls M.G., Holland L., Larsen M., Collier T.K., Scholz N.L., Incardona J.P. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat. Toxicol. 2008;88:121–127. doi: 10.1016/j.aquatox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Clark T.D., Jeffries K.M., Hinch S.G., Farrell A.P. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J. Exp. Biol. 2011;214:3074. doi: 10.1242/jeb.060517. [DOI] [PubMed] [Google Scholar]
- Convention on Biological Diversity, C . 2015. Nigeria: Fifth National Biodiversity Report; p. 89. [Google Scholar]
- Darwall W., Bremerich V., De Wever A., Dell A.I., Freyhof J., Gessner M.O., Grossart H.P., Harrison I., Irvine K., Jähnig S.C., Jeschke J.M., Lee J.J., Lu C., Lewandowska A.M., Monaghan M.T., Nejstgaard J.C., Patricio H., Schmidt-Kloiber A., Stuart S.N., Thieme M., Tockner K., Turak E., Weyl O. The Alliance for Freshwater Life: a global call to unite efforts for freshwater biodiversity science and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018;28:1015–1022. [Google Scholar]
- Dudgeon D., Arthington A.H., Gessner M.O., Kawabata Z.I., Knowler D.J., Lévêque C., Naiman R.J., Prieur-Richard A.H., Soto D., Stiassny M.L.J., Sullivan C.A. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 2005;81:163–182. doi: 10.1017/S1464793105006950. [DOI] [PubMed] [Google Scholar]
- Edmunds R.C., Gill J.A., Baldwin D.H., Linbo T.L., French B.L., Brown T.L., Esbaugh A.J., Mager E.M., Stieglitz J., Hoenig R., Benetti D., Grosell M., Scholz N.L., Incardona J.P. Corresponding morphological and molecular indicators of crude oil toxicity to the developing hearts of mahi mahi. Sci. Rep. 2015;5:17326. doi: 10.1038/srep17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.J., Ryder R.A., Marshall T.R. Using lake trout as a surrogate of ecosystem health for oligotrophic waters of the great lakes. J. Gt. Lakes Res. 1990;16:591–608. [Google Scholar]
- Eliason E.J., Clark T.D., Hague M.J., Hanson L.M., Gallagher Z.S., Jeffries K.M., Gale M.K., Patterson D.A., Hinch S.G., Farrell A.P. Differences in thermal tolerance among sockeye salmon populations. Science. 2011;332:109. doi: 10.1126/science.1199158. [DOI] [PubMed] [Google Scholar]
- Eliason E.J., Farrell A.P. Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J. Fish Biol. 2016;88:359–388. doi: 10.1111/jfb.12790. [DOI] [PubMed] [Google Scholar]
- Elyachar J. 230,000 gallons of crude oil spills into Iowa river following train derailment. Tech Times. 2018 [Google Scholar]
- Etheridge S.P., Asaki S.Y., Niu M.C.I. A personalized approach to long QT syndrome. Curr. Opin. Cardiol. 2019;34:46–56. doi: 10.1097/HCO.0000000000000587. [DOI] [PubMed] [Google Scholar]
- Farrell A.P. Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 2009;212:3771. doi: 10.1242/jeb.023671. [DOI] [PubMed] [Google Scholar]
- Fermini B., Fossa A.A. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat. Rev. Drug Discov. 2003;2:439. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- Gentes M.L., McNabb A., Waldner C., Smits J.E.G. Increased thyroid hormone levels in tree swallows (tachycineta bicolor) on reclaimed wetlands of the athabasca oil sands. Arch. Environ. Contam. Toxicol. 2007;53:287–292. doi: 10.1007/s00244-006-0070-y. [DOI] [PubMed] [Google Scholar]
- Gordan R., Gwathmey J.K., Xie L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015;7:204–214. doi: 10.4330/wjc.v7.i4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.S., Farrell A.P. The effect of temperature acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 1989;62:38–61. [Google Scholar]
- Hazilawati H., Mohd Azmi M.L., Noordin M.M. DNA damage and adduct formation in immune organs of developing chicks by polycyclic aromatic hydrocarbons AU - nisha, A. R. Toxicol. Mech. Methods. 2017;27:215–222. doi: 10.1080/15376516.2016.1273432. [DOI] [PubMed] [Google Scholar]
- Huang L., Xi Z., Wang C., Zhang Y., Yang Z., Zhang S., Chen Y., Zuo Z. Phenanthrene exposure induces cardiac hypertrophy via reducing miR-133a expression by DNA methylation. Sci. Rep. 2016;6 doi: 10.1038/srep20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona J.P. Molecular mechanisms of crude oil developmental toxicity in fish. Arch. Environ. Contam. Toxicol. 2017;73:19–32. doi: 10.1007/s00244-017-0381-1. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Carls M.G., Day H.L., Sloan C.A., Bolton J.L., Collier T.K., Scholz N.L. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ. Sci. Technol. 2009;43:201–207. doi: 10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Carls M.G., Holland L., Linbo T.L., Baldwin D.H., Myers M.S., Peck K.A., Tagal M., Rice S.D., Scholz N.L. Very low embryonic crude oil exposures cause lasting cardiac defects in salmon and herring. Sci. Rep. 2015;5:13499. doi: 10.1038/srep13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona J.P., Carls M.G., Teraoka H., Sloan C.A., Collier T.K., Scholz N.L. Environmental health perspectives; 2005. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development; pp. 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona J.P., Collier T.K., Scholz N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Day H.L., Collier T.K., Scholz N.L. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Incardona J.P., Swarts T.L., Edmunds R.C., Linbo T.L., Aquilina-Beck A., Sloan C.A., Gardner L.D., Block B.A., Scholz N.L. Exxon Valdez to Deepwater Horizon: comparable toxicity of both crude oils to fish early life stages. Aquat. Toxicol. 2013;142–143:303–316. doi: 10.1016/j.aquatox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Jarić I., Lennox R.J., Kalinkat G., Cvijanović G., Radinger J. Susceptibility of European freshwater fish to climate change: species profiling based on life-history and environmental characteristics. Glob. Chang. Biol. 2019;25:448–458. doi: 10.1111/gcb.14518. [DOI] [PubMed] [Google Scholar]
- Jiang B., Zheng H.l., Huang G.q., Ding H., Li X.g., Suo H.t., Li R. Characterization and distribution of polycyclic aromatic hydrocarbon in sediments of Haihe River, Tianjin, China. J. Environ. Sci. 2007;19:306–311. doi: 10.1016/s1001-0742(07)60050-3. [DOI] [PubMed] [Google Scholar]
- Keshavarzifard M., Zakaria M.P., Hwai T.S., Yusuff F.M., Mustafa S. Distributions and source apportionment of sediment-associated polycyclic aromatic hydrocarbons (PAHs) and hopanes in rivers and estuaries of Peninsular Malaysia. Environ. Sci. Pollut. Control Ser. 2015;22:9424–9437. doi: 10.1007/s11356-015-4093-7. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Kabir E., Kabir S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Laffon B., Fraga-Iriso R., Pérez-Cadahía B., Méndez J. Genotoxicity associated to exposure to Prestige oil during autopsies and cleaning of oil-contaminated birds. Food Chem. Toxicol. 2006;44:1714–1723. doi: 10.1016/j.fct.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Layland J., Young I.S., Altringham J.D. The effect of cycle frequency on the power output of rat papillary muscles in vitro. J. Exp. Biol. 1995;198:1035. doi: 10.1242/jeb.198.4.1035. [DOI] [PubMed] [Google Scholar]
- Le Bihanic F., Clérandeau C., Le Menach K., Morin B., Budzinski H., Cousin X., Cachot J. Developmental toxicity of PAH mixtures in fish early life stages. Part II: adverse effects in Japanese medaka. Environ. Sci. Pollut. Control Ser. 2014;21:13732–13743. doi: 10.1007/s11356-014-2676-3. [DOI] [PubMed] [Google Scholar]
- Li R., Zuo Z., Chen D., He C., Chen R., Chen Y., Wang C. Inhibition by polycyclic aromatic hydrocarbons of ATPase activities in Sebastiscus marmoratus larvae: relationship with the development of early life stages. Mar. Environ. Res. 2011;71:86–90. doi: 10.1016/j.marenvres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Lima A.L.C., Eglinton T.I., Reddy C.M. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ. Sci. Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Lindén O., Pålsson J. Oil contamination in Ogoniland, Niger Delta. Ambio. 2013;42:685–701. doi: 10.1007/s13280-013-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J., Perrichon P., Nouhaud M., Audras A., Leguen I., Lefrancois C. Aerobic metabolism and cardiac activity in the descendants of zebrafish exposed to pyrolytic polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Control Ser. 2014;21:13888–13897. doi: 10.1007/s11356-014-3116-0. [DOI] [PubMed] [Google Scholar]
- Mahler B.J., Van Metre P.C., Bashara T.J., Wilson J.T., Johns D.A. Parking lot Sealcoat: an unrecognized source of urban polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2005;39:5560–5566. doi: 10.1021/es0501565. [DOI] [PubMed] [Google Scholar]
- Mc Donald D.G., Milligan C.L. 2 chemical properties of the blood. In: Hoar W.S., Randall D.J., Farrell A.P., editors. Fish Physiology. Academic Press; 1992. pp. 55–133. [Google Scholar]
- Mercier C., Axelsson M., Imbert N., Claireaux G., Lefrancois C., Altimiras J., Farrell A. In vitro cardiac performance in triploid brown trout at two acclimation temperatures. J. Fish Biol. 2002;60:117–133. [Google Scholar]
- Michel J., Henry C.B., Thumm S. Shoreline assessment and environmental impacts from the M/T Westchester oil spill in the Mississippi River. Spill Sci. Technol. Bull. 2002;7:155–161. [Google Scholar]
- Michel J., Ploen M. Options for minimizing environmental impacts of inland spill response: new guide from the American petroleum institute. International Oil Spill Conference Proceedings 2017. 2017:1770–1783. [Google Scholar]
- Moeckel C., Monteith D.T., Llewellyn N.R., Henrys P.A., Pereira M.G.r. Relationship between the concentrations of dissolved organic matter and polycyclic aromatic hydrocarbons in a typical UK upland stream. Environ. Sci. Technol. 2013;48:130–138. doi: 10.1021/es403707q. [DOI] [PubMed] [Google Scholar]
- Moorthy B., Chu C., Carlin D.J. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol. Sci. 2015;145:5–15. doi: 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Oil in the Sea . National Academies Press (US); 2003. Inputs, Fates, and Effects. Oil in the Sea III: Inputs, Fates, and Effects. [PubMed] [Google Scholar]
- Nelson D., Heuer R.M., Cox G.K., Stieglitz J.D., Hoenig R., Mager E.M., Benetti D.D., Grosell M., Crossley D.A. Effects of crude oil on in situ cardiac function in young adult mahi–mahi (Coryphaena hippurus) Aquat. Toxicol. 2016;180:274–281. doi: 10.1016/j.aquatox.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Peterson C.H., Rice S.D., Short J.W., Esler D., Bodkin J.L., Ballachey B.E., Irons D.B. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003;302:2082–2086. doi: 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O., Farrell A.P. Physiology and climate change. Science. 2008;322:690. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Reddy C.M., Eglinton T.I., Hounshell A., White H.K., Xu L., Gaines R.B., Frysinger G.S. The West Falmouth oil spill after thirty years: the persistence of petroleum hydrocarbons in marsh sediments. Environ. Sci. Technol. 2002;36:4754–4760. doi: 10.1021/es020656n. [DOI] [PubMed] [Google Scholar]
- Shiels H., Farrell A. The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J. Exp. Biol. 1997;200:1607–1621. doi: 10.1242/jeb.200.11.1607. [DOI] [PubMed] [Google Scholar]
- Shiels H., Stevens E., Farrell A. Effects of temperature, adrenaline and ryanodine on power production in rainbow trout oncorhynchus mykiss ventricular trabeculae. J. Exp. Biol. 1998;201:2701–2710. doi: 10.1242/jeb.201.19.2701. [DOI] [PubMed] [Google Scholar]
- Shiels H.A., Freund E.V., Farrell A.P., Block B.A. The sarcoplasmic reticulum plays a major role in isometric contraction in atrial muscle of yellowfin tuna. J. Exp. Biol. 1999;202:881. doi: 10.1242/jeb.202.7.881. [DOI] [PubMed] [Google Scholar]
- Shiels H.A., Galli G.L. The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology. 2014;29:456–469. doi: 10.1152/physiol.00015.2014. [DOI] [PubMed] [Google Scholar]
- Shiels H.A., Paajanen V., Vornanen M. Sarcolemmal ion currents and sarcoplasmic reticulum Ca2+ content in ventricular myocytes from the cold stenothermic fish, the burbot (Lota lota) J. Exp. Biol. 2006;209:3091–3100. doi: 10.1242/jeb.02321. [DOI] [PubMed] [Google Scholar]
- Shiels H.A., Vornanen M., Farrell A.P. Temperature-dependence of L-type Ca(2+) channel current in atrial myocytes from rainbow trout. J. Exp. Biol. 2000;203:2771. doi: 10.1242/jeb.203.18.2771. [DOI] [PubMed] [Google Scholar]
- Shiels H.A., Vornanen M., Farrell A.P. The force–frequency relationship in fish hearts—a review. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2002;132:811–826. doi: 10.1016/s1095-6433(02)00050-8. [DOI] [PubMed] [Google Scholar]
- Short J.W., Lindeberg M.R., Harris P.M., Maselko J.M., Pella J.J., Rice S.D. Estimate of oil persisting on the beaches of prince william sound 12 years after the Exxon Valdez oil spill. Environ. Sci. Technol. 2004;38:19–25. doi: 10.1021/es0348694. [DOI] [PubMed] [Google Scholar]
- Sorhus E., Incardona J.P., Karlsen O., Linbo T., Sorensen L., Nordtug T., van der Meeren T., Thorsen A., Thorbjornsen M., Jentoft S., Edvardsen R.B., Meier S. Crude oil exposures reveal roles for intracellular calcium cycling in haddock craniofacial and cardiac development. Sci. Rep. 2016;6:31058. doi: 10.1038/srep31058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.L., Picard C.R., Chan H.M. Polycyclic aromatic hydrocarbons (PAHs) in traditionally harvested bivalves in northern British Columbia, Canada. Mar. Pollut. Bull. 2017;121:390–399. doi: 10.1016/j.marpolbul.2017.06.018. [DOI] [PubMed] [Google Scholar]
- USFWS . U.S. Fish and Wildlife Service; 2015. Enbridge Must Restore Environment Injured by 2010 Kalamazoo River Oil Spill. [Google Scholar]
- Van Metre P.C., Mahler B.J. The contribution of particles washed from rooftops to contaminant loading to urban streams. Chemosphere. 2003;52:1727–1741. doi: 10.1016/S0045-6535(03)00454-5. [DOI] [PubMed] [Google Scholar]
- Vane C.H., Harrison I., Kim A.W. Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in sediments from the Mersey Estuary, U.K. Sci. Total Environ. 2007;374:112–126. doi: 10.1016/j.scitotenv.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Wood C.M., Pieprzak P., Trott J.N. The influence of temperature and anaemia on the adrenergic and cholinergic mechanisms controlling heart rate in the rainbow trout. Can. J. Zool. 1979;57:2440–2447. [Google Scholar]
- Yue D.T. Intracellular [Ca2+] related to rate of force development in twitch contraction of heart. Am. J. Physiol. Heart Circ. Physiol. 1987;252:H760–H770. doi: 10.1152/ajpheart.1987.252.4.H760. [DOI] [PubMed] [Google Scholar]
- Zenetos A., Hatzianestis J., Lantzouni M., Simboura M., Sklivagou E., Arvanitakis G. The Eurobulker oil spill: mid-term changes of some ecosystem indicators. Mar. Pollut. Bull. 2004;48:122–131. doi: 10.1016/S0025-326X(03)00370-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Dong S., Wang H., Tao S., Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ. Pollut. 2016;213:809–824. doi: 10.1016/j.envpol.2016.03.050. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Huang L., Wang C., Gao D., Zuo Z. Phenanthrene exposure produces cardiac defects during embryo development of zebrafish (Danio rerio) through activation of MMP-9. Chemosphere. 2013;93:1168–1175. doi: 10.1016/j.chemosphere.2013.06.056. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Huang L., Zuo Z., Chen Y., Wang C. Phenanthrene exposure causes cardiac arrhythmia in embryonic zebrafish via perturbing calcium handling. Aquat. Toxicol. 2013;142–143:26–32. doi: 10.1016/j.aquatox.2013.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.