Abstract
Background
Renal stones are the accumulated or deposited crystals that form and appear in supersaturated urine. This study aimed to the investigate the therapeutic effects of Huashi Pill on clearance of renal stones.
Material/Methods
Sprague Dawley rats were divided into normal control, positive control, low-dosage Huashi Pill, medium-dosage Huashi Pill, and high-dosage Huashi Pill groups. A renal rat model was established by using ethylene glycol, ammonium chloride, and calcium gluconate. The urinary pH, urine protein, and uric acid levels, as well as the calcium, magnesium, and phosphorus levels were examined. The blood urea nitrogen (BUN) and creatinine (Cr) levels were also evaluated. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (TBIL) levels were evaluated. Crystal formation and calcium deposits were examined using hematoxylin and eosin (H and E) staining and von Kossa staining, respectively. Osteopontin (OPN) expression was evaluated with quantitative real-time polymerase chain reaction assay and immunohistochemical assay.
Results
A renal stone rat model was successfully established. Huashi Pill significantly improved water and food intake and enhanced pH value of urine (P<0.05). Huashi Pill significantly improved the liver functions by decreasing ALT and TBIL levels (P<0.05). Huashi Pill regulated the amounts of microelements. Huashi Pill significantly decreased the urine protein, uric acid, and Cr levels (P<0.05). Huashi Pill inhibited formation of stone crystals and reduced the insoluble calcium deposition. Huashi Pill significantly downregulated expression of OPN in the kidney tissues of renal rat models (P<0.05).
Conclusions
Huashi Pill inhibited stone formation by regulating urine biochemical indexes and reducing OPN expression in kidney tissue in a renal stone rat model.
MeSH Keywords: Calcium Oxalate; Calreticulin; Liquid Crystals; Medicine, Chinese Traditional
Background
Renal stones are the accumulated or deposited crystals that form and appear in supersaturated urine [1,2]. In recent years, the prevalence and incidence of kidney stones has been increasing year by year worldwide [3,4]. Clinically, approximate 75% of renal stones are caused by calcium oxalate (CaOx) [5], which is caused by hypercalciuria and induces pain [6,7]. A previous study [8] reported that CaOx is closely related to free radicals that induced renal tubular cell injury and stone formation. Another study [9] documented that the oxidative stress induced renal tubular cell injury and eventually caused renal stone formation.
Several previous studies [10,11] reported on several approaches for establishing a renal stone model. These approaches for establishing renal stone models were conducted mainly by downregulating pH values and increasing levels of uric acid and calcium excretion. In our study, we established a renal stone rat model by applying ethylene glycol, ammonium chloride, and calcium gluconate, and then the urinary protein and uric acid levels, as well as calcium, magnesium, and phosphorus levels were evaluated in the rat model. Therefore, the model was appropriate for investigating the effects of drug-administration in a laboratory setting. The interstitial and intraluminal renal mineralization can be regulated by osteopontin (OPN), which has a higher affinity to calcium and inhibits the intraluminal crystal growth [12]. Therefore, OPN was also examined to confirm the renal stone model and effects of drugs.
Until now, plenty of drugs have been used to treat or remove renal stones in the clinical setting, and most of the drugs are derived from plants [13]. However, the effects of the aforementioned drugs always need a long-term treatment period and the efficacy is relatively low. Moreover, some of the drugs exhibit a few side effects which inhibit long-term administration. Meanwhile, open renal surgery is also used for managing the urolithiasis [14], but it’s rarely used in clinical settings due to the often unavailable standard procedure and the high cost. Therefore, discovering effective anti-renal stone drugs is critical and urgently needed for renal stone treatment.
Huashi Pill is a novel drug-formula in traditional Chinese medicine and has been assigned as a “National Medicine Permission Drug”. Huashi Pill is mainly composed of Desmodium styracifolium, Ventriculi galli mucosa, Alisma orientalis, Sand cattle, Astragalus, Plantago seed, Corydalis corydalis, and licorice. Our clinical practice found that Huashi Pill could inhibit oxidative stress caused by renal tubular cell injury and suppress inflammation in tissues. Also, Huashi Pill is benefit to the digestive functions and could improve the status of the stomach. Therefore, we speculated that Huashi Pill might potentiate the anti-stones effects and increase the oxalate excretion, when administered.
In this study, the renal model was established by applying a novel approach and using ethylene glycol, ammonium chloride, and calcium gluconate. Then, therapeutic effects of Huashi Pill on clearance of renal stones were observed. This research could identify a promising drug-selection for renal stones therapy for clinical practice.
Material and Methods
Animals
Sixty specific pathogen free (SPF) Sprague Dawley (SD) rats (6 to 8 weeks old, weighting from 250 to 300 g) were purchased from Tengxin Biotech Co., Ltd. (Chongqing, China) and used in this study. SD rats were maintained at 25±2°C and with light/dark cycle of 12 hours/12 hours; rats were fed with a normal diet (CLEA Japan Inc., Shizuoka, Japan) and had accessed to water freely.
The present study was conducted following the Guidance of Care and Use of Laboratory Animals of HNI. Meanwhile, this study was approved by the Ethics Committee of Southwest Medical University, Luzhou, China.
Renal rat model establishment and trial grouping
The SD rats were divided into a normal control group (NC group, n=10), renal stone group (Model group, n=10, intragastrically administrated with 1% ethylene glycol (SinoPharm Co., Ltd., Shanghai, China), 2% ammonium chloride (BBI Life Sci., Shanghai, China) and 10% calcium gluconate (Sangon Biotech, Co., Ltd., Shanghai, China) 1 time per day for 28 days), Positive control group (intragastrically administrated with potassium sodium hydrogen citrate granules (Madaus AG, Koln, Germany) at concentration of 0.6 g/kg weight body, n=10), low-dosage Huashi Pill (Low-Huashi Pill group, intragastrically administrated with Huashi Pill (Qingdao Gla Dandong Pharm Co., Ltd., Ge’ermu, China) at concentration of 0.01 g/kg weight body, n=10), medium-dosage Huashi Pill (Medium-Huashi Pill group, intragastrically administrated with 0.02 g/kg weight body, n=10) and high-dosage Huashi Pill (High-Huashi Pill group, intragastrically administrated with 0.04 g/kg weight body, n=10).
Urine routine and urine biochemical analysis
On Day 0 (for identifying the model establishment) or Day 28 (for evaluating the effects of Huashi Pill), the rats were fed in metabolic-cages and the 24-hour urine was harvested by incubating with 0.02% sodium azide (SinoPharm Co., Ltd., Shanghai, China) to prevent the appearance of bacteria. The urinary pH and urinary volume were evaluated and then the urine was used for the other urine biochemical analysis. The urine protein (urine protein quantitative kit, Cat. No. C035-2, Nanjing Jiancheng Bioengineering Inst., Nanjing, China), uric acid (uric acid assay kit, Cat. No. C012-1, Nanjing Jiancheng Bioengineering Inst.), calcium (calcium assay kit, Cat. No. C004-3, Nanjing Jiancheng Bioengineering Inst.), magnesium (magnesium assay kit, Cat. No. C005, Nanjing Jiancheng Bioengineering Inst.), phosphorus (phosphate assay kit, Cat. No. C006, Nanjing Jiancheng Bioengineering Inst.) were examined by utilizing the commercial kit on the semiautomatic photometer (Mode: 750, Hitachi, Tokyo, Japan) following to the instructions of the manufacturers.
Moreover, the levels of serum urea nitrogen (BUN) and creatinine (Cr) were also evaluated using the urea assay kit (Cat. No. C013-1, Nanjing Jiancheng Bioengineering Inst.) and creatinine assay kit (Cat. No. C011-2, Nanjing Jiancheng Bioengineering Inst.), according to the manufacturer’s instructions.
Liver function evaluation
On Day 28 (for evaluating the effects of Huashi Pill) post Huashi Pill administration, the plasma was obtained from the blood treated with 11% trisodium citrate (Sangon Biotech Co., Ltd., Shanghai, China) using centrifugation. The aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (TBIL) in plasma were evaluated with commercial kits according to the manufacturers’ instructions and standard methods [15].
Histopathological evaluation
The kidney was resected from the rats in each group, incubated with the 4% paraformaldehyde (Sangon Biotech Co., Ltd., Shanghai, China) and embedded in the paraffin (Sangon Biotech Co., Ltd., Shanghai, China). The kidney tissues were cut into 4-μm thick sections and stained with hematoxylin (Nanjing Jiancheng Bioengineering Inst., Nanjing, China) and eosin (Biyotime Biotech Shanghai, China) and also sections were stained using a von Kossa staining kit (Beijing Leagene Biotech Co., Ltd., Beijing, China). The sections were observed by employing the polarized-light optical micro-photography (Mode: AX80, Olympus, Tokyo, Japan) to better confirm that the stained materials were the crystals. The formed crystals were evaluated using the professional image analysis software (NIH Image, version: 1.61, Scion Inc., Bethesda, MD, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Kidney tissues were lysed with radio-immunoprecipitation assay solution (RIPA, Tiangen Biotech Co., Ltd., Beijing, China) according to instructions of the manufacturer. The RNAs were extracted using RNAiso Plus Kit (Cat. No. 9108Q, TaKaRa, Dalian, China) and transcribed reversely with PrimeScript II 1st strand cDNA synthesis kit (Cat. No. 6210A/B, TaKaRa, Dalian, China) to synthesize complementary DNAs (cDNAs). The primers for the OPN gene are listed in Table 1. The amplification was conducted by using SYBR Green I real-time PCR kit (Cat. No. DRR820A, Takara, Dalian, China) according to instruction of the manufacturer. Amplification conditions were listed as follows: 35 cycles of 30 seconds at 95°C, 20 seconds at 60°C, and 60 seconds at 72°C. The qRT-PCR results were evaluated using method of 2−ΔΔCt.
Table 1.
Primers for the real-time polymerase chain reaction assay.
| Genes | Sequences | |
|---|---|---|
| Osteopontin | Forward | 5′-ATCAGGACAGCAACGGGAAG-3′ |
| Reverse | 5′-TGATGTTCCAGGCTGGCTTT-3′ | |
| β-actin | Forward | 5′-GACCCAGATCATGTTTGAGACC-3′ |
| Reverse | 5′-AGTACTTGCGCTCAGGAGGA-3′ |
Statistical analysis
Data were represented as mean ± standard deviation and analyzed using SPSS software 19.0 (SPSS Inc., Chicago, IL, USA). Tukey’s post-hoc test was employed to validate analysis of variance (ANOVA) for comparing data among multiple groups. Student’s t-test was utilized to analyze differences between 2 groups. P<0.05 was assigned as statistical significance. All experiments or tests repeated at least 6 times.
Results
Identification for renal stone rat model
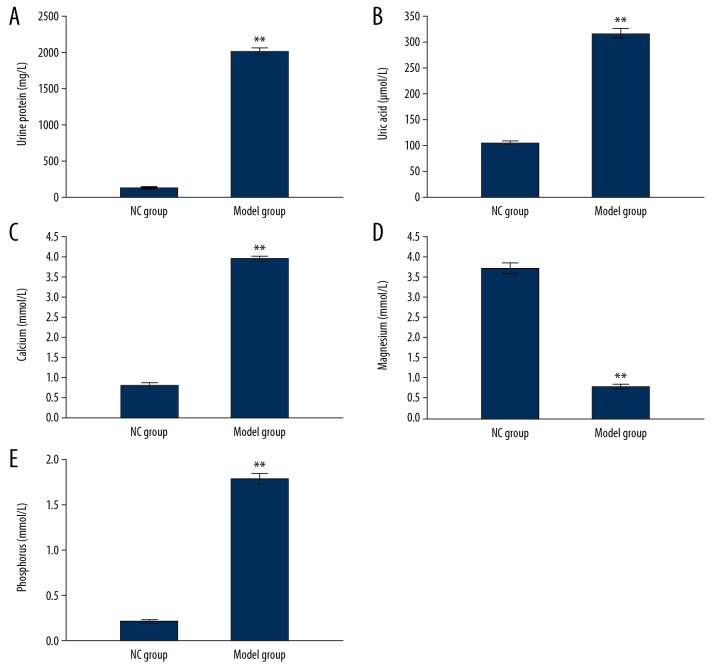
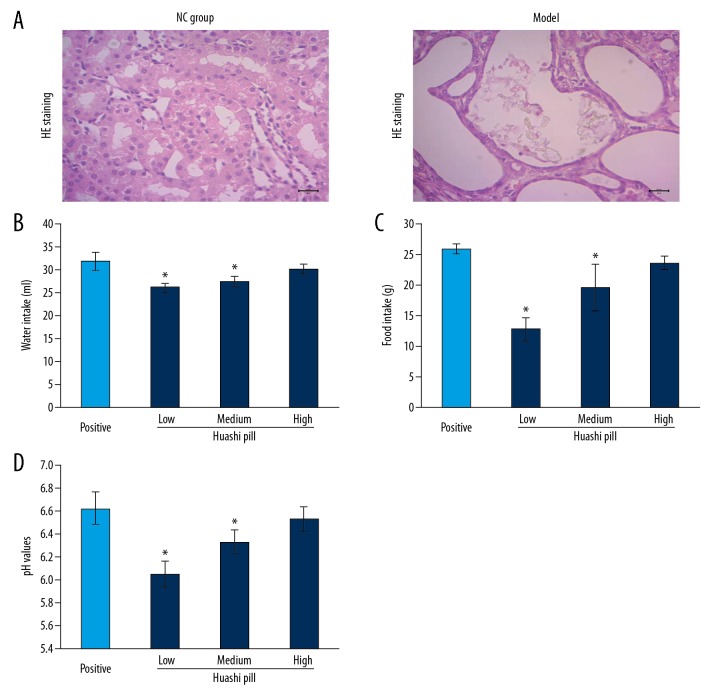
In order to identify the establishment of renal stone rat model, the biochemical parameters, including urine protein, uric acid, and urine routine tests including calcium, magnesium, and phosphorus levels were analyzed. The results showed that the levels of urine protein (Figure 1A) and uric acid (Figure 1B) were significantly higher in the Model group compared to the NC group (P<0.05). Meanwhile, the levels of calcium and phosphorus were significantly higher and magnesium levels were lower significantly in the Model group compared to the NC group (Figure 1C–1E, P<0.05). Moreover, H and E staining results also showed that there were many stone crystals in the intraluminal renal tubular in the Model group compared to the NC group (Figure 2A).
Figure 1.
Evaluation for urine protein (A), uric acid (B), calcium (C), phosphorus (D), and magnesium (E) for identifying renal stone rat model. ** P<0.01 versus NC group.
Figure 2.
Hematoxylin and eosin (H and E) staining observation and evaluation for water, food intake and pH value of urine: (A) H and E staining images. (B) Water intake. (C) Food intake. (D) pH values. * P<0.05 versus Positive group.
Huashi Pill improved water and food intake and enhanced pH value of urine
The results indicated that the Huashi Pill-Medium group and Huashi Pill-High group had significantly increased water intake (Figure 2B) and food intake (Figure 2C) compared to the Huashi Pill-Low group (P<0.05). Meanwhile, Huashi Pill-Medium group and Huashi Pill-High group also had significantly upregulated pH values compared to the Huashi Pill-Low group (Figure 2D, P<0.05). The effects of Huashi Pill on water intake, food intake, and pH values were increased following with concentration increasing.
Huashi Pill improved liver functions
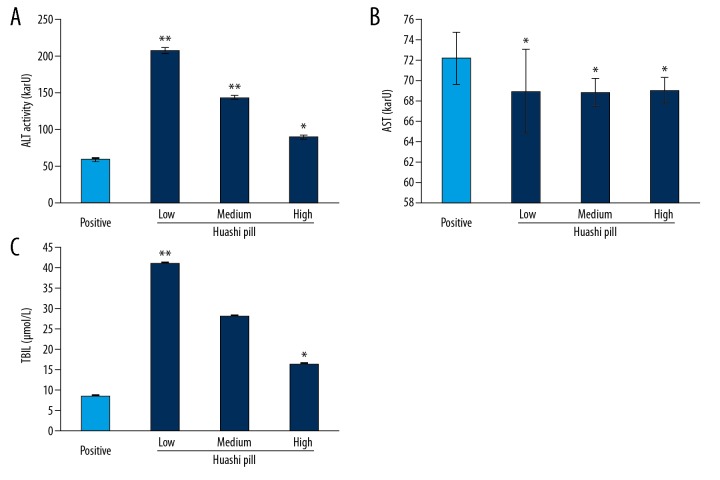
In order to observe the effects of Huashi Pill on liver function of renal stone rat models., the markers of liver function, including ALT (Figure 3A), AST (Figure 3B), and TBIL (Figure 3C) were examined. The results showed that the Huashi Pill-Medium group and the Huashi Pill-High group significantly reduced the activity of ALT and decreased levels of TBIL compared to that of the Huashi Pill-Low group (P<0.05). However, there were no effects of Huashi Pill on the activities of AST in the renal stone rat models.
Figure 3.
Examination for the liver functions of renal stone rat models. (A) ALT activity. (B) AST activity. (C) TBIL levels. * P<0.05 versus Positive group.
Huashi Pill regulated the amounts of microelements
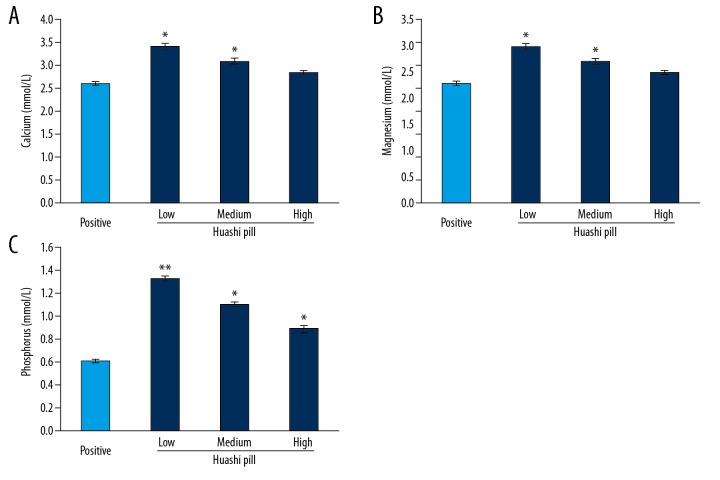
The results exhibited that the Huashi Pill-Medium group and the Huashi Pill-High group had significantly decreased calcium levels (Figure 4A), significantly increased magnesium levels (Figure 4B), and significantly reduced phosphorus levels (Figure 44C) compared to that of the Huashi Pill-Low group (P<0.05).
Figure 4.
Effects of Huashi Pill on the amounts of microelements. (A) Calcium levels. (B) Magnesium levels. (C) Phosphorus levels. * P<0.05, ** P<0.01 versus Positive group.
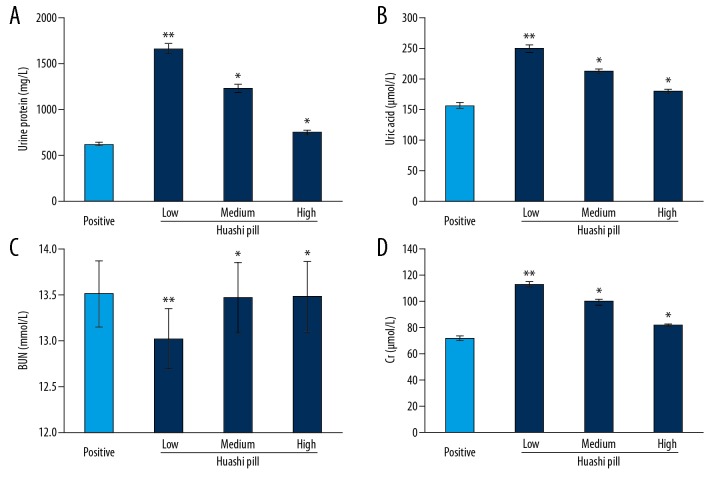
Huashi Pill improved urine routine and urine biochemical indexes
The urine protein (Figure 5A) and uric acid (Figure 5B) levels in the Huashi Pill-Medium group and the Huashi Pill-High group were significantly decreased compared to that of the Huashi Pill-Low group (P<0.05). Meanwhile, the BUN levels (Figure 5C) in Huashi Pill-Medium group and the Huashi PIll-High group were significantly increased compared to that of the Huashi Pill-Low group (P<0.05). The Cr levels (Figure 5D) in the Huashi Pill-Medium and the Huashi Pill-High group were significantly decreased compared to that of the Huashi Pill-Low group (P<0.05). Also, the effects of Huashi Pill on urine protein, uric acid, BUN, and Cr levels were enhanced following the increased concentrations.
Figure 5.
Improvement of Huashi Pill for the urine routine and urine biochemical indexes. (A) Levels of urine protein. (B) Levels of uric acid. (C) Levels of BUN. (D) Levels of Cr. * P<0.05, ** P<0.01 versus Positive group.
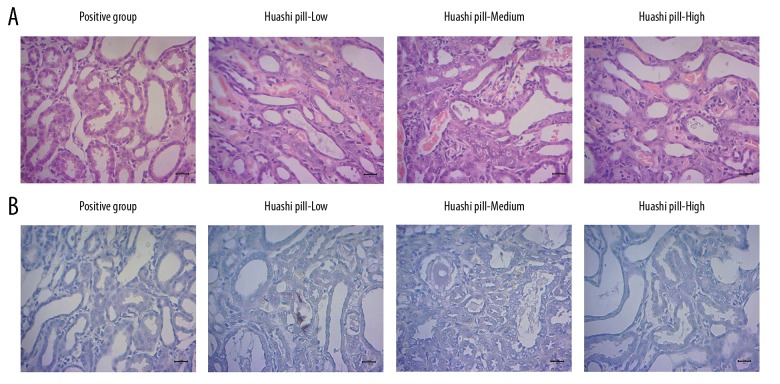
Huashi Pill inhibited formation of stone crystals
The H and E results showed that the stone crystals in the Huashi Pill-Medium group and the Huashi Pill-High group were significantly fewer compared to that of the Huashi Pill-Low group (Figure 6A, P<0.05). Meanwhile, the stone crystals in the Huashi Pill-High group were also significantly fewer compared to that of the Huashi Pill-Medium group (Figure 6A, P<0.05).
Figure 6.
Formation of stone crystals and insoluble calcium deposition was illustrated by using hematoxylin and eosin (H and E) staining and von Kossa staining, respectively. (A) Images for the H and E staining. (B) Images for the von Kossa staining.
Huashi Pill reduced the insoluble calcium deposition
The occurrence of the renal stones is associated with insoluble calcium deposition; therefore, we examined the levels of insoluble calcium in this study. The results indicated that compared with the Huashi Pill-Low group, the insoluble calcium deposition was significantly reduced in the Huashi Pill-Medium and the Huashi Pill-High group (Figure 6B, P<0.05). Also, the amount of insoluble calcium deposition in the Huashi Pill-High group was less compared to the Huashi Pill-Medium group (Figure 6B, P<0.05).
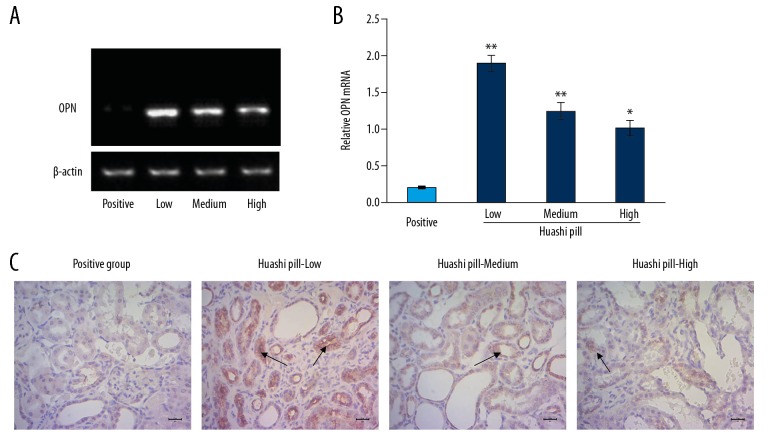
Huashi Pill downregulated expression of osteopontin (OPN)
A previous study reported that OPN is closely correlated with renal stone formation [16]; therefore, we evaluated OPN levels in our renal stone rat model. The RT-PCR data (Figure 7A) showed that the OPN mRNA levels in the Huashi Pill-Medium group and the Huashi Pill-High group were significantly decreased compared to the Huashi Pill-Low group (Figure 7B, P<0.05). Furthermore, the immunohistochemical assay also indicated that there were significantly fewer OPN staining positive cells in the Huashi Pill-Medium group and the Huashi Pill-High groups compared to the Huashi Pill-Low group (Figure 7C, P<0.05).
Figure 7.
Investigation for the osteopontin (OPN) expression by using real-time polymerase chain reaction assay and immunohistochemistry assay. (A) Images for the mRNAs. (B) Statistical analysis for the mRNA levels. (C) Immunohistochemistry assay for OPN expression. The black arrows represented the OPN staining positive cells.
Discussion
Recently, the epidemiological investigations demonstrated that the incidence rate of renal stones has been increasing year-by-year worldwide [3,17]. Renal stones are mainly caused by insoluble calcium deposition, especially by CaOx composition, which accounts for about 80% renal stones in clinical settings [18]. A previous study reported that CaOx-caused stone formation might be a direct result of urinary excretion of oxalate [8]. Actually, higher concentration of oxalate always participates in the onset and development of renal epithelium injury, which might be caused by oxidative stress [19]. Also, damage of epithelial cells is considered to be a risk factor for CaOx associated renal stone formation [9]. Therefore, discovering an effective drug that can reduce the oxidative damage of renal epithelial cells might inhibit the crystal deposition and renal stone formation in rat models.
Huashi Pill is a novel discovered Chinese medicine compound that initially was applied in clinical settings, in recent years. Huashi Pill has been proven to be effective for protecting epithelial cell from oxidative stress and also has been used for digestive diseases. In the present study, we applied Huashi Pill as treatment in a renal stone rat model and observed the effects of Huashi Pill on the insoluble calcium deposition and stone formation. At the begin of this study, we established the renal stone rat model by employing a novel method and compound, which was mainly compose of ethylene glycol, ammonium chloride, and calcium gluconate. Our renal stone model demonstrated the obvious characteristics of renal stones, including increased urine protein and uric acid, as well as enhanced calcium and magnesium, and decreased phosphorus [20]. Meanwhile, H and E staining also illustrated obvious stone crystals, which suggested that the renal rat model was appropriate.
Previous studies [21,22] reported that the urinary pH, water intake, and food intake could reflect the formation of renal stones. Our results showed that Huashi Pill significantly increased the water and food intake and enhanced the pH values, which suggested the severity of renal stones was alleviated. In the other hand, the liver function also reflects the digestive function and indirectly affects the formation of renal stones [23]. Therefore, we examined the levels of ALT, AST, and TBIL in the renal rat model. The results indicated that Huashi Pill significantly decreased the activity of ALT and decreased the levels of TBIL in a renal rat model, which suggested that Huashi Pill remarkably improved liver functions in a renal rat model and further improved clearance of the stones.
The critical affecter for renal stones, calcium and phosphorus have also been examined in rat models [24]. The data showed that Huashi Pill significantly decreased the amounts of calcium and phosphorus in renal stone rat models [25]. This result suggested that the Huashi Pill inhibited the accumulation of calcium and phosphorus, and further inhibited the formation of stones. A previous study [26] reported that the magnesium was effective in reducing oxalate absorption and urinary excretion and could reduce the risk of stone formation. However, our results showed that Huashi Pill decreased the levels of magnesium, which might promote the formation of stones. Therefore, in the future studies, the specific effects and associated mechanism of Huashi Pill on magnesium needed to be clarified. Moreover, the kidney function biomarkers [27], including urine protein, uric acid, BUN, and Cr levels, were also determined in our study. The results indicated that Huashi Pill significantly decreased the levels of urine protein, uric acid, and Cr in a renal stone rat model, all of which suggested that Huashi Pill significantly improved kidney functions.
In order to confirm the effects of Huashi Pill on the formation of stones, the stone crystals and insoluble calcium deposition in the kidney have also been observed in rat models [28,29]. Our results showed that Huashi Pill obviously inhibited formation of stone crystals and reduced the insoluble calcium deposition in a renal stone rat model. Furthermore, in order to clarify the mechanism of Huashi Pill triggered clearance of renal stones, the OPN protein levels were evaluated in the renal stone rat model. A previous study [30] reported that the OPN protein could promote crystal formation and induce renal stone formation. According to the present study, both the RT-PCR and immunohistochemical assay showed that Huashi Pill significantly reduced the OPN levels in a renal stone rat model. This result suggested that the Huashi Pill triggered clearance of stones might be triggered by reducing the OPN protein levels in kidney tissue. We must emphasize that the levels of OPN in the kidney tissue were taken into account in our study, because that the OPN level in kidney tissue and urinary OPN level have different effects on kidney stone formation [31]. For the urinary OPN, the lower OPN levels might be positively correlated with the increased risk of renal stone [31].
Conclusions
The data of this study showed that Huashi Pill could enhance water and food intake, improve liver functions, decrease amounts of calcium and phosphorus, reduce uric acid and Cr levels, and promote the pH values of urine. Huashi Pill also inhibited formation of stone crystals and reduced insoluble calcium deposition in our renal stone rat model. Meanwhile, Huashi Pill also significantly reduced the expression of OPN in kidney tissues. In summary, Huashi Pill inhibited stone formation by regulating urine biochemical indexes and reducing OPN expression in kidney tissue in a renal stone rat model.
Footnotes
Source of support: This study was granted by Preclinical Study on the Treatment of Renal Calculi with Recipe of Kui nonen (Grant No. 2015FZ0054)
Conflict of interest
None.
References
- 1.Bikulciene I, Vasiliauskaite L, Kucinskiene ZA, et al. Investigation of adipose tissue fatty acid composition in men with uronephrolithiasis and metabolic syndrome. Med Sci Monit. 2018;24:818–26. doi: 10.12659/MSM.906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byer K, Khan SR. Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol. 2005;173:640–66. doi: 10.1097/01.ju.0000143190.49888.c7. [DOI] [PubMed] [Google Scholar]
- 3.Yasui T, Iguchi M, Suzuki S, et al. Prevalence and epidemiological characteristics of urolithiasis in Japan: National trends between 1965 and 2005. Urology. 2008;71:209–13. doi: 10.1016/j.urology.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Zhang X. Potassium citrate is better in reducing salt and increasing urine pH than oral intake of lemonade: A cross-over study. Med Sci Monit. 2018;24:1924–29. doi: 10.12659/MSM.909319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesse A, Siener R. Current aspects of epidemiology and nutrition in urinary stone disease. World J Urol. 1997;15:165–71. doi: 10.1007/BF02201853. [DOI] [PubMed] [Google Scholar]
- 6.Goodman HO, Brommage R, Assimos DG, et al. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–94. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]
- 7.Pearle MS, Calhoun EA, Curhan GC, et al. Urologic diseases in America project: Urolithiasis. J Urol. 2005;173:848–57. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 8.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–57. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 9.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J Urol. 2013;189:803–11. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki Y, Kohjimoto Y, Iba A, et al. Weight loss intervention reduces the risk of kidney stone formation in a rat model of metabolic syndrome. Int J Urol. 2015;22:404–9. doi: 10.1111/iju.12691. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Xu XF, Feng Y, et al. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis. 2014;42:519–26. doi: 10.1007/s00240-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 12.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Al-Attar AM. Antilithiatic influence of spirulina on ethylene glycol-induced nephrolithiasis in male rats. Am J Biochem Biotech. 2010;6:25–31. [Google Scholar]
- 14.Nizami AN, Rahman MA, Ahmed NU, et al. Whole leea macrophylla ethanolic extract normalizes kidney deposits and recovers renal impairments in an ethylene glycol-induced urolithiasis model of rats. Asian Pac J Trop Med. 2012;5:533–38. doi: 10.1016/S1995-7645(12)60094-7. [DOI] [PubMed] [Google Scholar]
- 15.Selvam R, Kalaiselvi R, Govindaraj A, et al. Effect of A lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 16.Hirose M, Tozawa K, Okada A, et al. Role of osteopontin in early phase of renal crystal formation: Immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol Res. 2012;40:121–29. doi: 10.1007/s00240-011-0400-z. [DOI] [PubMed] [Google Scholar]
- 17.He XZ, Ou TW, Cui X, et al. Analysis of the safety and efficacy of combined extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy for the treatment of complex renal calculus. Eur Rev Med Pharmacol Sci. 2017;21:2567–71. [PubMed] [Google Scholar]
- 18.Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3:256–76. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackett RL, Shevock PN, Khan SR. Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res. 1994;22:197–203. doi: 10.1007/BF00541892. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Xu YF, Feng Y, et al. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis. 2014;42:519–26. doi: 10.1007/s00240-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 21.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:833–88. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 22.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–25. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed MH, Barakat S, Almobarak AO. The association between renal stone disease and cholesterol gallstones: The easy to believe and not hard to retrieve theory of the metabolic syndrome. Ren Fail. 2014;36:957–62. doi: 10.3109/0886022X.2014.900424. [DOI] [PubMed] [Google Scholar]
- 24.Arrabal-Polo MA, Sierra Giron-Prieto M, Orgaz-Molina J, et al. Calcium renal lithiasis and bone mineral density. Importance of bone metabolism in urinary lithiasis. Actas Urol Esp. 2013;37:362–67. doi: 10.1016/j.acuro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Gonullu E, Bilge NSY, Cansu DU, et al. Risk factor for urolithiasis in patients with ankylosing spondylitis: A prospective case-control study. Urolithiasis. 2017;45:353–57. doi: 10.1007/s00240-016-0911-8. [DOI] [PubMed] [Google Scholar]
- 26.Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol. 2000;163:1565–69. [PubMed] [Google Scholar]
- 27.Sasikumar P, Gomathi S, Anbazhagan K, et al. Recombinant lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. J Biomed Sci. 2014;21:86. doi: 10.1186/s12929-014-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal KP, Tandon S, Naik PK, et al. Peeping into human renal calcium oxalate stone matrix: characterization of novel proteins involved in the intricate mechanism of urolithiasis. PLoS One. 2013;8:e69916. doi: 10.1371/journal.pone.0069916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naghii MR, Eskandari E, Mofid M, et al. Antioxidant therapy prevents ethylene glycol-induced renal calcium oxalate crystal deposition in Wistar rats. Int Urol Nephrol. 2014;46:1231–38. doi: 10.1007/s11255-014-0658-5. [DOI] [PubMed] [Google Scholar]
- 30.Hirose M, Tozawa K, Okada A, et al. Role of osteopontin in early phase of renal crystal formation: immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol Res. 2012;40:121–29. doi: 10.1007/s00240-011-0400-z. [DOI] [PubMed] [Google Scholar]
- 31.Icer MA, Gezmen-Karadag M, Sozen S. Can osteopontin levels, which may be correlated with nutrition intake and body composition, be used as a new biomarker in the diagnosis of nephrolithiasis? Clin Biochem. 2018;60:38–43. doi: 10.1016/j.clinbiochem.2018.08.001. [DOI] [PubMed] [Google Scholar]