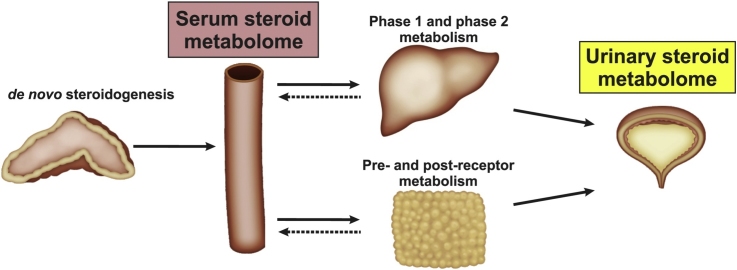
Graphical abstract
Keywords: Steroid metabolome, Steroid biosynthesis, Steroid metabolism, Urine metabolome, Serum metabolome
Highlights
-
•
The serum metabolome does not reflect steroids activated in an intracrine manner.
-
•
The urinary steroid metabolome reflects steroid biosynthesis and metabolism.
-
•
Defined pathways link the circulating and urinary steroid metabolomes.
-
•
Modern mass spectrometry techniques allow for comprehensive steroid profiling.
Abstract
Advances in technology have allowed for the sensitive, specific, and simultaneous quantitative profiling of steroid precursors, bioactive steroids and inactive metabolites, facilitating comprehensive characterization of the serum and urine steroid metabolomes. The quantification of steroid panels is therefore gaining favor over quantification of single marker metabolites in the clinical and research laboratories. However, although the biochemical pathways for the biosynthesis and metabolism of steroid hormones are now well defined, a gulf still exists between this knowledge and its application to the measured steroid profiles. In this review, we present an overview of steroid hormone biosynthesis and metabolism by the liver and peripheral tissues, specifically highlighting the pathways linking and differentiating the serum and urine steroid metabolomes. A brief overview of the methodology used in steroid profiling is also provided.
1. Introduction
Steroid hormones play an essential role in regulating water and salt balance, metabolism and stress response, and in initiating and maintaining sexual differentiation and reproduction. Researchers investigating steroid-related endocrine conditions have measured alterations in the steroid metabolome for several decades. While clinical laboratories have traditionally measured changes in individual diagnostic marker steroids, the quantification of steroid panels are now gaining widespread traction due to advances in technology, further driven by the emerging diagnostic power of steroid metabolomics, i.e. the combination of mass spectrometry-based steroid profiling with unbiased data analysis by machine learning approaches.
In most cases, alterations in steroid profiles associated with endocrine disorders were identified long before the responsible enzymes were identified or characterized following the advent of modern molecular techniques. While the biochemical pathways for the biosynthesis and metabolism of steroid hormones are now mostly well defined, a gulf still exists with regard to the application of this knowledge to the interpretation of the measured multi-steroid profiles in serum and urine. Researchers and clinicians are increasingly dependent on results obtained by steroid metabolome analysis, but are often unfamiliar with the metabolic pathways resulting in the observed steroid profile and the distinct metabolic pathways explaining the differences between serum and urine steroid metabolomes.
Therefore, it is the aim of this review to provide a comprehensive and up-to-date examination of our current knowledge of metabolic pathways underlying the serum and urine steroid metabolomes. We briefly review the origins of steroid hormones, and present the resulting serum metabolome of each of the main classes of steroids. Downstream metabolism of each of these steroid classes are subsequently presented and linked to the resulting urine steroid excretion patterns. Taken together this review provides a biochemical overview of the biosynthesis, metabolism and excretion of steroid hormones.
2. Origins of steroid hormones
2.1. Overview of de novo steroidogenesis
Steroid hormones are produced through de novo steroidogenesis in the adrenal cortex, the gonads and the placenta. In addition, a range of neurosteroids are produced in the brain [1], however these are beyond the scope of this review. Steroidogenic tissues are unique in their ability to utilize cholesterol as starting material for the mitochondrial biosynthesis of pregnenolone, the precursor steroid in the biosynthesis of all steroid hormones. Cholesterol can be obtained from multiple sources including de novo biosynthesis from acetate in the endoplasmic reticulum (ER) [[2], [3], [4]], the hydrolysis of cholesteryl esters stored in lipid droplets by cholesteryl ester hydrolases, exogenous lipoprotein-derived cholesterol esters from LDL receptor-mediated endocytic and/or SR-BI-mediated uptake pathways, and free cholesterol residing in the plasma membrane [[5], [6], [7], [8]]. All three primary steroidogenic organs, namely the adrenal cortex, gonads and placenta, can biosynthesize cholesterol de novo under the regulation of tropic hormones and plasma lipoproteins are widely accepted as the principal source of cholesterol used for steroid biosynthesis [[5], [6], [7], [8]].
2.2. Overview of steroidogenic enzymes
Two major functional classes of enzymes are involved in the biosynthesis of all steroid hormones, namely the cytochrome P450 (CYP) and hydroxysteroid dehydrogenase (HSD) enzymes. The heme-containing CYP enzymes activate molecular oxygen utilizing NADPH as an electron donor. During catalysis, they incorporate one oxygen atom into the substrate while the other oxygen atom is reduced to water. This catalytic potential allows CYPs to catalyze a wide range of reactions, with hydroxylation and C—C bond cleavage being relevant reactions in steroidogenesis [9,10]. CYP enzymes involved in steroidogenesis can be divided into two groups based on their intracellular location and mode of electron transfer. CYP type I enzymes are located within the inner mitochondrial membrane and are dependent on ferredoxin and ferredoxin reductase for the delivery of their electrons from NADPH. Ferredoxin reductase is a flavoprotein that oxidizes NADPH and transfers electrons to ferredoxin, a small iron-sulfur protein, which acts as a mobile electron carrier, delivering the electrons to the CYP. The adrenally located ferredoxin reductase and ferredoxin are often also referred to as adrenodoxin reductase (AdxR) and adrenodoxin (Adx), respectively. CYP type II enzymes are found in the ER and are dependent on the electron donor enzyme cytochrome P450 oxidoreductase (POR) for electron delivery. POR contains a flavin adenine dinucleotide (FAD) and a flavin mononucleotide (FMN) allowing the enzyme to oxidize NADPH and reduce the CYP enzyme in a stepwise manner. The availability of NADPH is a vital aspect of CYP-catalyzed reactions, with redox partner ratios differentially influencing CYP activities [[11], [12], [13]].
The other main functional class of enzymes involved in steroidogenesis are the HSD enzymes which are dependent on NAD(P)H and NAD(P)+ co-factors. HSDs are subdivided into two distinct enzyme superfamilies based on their structural fold. These are the short chain dehydrogenases and aldo-keto reductases (AKR). The function of the HSD enzymes from both families is to catalyze the conversion of a given hydroxysteroid to its corresponding ketosteroid counterpart and vice versa, and in doing so, regulate the activity of the steroid at specific steroid receptors [14]. Most HSD-catalyzed reactions are mechanistically reversible and can function bi-directionally, although a prominent directionality is observed in vivo as a result of co-factor affinity and cellular redox status. An exception to this rule are the two HSD3B isoforms, HSD3B1 and HSD3B2, which catalyze an irreversible reaction, directly linked to the isomerization of the Δ5 double bond. These enzymes have dual catalytic activity and not only transform the hydroxy group on carbon 3 to a keto group but additionally isomerize the double bond from Δ5 to Δ4 [[15], [16], [17], [18]].
2.3. Overview of adrenal steroidogenesis
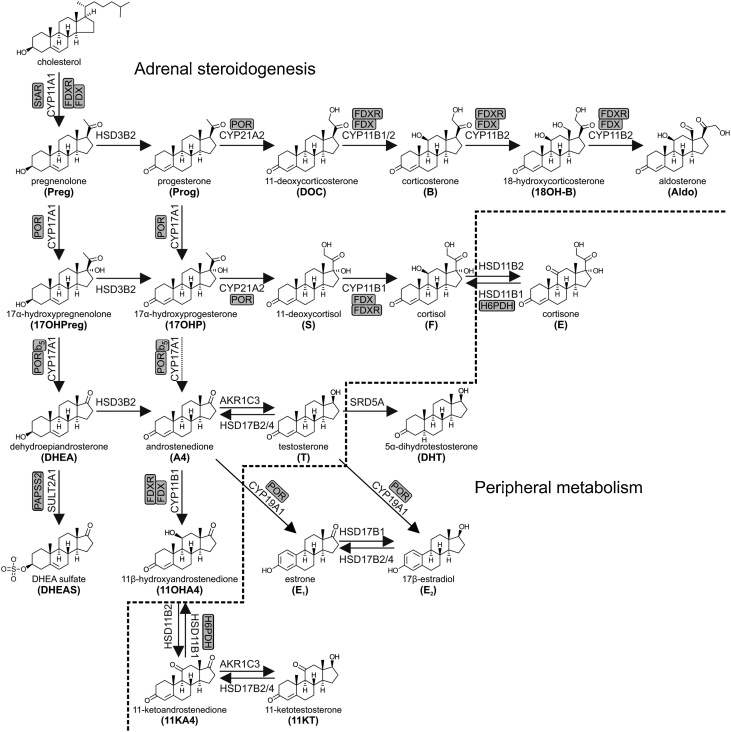
The cortex of the adrenal gland is responsible for the biosynthesis of mineralocorticoids and glucocorticoids, as well as the production of adrenal androgen precursors and androgens, a function unique to higher primates [19,20]. The cortex is subdivided into three functional zones, each responsible for the production of a distinct steroid class due to the zone-specific expression of steroidogenic enzymes. The outer zone of the adrenal is termed the zona glomerulosa and expresses enzymes that catalyze the production of the major mineralocorticoid aldosterone under the control of the renin-angiotensin-aldosterone system. The middle zone, the zona fasciculata, is responsible for the production of the primary glucocorticoid, cortisol. Finally, the innermost zone, the zona reticularis, contributes to the formation of C19 androgen precursors including dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), androstenedione (A4) and 11β-hydroxyandrostenedione (11OHA4) (Fig. 1). The hypothalamic-pituitary-adrenal (HPA) axis regulates the production of glucocorticoids and adrenal androgen precursors by the adrenal. In short, the hypothalamus produces corticotropin-releasing hormone (CRH) that stimulates corticotrope cells in the anterior pituitary to biosynthesize and release adrenocorticotropic hormone (ACTH) that in turn stimulates the adrenal gland to produce steroid hormones, specifically DHEA and cortisol [21,22]. Glucocorticoids complete the system by having a negative feedback effect on the pituitary, hypothalamus and the hippocampus, inhibiting further stimulation of the adrenal gland, while there is no feedback inhibition of the HPA axis by adrenal androgen precursors.
Fig. 1.
Schematic overview of adrenal steroidogenesis and peripheral modulation of steroid bioactivity. Arrows are labelled with the catalyzing enzyme and isoform where appropriate. Essential accessory proteins are also indicated: cytochrome b5 (b5); cytochrome P450 oxidoreductase (POR); ferredoxin (FDX); ferredoxin reductase (FDXR); hexose-6-phosphate dehydrogenase (H6PDH); PAPS synthase 2 (PAPSS2); steroidogenic acute regulatory protein (StAR).
2.4. Overview of gonadal steroidogenesis
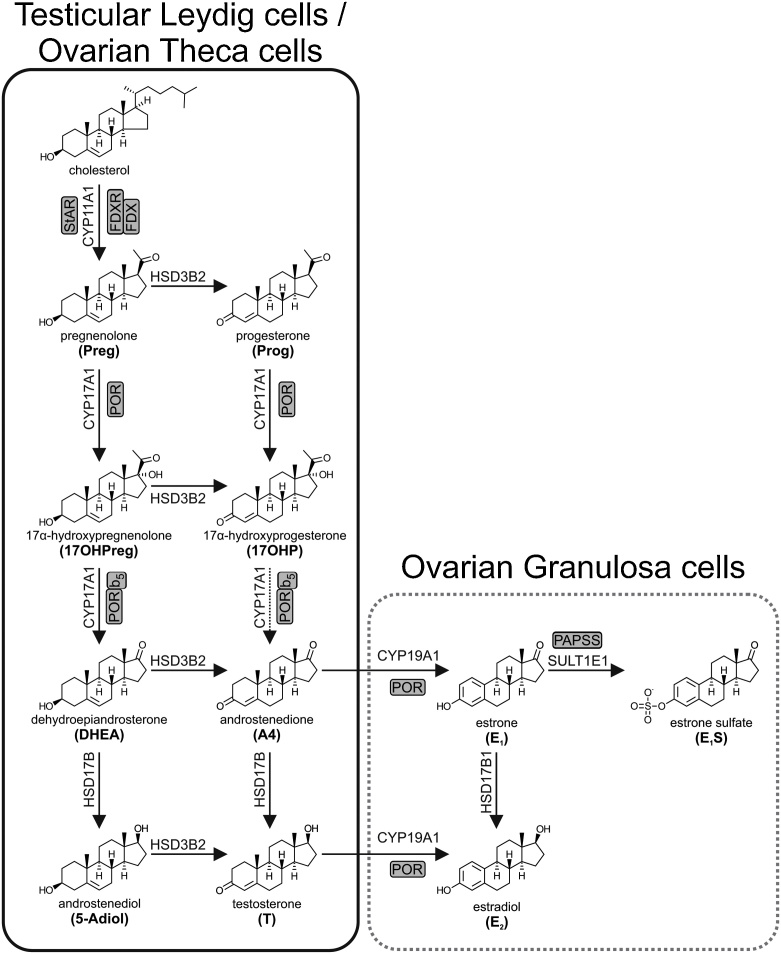
Steroidogenesis in the gonads is tailored to the production of androgens and estrogens, with the corpus luteum additionally playing an important role in the production of the major endogenous progestogen, progesterone. Similar to the zonation of the adrenal, it is the cell-specific expression pattern of steroidogenic enzymes within each cell type that dictates steroid output (Fig. 2). Gonadal steroidogenesis is initiated by the development of the hypothalamic-pituitary-gonadal axis at puberty. The hypothalamus produces and secretes gonadotropin-releasing hormone (GnRH) in a pulsatile fashion, which in turn stimulates the production and secretion of luteinizing hormone (LH) from the pituitary. Androgens and estrogens provide negative feedback at the hypothalamus and pituitary level to suppress LH in men and women, respectively [23]. Gonadal steroidogenesis is also active during ‘minipuberty’, a short period of hypothalamic-pituitary-gonadal axis activation during the neonatal period [24].
Fig. 2.
Schematic overview of steroidogenesis in the gonads. Steroidogenic pathways in the testicular Leydig cells are shown in the black box, while those in the ovaries are shown in the grey box and are further subdivided into the theca and granulosa cells. Arrows are labelled with the catalyzing enzyme and isoform where appropriate. Essential accessory proteins are also indicated: cytochrome b5 (b5); cytochrome P450 oxidoreductase (POR); ferredoxin (FDX); ferredoxin reductase (FDXR); PAPS synthase (PAPSS); steroidogenic acute regulatory protein (StAR).
2.5. Biosynthesis of specific steroid classes
2.5.1. Mineralocorticoid production in the adrenal zona glomerulosa
Enzyme expression in the zona glomerulosa is tailored to produce the C21 mineralocorticoid, aldosterone. CYP11A1 converts cholesterol to pregnenolone, followed by the HSD3B2-catalyzed conversion of pregnenolone to the Δ4 steroid, progesterone. HSD3B2 is present in both the mitochondria and ER of zona glomerulosa cells [25,26]. Progesterone is subsequently converted to 11-deoxycorticosterone (DOC) by CYP21A2, which is abundantly expressed in the ER of the zona glomerulosa [26,27]. The lack of CYP17A1 expression in the zona glomerulosa [25] together with the abundant expression of HSD3B2 ensures that all steroid intermediates are directed towards aldosterone biosynthesis. Two isoforms of CYP11B are expressed in the zona glomerulosa, both with the ability to catalyze the 11β-hydroxylation of DOC yielding corticosterone. CYP11B2, which is also known as aldosterone synthase, additionally exhibits 18-hydroxylase and 18-methyl oxidase activity, which are required to convert corticosterone to aldosterone via the 18-hydroxycorticosterone intermediate [26,28,29].
2.5.2. Glucocorticoid production in the adrenal zona fasciculata
The adrenal zona fasciculata is the site of glucocorticoid production. Pregnenolone, produced from the CYP11A1 catalyzed side-chain cleavage of cholesterol, is converted to 17α-hydroxyprogesterone (17OHP), the universal precursor of cortisol production, by HSD3B2 and CYP17A1 17α-hydroxylase activity. CYP21A2 subsequently catalyzes the conversion of 17OHP to 11-deoxycortisol, an obligatory step in the production of glucocorticoids. Finally, CYP11B1, located in the mitochondria of the zona fasciculata cells, facilitates the final step in glucocorticoid biosynthesis by catalyzing the conversion of 11-deoxycortisol to cortisol.
2.5.3. Androgen biosynthesis
2.5.3.1. The classic androgen biosynthesis pathway in the adrenal zona reticularis and the gonads
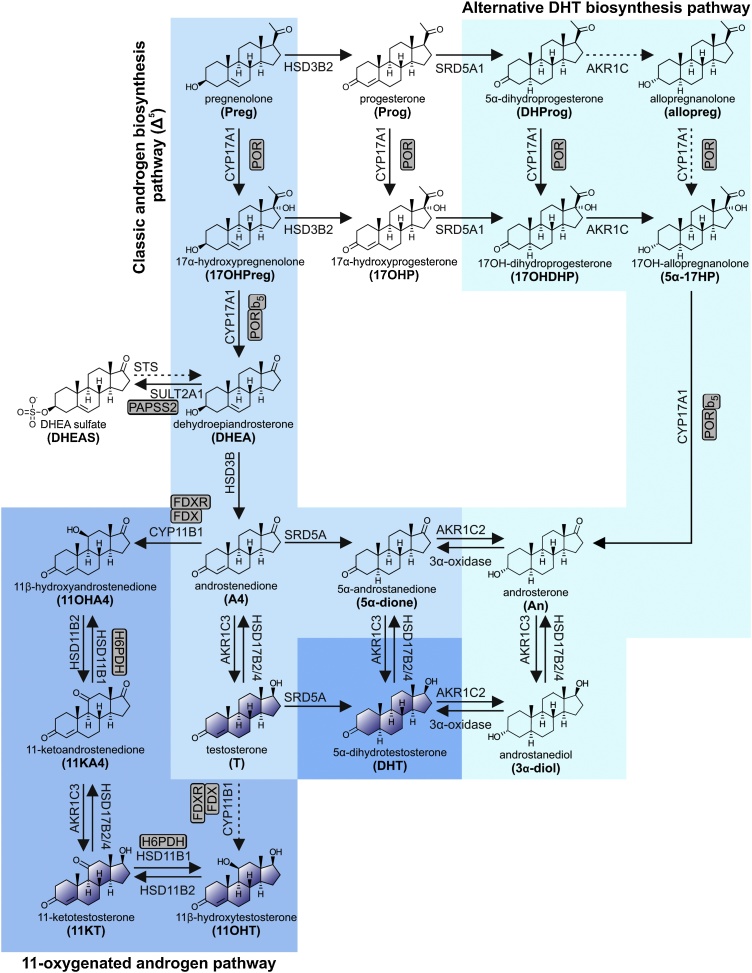
Androgen precursors and active androgens are produced by both the adult adrenal and gonads by the Δ5 pathway from pregnenolone to DHEA (Fig. 3). This pathway is also referred to as the ‘classic androgen biosynthesis pathway’. The CYP17A1-catalyzed 17α-hydroxylation of pregnenolone yields 17α-hydroxypregnenolone, which serves as the preferred substrate for the 17,20-lyase activity of CYP17A1, producing C19 steroids from C21 precursors [[30], [31], [32]]. It should be noted that the 17,20-lyase activity of CYP17A1 is dependent on augmentation by cytochrome b5 (CYB5A) in addition to electron transfer from POR [33].
Fig. 3.
Schematic overview of androgen biosynthesis. Bioactive androgens (testosterone (T), 5α-dihydrotestosterone (DHT), 11-ketotestosterone (11KT) and 11β-hydroxytestosterone (11OHT) can be generated by three partially independent pathways which operate across multiple tissues: (1) the classic Δ5 pathway, (2) the alternative DHT biosynthesis pathway, and (3) the 11-oxygenated androgen pathway. Arrows are labelled with the catalyzing enzyme and isoform where appropriate. Essential accessory proteins are indicated: cytochrome b5 (b5); cytochrome P450 oxidoreductase (POR); ferredoxin (FDX); ferredoxin reductase (FDXR); hexose-6-phosphate dehydrogenase (H6PDH); PAPS synthase 2 (PAPSS2); steroidogenic acute regulatory protein (StAR).
The Δ5 pathway is active in the zona reticularis of the adrenal cortex, which only develops during adrenarche between the ages of 6–10, a process unique to humans and higher primates. During that time, the development of a distinct zona reticularis is accompanied by an extreme increase in the adrenal androgen precursor production due to the decreased expression of HSD3B2 in conjunction with increased CYB5A expression [[34], [35], [36], [37], [38]]. Some of the resulting DHEA is converted to androstenediol by AKR1C3, which exhibits minor expression in the zona reticularis [39]. However, the majority of DHEA is efficiently sulfated by the major DHEA sulfotransferase (SULT2A1), which is abundantly expressed in the zona reticularis [40]. This results in significant DHEAS output and accounts for DHEAS being the most abundant steroid in circulation (Table 2) [39,41]. Other Δ5 steroids, e.g. pregnenolone, 17α-hydroxypregnenolone and androstenediol, can also be released in their respective sulfated form [[42], [43], [44]]. Moreover, DHEA and androstenediol can all be converted to their corresponding Δ4 products by the low levels of HSD3B2, yielding A4 and testosterone, respectively. A4 can serve as an additional substrate for AKR1C3, yielding testosterone [45]. An age-related gradual decrease in adrenal androgen secretion and excretion, known as adrenopause, occurs starting in the fourth decade of life and is associated with a decrease in the zona reticularis cell layer and cell function. This reaches a minimum by the age of 70, with only about 5–10% of the peak levels observed in young adulthood [46].
Table 2.
Graphical representation of the circulating serum steroid metabolome. Major circulating steroids are shown divided into six concentration ranges illustrating their relative contribution to the total circulating steroid pool.
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
| 1-10 μmol/L | 100-1000 nmol/L | 10-100 nmol/L | 1-10 nmol/L | 100-1000 pmol/L | <100 pmol/L |
| DHEAS | cortisol | corticosterone | 17OHP | 17OHP | 17β-estradiol |
| cortisone | (male) | (female, follicular phase) | (male) | ||
| DHEA | 17OHP | DOC | 17β-estradiol | ||
| testosterone | (female, luteal phase) | 18OH-corticosterone | (female, postmenopausal) | ||
| (male) | pregnenolone | aldosterone | estrone | ||
| progesterone | 17OH-pregnenolone | 18-oxocortisol | (male) | ||
| (female, luteal phase) | 11-deoxycortisol | 11OHT | estrone | ||
| 18-hydroxycortisol | progesterone | (female, postmenopausal) | |||
| A4 | (male) | ||||
| 11OHA4 | progesterone | ||||
| 11KA4 | (female, postmenopausal) | ||||
| testosterone | 17β-estradiol | ||||
| (female) | (female, premenopausal) | ||||
| 11KT | estrone | ||||
|
progesterone (female, follicular phase) |
(female, premenopausal) |
Like in the zona reticularis, the Leydig cells of the testes follow the Δ5 pathway due to the co-expression of CYP17A1 and CYB5A (Fig. 2). However, in the Leydig cells subsequent metabolism is directed at testosterone biosynthesis due to the absence of SULT2A1 and the expression of HSD3B2 and HSD17B3. DHEA is then converted to testosterone via A4 or androstenediol through the action of HSD3B2 and HSD17B3, respectively, with HSD17B3 playing a key role in testicular androgen biosynthesis [47,48]. AKR1C3 expression has been reported in Leydig cells and may also contribute to testosterone production [49,50]. Testicular steroid output is predominantly testosterone, with lower levels of A4 and DHEA also being released into circulation [[51], [52], [53]]. Androgen and androgen precursor production by the ovary follows a similar route to that of the testes (Fig. 2).
2.5.3.2. Peripheral tissue activation of androgen precursors
It should be highlighted that with the exception of testosterone produced by the testes, the vast majority of C19 steroids produced by the adrenal and ovaries are inactive androgen precursors. These can, however, subsequently be converted to active androgens in target cells of androgen action that express the required enzymatic machinery (Fig. 3) [45]. More specifically, DHEA can be converted to A4 by peripheral HSD3B1, with A4 serving as the substrate for the production of testosterone by AKR1C3 [54]. Subsequent 5α-reduction of testosterone yields the more potent androgen 5α-dihydrotestosterone (DHT), with this step therefore serving as a target-specific amplification of the androgen signal, a pre-receptor activation of testosterone to DHT [45]. A4 can also be 5α-reduced to 5α-androstanedione prior to conversion to DHT by AKR1C3, thereby bypassing testosterone (Fig. 3) [45,55]. Indeed, the enzyme steroid 5α-reductase 1 (SRD5A1) catalyzes the 5α-reduction of A4 more efficiently than that of testosterone [56,57]. This so called “alternate 5α-androstanedione” pathway is favored in tissues with predominant SRD5A1 expression and in conditions such as castration-resistant prostate cancer in which the expression of SRD5A1 is upregulated and that of SRD5A2 is downregulated [55,58]. As SRD5A2 does not demonstrate the same substrate preference, tissues expressing this isoform are thought to follow the more conventional pathway of conversion to testosterone, prior to 5α-reduction [56]. Moreover, although the liver undoubtedly makes the major contribution to steroid metabolism, most peripheral tissues also possess enzymatic machinery for inactivation of androgens (both those obtained from circulation and those produced from inactive precursors) by phase 1 and 2 metabolism (Section 3). This process of peripheral cell specific activation and inactivation is known as pre- and post-receptor steroid metabolism or steroid ” intracrinology” [45,[59], [60], [61]].
2.5.3.3. The alternative DHT biosynthesis pathway
In selected circumstances, such as CYP21A2 deficiency and during fetal development (Section 2.6), accumulation of progesterone and 17OHP in circulation can lead to the activation of an alternative pathway of DHT biosynthesis (Fig. 3). This is sometimes referred to as the “backdoor pathway” to DHT [62]. To enter this pathway, progesterone and 17OHP are 5α-reduced by SRD5A1 to yield 5α-dihydroprogesterone and 17α-hydroxydihydroprogesterone, respectively. These are then subsequently converted to allopregnanolone and 17α-hydroxyallopregnanolone by the 3α-reductase activity of AKR1C enzymes. Allopregnanolone can then be converted to 17α-hydroxyallopregnanolone, which serves as an excellent substrate for the 17,20-lyase activity of CYP17A1 [30], yielding androsterone. Androsterone, considered an inactive metabolite of DHT under normal circumstances, can then be reactivated by the sequential 17β-reduction and 3α-oxidase reactions [[63], [64], [65]]. Androsterone has been shown to be the principle circulating androgen precursor for the alternative DHT biosynthesis pathway in the male fetus during the second trimester. Interestingly, placental progesterone has been suggested to serve as substrate for androsterone biosynthesis in the male fetus via the alternative pathway which occurs across several non-gonadal fetal tissues [66].
2.5.3.4. The 11-oxygenated androgen biosynthesis pathway
Within the adrenal, A4 can serve as a substrate for CYP11B1, yielding the 11-oxygenated androgen precursor, 11OHA4 [67], with conversion of testosterone to 11β-hydroxytestosterone (11OHT), also occurring (Fig. 3). However, 11OHA4 is by far the predominant product due to the significantly higher levels of A4 produced in the zona reticularis [39]. Notably, the addition of exogenous testosterone does not lead to increased 11-oxygenated androgen output, thereby confirming that only locally produced substrates (primarily A4) can be 11β-hydroxylated [68]. Low levels of 11-ketoandrostenedione (11KA4) and 11-ketotestosterone (11KT) have also been reported in adrenal vein samples and are suggested to result from some HSD11B2 activity in the adrenal [39]. However, differences in the concentration of 11KA4 measured in the adrenal vein and inferior vena cava suggest that 11KA4 is predominantly produced from conversion of 11OHA4 in peripheral tissue expressing HSD11B2 such as the kidney [39,69]. 11KA4 in turn serves as a substrate for AKR1C3 expressed in peripheral tissues such as adipose tissue, yielding 11KT, which binds and activates the human androgen receptor with an affinity and potency similar to that of testosterone [45,[70], [71], [72]]. Indeed, a recent study has shown that AKR1C3 catalyzes the conversion of 11KA4 to 11KT with an 8-fold higher efficiency than that of A4 to testosterone, which may account for higher levels of peripheral activation [73]. Peripheral or intracrine activation of 11-oxygenated androgens may therefore play a vital role in regulating their physiological activity. Interestingly, activation/inactivation of glucocorticoids and 11-oxygenated androgens work in an antiparallel manner, with the 11β-hydroxy derivative being the active glucocorticoid [74,75], while the 11-keto androgens are more potent than their 11β-hydroxy counterparts [76].
2.5.4. Progestogen and estrogen biosynthesis by the ovary
Steroidogenesis within the ovary is compartmentalized in a cell-specific manner, with the theca cells mainly producing A4 and progesterone, while the granulosa cells complete the biosynthesis of 17β-estradiol (Fig. 2). Ovarian steroidogenesis originates in the theca cells with the production of A4 via the Δ5 pathway. The resulting A4 (and testosterone) enters the granulosa cells where the expression of CYP19A1 (aromatase) results in estrogen biosynthesis. Ovarian steroid output varies considerably during the course of the menstrual cycle – 17β-estradiol is the primary steroid produced during the follicular phase, while progesterone is the principal steroid during the luteal phase [[77], [78], [79]].
In addition to de novo steroidogenesis, it is important to note that the ovary also utilizes circulating DHEA and A4 of adrenal origin for the biosynthesis of androgens and estrogens [80]. Suppression of adrenal androgen output by dexamethasone in healthy, young women with a regular menstrual cycle leads to a 90% decrease in DHEA(S) output, but also reduces circulating testosterone and DHT concentrations to one third of their respective baseline concentrations [81]. While A4 can be metabolized to testosterone by AKR1C3 in the theca cells, the majority diffuses to the granulosa cells where the high expression levels of CYP19A1 results in the production of estrone. HSD17B1 subsequently catalyzes the conversion of estrone to 17β-estradiol, under the regulation of FSH. Testosterone diffusing from the theca cells also serves as the substrate for CYP19A1, directly contributing to 17β-estradiol production. Theca cells also express high levels of the estrogen sulfotransferase enzyme SULT1E1, which preferentially sulfates estrone yielding the relatively abundant estrone sulfate measured in circulation [[82], [83], [84]]. However, quantitatively estrogens circulate at significantly lower levels than androgens. Indeed, androgen secretion by the theca cells surpasses the secretion of estrogens, while progesterone is the primary progestogen produced [85]. It should also be noted that the peripheral aromatization of C19 steroids plays an important role in peripheral estrogen production, particularly following menopause as outlined in Section 4.4 below.
3. Principles of steroid metabolism and excretion
Steroids are inherently lipophilic molecules. Metabolic conversions are therefore required to increase their water-solubility and enable efficient excretion in urine and bile (Table 1). This metabolism is traditionally divided into two sequential stages, namely phase 1 and phase 2 reactions [86]. Phase 1 reactions alter the biological activity and at the same time add or reveal functional groups that function as targets for subsequent phase 2 reactions. Phase 2 reactions are conjugation reactions that ultimately inactivate the compound and increase polarity and water solubility, thereby facilitating urinary and biliary excretion. Additionally, conjugation with a charged group limits transport over membranes to active transport, thereby allowing for the concentration of the metabolite on one side [87,88]. The major phase 1 reactions for steroids are the reduction of the 3-keto-Δ4 motif, the interconversion of hydroxy- and keto-groups by HSDs/oxoreductases and additional hydroxylations by CYPs.
Table 1.
List of common circulating steroids and their major urine metabolites. Common abbreviations are shown in brackets.
| Serum steroid and abbreviation | Major urine metabolite and abbreviation (unconjugated form) |
|---|---|
| General precursors | |
| pregnenolone, 5-pregnen-3β-ol-20-one (PREG; P5) | 5-pregnenediol, 5-pregnen-3β,20α-diol (5PD) |
| progesterone, 4-pregnen-3,20-dione (PROG; P4) | pregnanediol, 5β-pregnan-3α,20α-diol (PD) |
| 17α-hydroxypregnenolone, 5-pregnen-3β,17α-diol-20-one (17Preg; 17OHPreg; 17P5) | 5-pregnenetriol, 5-pregnen-3β,17α,20α-triol (5 P T) |
| 17α-hydroxyprogesterone, 4-pregnen-17α-ol-3,20-dione (17OHP; 17OHProg; 17P4) | pregnanetriol, 5β-pregnan-3α,17α,20α-triol (PT) |
| 17α-hydroxypregnanolone, 5β-pregnan-3α,17α-diol-20-one (17HP) | |
| Mineralocorticoids and their precursors | |
| 11-deoxycorticosterone, 4-pregnen-21-ol-3,20-dione (DOC) | tetrahydro-11-deoxycorticosterone, 5β-pregnan-3α,21-diol-20-one (THDOC) |
| corticosterone, 4-pregnene-11β,21-diol-3,20-dione (CORT; B) | tetrahydro-11-dehydrocorticosterone, 5β-pregnan-3α,21-diol-11,20-dione (THA) |
| 5α-tetrahydro-11-dehydrocorticosterone, 5α-pregnan-3α,21-diol-11,20-dione (5α-THA) | |
| 5β-tetrahydrocorticosterone, 5β-pregnan-3α,11β,21-triol-20-one (THB) | |
| 5α-tetrahydrocorticosterone, 5α-pregnan-3α,11β,21-triol-20-one (5α-THB) | |
| 18-hydroxycorticosterone, 4-pregnene-11β,18,21-triol-3,20-dione (18OHCORT; 18OHB; 18B) | 18-hydroxytetrahydro-11-dehydrocorticosterone, 5β-pregnan-3α,18,21-triol-11, 20-dione (18OHTHA) |
| aldosterone, 4-pregnene-11β,21-diol-3,20-dione-18-al (ALDO) | tetrahydroaldosterone, 5β-pregnan-3α,11β,21-triol-20-one-18-al (THAldo) |
| Glucocorticoids and their precursors | |
| 11-deoxycortisol, 4-pregnen-17α,21-diol-3,20-dione (S) | tetrahydro-11-deoxycortisol, 5β-pregnan-3α,17α,21-triol-20-one (THS) |
| 21-deoxycortisol, 4-pregnene-11β,17α-diol-320-dione | pregnanetriolone, 5β-pregnan-3α,17α,20α-triol-11-one (PTONE) |
| cortisol, 4-pregnene-11β,17α,21-triol-320-dione (F) | 6β-hydroxycortisol, 4-pregnen-6β,11β,17α,21-tetrol-3,20-dione (6β-OHF) |
| cortisol, 4-pregnene-11β,17α,21-triol-3,20-dione (F) | |
| tetrahydrocortisol, 5β-pregnan-3α,11β,17α,21-tetrol-20-one (THF) 5α-tetrahydrocortisol, 5α-pregnan-3α,11β,17α,21-tetrol-20-one (5α-THF) α-cortol, 5β-pregnan-3α,11β,17α,20α,21-pentol β-cortol, 5β-pregnan-3α,11β,17α,20β,21-pentol 11β-hydroxyeticholanolone, 5β-androstan-3α,11β-ol-17-one (11β-OHEt) |
|
| cortisone, 4-pregnene-17α,21-diol-3,11,20-trione (E) | cortisone (E) |
| tetrahydrocortisone (THE) | |
| α-cortolone, 5β-pregnan-3α,17α,20α,21-tetrol-11-one | |
| β-cortolone, 5β-pregnan-3α,17α,20β,21-tetrol-11-one | |
| 11-ketoetiocholanolone, 5β-androstan-3α-ol-17,11-dione (11ketoEt) | |
| “Hybrid steroids” | |
| 18-hydroxycortisol, 4-pregnene-11β,17α,18,21-tetrol-3,20-dione (18OHF) | 18-hydroxycortisol, 4-pregnene-11β,17α,18,21-tetrol-3,20-dione (18OHF) |
| 18-oxo-cortisol, 4-pregnene-11β,17α,21-triol-3,20-dione-18-al (18oxoF) | 18-oxo-tetrahydrocortisol, 4-pregnene-11β,17α,21-triol-3,20-dione-18-al (18oxoTHF) |
| Androgen precursor metabolites | |
| dehydroepiandrosterone sulfate, 5-androsten-3β-sulfate-17-one (DHEAS) | dehydroepiandrosterone, 5-androsten-3β-ol-17-one (DHEA) |
| dehydroepiandrosterone, 5-androsten-3β-ol-17-one (DHEA) | dehydroepiandrosterone, 5-androsten-3β-ol-17-one (DHEA) |
| 16α-hydroxydehydroepiandrosterone (16α-DHEA) | |
| androstenedione, 4-androsten-3,17-dione (A4) | androsterone, 5α-androstan-3α-ol-17-one (An; AST) |
| etiocholanolone, 5β-androstan-3α-ol-17-one (Et) | |
| 11β-hydroxyandrostenedione, 4-androsten-11β-ol-3,17-dione (11OHA4; 11β-OHA4) | 11β-hydroxyandrosterone, 5α-androstan-3α,11β-diol-17-one (11β-OHAn; 11βOHAST)) |
| 17-hydroxyallopregnanolone, 5α-pregnane-3α,17α-diol-20-one (5α-17HP) | 17-hydroxyallopregnanolone, 5α-pregnan-3α,17α-diol-20-one (5α-17HP) |
| Androgen metabolites | |
| testosterone, 4-androsten-17β-ol-3-one (T) | androsterone, 5α-androstan-3α-ol-17-one (An; AST) |
| etiocholanolone, 5β-androstan-3α-ol-17-one (Et) | |
| 5α-dihydrotestosterone, 5α-androstan-17β-ol-3-one (DHT; 5α-DHT) | androsterone, 5α-androstan-3α-ol-17-one (An; AST) |
Although the liver undoubtedly makes the major contribution to steroid metabolism, most peripheral tissues also possess enzymatic machinery for aspects of both steroid activation and subsequent inactivation by phase 1 and 2 metabolism. This localized enzyme expression controls the local steroid milieu by precursor activation and inactivation according to tissue-specific needs, a mechanism known as intracellular pre-and post-receptor metabolism or “intracrinology” [59].
While phase 1 and phase 2 reactions are classically believed to be sequential, more recent studies have shown the metabolism of conjugated steroids by phase 1 enzymes [89]. Additionally, some steroids can directly undergo phase 2 metabolism without being subjected to a phase 1 reaction, e.g. testosterone can be directly conjugated at its 17β-hydroxy group and corticosteroids through 21-sulfation [90]. Despite these and other shortcomings [91], the traditional classification in phase 1 and 2 reactions remains helpful to structure the wide range of reactions and this classification is used below to guide the reader through the metabolism of endogenous steroids.
3.1. Phase 1 metabolism of steroids
3.1.1. Steroid A-ring reduction
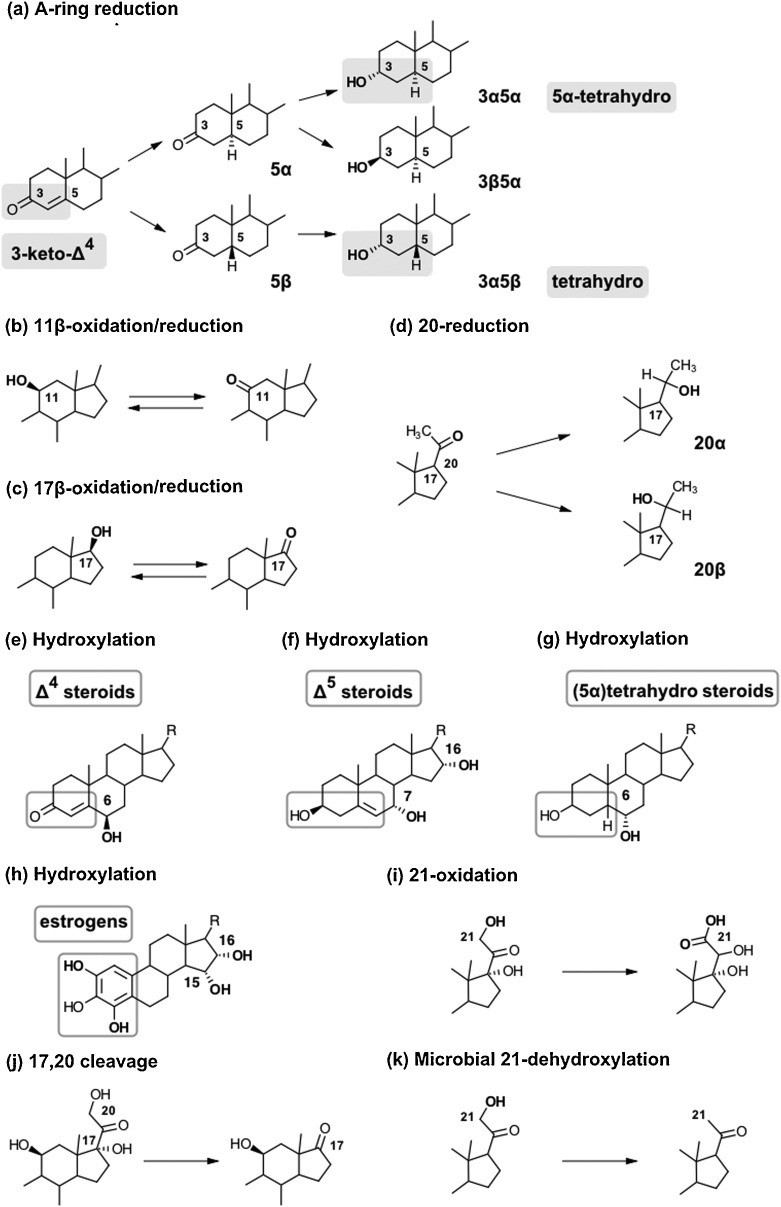
Reduction of the steroid A-ring 3-keto-Δ4 motif is an essential step for the inactivation of gluco- and mineralocorticoids and controls the peripheral activation and inactivation of androgens. A-ring reduction consists of two sequential reductions, namely the reduction of the Δ4-double bond followed by the reduction of the 3-keto group to a hydroxy group [92] (Fig. 4(a)). This leads to the production of a 3α/β-hydroxy-5α/βH motif common to the biologically inactive, excreted metabolites. Among these, the 5β/3α-metabolites are referred to as “tetrahydro”, while 5α/3α-metabolites are referred to as “5α-tetrahydro”. Importantly, 5α/β-reduction is irreversible, with the stereochemistry of this reduction playing an important role in regulating the biological activity of androgens (Section 4.3.2) [93].
Fig. 4.
Schematic overview of the major phase 1 reactions contributing to steroid metabolism. (a) A-ring reduction to (5α)tetrahydro metabolites. The formation of 3β,5β-tetrahydro metabolites is sterically unfavorable (not shown). (b) 11β-oxidation/reduction by HSD11B1 modulates the bioactivity of glucocorticoids, mineralocorticoids and 11-oxygenated androgens. (c) 17β-oxidation/reduction regulates the bioactivity of androgens and estrogens. (d) 20-reduction to a hydroxy group with α- or β-stereochemistry. (e–h) Hydroxylations: major positions are indicated for different structural steroid classes. (i) 21-oxidation leading to the formation of the so-called cortolic acids from cortisol. (j)17,20-cleavage: cortisol, cortisone and their metabolites can undergo metabolism by 17,20-lyase activity. (k) Microbial 21-dehydroxylation: steroids excreted with bile can undergo metabolism by the gut microbiome prior to reabsorption.
5α-Reduction is catalyzed by steroid 5α-reductase (SRD5A) enzymes of which there are three main isozymes. However, only two of these, SRD5A1 and SRD5A2, function as genuine steroid 5α-reductases. SRD5A1 is mainly expressed in the liver and peripheral tissues [94], while SRD5A2 is expressed mainly in male reproductive and genital tissues, with its disruption leading to disordered sex development in 46,XY individuals [95]. SRD5A3 appears to have only minor steroid 5α-reductase activity, but has been shown to play an important role in N-linked protein glycosylation [[96], [97], [98]]. In addition, two partially homologous SRD5A genes have been identified (SRD5A2L2 and GPSN2), but have been shown to be involved in the elongation of very long chain fatty acids [99]. Steroid 5β-reduction is catalyzed by the aldo-keto-reductase (AKR) family member AKR1D1, which is primarily expressed in the liver. It is the only human enzyme catalyzing the 5β-reduction of 3-keto-Δ4 steroids and bile acids [100]. AKR1D1 deficiency leads to severely reduced or abolished urinary 5β-reduced steroid excretion and hepatic failure [101].
Due to differential tissue expression patterns, with SRD5A isoforms being widely expressed in peripheral tissues including the liver and AKR1D1 expression being limited to the liver, 5α-reduced metabolites inform about global metabolism while 5β-reduced metabolites predominantly reflect hepatic reduction only. Moreover, SRD5As and AKR1D1 exhibit different catalytic efficiencies towards structurally different steroids [57,100], with the result that 5α- and 5β-reduced metabolites are produced with different ratios for different structural classes of steroids.
The second step of the A-ring reduction is the reduction of the 3-keto group to a hydroxy group. These reactions are catalyzed by members of the aldo-keto reductase family, namely AKR1C1, AKR1C2, AKR1C3 and AKR1C4. Of these, AKR1C4 is thought to be a liver-specific enzyme which works in concert with AKR1D1, yielding 5β,3α-metabolites. The other isozymes are expressed in different peripheral tissue in an tissue-specific manner [54].
While 5α-reduced steroids can be converted to both their 3α- or 3β-hydroxy epimers (with the 3α-reduction generally being more efficient), 5β-reduced steroids are predominantly converted to the 3α-hydroxy epimer as the 5β-reduced bent confirmation of the A/B-ring sterically does not allow binding in the AKR1C active site for 3β-reduction [102,103]. AKR1C2 is the major isoform for 3-reduction to the 3α-hydroxyepimer in peripheral tissue, while AKR1C1 is the most important isoform for the formation of the 3β-hydroxyepimer [102]. Moreover, AKR1C enzymes are multifunctional and also function as 20α- and 17β-HSDs, with different efficiencies, stereoselectivities and tissue specific expression [104]. For a comprehensive review of these enzymes see [54].
3.1.2. Hydroxysteroid dehydrogenation and reduction
The interconversion of hydroxy- and keto-groups (Fig. 4(b–d)) at positions 11, 17 and 20, greatly contribute to the regulation of steroid activity via their receptors. These reactions are catalyzed by members of the short-chain dehydrogenase/reductase (SDR) superfamily and the AKR superfamily using NAD(P)+/H and are typically reversible. While most of these enzymes are bidirectional in vitro, in vivo directionality is dictated by co-factor affinity, cellular redox status and pH [54,[105], [106], [107]].
3.1.2.1. 11β-hydroxysteroid dehydrogenases
Two isoforms of HSD11B play a key role in regulating glucocorticoid inactivation and reactivation by catalyzing the interconversion of 11β-hydroxy- and 11-ketosteroids (Fig. 4(b)). Thereby, they modulate systemic and tissue-specific glucocorticoid action [74,108]. Additionally, they are involved in the regulation of mineralocorticoid and 11-oxygenated androgen activity.
HSD11B1 is a bidirectional enzyme, but primarily catalyzes the reduction of 11-ketosteroids in vivo as colocalized hexose-6-phosphate dehydrogenase (H6PDH) regenerates NADPH required for its cortisone reductase activity, mainly activating cortisone to cortisol [109]. Conversely, HSD11B2 functions exclusively as an oxidative enzyme, inactivating cortisol to cortisone [108]. Both isoforms are involved in the metabolism of glucocorticoids and 11-oxygenated androgens as described in Sections 4.2.1 and 4.3.2. Moreover, both HSD11B isoforms also act on 7-oxygenated C19 steroids whereby HSD11B1 functions as an epimerase interconverting the 7α- and 7β-hydroxy-stereoisomers via a 7-keto-intermediate, while HSD11B2 only oxidizes the 7α-hydroxy-stereoisomer [110,111].
3.1.2.2. 17β-hydroxysteroid dehydrogenases
Enzymes from the SDR and AKR superfamilies regulate the activity of androgens and estrogens by catalyzing the interconversion of bioactive 17β-hydroxy- and inactive keto- containing forms (Fig. 4(c)). Excreted metabolites are therefore predominantly in the keto-form [112]. To date, 14 human enzymes with 17β-HSD/oxoreductase activities have been identified [105,106,113]. Generally, these enzymes are multi-functional and often have overlapping substrate specificities and expression patterns, allowing for redundant enzymes to cover in case of deficiency of another enzyme [107,114]. However, certain enzymes have been identified as major catalysts for specific reactions in androgen and estrogen metabolism as described in Sections 4.3.2 and 4.4.1.
3.1.2.3. 20-reduction
Glucocorticoids and progesterone can be modified by 20-reduction with α- or β-stereochemistry prior to excretion (Fig. 4(d)). Of note, the direct 20α-reduction of progesterone terminates its progestogenic activity and is predominantly catalyzed by AKR1C1 [104,115]. 20-reduction of glucocorticoids is primarily observed for downstream tetrahydrometabolites as described in Section 4.2.2.
3.1.3. Cytochrome P450-catalyzed steroid oxidations
In addition to the steroidogenic CYP enzymes described in Section 2.2, hepatic xenobiotic-metabolizing members of the CYP superfamily are able to modify steroid hormones and generate a plethora of minor steroid metabolites [116]. The reaction repertoire of these enzymes for steroids includes hydroxylation reactions, further oxidations, and C—C bond cleavages [9,10]. Hepatic CYPs are promiscuous enzymes accepting a wide range of substrates with low stereo- and regioselectivity compared to their steroidogenic counterparts. Therefore, several CYPs can contribute to the same reaction and the high variation of their expression levels can make it difficult to assess the enzyme(s) making the dominant contribution to a specific reaction, with at least 17 hepatic CYPs potentially participating in the metabolism of steroids [[116], [117], [118]].
A high inter- and intra-individual variability of hepatic CYP activity results from the strong potential for induction by pharmacological and natural compounds, the high frequency and number of polymorphisms, and promoter and copy number variants [119,120]. In addition, differential expression profiles of functionally different isoforms of the CYP3A subfamily during prenatal and early postnatal life complicate the assessment of hepatic steroid metabolism. CYP3A4 is the most abundant CYP expressed in the adult liver [121,122] and makes the major contribution to steroid metabolism. CYP3A5 catalyzes a comparable range of reactions as CYP3A4 but its role in drug and steroid metabolism is limited due to its generally low activity and expression in a relatively small percentage of individuals [[123], [124], [125]]. CYP3A7 is the major CYP3A isoform in prenatal and early postnatal life and differs from CYP3A4 in terms of expression and function. CYPs also contribute to steroid metabolism in several extra-hepatic tissues, including the brain, breast and prostate [[126], [127], [128], [129]].
3.1.3.1. Steroid hydroxylations
The hydroxylation by hepatic CYPs inactivates the steroids and increases their polarity and water solubility. In some cases, the additional hydroxy groups also serve as sites for conjugation by phase 2 metabolism. CYP3A4-catalyzed 6β-hydroxylation is the most common hydroxylation for Δ4 steroids, e.g. cortisol (Fig. 4(e)), while 6α-hydroxylated pregnanolones are quantitatively important urine steroid metabolites during pregnancy. Tetrahydro and hexahydro C21 steroids (e.g. THE and the cortolones) hydroxylated at 1β- and 6α- are quantitatively important during the perinatal period [130]. The enzymes responsible for these hydroxylations is uncertain. The differential substrate specificity, regioselectivity and catalytic activity of CYP3A4 and CYP3A7 and the dynamic expression pattern of the two isoforms throughout fetal development and the first year of life lead to substantial changes in the hepatic steroid metabolome during this period of life. CYP3A7 is highly expressed in fetal liver and up to 6 months postnatal but expression levels gradually decrease over this time. CYP3A4 levels are low in the fetus and newborn compared to the adult. Thus, there is a switch from CYP3A7 to CYP3A4 during the first months after birth. Additionally, the total liver CYP3A content is significantly higher prenatally followed by a reduction after birth reaching plateau at 6 months [131,132].
In terms of Δ5 steroids, e.g. DHEA, 16α-hydroxylation is the most frequent hydroxylation detected in adults followed by 7α-hydroxylation (Fig. 4(f)), while 16β-, 21-, 18- and 15β-hydroxylation are also observed in neonates [[133], [134], [135], [136]].
Interestingly, hepatic CYPs can also perform some reactions that are classically catalyzed by steroidogenic CYPs (11β-, 17α- and 21-hydroxylation) [[137], [138], [139]]. In fact, CYP2C19 and CYP3A4 can 21-hydroxylate progesterone and pregnenolone, possibly partially compensating mineralocorticoid deficiency in CAH due to 21-hydroxylase deficiency; however, the two enzymes are not capable of catalyzing the 21-hydroxylation of 17OHP to 11-deoxycortisol [140].
Estrogens can be hydroxylated in various different positions by a number of CYPs [141]. The formation of catecholestrogens by 2- or 4-hydroxyation are the dominant reactions (Fig. 4(h)). However, during pregnancy, estriol, which has a 16α-hydroxygroup, is the main metabolite of fetal DHEA. Estriol predominately originates from the aromatization of 16α-hydroxy C19 steroids by the placenta (Section 2.6.2).
3.1.3.2. Additional steroid oxidations
CYPs can further oxidize hydroxy groups to their respective keto, aldehyde and carboxylic acids. For example, 6-keto metabolites can be produced from their hydroxy precursors [141]. 21-carboxylic acid formation from 21-hydroxysteroids is also possible (Fig. 4(i)) [[142], [143], [144], [145], [146]].
3.1.3.3. Could a empty line be added before these subheadings as the heading is difficult to distinguish in the pdf C—C bond cleavages
CYPs can also catalyze oxidative C—C bond cleavages in multi-step reactions [10]. Examples for such reactions from steroid biosynthesis are the side-chain cleavage of cholesterol catalyzed by CYP11A1 and the 17,20-lyase activity of CYP17A1 producing C19 steroids from C21 substrates (Section 2.5.3) [113]. Hepatic CYPs may employ similar mechanisms to catalyze the 17–20 cleavage of 17α,21-dihydroxypregnanes (Fig. 4(j)).
3.1.3.4. Could a empty line be added before these subheadings as the heading is difficult to distinguish in the pdf Contributions of the gut microbiome
Metabolism by the gut microbiome is relevant for C17-deoxy corticosteroids, e.g. mineralocorticoids and their precursors, which have a high biliary excretion [147]. The resulting metabolites can be reabsorbed into the portal system and undergo further metabolism in the liver and kidney before being excreted with the urine. Reactions catalyzed by different strains of gut bacteria include (1) A-ring reduction, (2) reduction of the Δ5-double bond, (3) reduction of 17-keto estrogens and 17-keto androstenes, (4) 17,20-cleavage of 17α-hydroxysteroids, and (5) 16α- and 21-dehydroxylation (Fig. 4(k)) [[148], [149], [150], [151], [152], [153], [154], [155], [156]]. Additionally, reductive 20α/β-HSDs are active in gut bacteria [151]. Unsurprisingly, steroid metabolism by gut bacteria has been shown to be influenced by the administration of antibiotics [157].
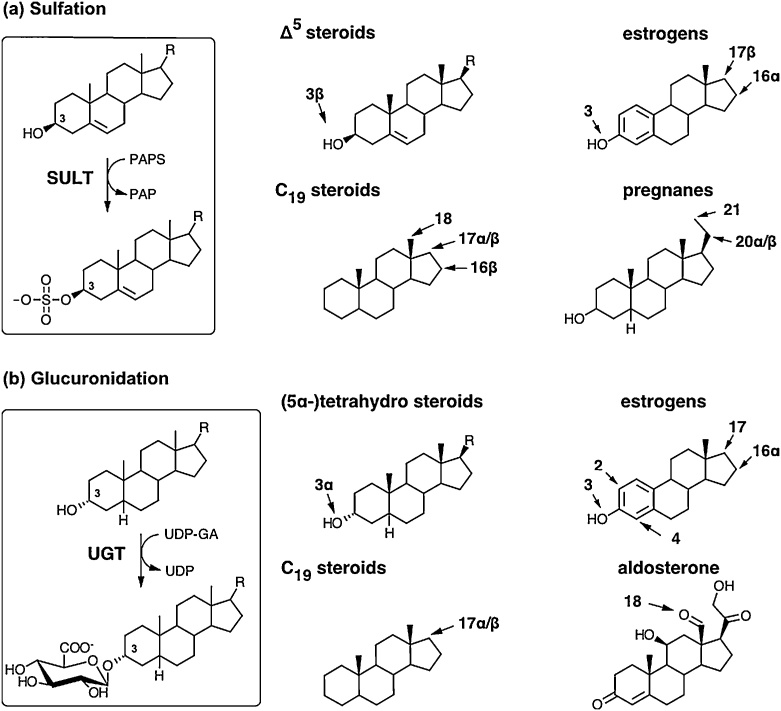
3.2. Phase 2 metabolism of steroids
The classic phase 2 conjugation reactions – sulfation and glucuronidation – increase the polarity and water solubility of the steroids and thereby facilitate their excretion and concentration in the urine. Mechanistically, these conjugation reactions proceed via two subsequent, enzymatically catalyzed reactions: (1) the activation of the moiety to be attached and (2) the transfer of the moiety from the activated donor onto a hydroxy group of the steroid. While the conjugated product is generally considered to be biologically inactive, rare exceptions have been identified [91]. Importantly, steroid sulfation is reversible and sulfated steroids can be hydrolyzed to free steroids by steroid sulfatase (STS), while glucuronidation is irreversible in humans, with the exception of the activity of some gut bacteria (Section 3.1.3.4). Notably, bis-conjugation with the same (e.g. bis-sulfation) [[158], [159], [160], [161], [162]] or two different groups (e.g. sulfate and glucuronic acid) are possible [163]. Other conjugation reactions include the methylation of catechol estrogens, conjugation with cysteine or glutathione, and esterification with fatty acids as outlined below.
3.2.1. Steroid sulfation and desulfation
Sulfation and desulfation play an essential role in mediating the activity of selected steroids, specifically Δ5 steroids and estrogens, as has been comprehensively reviewed recently [40]. Sulfation is the result of two consecutive enzymatic reactions. Firstly, the inert sulfate anion is activated by conversion to the universal sulfate donor 3′-phospho-adenosine-5′-phosphosulfate (PAPS), which is catalyzed by two human PAPS synthase isoforms, PAPSS1 and PAPSS2 [164]. Secondly, the sulfate moiety is transferred onto hydroxy or amino groups by sulfotransferases (SULTs) whereby stereochemistry is retained (Fig. 5(a)). Sulfation is of particular relevance for Δ5 steroids which are almost exclusively excreted as their sulfates. Five cytoplasmic SULTs are involved in the sulfation of steroids in humans: SULT1A1, SULT2E1, SULT2A1 and two isoforms of SULT2B1 (SULT2B1a and SULT2B1b) [40,165]. These SULTs have overlapping substrate spectra, but the major enzymes responsible for the sulfation of selected steroids have been identified and are presented in Section 4 below.
Fig. 5.
Schematic overview of the major phase 2 reactions contributing to steroid metabolism – sulfation (a) and glucuronidation (b). Important target positions of steroid conjugation are indicated, with stereochemistry for the different structural classes of steroids.
Notably, although the contribution of bis-sulfates to the steroid metabolomes of urine, blood and bile was first established in the 1960s [159,160,[166], [167], [168]], interest in these species has only recently re-emerged with the development of new methodological approaches [161,169].
Importantly, sulfation is reversible and unconjugated bioactive steroids can be regenerated from their sulfates by STS, which is ubiquitously expressed in all tissues [40]. STS activity is upregulated in several steroid-dependent cancers [40] and has been evaluated as drug target [170,171]. The 17α- and 20α-sulfates of steroid bis-sulfates are not substrates for STS [172].
3.2.2. Steroid glucuronidation
Glucuronidation makes a substantial contribution to the phase 2 metabolism of Δ4 steroids. UDP-glucuronic acid is the activated donor molecule for glucuronidation. Subsequently, the glucuronic acid is coupled with a steroid hydroxy group leading to the formation of the steroid β-D-glucuronide, whereby the stereochemistry of the steroid in the respective position is preserved (Fig. 5(b)) [173,174]. These reactions are catalyzed by enzymes of the UGT-glucuronosyltransferase superfamily (UGTs), with the UGT1A and UGT2B subfamily catalyzing the glucuronidation of steroids [175]. These UGTs are expressed in the liver, as well as in a range of extrahepatic tissues [176,177]. A-ring reduced steroid metabolites are predominantly excreted as 3-glucuronides. Notably, the formation of linked di-glucuronides and bis-glucuronides is also possible [178].
3.2.3. Methylation of catecholestrogens
The O-methylation of catechols plays an important role in the phase 2 metabolism of estrogens (Section 4.4.1) and is catalyzed by the enzyme catechol-O-methyltransferase (COMT) [179]: COMT methylates 2- and 4-hydroxyestrogens, thereby producing the so-called methoxyestrogens [180]. The donor molecule for the methyltransfer is S-adenosylmethionine, which is synthesized from methionine and ATP. COMT primarily methylates the 2 or 4 position of the catechol substrate [181,182]. The highest levels of COMT are found in the liver, brain, kidney, adrenal and lungs [183]. While methylation plays an important role in inactivating catecholestrogens, this phase 2 conjugation reduces water solubility as opposed to the classic phase 2 reactions described above [91].
3.2.4. Steroid thioether formation
Cysteine conjugates of androgens, cortisol and progesterone have recently been detected in human urine and plasma [[184], [185], [186]]. The authors proposed a metabolic pathway starting with a dehydrogenation of the steroid in the liver [187], followed by glutathione S-conjugation of the steroid and subsequent extracellular degradation of the glutathione moiety leading to a cysteine conjugate which is excreted.
3.2.5. Fatty acid esterification of steroids
Although the physiological relevance has yet to be determined, fatty acid esterification of pregnenolone, DHEA and androstenediol has been described [188,189]. Plasma lecithin:cholesterol acyltransferase located on high-density lipoproteins can acylate steroids using acyl-CoA as donor [190]. The steroid fatty acid ester can then be transferred to other lipoproteins and be taken up by peripheral cells via lipoprotein receptors [191,192]. Additionally, steroids can be esterified with fatty acids in peripheral tissues [193].
3.3. Steroid excretion
Steroids are excreted predominantly as their conjugates in the urine and bile, with urine excretion accounting for approximately 80% of excretion following exogenous administration [194]. The clearance of steroid glucuronides generally proceeds faster than the clearance of steroid sulfates [195], presumably due to the irreversible nature of glucuronidation in humans.
3.3.1. Urinary steroid excretion
In the kidney, steroid conjugates are transported from the blood filtrate over the epithelium into the lumen of the nephron. Cellular uptake is mediated by organic anion symport or exchange. The efflux from the cell into the lumen is carrier-mediated and makes use of an electrochemical gradient [196]. The active nature of transport across the epithelial cells allows for the concentration of steroid conjugates in the lumen. Urinary excretion of unconjugated steroids is low, accounting for only 5–10% of the total urine steroid pool. The urine metabolomes of all classes of steroids will be discussed in detail in Section 4. Interestingly, enzymes with 20β-HSD and 17,20-lyase activity have been recently identified in a microbial inhabitant of the urinary tract [197].
3.3.2. Biliary excretion of steroids
Steroids passing the canalicular membrane of the hepatocyte are excreted in the bile. The rate of biliary excretion determines the quantitative contribution of the gut microbiome to the metabolism of the respective steroid. Excretion with the feces is low as steroids are reabsorbed in the gut [198]. 17-deoxysteroids (mineralocorticoids and precursors) have high biliary excretion as opposed to 17-hydroxy C21 steroids like cortisol [147]. Steroid conjugates represent the major fraction of biliary excreted steroids [147,[199], [200], [201], [202]] and the dominance of bis-sulfates [168] led to the hypothesis that bis-sulfates originating from the liver are preferably excreted with the bile, while glucuronides preferably undergo renal excretion [200]. Biliary excretion is increased during pregnancy [201,203,204] and an equal quantitative contribution of urinary and fecal excretion has been suggested during the newborn period [204]. The steroid metabolome in the feces comprises unconjugated, mono- and bis-sulfated steroids [203] and up to 90% of steroids in feces during pregnancy are unconjugated [205]. Notably, estrogens seem to undergo higher biliary excretion than other steroids, but also higher reabsorption leading to low fecal excretion [194,206]. Gut microbiota have hydrolase activity for steroid conjugates and glucuronides in particular [148], leading to the high proportion of unconjugated steroids compared to bile, and conjugate hydrolysis might be a prerequisite for reabsorption [207].
3.3.3. Salivary steroids
Steroids are also excreted in saliva. Indeed, the measurement of salivary steroids is becoming an emerging tool for the diagnosis and treatment monitoring of steroidogenic disorders due to the ease of saliva collection [208]. Unconjugated steroids passively diffuse over the membranes of the acinar cells in the salivary glands independent of salivary flow rate [209]. Their levels in saliva therefore provide a measure of their free concentrations in serum [210]. However, steroids can be subject to metabolism while crossing the acinar cells, which affects their levels in saliva. For example, the presence of HSD11B2 in the parotid gland makes salivary cortisone a useful marker for serum free cortisol and adrenal stimulation [[211], [212], [213]]. Conjugated steroids enter saliva by ultrafiltration through the extracellular space between the acinar cells and their salivary levels are highly flow rate dependent as has been shown for DHEAS [209].
4. Serum and urine steroid metabolomes
4.1. The mineralocorticoid metabolome
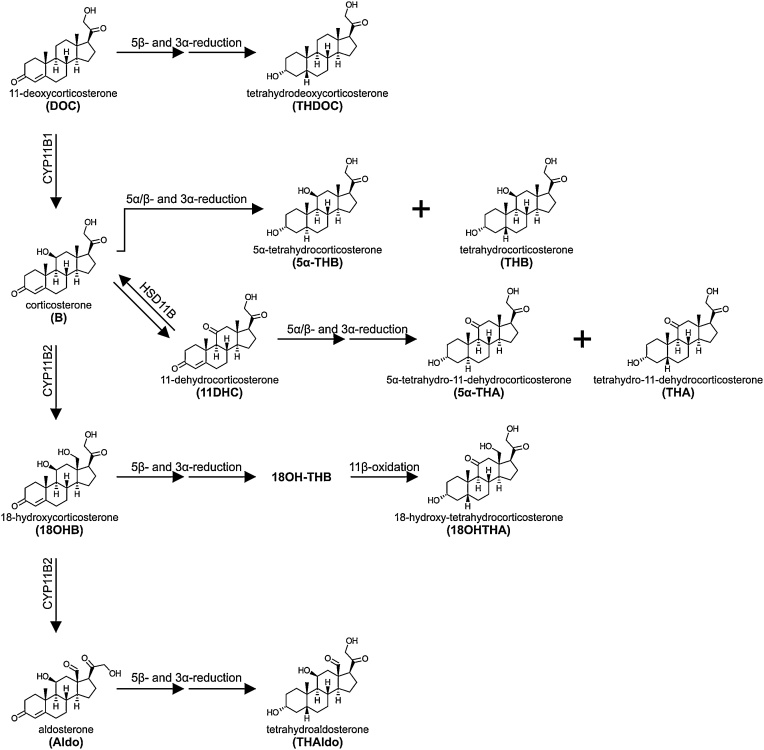
The primary mineralocorticoid in circulation is aldosterone. It is estimated that approximately one third of aldosterone circulates in the free form, with the remainder bound to corticosteroid binding globulin (CBG) and serum albumin [214]. Serum mineralocorticoid assays primarily focus on the measurement of aldosterone only, although the precursors DOC, corticosterone and 18-hydroxycorticosterone are also detectable in serum (Table 2) [215].
Aldosterone, as well as its immediate precursors, contain a Δ4 moiety. Metabolism of these steroids is therefore primarily by sequential 5α/β- and 3α-reductions in the liver (Fig. 6). DOC and aldosterone are both preferentially 5β-reduced by AKR1D1 followed by 3α-reduction yielding the tetrahydro metabolites, tetrahydrodeoxycorticosterone (THDOC) and tetrahydroaldosterone (THAldo), respectively. Similarly, 18-hydroxycorticosterone is converted to the tetrahydro metabolite, 18-hydroxy-tetrahydro-11-dehydrocorticosterone (18OHTHA), but this requires the additional conversion of the 11β-hydroxy group to an 11-keto group, a reaction catalyzed by HSD11B2 [[216], [217], [218]]. Corticosterone can be converted to 11-dehydrocorticosterone by the action of HSD11B2 in the kidney, which prevents the activation of the mineralocorticoid receptor (MR) by corticosterone though corticosterone’s MR-activating potency is considerably lower than that of aldosterone [219]. Unlike the other mineralocorticoid precursors, both corticosterone and 11-dehydrocorticosterone can either be 5α- or 5β-reduced, prior to 3α-reduction leading to a tetrahydro and a 5α-tetrahydro metabolite for each. These are tetrahydro-11-dehydrocorticosterone (THA) and 5α-tetrahydro-11-dehydrocorticosterone (5α-THA) for 11-dehydrocorticosterone, and tetrahydrocorticosterone (THB) and 5α-tetrahydrocorticosterone (5α-THB) for corticosterone, the latter being dominant (Fig. 6). Importantly, during neonatal life, THA, 5α-THA and the polar metabolite 6α-hydroxy-THA are the more relevant corticosterone metabolites (Fig. 4(g)) [130]. As with most steroid metabolites, the majority of these are subsequently glucuronidated in the liver and excreted in urine [[220], [221], [222]]. Aldosterone is preferentially 18-glucuronidated in the liver by UGT2B7 and UGT1A10 [223]. UGT2B7 also efficiently conjugates 5α-dihydroaldosterone and THAldo, while UGT2B4 glucuronidates only THAldo [224]. It should, however, be noted that aldosterone is also glucuronidated directly to aldosterone-18β-glucuronide within the kidney, which has been proposed to be catalyzed by UGT2B7 [225]. Indeed, it has been estimated that aldosterone-18β-glucuronide and THAldo-glucuronides contribute 5–15% and 15–40% towards the daily urinary excretion of aldosterone [221,226]. It has also been found that THAldo-glucuronides are consistently five-fold more prevalent than aldosterone-18β-glucuronide irrespective of sodium intake [221]. Finally, it should be noted that enzymes expressed by anaerobic bacteria in the human gut have been shown to be able to convert aldosterone to THAldo, 3β,5α-THAldo, 3α,5α-THAldo as well as 20β-dihydroaldosterone in a species-specific manner [150]. Biliary excretion and metabolism by the gut microbiome is also relevant for the 17-deoxy mineralocorticoid precursors and their metabolites as described in Section 3.1.3.4 [227].
Fig. 6.
Schematic overview of the pathways linking mineralocorticoids and their precursors to their urine metabolites. The pathway of mineralocorticoid biosynthesis is indicated on the left. The metabolism of each steroid is shown from left to right and the structures of the major urine products are shown. Phase 2 conjugation reactions are not indicated in the figure.
4.2. The glucocorticoid metabolome
4.2.1. Cortisol and cortisone interconversion
The primary active glucocorticoid in circulation is cortisol, which is produced by the adrenal cortex (Section 2.5.2). Inactivation of cortisol to cortisone, which cannot activate the glucocorticoid receptor or the MR, subsequently occurs in peripheral mineralocorticoid target tissue, such as the kidney, the colon and the salivary glands all of which express HSD11B2 [74]. HSD11B2 converts cortisol to cortisone to protect the MR from activation by cortisol, thus allowing the dedicated MR agonist aldosterone to bind [[228], [229], [230], [231]]. The placenta is another important tissue expressing HSD11B2 as to inactivate maternal cortisol, thereby limiting fetal exposure [74].
Cortisone is in-turn reactivated to cortisol by the action of HSD11B1 expressed predominantly in the liver, as well as some peripheral tissues such as adipose tissue, muscle, skin and bone [74,[232], [233], [234], [235]]. Although the ratio of cortisol to cortisone remains relatively constant in circulation, studies with radiolabeled tracers have shown that there is constant interconversion of cortisol and cortisone [236]. Tissue-specific expression of HSD11B1 allows for local intracellular cortisol reactivation independently of circulating cortisol levels [74]. Interestingly, HSD11B1 expression is low/undetectable at birth, but thereafter increases rapidly, with adult levels reached after 6–12 months [237]. As a result, cortisone and the resulting 11-keto-metabolites (e.g. tetrahydrocortisone) are substantially increased during the neonatal period [74].
While HSD11B1 can function bi-directionally in vitro, it acts predominantly as a reductase in vivo due to localized co-expression on the ER membrane with H6PDH, which produces NADPH that drives the reductase activity of HSD11B1. A deficiency of H6PDH therefore leads to an impairment of HSD11B1 reductive function and apparent cortisone reductase deficiency [238].
A vital aspect to consider when measuring cortisol and cortisone is their diurnal secretion rhythm [239]. This follows a distinct pattern with nadir concentrations around midnight and the highest levels observed between 3 and 5 a.m., although the exact timings can show inter-individual variability [[240], [241], [242]]. Any healthy individual’s serum cortisol concentration will be significantly higher in the morning than at midnight. This rhythm can be lost in times of severe illness or stress, with significant increases in circulating cortisol throughout the entire 24-h period observed in patients with sepsis and during surgical procedures [243,244]. To account for the diurnal rhythm of glucocorticoids, when assessing serum glucocorticoid concentrations, comparator samples should be drawn at the same time of day. With urine collections, the time of collection is similarly important – a one-off spot urine collected in the morning will differ greatly from a spot urine collected in the afternoon, a problem that can be overcome by 24-h urine collections, which provide output data for the entire 24-h period independent of diurnal variation.
The majority of cortisol circulates bound to proteins, with 80–90% bound to CBG. CBG also binds other steroids such as cortisone, 17OHP, progesterone, DOC, corticosterone, and, to a lesser degree, aldosterone, testosterone and 17β-estradiol. The remaining cortisol is either bound to albumin (5–10%) or circulates in its free (active) form (<10%) [[245], [246], [247]].
The serum levels of the glucocorticoid precursors, progesterone, 17OHP and 11-deoxycortisol are all ≤10nM in the healthy population and therefore significantly lower than that of cortisol (ranging from 100 to 600 nM) and cortisone (ranging from 30 to 100 nM) (Table 2). The concentrations of cortisol, cortisone and 11-deoxycortisol are similar in men and women, though women who have increased estrogenlevels due to oral or transdermal contraceptives or pregnancy, have increased total serum cortisol due to an increase in CBG (and in pregnancy, also an increase in total cortisol production from the 22nd week of gestation onwards) [[248], [249], [250]].
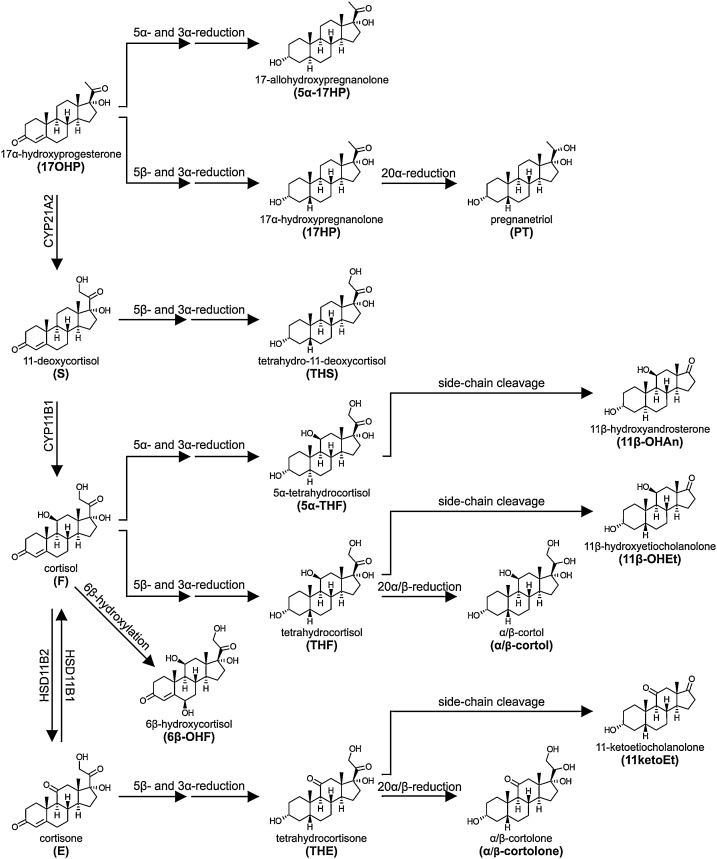
4.2.2. Downstream glucocorticoid metabolism
As with all steroids containing the Δ4 steroid moiety, the dominant first steps in the metabolism of glucocorticoids is the 5α/5β-reduction in the liver (Section 3.1.1). Interestingly, while 17OHP and cortisol can be either 5α- or 5β-reduced, 11-deoxycortisol and cortisone are predominantly 5β-reduced [108]. All products are subsequently 3α-reduced yielding the respective tetrahydro or 5α-tetrahydro metabolites (Fig. 7), which are detectable in urine. A number of the tetrahydro metabolites can also be further metabolized by 20α- or 20β-HSDs [251,252]. Of quantitative importance is the 20α/β-reduction of tetrahydrocortisol (THF), yielding the so called cortols (α- and β-cortol), and that of tetrahydrocortisone (THE), which yields the equivalent cortolones (α- and β-cortolone) [253]. While all four members of the human AKR1C enzyme subfamily can catalyze the reduction to the 20α-hydroxy group, AKR1C1 is the predominant 20-ketosteroid reductase in human [104]. Although a 20β-HSD reducing cortisone in zebrafish has been characterized as a member of the SDR family [254] and carbonyl reductase 1 has been described a relevant 20β-HSD for cortisol in humans [255], the human enzyme(s) responsible for the formation of the 20β-hydroxy isomers of the cortols and cortolones has not yet been identified. Cortisone and cortisol reduced at 20α- and 20β- while retaining the Δ4 moiety are also excreted in significant amounts, i.e. 20α(andβ)-dihydrocortisone and 20α-(andβ)-dihydrocortisol [256].
Fig. 7.
Schematic overview of the pathways linking glucocorticoids and their precursors to their urine metabolites. The glucocorticoid biosynthetic pathway is shown on the left. The metabolism of each steroid is shown from left to right and the structures of the major urine products are shown. Phase 2 conjugation reactions are not indicated in the figure.
THF and THE can also be subject to an elusive side-chain cleavage reaction not catalyzed by CYP17A1, producing C19 metabolites of glucocorticoid origin. Thereby, glucocorticoids contribute predominantly to urinary excretion of 11β-hydroxyetiocholanolone and 11-ketoetiocholanolone. While 5α-THF is also a substrate for this side-chain cleavage reaction, this reaction is catalyzed less efficiently [257]. As a result, the glucocorticoid contribution to urine 11β-OHAn is very low in healthy individuals, with the majority originating from the metabolism of the androgen 11OHA4 (Section 4.3.2) [258].
Cortisol can also be 6β-hydroxylated by CYP3A4 expressed in the liver, resulting in 6β-hydroxycortisol (6β-OHF) [259,260]. Orally administered hydrocortisone results in relatively increased circulating 6β-OHF, in comparison to the other glucocorticoid metabolites, due to the hepatic first pass effect after the oral ingestion. 18-Hydroxycortisol is also a product formed in zona fasciculata of the adrenal, with the minor downstream product 18-oxocortisol also being produced. These metabolites are often referred to as “hybrid steroids” as they require enzymatic machinery from both the glucocorticoid and mineralocorticoid pathways [[261], [262], [263]]. They are particularly important in patients in glucocorticoid remediable aldosteronism or with aldosterone-producing adenomas associated with KCNJ5 mutations [261,[264], [265], [266], [267], [268], [269], [270], [271], [272]]. Hydroxylation of the tetra- and hexahydro-metabolites of cortisol (e.g., THE and the cortolones) at 1β- and 6α-carbon position is quantitatively important in the neonatal period. [130]. The glucocorticoid precursor 17OHP can be converted to 17α-hydroxypregnanolone (17HP) via 5β-reductase and 3αHSD activities. The subsequent 20α-reduction of 17HP yields the metabolite pregnanetriol (PT, 5β-pregnane-3α,17α,20α-triol).
The majority (>90%) of the glucocorticoid metabolites described above are glucuronidated in the liver prior to urinary excretion as mono-glucuronides with glycosidic bonds added at positions 3 or 21. UGT2B7 has been shown to efficiently catalyze the conjugation of glucocorticoids [224]. Metabolites retaining the Δ4 moiety are excreted to a greater degree unconjugated. Two studies report unconjugated excretion of the following individual steroids: cortisol (30%), cortisone, 20αDHE, 20βDHE, 20βDHF (40–60%); 20αDHF, 6β-OH-cortisol, 6β-OH-E and 18-OH-cortisol (80–100%) [273,274]. Urine free cortisol, i.e. the free fraction of total, non-metabolized urine cortisol, is commonly measured in clinical chemistry laboratories for the diagnosis of Cushing’s syndrome [275] whereas gas chromatography-mass spectrometry (GC-MS) measures total urine cortisol following deconjugation.
4.3. The androgen metabolome
4.3.1. Androgens in circulation
Androgens and their precursors are derived from both the adrenal cortex and the gonads as described in Section 2.5.3 above. It is important to note the that circulating androgen metabolome consist of both active androgens and androgen precursors, with both of these contributing to androgen action in target tissues [45]. Downstream metabolites can also be measured in circulation [60]. The best-known circulating androgen in both men and women of reproductive age is testosterone (Table 2). Circulating testosterone concentrations in men are approximately 10-fold higher than those of women, due to the dedicated biosynthesis in the testes, together with a very minor contribution from the adrenals (Section 2.5.3.1). Conversely, female androgens are equally derived from the adrenal glands and the ovaries (Section 2.5.3.1), which are each estimated to contribute 25% towards the circulating levels of testosterone in both pre- and postmenopausal women. The remaining 50% originates from the peripheral conversion of androgen precursors such as A4 to testosterone [[276], [277], [278]]. Androgen precursors in circulation include DHEA, its sulfate ester DHEAS, A4, 11OHA4, androstenediol and androstenediol sulfate. In fact, the circulating levels of DHEAS dwarf those of any other steroid in circulation (Table 2) and DHEAS is the only human steroid that circulates in micromolar concentrations. However, it is primarily thought to serve as an inactive waste product of adrenal steroidogenesis, produced to prevent an excessive androgen load [39,40,279]. The production of adrenal androgen precursors increases at adrenarche at 6–9 years of age, peak between 20 to 30 years of age, and subsequently decline gradually with age (Section 2.5.3.1). Gonadal androgen production is initiated for a short period of time during minipuberty in infancy, but then remains dormant until puberty. Following full initiation at puberty gonadal androgen production decreases significantly after menopause in women, while in men the testicular output of testosterone gradually decreases with age, resulting in significantly lower combined androgen levels in men aged 60 and over [24,[280], [281], [282]]. In women, testosterone and A4 demonstrate cyclic changes in concentration during the course of the menstrual cycle due to the ovarian contribution, with levels peaking mid-cycle [283,284].
Within circulation, most active sex steroids are bound to the plasma proteins sex hormone binding globulin (SHBG) or albumin and only a small fraction (1–2%) circulates unbound, which is the only form in which testosterone is accessible to the target tissues. Sex steroid-binding plasma proteins, therefore, play a key role in the regulation of androgen action. SHBG binds sex steroids (including active androgens and estrogens) with high affinity (nanomolar ranges) and specificity [[285], [286], [287]]. Although albumin binds all unconjugated steroids with low affinities (micromolar ranges), it makes a significant contribution to steroid binding due to its high abundance [45,287]. While SHBG binds the active androgens DHT and testosterone with high affinity, the affinity of SHBG for androgen precursors such as DHEA is substantially lower. Moreover, the conjugated precursor, DHEAS, circulates only in its free form.
While the levels of 11-oxygenated androgens and their precursors have been shown to be significantly elevated in patients with polycystic ovary syndrome (PCOS) and 21-hydroxylase deficiency [41,69], one study reported that the circulating levels of 11KT were also higher than that of testosterone in a healthy female control group (BMI 21.2–26.1 kg/m2) [41]. This and other recent findings have led some to suggest that 11KT may in fact be the most physiologically relevant androgen in women, though more work is needed to investigate this [288]. However, although multiple studies have measured the circulating concentrations of 11-oxygenated androgens in healthy control groups, there are significant variations in the levels reported and as such, no reference ranges have been established to date [39,41,68,69,289]. Nonetheless, it is clear that 11OHA4, the major 11-oxygenated androgen precursor produced by the adrenal, circulates at higher levels than A4. 11KA4 is the next most abundant 11-oxygenated androgen precursor in circulation (Table 2) [39,41,68,69,289]. Significantly, a recent study has revealed that unlike the classical androgens, the circulating levels of 11-oxygenated androgens do not decrease with age in women, suggested to be due to the involution of the zona reticularis with age and the appearance of areas co-expressing HSD3B2 and CYB5A [289].
4.3.2. Downstream androgen metabolism
The contributions of androgen precursors of adrenal and gonadal origin are often overlooked when considering the total androgen pool. The primary reason for this is that, while androgen precursors are activated in peripheral target tissues, this is often, but not always, followed by subsequent inactivation within the same tissue, thus with the result that much of the active androgen is never accounted for in circulation (Section 2.5.3.2). It is therefore important to consider both androgen precursors and metabolites when accessing androgen action. While androgen precursors and active androgens can be measured in serum, it is often more convenient to measure their metabolites in urine (Table 3).
Table 3.
Graphical representation of the targeted urine steroid metabolome. Major urine steroids are shown divided into six concentration ranges illustrating their relative contribution to total 24 h urine steroid metabolite excretion. Divisions are based on respective median values as urine metabolites demonstrate substantial variation between individuals and the 25-75th percentiles may overlap groups.
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
| 1000-3000 μg/24 h | 700-1000 μg/24 h | 400-700 μg/24 h | 150-400 μg/24 h | 20-150 μg/24 h | <20 μg/24 h |
| 5α-THF (male) | 5α-THF (female) | β-cortol (male) | 17HP (male) | 17HP (female) | PTONE |
| THF | α-cortolone (female) | PT (male) | PD | 5 P T (female) | THDOC |
|
α-cortolone (male) |
Et (female) | 11β-OHAn (male) | 5 P T (male) | THS | 5α-17HP |
| An (female) | PT (female) | THAldo | |||
| THE | 5α-THB | 18OHTHA | |||
| An (male) | α-cortol | THA | |||
| Et (male) | 11β-OHEt | 5α-THA | |||
| β-cortol (female) | THB | ||||
| 11ketoEt | cortisol | ||||
| β-cortolone | cortisone | ||||
| DHEA | 6β-OHF | ||||
|
16α-DHEA 11β-OHAn (female) |
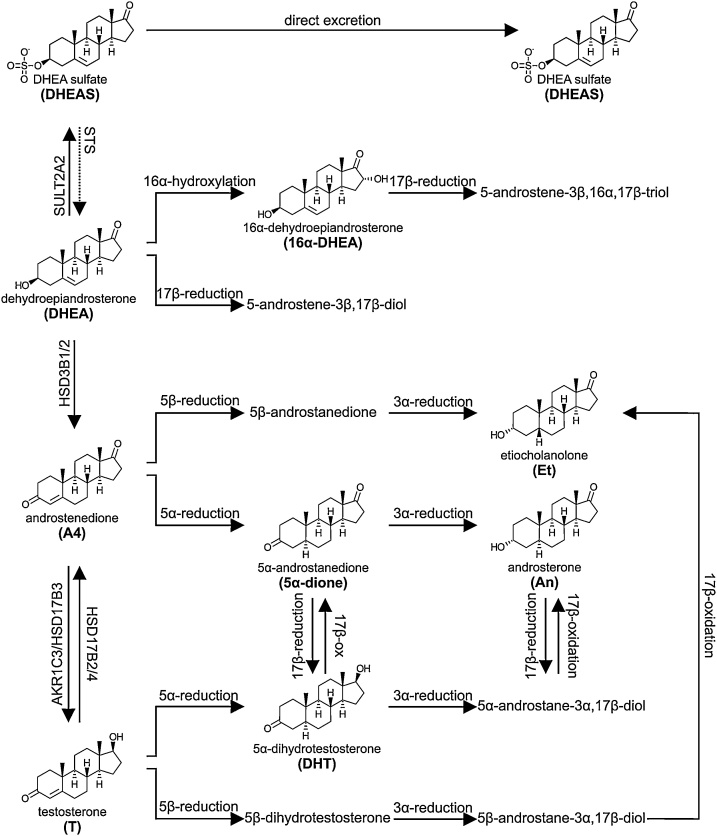
Undoubtedly, the most important step in androgen activation and inactivation is the 5α-/5β-reduction of the Δ4 steroid moiety common to all androgen precursors as well as the potent androgen testosterone (Fig. 8). This moiety is selectively 5α-reduced by the action of steroid 5α-reductase enzymes within target tissues. Those androgens and precursors that escape the tissue specific activation via 5α-reduction are metabolized within the liver, which expresses both 5α- and 5β-reductases [54,94,100]. Unlike 5α-reduction, which is required to produce the potent androgen DHT, AKR1D1-catalyzed 5β-reduction acts only as an inactivation step. Even 5β-DHT, the product of the 5β-reduction of testosterone, is an inactive androgen metabolite [54]. Following 5α/5β-reduction, androgen metabolites are subject to reduction of the 3-keto group with predominant 3α-stereoselectivity [54]. Importantly, 3α,5α-reduced metabolites can potentially be converted back to the 3-keto metabolite by oxidative 3α-HSDs such as in the alternative DHT biosynthesis pathway (Section 2.5.3.3).
Fig. 8.
Schematic overview of the pathways linking androgens and their precursors to their urine metabolites. Major serum androgen precursors and androgens are shown on the left. The metabolism of each steroid is shown from left to right and the structures of the major urine products are shown. 5α-dihydrotestosterone (DHT), the most potent androgen, is derived from testosterone by 5α-reduction and, thus, its formation is only reflected by urine androsterone. Phase 2 conjugation reactions are not indicated in the figure.
The majority of 5α/β-3α-metabolites of testosterone and DHT, which contain a 17β-hydroxy, are converted to 17-keto steroids by the action of the oxidative 17β-HSDs, HSD17B2 and HSD17B4 [106]. As a result, androgen metabolites are excreted with a 17-keto/17β-hydroxy ratio of approximately 10:1 [112].
Therefore, the primary urine androgen metabolites are androsterone (An; 5α-androstan-3α-ol-17-one) and etiocholanolone (Et; 5β-androstan-3α-ol-17-one) (Table 3). While, A4 and testosterone can be metabolized to either androsterone or etiocholanolone, DHT is 5α-reduced and thus only reflected in the androsterone fraction.
Both An and Et are subject to glucuronidation at the 3 position. This phase 2 metabolism can occur in the liver or within peripheral target tissues. The glucuronidation of C19 steroids is catalyzed by three members of the UGT2B subfamily, namely: UGT2B7, UGT2B15 and UGT2B17 [175]. The three enzymes have differential regioselectivity and substrate specificity for the 5α/β-stereoisomers [290]. UGT2B7 glucuronidates only the hydroxy group at position 3, but not in position 17 and preferentially conjugates 5α- over 5β-androstanes. UGT2B7 is the most efficient UGT for androstanediol conjugation [176]. UGT2B15 does not target the 3-hydroxy group, but conjugates the 17-hydroxy group in the androstane-3α,17β-diols, such as testosterone or DHT, and prefers the 5α-stereoisomers. Similarly, UGT2B17 has a preference for the 17β-hydroxy group in the androstane-3α,17β-diols, but conjugates the 3α-hydroxy group of An and Et with Et being the preferred substrate [290]. UGT2B17 has highest activity of all UGTs towards An, testosterone and DHT. UGT2A1 may also contribute to the glucuronidation of testosterone [291]. Interestingly, UGT2B15 which is expressed in adipose tissue has been shown to demonstrate a higher activity in obese individuals, which may contribute to the increased levels of 3α-androstanediol glucuronide observed in obesity [292,293]. While Δ5 steroids like DHEA and pregnenolone are excreted almost exclusively as sulfates, sulfation of other C19 steroid metabolites are considered minor phase 2 reactions. SULT2A2 can target 3α- and 17β-hydroxyl groups and has been shown to sulfate An, testosterone and DHT [294,295]. Hydroxy groups in positions 16β, 17α/β and 18 are also important targets for sulfation of C19 steroids [159,[296], [297], [298]].
Major urine androgen precursor metabolites include DHEA and 16α-hydroxy-DHEA. Circulating DHEA is readily 16α-hydroxylated by CYP3A4/7 within the liver [136,299]. The abundant conjugated androgen precursor, DHEAS, is water-soluble and is largely excreted in an unmodified form as represented by the urinary DHEA fraction following deconjugation.
Urinary metabolite excretion deriving from the 11-oxygenated androgen precursor 11OHA4 is well understood. 11OHA4 undergoes sequential 5α- and 3α- reduction yielding 11β-OHAn, which is readily quantifiable in urine (Table 3). It should be noted that although 11β-OHAn can also derive from cortisol metabolism (Section 4.2.2), this only contributes to approximately 5–10% of the measured levels, with at least 90% originating from 11OHA4 [258]. The metabolism of the active 11-oxygenated androgen, 11KT, has yet to be fully elucidated. Similarly, only a few studies have investigated the potential conjugation of 11-oxygenated steroids. While these steroids do appear to be glucuronidated, the limited data at hand suggests that glucuronidation of these steroids is less efficient than what is observed for the classic androgens [300].
4.4. The estrogen and progestogen metabolomes
4.4.1. The estrogen metabolome
The primary estrogens in circulation are estrone, estrone sulfate and 17β-estradiol, with 17β-estradiol considered the biologically active form [82,[301], [302], [303]]. In premenopausal women, these estrogens are predominantly produced by the ovaries (Section 2.5.4), but estrogens are also synthesized in peripheral tissues expressing aromatase, such as adipose tissue, using adrenal-derived androgen precursors. This peripheral production of estrogens is especially important in postmenopausal women and men [61]. It should be noted that this peripheral estrogen production often functions in a paracrine and intracrine manner and as such circulating concentrations are not reflective of the concentrations achieved locally [304,305]. Circulating levels of estrogens vary greatly during the course of the menstrual cycle and decrease significantly in postmenopausal women (Table 3) [[306], [307], [308]]. Notably, estrone sulfate is the predominant estrogen in circulation for both men and premenopausal women and serves as a biologically inactive reservoir for the generation of active estrogens in target tissues [40,309,310]. Like with androgens, the majority of unconjugated estrogen circulates bound to SHBG with high affinity and albumin with low affinity [[285], [286], [287]]. Another similarity to androgens is the regulation of estrogen potency by HSD17B enzymes, with HSD17B1 and HSD17B2 being the two most prominent isoforms involved in estrogen metabolism. HSD17B1 reduces estrone to the most active estrogen, 17β-estradiol. HSD17B2 catalyzes the reverse oxidative reaction of 17β-estradiol to estrone in addition to its high activity towards androgens. Further metabolism of both estrone and 17β-estradiol can yield estriol. Estrone undergoes 16α-hydroxylation and HSD17B1 catalyzed reduction, while 17β-estradiol only requires 16α-hydroxylation [[311], [312], [313], [314]]. CYP3A4 is the major enzyme responsible for the 16α-hydroxylation of estrone in adults, though CYP1A1, CYP2C19 and CYP3A5 can also catalyze the reaction [315,316]. Conversely, CYP1A2 is the dominant enzyme catalyzing the 16α-hydroxylation of 17β-estradiol, with CYP3A4, CYP1A1 and CYP1B1 also demonstrating this activity [317]. Estriol is rapidly excreted in urine and, as a result, serum levels are low to undetectable [318].
Both estrone and 17β-estradiol can also undergo hydroxylation at position 2 and 4 [141,317,[319], [320], [321]]. These reactions are catalyzed by a variety of CYPs, including CYP3A4 and CYP1A2 in the liver, or CYP1A1 and CYP3A4 in peripheral tissues. In the liver approximately 80% of 17β-estradiol is hydroxylated to the 2 position and 20% at the 4 position [322]. 2- and 4-hydroxy groups on the A-ring can be methylated as introduced in Section 3.2.3. Other reported hydroxylations include those at 6α, 6β, 7α, 12β, 15α, 15β, 16α and 16β positions as well as further oxidation to a 6-ketone or 9-11-dehydrogenation [141,[322], [323], [324]].
Estrogens and catecholestrogens are efficiently sulfated at several positions [[325], [326], [327]]. SULT1E1 is the major SULT for estrogen sulfation [328,329], while SULT1A1 and SULT1A3 also sulfate estrogens, but with a lower affinity [327].
Glucuronidation of estrogens is catalyzed by members of the UGT1A and UGT2B7 subfamilies with the UGT1A isoforms making the largest contribution to the glucuronidation of estrone and 17β-estradiol. Estriol and 16α-hydroxyestrone are conjugated at the 3-hydroxygroup by UGT1A10 and at the 16α-hydroxy group by UGT2B7 [330,331]. Catcholestrogens can additionally be glucuronidated in positions 2 and 4 [332].
4.4.2. The progestogen metabolome
Progestogens are compounds with progestational activity, referring to their induction of a secretory endometrium to support gestation [333]. The only true natural progestogen is progesterone. Levels change substantially during the course of the menstrual cycle, peaking during the luteal phase (Table 3). Low levels of circulating progesterone are also detectable in men [334]. Progesterone primarily circulates bound to CBG. During the second and third trimesters of pregnancy placental trophoblasts produce large amounts of progesterone, which displaces glucocorticoids from CBG [287,335].
Progestogens are primarily metabolized by the liver largely to form pregnanediols and pregnanolones [336,337]. Progesterone is metabolized to pregnanediol (PD, 5β-pregnane-3α,20α-diol) in three steps. AKR1D1 catalyzes the 5β-reduction followed by members of the AKR1C enzyme family catalyzing subsequent 3α- and 20α-reductions. Alternatively, progesterone can first be reduced to 20α-hydroxyprogesterone, which can then be further 5β-reduced by AKR1D1 and 3α-reduced by AKR1C1–4 [103]. PD is efficiently glucuronidated at position 3, resulting in pregnanediol-3-glucuronide being the major progesterone metabolite identified in urine. Progesterone metabolites reduced at 5α position are subject to extrahepatic 6α-hydroxylation, which is distinct from the hepatic 6α-hydroxylation active on Δ4 steroids [338].
5. Steroid metabolome profiling by mass spectrometry
5.1. Current state-of-the-art techniques in steroid analysis
Mass spectrometry is a powerful technique with which multiple steroids can be measured within a single analytical run. Despite the wealth of information that can be achieved by these methods, uptake in the clinical setting is still limited, primarily due to the cost of the technology and the limited availability of the required expertise.
Currently, gas chromatography-mass spectrometry (GC-MS) is the preferred method for the analysis of urine steroids in research laboratories due to the unparalleled resolution offered by this technique [339,340]. However, of late, there are increasing efforts to develop both ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry (UPHSFC-MS/MS) methods for the screening of multiple urine steroids [341,342]. An advantage of these techniques is that deconjugation is not mandatory, unlike with GC—MS. The idea of quantifying conjugated urine steroid metabolites is therefore gaining momentum in the field. This may be advantageous as some steroids with secondary sulfate groups (bis-sulfates), or glucuronides can be resistant to common hydrolysis procedures.
The introduction of high throughput UHPLC-MS/MS has led to a substantial increase in the use of mass spectrometry-based assays for steroid profiling, especially in serum, as UHPLC-MS/MS is a more accurate and reliable technique without the cross-reactivity issues that plague immuno-based assays. Indeed, there is a drive within the endocrine community to phase out immunoassays where possible [343]. Moreover, the use of high-resolution accurate mass (HRAM) mass spectrometry coupled to liquid chromatography systems is being explored as an alternative to traditional MS/MS systems as accurate mass quantification offers the potential to resolve all steroid metabolites with the exception of steroid isomers, unless they are separated chromatographically [344].
It should, however, be noted that despite the advantages of mass spectrometry techniques, these are not without their challenges. Perhaps the biggest challenge to the endocrine community is the cross validation of methodologies employed in different laboratories. Currently differences in sample work-up methodologies and/or instrumentation and settings can result in reference ranges that vary between laboratories. Moving forward methods therefore ideally need to be validated both internally according to set standards and subsequently compared using standardized reference material and quality controls [[345], [346], [347]].
5.2. Steroid metabolomics
Steroid metabolomics is defined as the combination of steroid metabolome profiling by mass spectrometry with computational machine learning-based analysis of the mass spectrometry data. Such sophisticated and unbiased computational analysis techniques have shown potential for assisting and even automating analysis of large or highly heterogeneous datasets, making it an ideal resource for use in metabolomics. Machine learning involves training a computer program to recognize patterns within large-scale data - the more data it is exposed to, the greater the learning capability. This generates a tailor-made diagnostic algorithm that can be prospectively applied to newly recorded steroid data. Interpretable models can help to understand underlying mechanisms, categorize and classify, or even make predictions based on observed patterns in the data. As an example, this approach has been used for automating differentiation of adrenocortical carcinoma (ACC) from benign adrenocortical tumors based on the detection of a “malignant steroid fingerprint”, a distinct set of urine steroid metabolites characteristically increased in ACC [348]. The principle established in this example has opened the door for the application of this approach to other steroidogenic disorders that create a unique steroid “fingerprint”.
6. Conclusion
The biosynthesis and metabolism of steroid hormones is complex. Although the measurement of individual steroids has routinely been employed for the diagnosis of endocrine conditions for many years, advances in technology now allow for the high throughput profiling of comprehensive steroid panels, thereby offering significantly more information and diagnostic power. Furthermore, the use of unbiased computational approaches such as machine learning allows for the development and implementation of steroid metabolomics analysis, which has the potential to not only improve, accelerate and automate diagnostics, but also to lead to improvements in treatment monitoring and prognostic prediction. Nonetheless, a detailed understanding of steroid biosynthesis and the principles that govern steroid metabolism and excretion remains fundamental to the accurate interpretation of metabolomics data as well as the improvement of our understanding of associated disorders.
Declaration of Competing Interest
W.A. is an inventor on a patent for the use of steroid profiling as a biomarker tool in the differential diagnosis of steroid-producing and steroid-dependent tumors (PCT/GB2010/000274). All other authors did not declare a conflict of interest.
Acknowledgements
We would like to thank Trish Storbeck for the preparation of the figures. This work was supported by the Wellcome Trust (Investigator Grant WT209492/Z/17/Z, to W.A.), the Medical Research Council UK (MR/R017913/1, to E.S.B.), and the Academy of Medical Sciences UK (Newton Advanced Fellowship NAF004\1002, to K.-H.S.). W.A. receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (Grant Reference Number BRC-1215-20009). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- 1.Porcu P., Barron A.M., Frye C.A., Walf A.A., Yang S.Y., He X.Y., Morrow A.L., Panzica G.C., Melcangi R.C. Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J. Neuroendocrinol. 2016;28 doi: 10.1111/jne.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rittenberg D., Bloch K. The utilization of acetic acid for the synthesis of fatty acids. J. Biol. Chem. 1945;160:417–424. [PubMed] [Google Scholar]
- 3.Bloch K., Rittenberg D. On the utilization of acetic acid for cholesterol formation. J. Biol. Chem. 1942;145:625–636. [Google Scholar]
- 4.Little H.H.N., Bloch K. Studies on the utilization of acetic acid for the biological synthesis of cholesterol. J. Biol. Chem. 1950;183:33–46. [Google Scholar]
- 5.Gwynne J.T., Strauss J.F. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 6.Azhar S., Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol. Cell. Endocrinol. 2002;195:1–26. doi: 10.1016/s0303-7207(02)00222-8. [DOI] [PubMed] [Google Scholar]
- 7.Azhar S., Leers-Sucheta S., Reaven E. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and “selective” pathway connection. Front. Biosci. 2003;8:998–1029. doi: 10.2741/1165. http://www.ncbi.nlm.nih.gov/pubmed/12957864 [DOI] [PubMed] [Google Scholar]
- 8.Kraemer F.B. Adrenal cholesterol utilization. Mol. Cell. Endocrinol. 2007;265–266:42–45. doi: 10.1016/j.mce.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich F.P., Yoshimoto F.K. Formation and cleavage of C–C bonds by enzymatic oxidation–reduction reactions. Chem. Rev. 2018;118:6573–6655. doi: 10.1021/acs.chemrev.8b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin D., Black S.M., Nagahama Y., Miller W.L. Steroid 17 alpha-hydroxylase and 17, 20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology. 1993;132:2498–2506. doi: 10.1210/endo.132.6.8504753. [DOI] [PubMed] [Google Scholar]
- 12.Yamada M., Ohta Y., Bachmanova G.I., Nishimoto Y., Archakov A.I., Kawato S. Dynamic interactions of rabbit liver cytochromes P450IA2 and P450IIB4 with cytochrome b5 and NADPH-cytochrome P450 reductase in proteoliposomes. Biochemistry. 1995;34:10113–10119. doi: 10.1021/bi00032a003. [DOI] [PubMed] [Google Scholar]
- 13.Zöllner A., Kagawa N., Waterman M.R., Nonaka Y., Takio K., Shiro Y., Hannemann F., Bernhardt R. Purification and functional characterization of human 11β hydroxylase expressed in Escherichia coli. FEBS J. 2008;275:799–810. doi: 10.1111/j.1742-4658.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- 14.Penning T.M. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr. Rev. 1997;18:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 15.Luu-The V., Takahashi M., de Launoit Y., Dumont M., Lachance Y., Labrie F. Evidence for distinct dehydrogenase and isomerase sites within a single 3β-hydroxysteroid dehydrogenase/5-ene-4-ene isomerase protein. Biochemistry. 1991;30:8861–8865. doi: 10.1021/bi00100a019. [DOI] [PubMed] [Google Scholar]
- 16.Thomas J.L., Frieden C., Nash W.E., Strickler R.C. An NADH-induced conformational change that mediates the sequential 3β-hydroxysteroid dehydrogenase/isomerase activities Is supported by affinity labeling and the time-dependent activation of isomerase. J. Biol. Chem. 1995;270:21003–21008. doi: 10.1074/jbc.270.36.21003. [DOI] [PubMed] [Google Scholar]
- 17.Thomas J.L., Berko E.A., Faustino A., Myers R.P., Strickler R.C. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5→4-ene-isomerase: purification from microsomes, substrate kinetics and inhibition by product steroids. J. Steroid Biochem. 1988;31:785–793. doi: 10.1016/0022-4731(88)90287-7. [DOI] [PubMed] [Google Scholar]
- 18.Thomas L., Mvers R.P., Strickler R.C. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5→4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J. Steroid Biochem. 1989;33:209–217. doi: 10.1016/0022-4731(89)90296-3. [DOI] [PubMed] [Google Scholar]
- 19.Cutler G.B., Glenn M., Bush M., Hodgen G.D., Graham C.E., Loriaux D.L. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 20.Arlt W., Martens J.W.M., Song M., Wang J.T., Auchus R.J., Miller W.L. Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143:4665–4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- 21.Xing Y., Edwards M.A., Ahlem C.N., Kennedy M., Cohen A., Gomez-sanchez C.E., Rainey W.E. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J. Endocrinol. 2011;209:327–335. doi: 10.1530/JOE-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson E.R., Waterman M.R. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu. Rev. Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- 23.Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo – pituitary – adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti L., Cofini M., Leonardi A., Penta L., Esposito S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front. Endocrinol. (Lausanne) 2018;9:410. doi: 10.3389/fendo.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T., Sasano H., Takeyama J., Kaneko C., Freije W.A., Carr B.R., Rainey W.E. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin. Endocrinol. (Oxf.) 2000:739–748. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 26.Pezzi V., Mathis J.M., Rainey W.E., Carr B.R. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J. Steroid Biochem. Mol. Biol. 2003;87:181–189. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Coulter C.L., Jaffe R.B. Functional maturation of the primate fetal adrenal in vivo : 3. specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroi. Endocrinology. 1998;139:5144–5150. doi: 10.1210/endo.139.12.6333. [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto T., Mitsuuchi Y., Toda K., Yokoyama Y., Miyahara K., Miura S., Ohnishi T., Ichikawa Y., Makao K., Mura H., Ulick S., Shizuta Y. Role of steroid 11β-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1458–1462. doi: 10.1073/pnas.89.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto T., Mitsuuchi Y., Ohnishi T., Ichikawa Y., Yokoyama Y., Sumimoto H., Toda K., Miyahara K., Kuribayashi I., Nakao K. Cloning and expression of a cDNA for human cytochrome P-450aldo as related to primary aldosteronism. Biochem. Biophys. Res. Commun. 1990;173:309–316. doi: 10.1016/s0006-291x(05)81058-7. [DOI] [PubMed] [Google Scholar]
- 30.Gupta M.K., Guryev O.L., Auchus R.J. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch. Biochem. Biophys. 2003;418:151–160. doi: 10.1016/j.abb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Petrunak E.M., DeVore N.M., Porubsky P.R., Scott E.E. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J. Biol. Chem. 2014;289:32952–32964. doi: 10.1074/jbc.M114.610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory M.C., Denisov I.G., Grinkova Y.V., Khatri Y., Sligar S.G. Kinetic solvent isotope effect in human P450 CYP17A1-mediated androgen formation: evidence for a reactive peroxoanion intermediate. J. Am. Chem. Soc. 2013;135:16245–16247. doi: 10.1021/ja4086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auchus R.J., Lee T.C., Miller W.L. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. http://www.ncbi.nlm.nih.gov/pubmed/9452426 [DOI] [PubMed] [Google Scholar]
- 34.Kohara T., Matsui Y., Fujii T.K., Tanaka N. Cefpirome in obstetrics and gynecology. Chemotherapy. 1991;39:452–457. [Google Scholar]
- 35.Nakamura Y., Gang H.X., Suzuki T., Sasano H., Rainey W.E. Adrenal changes associated with adrenarche. Rev. Endocr. Metab. Disord. 2009;10:19–26. doi: 10.1007/s11154-008-9092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auchus R.J., Rainey W.E. Adrenarche – physiology, biochemistry and human disease. Clin. Endocrinol. (Oxf.) 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 37.Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr. Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- 38.Rainey W.E., Carr B.R., Sasano H., Suzuki T., Mason J.I. Dissecting human adrenal androgen production. Trends Endocrinol. Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 39.Rege J., Nakamura Y., Satoh F., Morimoto R., Kennedy M.R., Layman L.C., Honma S., Sasano H., Rainey W.E. Liquid chromatography – tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 2013;98:1182–1188. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller J.W., Gilligan L.C., Idkowiak J., Arlt W., Foster P.A. The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 2015;36:526–563. doi: 10.1210/er.2015-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Reilly M.W., Kempegowda P., Jenkinson C., Taylor A.E., Quanson J.L., Storbeck K.-H.H., Arlt W., O’Reilly M.W., Kempegowda P., Jenkinson C., Taylor A.E., Quanson J.L., Storbeck K.-H.H., Arlt W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017;102:840–848. doi: 10.1210/jc.2016-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strott C.A. Sulfonation and molecular action. Endocr. Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 43.Rege J., Nanba A.T., Auchus R.J., Ren J., Peng H.-M., Rainey W.E., Turcu A.F. Adrenocorticotropin acutely regulates pregnenolone sulfate production by the human adrenal in vivo and in vitro. J. Clin. Endocrinol. Metab. 2018;103:320–327. doi: 10.1210/jc.2017-01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rege J., Karashima S., Lerario A.M., Smith J.M., Auchus R.J., Kasa-Vubu J.Z., Sasano H., Nakamura Y., White P.C., Rainey W.E. Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J. Clin. Endocrinol. Metab. 2016;101:4585–4593. doi: 10.1210/jc.2016-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffer L., Arlt W., Storbeck K.H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell. Endocrinol. 2018;465:4–26. doi: 10.1016/j.mce.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papierska L. Adrenopause – does it really exist? Menopausal Rev. 2017;2:57–60. doi: 10.5114/pm.2017.68593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geissler W.M., Davis D.L., Wu L., Bradshaw K.D., Patel S., Mendonca B.B., Elliston K.O., Wilson J.D., Russell D.W., Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat. Genet. 1994;7:34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 48.Andersson S., Geissler W.M., Wu L., Davis D.L., Grumbach M.M., New M.I., Schwarz H.P., Blethen S.L., Mendonca B.B., Bloise W., Witchel S.F., Cutler G.B., Griffin J.E., Wilson J.D., Russell D.W. Molecular genetics and pathophysiology of 17β-hydroxysteroid dehydrogenase 3 deficiency. J. Clin. Endocrinol. Metab. 1996;81:130–136. doi: 10.1210/jcem.81.1.8550739. [DOI] [PubMed] [Google Scholar]
- 49.Pelletier G., Luu-The V., Têtu B., Labrie F. Immunocytochemical localization of type 5 17β-hydroxysteroid dehydrogenase in human reproductive tissues. J. Histochem. Cytochem. 1999;47:731–737. doi: 10.1177/002215549904700602. [DOI] [PubMed] [Google Scholar]
- 50.Werner R., Kulle A., Sommerfeld I., Riepe F.G., Wudy S., Hartmann M.F., Merz H., Döhnert U., Bertelloni S., Holterhus P.M., Hiort O. Testosterone synthesis in patients with 17β-hydroxysteroid dehydrogenase 3 deficiency. Sex. Dev. 2012;6:161–168. doi: 10.1159/000336605. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein R.L., Kelch R.P., Jenner M.R., Kaplan S.L., Grumbach M.M. Secretion of unconjugated androgens and estrogens by the normal and abnormal human testis before and after human chorionic gonadotropin. J. Clin. Invest. 1974;53:1–6. doi: 10.1172/JCI107526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammond G.L., Ruokonen A., Kontturi M., Koskela E., Vihko R. The simultaneous radioimmunoassay of seven steroids in human spermatic and peripheral venous blood. J. Clin. Endocrinol. Metab. 1977;45:16–24. doi: 10.1210/jcem-45-1-16. [DOI] [PubMed] [Google Scholar]
- 53.Ishida H., Tashiro H., Watanabe M., Fujii N., Yoshida H., Imamura K., Minowada S., Shinohara M., Fukutani K., Aso Y., Kretser D.M.D.E. Measurement of inhibin concentrations in men: study of changes after castration and comparison with androgen levels in testicular tissue, spermatic venous blood, and peripheral venous blood. J. Clin. Endocrinol. Metab. 2015;70:1019–1022. doi: 10.1210/jcem-70-4-1019. [DOI] [PubMed] [Google Scholar]
- 54.Penning T.M., Wangtrakuldee P., Auchus R.J. Structural and functional biology of aldo-keto reductase steroid transforming enzymes. Endocr. Rev. 2018;40(2):447–475. doi: 10.1210/er.2018-00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharifi N. The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J. Invest. Med. 2012;60:504–507. doi: 10.231/JIM.0b013e31823874a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thigpen A.E., Cala K.M., Russell D.W. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J. Biol. Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- 57.Andersson S., Russell D.W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang K.H., Li R., Papari-zareei M., Watumull L., Zhao Y.D., Auchus R.J., Sharifi N., Daniel Y. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13728–13733. doi: 10.1073/pnas.1107898108. /-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labrie F. Intracrinology. Mol. Cell. Endocrinol. 1991;78:13–18. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 60.Labrie F., Bélanger A., Simard J., Luu-The V., Labrie C. DHEA and peripheral androgen and estrogen formation: intracrinology. Ann. N. Y. Acad. Sci. 1995;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- 61.Labrie F. Intracrinology in action: importance of extragonadal sex steroid biosynthesis and inactivation in peripheral tissues in both women and men. J. Steroid Biochem. Mol. Biol. 2015;145:131–132. doi: 10.1016/j.jsbmb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Auchus R.J. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Kamrath C., Hochberg Z., Hartmann M.F., Remer T., Wudy S.A. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J. Clin. Endocrinol. Metab. 2012;97:E367–E375. doi: 10.1210/jc.2011-1997. [DOI] [PubMed] [Google Scholar]
- 64.Fukami M., Homma K., Hasegawa T., Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev. Dyn. 2013;242:320–329. doi: 10.1002/dvdy.23892. [DOI] [PubMed] [Google Scholar]
- 65.Bauman D.R., Steckelbroeck S., Williams M.V., Peehl D.M., Penning T.M. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha,17beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol. Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 66.O’Shaughnessy P.J., Antignac J.P., Le Bizec B., Morvan M.L., Svechnikov K., Söder O., Savchuk I., Monteiro A., Soffientini U., Johnstonid Z.C., Bellingham M., Hough D., Walker N., Filis P., Fowler P.A. Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 2019;17(2) doi: 10.1371/journal.pbio.3000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swart A.C., Schloms L., Storbeck K.-H., Bloem L.M., Du Toit T., Quanson J.L., Rainey W.E., Swart P. 11β-Hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione. J. Steroid Biochem. Mol. Biol. 2013;138:132–142. doi: 10.1016/j.jsbmb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Davis S.R., Turcu A.F., Robinson P.J., Bell R.J. Exogenous testosterone does not influence 11-oxygenated C19 steroid concentrations in healthy postmenopausal women. J. Endocr. Soc. 2019;3:670–677. doi: 10.1210/js.2018-00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turcu A.F., Nanba A.T., Chomic R., Upadhyay S.K., Giordano T.J., Shields J.J., Merke D.P., Rainey W.E., Auchus R.J. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol. 2016;174:601–609. doi: 10.1530/EJE-15-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pretorius E., Arlt W., Storbeck K.-H. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol. Cell. Endocrinol. 2017;441:76–85. doi: 10.1016/j.mce.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Pretorius E., Africander D.J., Vlok M., Perkins M.S., Quanson J.L., Storbeck K.-H. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Reilly M.W., Kempegowda P., Walsh M., Taylor A.E., Manolopoulos K.N., Allwood J.W., Semple R.K., Hebenstreit D., Dunn W.B., Tomlinson J.W., Arlt W., Reilly M.W.O., Manolopoulos N., Warwick B. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017;102:3327–3339. doi: 10.1210/jc.2017-00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnard M., Quanson J.L., Mostaghel E., Pretorius E., Snoep J.L., Storbeck K.-H. 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): implications for castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2018;183:192–201. doi: 10.1016/j.jsbmb.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gathercole L.L., Lavery G.G., Morgan S.A., Cooper M.S., Sinclair A.J., Tomlinson J.W., Stewart P.M. 11β-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr. Rev. 2013;34:525–555. doi: 10.1210/er.2012-1050. [DOI] [PubMed] [Google Scholar]
- 75.Grossmann C., Scholz T., Rochel M., Bumke-Vogt C., Oelkers W., Pfeiffer A.F.H., Diederich S., Bähr V. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur. J. Endocrinol. 2004;151(3):397–406. doi: 10.1530/eje.0.1510397. [DOI] [PubMed] [Google Scholar]
- 76.Storbeck K.-H., Bloem L.M., Africander D.J., Schloms L., Swart P., Swart A.C. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol. Cell. Endocrinol. 2013;377:135–146. doi: 10.1016/j.mce.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Short R.V. Steroids in the follicular fluid and the corpus luteum of the mare. A “two-cell type” theory of ovarian steroid synthesis. J. Endocrinol. 1962;24:59–63. doi: 10.1677/joe.0.0240059. [DOI] [PubMed] [Google Scholar]
- 78.Mikhail G. Hormone secretion by the human ovaries. Gynecol. Obstet. Invest. 1970;1:5–20. doi: 10.1159/000301902. [DOI] [PubMed] [Google Scholar]
- 79.Baird D.T. The endocrinology of ovarian steroid secretion. Eur. J. Obstet. Gynecol. Reprod. Biol. 1974:31–39. doi: 10.1016/0028-2243(74)90006-9. [DOI] [PubMed] [Google Scholar]
- 80.Haning R.V., Austin C.W., Carlson I.H., Kuzma D.L., Zweibel W.J. Role of dehydroepiandrosterone sulfate as a prehormone for ovarian steroidogenesis. Obstet. Gynecol. 1985;65:199–205. http://www.ncbi.nlm.nih.gov/pubmed/3155830 [PubMed] [Google Scholar]
- 81.Arlt W., Justl H.-G., Callies F., Reincke M., Hübler D., Oettel M., Ernst M., Schulte H.M., Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J. Clin. Endocrinol. Metab. 1998;83:1928–1934. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- 82.Noel T., Noel C.T., Reed M.J., Jacobs H.S., James V.H.T. The plasma concentration of oestrone sulphate in postmenopausal women: lack of diurnal variation, effect of ovariectomy, age and weight. J. Steroid Biochem. 1981;14:1101–1105. doi: 10.1016/0022-4731(81)90039-x. [DOI] [PubMed] [Google Scholar]
- 83.Rezvanpour A., Don-Wauchope A.C. Critical reviews in clinical laboratory sciences clinical implications of estrone sulfate measurement in laboratory medicine clinical implications of estrone sulfate measurement in laboratory medicine *. Crit. Rev. Clin. Lab. Sci. 2016;0:000. doi: 10.1080/10408363.2016.1252310. [DOI] [PubMed] [Google Scholar]
- 84.Mungenast F., Aust S., Vergote I., Vanderstichele A., Sehouli J., Braicu E., Mahner S., Cacsire D.A.N., Tong C., Zeillinger R., Thalhammer T. Clinical significance of the estrogen-modifying enzymes steroid sulfatase and estrogen sulfotransferase in epithelial ovarian cancer. Oncol. Lett. 2017;13:4047–4054. doi: 10.3892/ol.2017.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNatty K.P., Makris A., DeGrazia C., Osathanondh R., Ryan K.J. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro*. J. Clin. Endocrinol. Metab. 1979;49:687–699. doi: 10.1210/jcem-49-5-687. [DOI] [PubMed] [Google Scholar]
- 86.Williams R.T. Wiley; New York: 1959. Detoxication Mechanisms; the Metabolism and Detoxication of Drugs, Toxic Substances, and Other Organic Compounds. [Google Scholar]
- 87.Hachey D.L., Dawling S., Roodi N., Parl F.F. Sequential action of phase I and II enzymes cytochrome p450 1B1 and glutathione S-transferase P1 in mammary estrogen metabolism. Cancer Res. 2003;63:8492–8499. [PubMed] [Google Scholar]
- 88.Hevir N., Sinkovec J., Rizner T.L. Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in endometrial cancer: lower levels of CYP1B1 and increased expression of S-COMT. Mol. Cell. Endocrinol. 2011;331:158–167. doi: 10.1016/j.mce.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Jin Y., Duan L., Lee S.H., Kloosterboer H.J., Blair I.A., Penning T.M. Human cytosolic hydroxysteroid dehydrogenases of the aldo-ketoreductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism. J. Biol. Chem. 2009;284:10013–10022. doi: 10.1074/jbc.M809465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kornel L., Patel S.K., Ezzeraimi E., Shackleton C.H. Corticosteroids in human blood: IX. Evidence for adrenal secretion of sulfate-conjugated cortisol, 11 beta,17 alpha-dihydroxy-4-pregnene-3,20-dione-21-yl-sulfate. Steroids. 1995;60:817–823. doi: 10.1016/0039-128x(95)00141-c. [DOI] [PubMed] [Google Scholar]
- 91.David Josephy P., Peter Guengerich F., Miners J.O. “Phase I and phase II” drug metabolism: terminology that we should phase out? Drug Metab. Rev. 2005;37:575–580. doi: 10.1080/03602530500251220. [DOI] [PubMed] [Google Scholar]
- 92.Penning T.M. New frontiers in androgen biosynthesis and metabolism. Curr. Opin. Endocrinol. Diab. Obes. 2010;17:233–239. doi: 10.1097/MED.0b013e3283381a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen M., Penning T.M. 5β-Reduced steroids and human Δ4-3-ketosteroid 5β-reductase (AKR1D1) Steroids. 2014;83:17–26. doi: 10.1016/j.steroids.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thigpen A.E., Silver R.I., Guileyardo J.M., Casey M.L., McConnell J.D., Russell D.W. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J. Clin. Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson J.D., Griffin J.E., Russell D.W. Steroid 5 alpha-reductase 2 deficiency. Endocr. Rev. 1993;14:577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 96.Uemura M., Tamura K., Chung S., Honma S., Okuyama A., Nakamura Y., Nakagawa H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99:81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stiles A.R., Russell D.W. SRD5A3: a surprising role in glycosylation. Cell. 2010;142:196–198. doi: 10.1016/j.cell.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantagrel V., Lefeber D.J., Ng B.G., Guan Z., Silhavy J.L., Bielas S.L., Lehle L., Hombauer H., Adamowicz M., Swiezewska E., De Brouwer A.P., Blümel P., Sykut-Cegielska J., Houliston S., Swistun D., Ali B.R., Dobyns W.B., Babovic-Vuksanovic D., van Bokhoven H., Wevers R.A., Raetz C.R.H., Freeze H.H., Morava É., Al-Gazali L., Gleeson J.G. SRD5A3 Is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142(2):203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moon Y.A., Horton J.D. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J. Biol. Chem. 2003 doi: 10.1074/jbc.M211684200. [DOI] [PubMed] [Google Scholar]
- 100.Chen M., Drury J.E., Penning T.M. Substrate specificity and inhibitor analyses of human steroid 5beta-reductase (AKR1D1) Steroids. 2011;76:484–490. doi: 10.1016/j.steroids.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palermo M., Marazzi M.G., Hughes B.A., Stewart P.M., Clayton P.T., Shackleton C.H. Human delta4-3-oxosteroid 5beta-reductase (AKR1D1) deficiency and steroid metabolism. Steroids. 2008;73:417–423. doi: 10.1016/j.steroids.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Steckelbroeck S., Jin Y., Gopishetty S., Oyesanmi B., Penning T.M. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J. Biol. Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 103.Jin Y., Mesaros A.C., Blair I.A., Penning T.M. Stereospecific reduction of 5beta-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5beta-reductase pathway. Biochem. J. 2011;437:53–61. doi: 10.1042/BJ20101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Penning T.M., Burczynski M.E., Jez J.M., Hung C.F., Lin H.K., Ma H., Moore M., Palackal N., Ratnam K. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mindnich R., Möller G., Adamski J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004;218:7–20. doi: 10.1016/j.mce.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Moeller G., Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2009;301:7–19. doi: 10.1016/j.mce.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 107.Marchais-Oberwinkler S., Henn C., Moller G., Klein T., Negri M., Oster A., Spadaro A., Werth R., Wetzel M., Xu K., Frotscher M., Hartmann R.W., Adamski J. 17beta-hydroxysteroid dehydrogenases (17beta-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J. Steroid Biochem. Mol. Biol. 2011;125:66–82. doi: 10.1016/j.jsbmb.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 108.Tomlinson J.W., Walker E.A., Bujalska I.J., Draper N., Lavery G.G., Cooper M.S., Hewison M., Stewart P.M. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 109.Bujalska I.J., Draper N., Michailidou Z., Tomlinson J.W., White P.C., Chapman K.E., a Walker E., Stewart P.M. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11 beta-hydroxysteroid dehydrogenase type 1. J. Mol. Endocrinol. 2005;34:675–684. doi: 10.1677/jme.1.01718. [DOI] [PubMed] [Google Scholar]
- 110.Hennebert O., Montes M., Favre-Reguillon A., Chermette H., Ferroud C., Morfin R. Epimerase activity of the human 11beta-hydroxysteroid dehydrogenase type 1 on 7-hydroxylated C19-steroids. J. Steroid Biochem. Mol. Biol. 2009;114:57–63. doi: 10.1016/j.jsbmb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 111.Muller C., Pompon D., Urban P., Morfin R. Inter-conversion of 7alpha- and 7beta-hydroxy-dehydroepiandrosterone by the human 11beta-hydroxysteroid dehydrogenase type 1. J. Steroid Biochem. Mol. Biol. 2006;99:215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Shackleton C.H.L., Marcos P. GC/MS steroid profiling: diagnosis of disorders affecting steroid synthesis and metabolism. Encycl. Mass Spectrom. 2011;8:789–813. [Google Scholar]
- 113.Miller W.L., Auchus R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moeller G., Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2009;301:7–19. doi: 10.1016/j.mce.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 115.Rižner T.L., Šmuc T., Rupreht R., Šinkovec J., Penning T.M. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol. Cell. Endocrinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Niwa T., Murayama N., Imagawa Y., Yamazaki H. Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev. 2015;47:89–110. doi: 10.3109/03602532.2015.1011658. [DOI] [PubMed] [Google Scholar]
- 117.Waxman D.J. Interactions of hepatic cytochromes P-450 with steroid hormones: regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem. Pharmacol. 1988;37:71–84. doi: 10.1016/0006-2952(88)90756-3. [DOI] [PubMed] [Google Scholar]
- 118.You L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem. Biol. Interact. 2004;147:233–246. doi: 10.1016/j.cbi.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 120.Zhou Y., Ingelman-Sundberg M., Lauschke V.M. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin. Pharmacol. Ther. 2017;102:688–700. doi: 10.1002/cpt.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imaoka S., Yamada T., Hiroi T., Hayashi K., Sakaki T., Yabusaki Y., Funae Y. Multiple forms of human P450 expressed in Saccharomyces cerevisiae. Systematic characterization and comparison with those of the rat. Biochem. Pharmacol. 1996;51:1041–1050. doi: 10.1016/0006-2952(96)00052-4. [DOI] [PubMed] [Google Scholar]
- 122.Shimada T., Yamazaki H., Mimura M., Inui Y., Guengerich F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 123.Williams J.A., Ring B.J., Cantrell V.E., Jones D.R., Eckstein J., Ruterbories K., Hamman M.A., Hall S.D., Wrighton S.A., Kitada M. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002;30:883–891. doi: 10.1124/dmd.30.8.883. [DOI] [PubMed] [Google Scholar]
- 124.Westlind-Johnsson A., Malmebo S., Johansson A., Otter C., Andersson T.B., Johansson I., Edwards R.J., Boobis A.R., Ingelman-Sundberg M. Comparative analysis of CYP3A expression in human liver suggests only a minor role for CYP3A5 in drug metabolism. Drug Metab. Dispos. 2003;31:755–761. doi: 10.1124/dmd.31.6.755. [DOI] [PubMed] [Google Scholar]
- 125.Hakkola J., Raunio H., Purkunen R., Saarikoski S., Vahakangas K., Pelkonen O., Edwards R.J., Boobis A.R., Pasanen M. Cytochrome P450 3A expression in the human fetal liver: evidence that CYP3A5 is expressed in only a limited number of fetal livers. Biol. Neonate. 2001;80:193–201. doi: 10.1159/000047142. [DOI] [PubMed] [Google Scholar]
- 126.Miyoshi Y., Taguchi T., Kim S.J., Tamaki Y., Noguchi S. Prediction of response to docetaxel by immunohistochemical analysis of CYP3A4 expression in human breast cancers. Breast Cancer. 2005;12:11–15. doi: 10.2325/jbcs.12.11. http://www.ncbi.nlm.nih.gov/pubmed/15657517 [DOI] [PubMed] [Google Scholar]
- 127.Dutheil F., Beaune P., Loriot M.-A. Xenobiotic metabolizing enzymes in the central nervous system: contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie. 2008;90:426–436. doi: 10.1016/j.biochi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Hayes C.L., Spink D.C., Spink B.C., Cao J.Q., Walker N.J., Sutter T.R. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maguire O., Pollock C., Martin P., Owen A., Smyth T., Doherty D., Campbell M.J., McClean S., Thompson P. Regulation of CYP3A4 and CYP3A5 expression and modulation of “intracrine” metabolism of androgens in prostate cells by liganded vitamin D receptor. Mol. Cell. Endocrinol. 2012;364:54–64. doi: 10.1016/j.mce.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 130.Shackleton C.H.L., Honour J.W., Taylor N.F. Metabolism of fetal and neonatal adrenal steroids. J. Steroid Biochem. 1979;11:523–529. doi: 10.1016/0022-4731(79)90077-3. [DOI] [PubMed] [Google Scholar]
- 131.Stevens J.C., Hines R.N., Gu C., Koukouritaki S.B., Manro J.R., Tandler P.J., Zaya M.J. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther. 2003;307:573–582. doi: 10.1124/jpet.103.054841. [DOI] [PubMed] [Google Scholar]
- 132.Lacroix D., Sonnier M., Moncion A., Cheron G., Cresteil T. Expression of CYP3A in the human liver - evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur. J. Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 133.Shackleton C.H., Taylor N.F. Identification of the androstenetriolones and androstenetetrols present in the urine of infants. J Steroid Biochem. 1975;6:1393–1399. doi: 10.1016/0022-4731(75)90075-8. [DOI] [PubMed] [Google Scholar]
- 134.Stárka L. The origin of 7α-hydroxy-dehydroepiandrosterone and its physiological role: a history of discoveries. Physiol. Res. 2017;66:285–294. doi: 10.33549/physiolres.933717. [DOI] [PubMed] [Google Scholar]
- 135.Stárka L., Hill M., Kolatorova L., Dušková M. Androst-5-ene-3β,7α/β,17β-triols, their plasma levels and dependence on the hypothalamic–pituitary–adrenal axis. Steroids. 2018;134:88–95. doi: 10.1016/j.steroids.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 136.Miller K.K.M., Cai J., Ripp S.L., Pierce W.M., Rushmore T.H., Prough R.A. Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab. Dispos. 2004;32:305–313. doi: 10.1124/dmd.32.3.305. [DOI] [PubMed] [Google Scholar]
- 137.Choi M.H., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Characterization of testosterone 11 beta-hydroxylation catalyzed by human liver microsomal cytochromes P450. Drug Metab Dispos. 2005;33:714–718. doi: 10.1124/dmd.104.003327. [DOI] [PubMed] [Google Scholar]
- 138.Niwa T., Yabusaki Y., Honma K., Matsuo N., Tatsuta K., Ishibashi F., Katagiri M. Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica. 1998;28:539–547. doi: 10.1080/004982598239290. [DOI] [PubMed] [Google Scholar]
- 139.Yamazaki H., Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- 140.Gomes L.G., Huang N., Agrawal V., Mendonca B.B., Bachega T.A., Miller W.L. Extraadrenal 21-hydroxylation by CYP2C19 and CYP3A4: effect on 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2009;94:89–95. doi: 10.1210/jc.2008-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee A.J., Cai M.X., Thomas P.E., Conney A.H., Zhu B.T. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 142.Bradlow H.L., Zumoff B., Monder C., Lee H.J., Hellman L. Isolation and identification of four new carboxylic acid metabolites of cortisol in man. J. Clin. Endocrinol. Metab. 1973;37:811–818. doi: 10.1210/jcem-37-5-811. [DOI] [PubMed] [Google Scholar]
- 143.Bradlow H.L., Monder C., Zumoff B. Studies in the biotransformation of cortisol to cortoic acids in man. III. 21-oxidation of 4-14C,21-3H-desoxycorticosterone. J. Clin. Endocrinol. Metab. 1977;45:960–964. doi: 10.1210/jcem-45-5-960. [DOI] [PubMed] [Google Scholar]
- 144.Zumoff B., Monder C., Bradlow H.L. Studies in the biotransformation of cortisol to the cortoic acids in man. II. The central role of tetrahydrocortisol and tetrahydrocortisone as intermediates. J. Clin. Endocrinol. Metab. 1977;44:647–650. doi: 10.1210/jcem-44-4-647. [DOI] [PubMed] [Google Scholar]
- 145.Monder C., Bradlow H.L. Carboxylic acid metabolites of steroids. J. Steroid Biochem. 1977;8:897–908. doi: 10.1016/0022-4731(77)90101-7. http://www.ncbi.nlm.nih.gov/pubmed/338990 [DOI] [PubMed] [Google Scholar]
- 146.Shackleton C.H.L., Homoki J., Taylor N.F. A paradox: elevated 21-hydroxypregnenolone production in newborns with 21-hydroxylase deficiency. Steroids. 1987;49:295–311. doi: 10.1016/0039-128x(87)90006-7. [DOI] [PubMed] [Google Scholar]
- 147.Laatikainen T. Identification of C21-O3 and C21-O4 steroids in human bile. Eur J Biochem. 1970;14:372–378. doi: 10.1111/j.1432-1033.1970.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 148.Winter J., Bokkenheuser V.D. Bacterial metabolism of natural and synthetic sex hormones undergoing enterohepatic circulation. J Steroid Biochem. 1987;27:1145–1149. doi: 10.1016/0022-4731(87)90201-9. [DOI] [PubMed] [Google Scholar]
- 149.Bokkenheuser V.D., Winter J., Honour J.W., Shackleton C.H. Reduction of aldosterone by anaerobic bacteria: origin of urinary 21-deoxy metabolites in man. J Steroid Biochem. 1979;11:1145–1149. doi: 10.1016/0022-4731(79)90166-3. [DOI] [PubMed] [Google Scholar]
- 150.Winter J., Shackleton C.H.L., O’rourke S., Bokkenheuser V.D. Bacterial formation of aldosterone metabolites. J. Steroid Biochem. 1984;21:563–569. doi: 10.1016/0022-4731(84)90332-7. [DOI] [PubMed] [Google Scholar]
- 151.Winter J., Cerone-McLernon A., O’Rourke S., Ponticorvo L., Bokkenheuser V.D. Formation of 20 beta-dihydrosteroids by anaerobic bacteria. J Steroid Biochem. 1982;17:661–667. doi: 10.1016/0022-4731(82)90568-4. [DOI] [PubMed] [Google Scholar]
- 152.Bokkenheuser V.D., Winter J., Hylemon P.B., Ayengar N.K., Mosbach E.H. Dehydroxylation of 16 alpha-hydroxyprogesterone by fecal flora of man and rat. J Lipid Res. 1981;22:95–102. [PubMed] [Google Scholar]
- 153.Bokkenheuser V.D., Winter J., O’Rourke S., Ritchie A.E. Isolation and characterization of fecal bacteria capable of 16 alpha-dehydroxylating corticoids. Appl Env. Microbiol. 1980;40:803–808. doi: 10.1128/aem.40.4.803-808.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winter J., Bokkenheuser V.D. 21-dehydroxylation of corticoids by anaerobic bacteria isolated from human fecal flora. J Steroid Biochem. 1978;9:379–384. doi: 10.1016/0022-4731(78)90604-0. [DOI] [PubMed] [Google Scholar]
- 155.Feighner S.D., Hylemon P.B. Characterization of a corticosteroid 21-dehydroxylase from the intestinal anaerobic bacterium, Eubacterium lentum. J Lipid Res. 1980;21:585–593. [PubMed] [Google Scholar]
- 156.Ridlon J.M., Ikegawa S., Alves J.M., Zhou B., Kobayashi A., Iida T., Mitamura K., Tanabe G., Serrano M., De Guzman A., Cooper P., Buck G.A., Hylemon P.B. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013;54:2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adlercreutz H., Pulkkinen M.O., Hamalainen E.K., Korpela J.T. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984;20:217–229. doi: 10.1016/0022-4731(84)90208-5. [DOI] [PubMed] [Google Scholar]
- 158.Setchell K.D.R., Almé B., Axelson M., Sjövall J. The multicomponent analysis of conjugates of neutral steroids in urine by lipophilic ion exchange chromatography and computerised gas chromatography-mass spectrometry. J. Steroid Biochem. 1976;7:615–629. doi: 10.1016/0022-4731(76)90086-8. [DOI] [PubMed] [Google Scholar]
- 159.Janne O., Vihko R., Sjovall J., Sjovall K., Jänne O., Vihko R., Sjövall J., Sjövall K. Determination of steroid mono- and disulfates in human plasma. Clin Chim Acta. 1969;23:405–412. doi: 10.1016/0009-8981(69)90340-4. [DOI] [PubMed] [Google Scholar]
- 160.Pasqualini J.R., Jayle M.F. Identification of 3beta,21-dihydroxy-5-pregnene-20-one disulfate in human urine. J Clin Invest. 1962;41:981–987. doi: 10.1172/JCI104577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pozo O.J., Marcos J., Khymenets O., Pranata A., Fitzgerald C.C.C., McLeod M.M.D., Shackleton C. Alternate steroid sulfation pathways targeted by LC-MS/MS analysis of disulfates: application to prenatal diagnosis of steroid synthesis disorders. J Mol Endocrinol. 2018;61:M1–M12. doi: 10.1530/JME-17-0286. [DOI] [PubMed] [Google Scholar]
- 162.Kornel L., Saito Z. Studies on steroid conjugates-VIII: isolation and characterization of glucuronide-conjugated metabolites of cortisol in human urine. J. Steroid Biochem. 1975;6:1267–1284. doi: 10.1016/0022-4731(75)90118-1. [DOI] [PubMed] [Google Scholar]
- 163.Miyabo S., Kornel L. Corticosteroids in human blood—VI. Isolation, characterization and quantitation of sulfate conjugated metabolites of cortisol in human plasma. J. Steroid Biochem. 1974;5:233–247. doi: 10.1016/0022-4731(74)90137-x. [DOI] [PubMed] [Google Scholar]
- 164.Venkatachalam K.V. Human 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase: biochemistry, molecular biology and genetic deficiency. IUBMB Life. 2003;55:1–11. doi: 10.1080/1521654031000072148. [DOI] [PubMed] [Google Scholar]
- 165.Coughtrie M.W.H. Function and organization of the human cytosolic sulfotransferase (SULT) family. Chem Biol Interact. 2016;259:2–7. doi: 10.1016/j.cbi.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 166.Shackleton C.H., Livingstone J.R., Mitchell F.L. The conjugated 17-hydroxy epimers of delta5-androstene-3beta-17-diol in infant and adult urine and umbilical cord plasma. Steroids. 1968;11:299–311. doi: 10.1016/s0039-128x(68)80142-4. [DOI] [PubMed] [Google Scholar]
- 167.Shackleton C.H., Kelly R.W., Adhikary P.M., Brooks C.J., Harkness R.A., Sykes P.J., Mitchell F.L. The identification and measurement of a new steroid 16 beta-hydroxydehydroepiandrosterone in infant urine. Steroids. 1968;12:705–716. doi: 10.1016/s0039-128x(68)80025-x. [DOI] [PubMed] [Google Scholar]
- 168.Laatikainen T., Vihko R. Quantitation of C19O2 and C21O2 steroid mono- and disulfates in human bile. Steroids. 1969;14:119–131. doi: 10.1016/0039-128x(69)90027-0. [DOI] [PubMed] [Google Scholar]
- 169.McLeod M.D., Waller C.C., Esquivel A., Balcells G., Ventura R., Segura J., Pozo O.J. Constant ion loss method for the untargeted detection of bis-sulfate metabolites. Anal Chem. 2017;89:1602–1609. doi: 10.1021/acs.analchem.6b03671. [DOI] [PubMed] [Google Scholar]
- 170.Thomas M.P., Potter B.V. Estrogen O-sulfamates and their analogues: clinical steroid sulfatase inhibitors with broad potential. J Steroid Biochem Mol Biol. 2015;153:160–169. doi: 10.1016/j.jsbmb.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 171.Purohit A., Foster P.A. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J. Endocrinol. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]
- 172.Cook I.T., Duniec-Dmuchowski Z., Kocarek T.A., Runge-Morris M., Falany C.N. 24-hydroxycholesterol sulfation by human cytosolic sulfotransferases: formation of monosulfates and disulfates, molecular modeling, sulfatase sensitivity, and inhibition of liver x receptor activation. Drug Metab Dispos. 2009;37:2069–2078. doi: 10.1124/dmd.108.025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bélanger A., Hum D.W., Beaulieu M., Lévesque É., Guillemette C., Tchernof A., Bélanger G., Turgeon D., Dubois S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J. Steroid Biochem. Mol. Biol. 1998;65:301–310. doi: 10.1016/s0960-0760(97)00183-0. [DOI] [PubMed] [Google Scholar]
- 174.Burchell B., Coughtrie M.W.H. UDP-glucuronosyltransferases. Pharmacol. Ther. 1989;43:261–289. doi: 10.1016/0163-7258(89)90122-8. [DOI] [PubMed] [Google Scholar]
- 175.Bélanger A., Pelletier G., Labrie F., Barbier O., Chouinard S. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol. Metab. 2003;14:473–479. doi: 10.1016/j.tem.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 176.Turgeon D., Carrier J.-S., Lévesque É., Hum D.W., Bélanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- 177.Strassburg C.P., Oldhafer K., Manns M.P., Tukey R.H. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol. 1997;52:212–220. doi: 10.1124/mol.52.2.212. [DOI] [PubMed] [Google Scholar]
- 178.Murai T., Iwabuchi H., Ikeda T. Repeated glucuronidation at one hydroxyl group leads to structurally novel diglucuronides of steroid sex hormones. Drug Metab. Pharmacokinet. 2005;20:282–293. doi: 10.2133/dmpk.20.282. [DOI] [PubMed] [Google Scholar]
- 179.Axelrod J., Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 180.Ball P., Knuppen R., Haupt M., Breuer H. Interactions between estrogens and catechol amines. 3. Studies on the methylation of catechol estrogens, catechol amines and other catechols by the ctechol-O-methyltransferases of human liver. J Clin Endocrinol Metab. 1972;34:736–746. doi: 10.1210/jcem-34-4-736. [DOI] [PubMed] [Google Scholar]
- 181.Dawling S., Roodi N., Mernaugh R.L., Wang X., Parl F.F. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 182.Bai H.W., Shim J.Y., Yu J., Zhu B.T. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem Res Toxicol. 2007;20:1409–1425. doi: 10.1021/tx700174w. [DOI] [PubMed] [Google Scholar]
- 183.Tenhunen J., Salminen M., Lundstrom K., Kiviluoto T., Savolainen R., Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 184.Marcos J., Pol M., Fabregat A., Ventura R., Renau N., Hanzu F.A., Casals G., Marfa S., Barcelo B., Barcelo A., Robles J., Segura J., Pozo O.J. Urinary cysteinyl progestogens: occurrence and origin. J Steroid Biochem Mol Biol. 2015;152:53–61. doi: 10.1016/j.jsbmb.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 185.Fabregat A., Marcos J., Garrostas L., Segura J., Pozo O.J., Ventura R. Evaluation of urinary excretion of androgens conjugated to cysteine in human pregnancy by mass spectrometry. J. Steroid Biochem. Mol. Biol. 2014;139:192–200. doi: 10.1016/j.jsbmb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 186.Fabregat A., Kotronoulas A., Marcos J., Joglar J., Alfonso I., Segura J., Ventura R., Pozo O.J. Detection, synthesis and characterization of metabolites of steroid hormones conjugated with cysteine. Steroids. 2013;78:327–336. doi: 10.1016/j.steroids.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 187.Fabregat A., Marcos J., Ventura R., Casals G., Jimenez W., Reichenbach V., Segura J., Pozo O.J. Formation of Delta(1) and Delta(6) testosterone metabolites by human hepatocytes. Steroids. 2015;95:66–72. doi: 10.1016/j.steroids.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 188.Jones D.L., James V.H. The identification, quantification and possible origin of non-polar conjugates in human plasma. J Steroid Biochem. 1985;22:243–247. doi: 10.1016/0022-4731(85)90119-0. [DOI] [PubMed] [Google Scholar]
- 189.Belanger B., Caron S., Belanger A., Dupont A. Steroid fatty acid esters in adrenals and plasma: effects of ACTH. J Endocrinol. 1990;127:505–511. doi: 10.1677/joe.0.1270505. [DOI] [PubMed] [Google Scholar]
- 190.Lavallee B., Provost P.R., Belanger A. Formation of pregnenolone- and dehydroepiandrosterone-fatty acid esters by lecithin-cholesterol acyltransferase in human plasma high density lipoproteins. Biochim Biophys Acta. 1996;1299:306–312. doi: 10.1016/0005-2760(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 191.Provost P.R., Lavallee B., Belanger A. Transfer of dehydroepiandrosterone- and pregnenolone-fatty acid esters between human lipoproteins. J Clin Endocrinol Metab. 1997;82:182–187. doi: 10.1210/jcem.82.1.3693. [DOI] [PubMed] [Google Scholar]
- 192.Lavallee B., Provost P.R., Roy R., Gauthier M.C., Belanger A. Dehydroepiandrosterone-fatty acid esters in human plasma: formation, transport and delivery to steroid target tissues. J Endocrinol. 1996;150(Suppl):S119–S124. [PubMed] [Google Scholar]
- 193.Vihma V., Tikkanen M.J. Fatty acid esters of steroids: synthesis and metabolism in lipoproteins and adipose tissue. J Steroid Biochem Mol Biol. 2011;124:65–76. doi: 10.1016/j.jsbmb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 194.van Kammen E., Thijssen J.H., Donker G.H., Schwarz F. The excretion of metabolites of testosterone and of estradiol in male patients with chronic renal failure. Steroids. 1975;26:508–515. doi: 10.1016/0039-128x(75)90070-7. [DOI] [PubMed] [Google Scholar]
- 195.Hellman L., Rosenfeld R.S. Metabolism of testosterone-1,2-3H in man. Distribution of the major 17-ketosteroid metabolites in plasma: relation to thyroid states. J Clin Endocrinol Metab. 1974;38:424–435. doi: 10.1210/jcem-38-3-424. [DOI] [PubMed] [Google Scholar]
- 196.Pelis R.M., Wright S.H. Renal transport of organic anions and cations. Compr Physiol. 2011;1:1795–1835. doi: 10.1002/cphy.c100084. [DOI] [PubMed] [Google Scholar]
- 197.Devendran S., Mendez-Garcia C., Ridlon J.M. Identification and characterization of a 20beta-HSDH from the anaerobic gut bacterium butyricicoccus desmolans ATCC 43058. J Lipid Res. 2017;58:916–925. doi: 10.1194/jlr.M074914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sanberg A., Slaunwhite W.R. The metabolic fate of C14 -progesterone in human subjects. J. Clin. Endocrinol. Metab. 1958;18:253–265. doi: 10.1210/jcem-18-3-253. [DOI] [PubMed] [Google Scholar]
- 199.Laatikaninen T., Peltokallio P., Vihko R. Steroid sulfates in human bile. Steroids. 1968;12:407–421. doi: 10.1016/0039-128x(68)90031-7. [DOI] [PubMed] [Google Scholar]
- 200.Laatikainen T. Quantitative studies on the excretion of glucuronide and mono- and disulphate conjugates of neutral steroids in human bile. Ann Clin Res. 1970;2:338–349. [PubMed] [Google Scholar]
- 201.Laatikainen T., Karjalainen O. Excretion of conjugates of neutral steroids in human bile during late pregnancy. Acta Endocrinol. 1972;69:775–788. doi: 10.1530/acta.0.0690775. [DOI] [PubMed] [Google Scholar]
- 202.Adlercreutz H., Martin F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J Steroid Biochem. 1980;13:231–244. doi: 10.1016/0022-4731(80)90196-x. [DOI] [PubMed] [Google Scholar]
- 203.Eriksson H., Gustafsson J.A. Excretion of steroid hormones in adults. Steroids in faeces from adults. Eur J Biochem. 1971;18:146–150. doi: 10.1111/j.1432-1033.1971.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 204.Gustafsson J.A., Shackleton C.H., Sjovall J. Steroids in newborns and infants. A semiquantitative analysis of steroids in faeces from infants. Acta Endocrinol. 1970;65:18–28. [PubMed] [Google Scholar]
- 205.Eriksson H., Gustafsson J.A., Sjovall J. Excretion of steroid hormones in adults. C19 and C21 steroids in faeces from pregnant women. Eur J Biochem. 1970;12:520–526. doi: 10.1111/j.1432-1033.1970.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 206.Sandberg A.A., Slaunwhite W.R. Studies on Phenolic Steroids in Human Subjects. II. The Metabolic Fate and Hepato-Biliary-Enteric Circulation of C14-Estrone and C14-Estradiol in Women. Clin Invest. 1957;36(8):1266–1278. doi: 10.1172/JCI103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huijghebaert S.M., Sim S.M., Back D.J., Eyssen H.J. Distribution of estrone sulfatase activity in the intestine of germfree and conventional rats. J Steroid Biochem. 1984;20:1175–1179. doi: 10.1016/0022-4731(84)90363-7. [DOI] [PubMed] [Google Scholar]
- 208.Keevil B.G. Novel liquid chromatography tandem mass spectrometry (LC-MS/MS) methods for measuring steroids, Best Pr. Res Clin Endocrinol Metab. 2013;27:663–674. doi: 10.1016/j.beem.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 209.Vining R.F., McGinley R.A., Symons R.G. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29:1752–1756. [PubMed] [Google Scholar]
- 210.Vining R.F., McGinley R.A., Maksvytis J.J., Ho K.Y. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20(Pt 6):329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 211.Perogamvros I., Keevil B.G., Ray D.W., Trainer P.J. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95:4951–4958. doi: 10.1210/jc.2010-1215. [DOI] [PubMed] [Google Scholar]
- 212.Debono M., Harrison R.F., Whitaker M.J., Eckland D., Arlt W., Keevil B.G., Ross R.J. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J. Clin. Endocrinol. Metab. 2016;101:1469–1477. doi: 10.1210/jc.2015-3694. [DOI] [PubMed] [Google Scholar]
- 213.Harrison R.F., Debono M., Whitaker M.J., Keevil B.G., Newell-Price J., Ross R.J. Salivary cortisone to estimate cortisol exposure and sampling frequency required based on serum cortisol measurements. J. Clin. Endocrinol. Metab. 2018;104(3):765–772. doi: 10.1210/jc.2018-01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Becker K.L., Bilezikian J.P., Bremner W.J., Hung W., Kahn R., Loriaux L., Nylén E.S., Rebar R.W., Robertson G.L., Snider R.H. Lippincott Williams & Wilkins; 2001. Principles and Practice of Endocrinology and Metabolism. [Google Scholar]
- 215.Hinchliffe E., Carter S., Owen L.J., Keevil B.G. Quantitation of aldosterone in human plasma by ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2013;913–914:19–23. doi: 10.1016/j.jchromb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 216.Ulick S., Kusch K., August J.T. Correction of the structure of a urinary metabolite of aldosterone. J. Amer. Chem. Soc. 1961;8:4482. [Google Scholar]
- 217.Ulick S., Vetter K. Identification of two C18 oxygenated corticosteroids isolated from human urine. J Biol Chem. 1962;237:3364–3374. http://www.jbc.org/content/237/11/3364.short [PubMed] [Google Scholar]
- 218.Shackleton C.H.L., Honour J.W. Identification and measurement of 18-hydroxycorticosterone metabolites by gas chromatography-mass spectrometry. J. Steroid Biochem. 1977;8:199–203. doi: 10.1016/0022-4731(77)90051-6. [DOI] [PubMed] [Google Scholar]
- 219.Gomez-Sanchez E., Gomez-Sanchez C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2014;4:965–994. doi: 10.1002/cphy.c130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Peterson R.E., Pierce C.E. The metabolism of corticosterone in man. J. Clin. Invest. 1960;39:741–757. doi: 10.1172/JCI104091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gomez-Sanchez C.E., Holland O.B. Urinary tetrahydroaldosterone and aldosterone-18-glucuronide excretion in white and black normal subjects and hypertensive patients. J Clin Endocrinol Metab. 1981;52:214–219. doi: 10.1210/jcem-52-2-214. https://academic.oup.com/jcem/article-abstract/52/2/214/2677463 [DOI] [PubMed] [Google Scholar]
- 222.Luetscher J.A., Hancock E.W., Camargo C.A., Dowdy A.J., Nokes G.W. Conjugation of 1,2-3H-aldosterone in human liver and kidneys and renal extraction of aldosterone and labeled conjugates from blood plasma. J Clin Endocrinol Metab. 1965;25:628–638. doi: 10.1210/jcem-25-5-628. [DOI] [PubMed] [Google Scholar]
- 223.Knights K.M., Winner L.K., Elliot D.J., Bowalgaha K., Miners J.O. Aldosterone glucuronidation by human liver and kidney microsomes and recombinant UDP-glucuronosyltransferases: inhibition by NSAIDs. Br. J. Clin. Pharmacol. 2009;68:402–412. doi: 10.1111/j.1365-2125.2009.03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Girard C., Barbier O., Veilleux G., El-Alfy M., Bélanger A. Human uridine diphosphate-glucuronosyltransferase UGT2B7 conjugates mineralocorticoid and glucocorticoid metabolites. Endocrinology. 2003:2659–2668. doi: 10.1210/en.2002-0052. [DOI] [PubMed] [Google Scholar]
- 225.Bledsoe T., Liddle G.W., Riondel A., Island D.P., Bloomfield D., Sinclair-Smith B. Comparative fates of intravenously and orally administered aldosterone: evidence for extrahepatic formation of acid-hydrolyzable conjugate in man. J. Clin. Invest. 1966;45:264–269. doi: 10.1172/JCI105339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Luetscher J.A., Camargo C.A., Cohn A.P., Dowdy A.J., Callaghan A.M. Observations on metabolism of aldosterone in man. Ann. Intern. Med. 1963;59:1–7. doi: 10.7326/0003-4819-59-1-1. http://annals.org/data/journals/aim/19349/aime196307010-00001.pdf [DOI] [PubMed] [Google Scholar]
- 227.Shackleton C.H.L., Biglieri E.G., Roitman E., Honour J.W. Metabolism of radiolabeled corticosterone in an adult with the 17α-hydroxylase deficiency syndrome. J. Clin. Endocrinol. Metab. 1979;48:976–982. doi: 10.1210/jcem-48-6-976. [DOI] [PubMed] [Google Scholar]
- 228.Arriza J.L., Weinberger C., Cerelli G., Glaser T.M., Handelin B.L., Housman D.E., Evans R.M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. http://www.ncbi.nlm.nih.gov/pubmed/3037703 [DOI] [PubMed] [Google Scholar]
- 229.Shimojo M., Stewart P.M. Apparent mineralocorticoid excess syndromes. J. Endocrinol. Invest. 1995;18:518–532. doi: 10.1007/BF03349763. [DOI] [PubMed] [Google Scholar]
- 230.Palermo M., Quinkler M., Stewart P.M. Apparent mineralocorticoid excess syndrome: an overview. Arq. Bras. Endocrinol. Metabol. 2004;48:687–696. doi: 10.1590/s0004-27302004000500015. doi:/S0004-27302004000500015. [DOI] [PubMed] [Google Scholar]
- 231.Stewart P.M., Murry B.A., Mason J.I. Human kidney 11 beta-hydroxysteroid dehydrogenase is a high affinity nicotinamide adenine dinucleotide-dependent enzyme and differs from the cloned type I isoform. J. Clin. Endocrinol. Metab. 1994;79:480–484. doi: 10.1210/jcem.79.2.8045966. [DOI] [PubMed] [Google Scholar]
- 232.Hardy R.S., Doig C.L., Hussain Z., O’Leary M., Morgan S.A., Pearson M.J., Naylor A., Jones S.W., Filer A., Stewart P.M., Buckley C.D., Lavery G.G., Cooper M.S., Raza K. 11β-hydroxysteroid dehydrogenase type 1 within muscle protects against the adverse effects of local inflammation. J. Pathol. 2016;240:472–483. doi: 10.1002/path.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cooper M.S., Rabbitt E.H., Goddard P.E., Bartlett W.A., Hewison M., Stewart P.M. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Miner. Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 234.Tannin G.M., Agarwal A.K., Monder C., New M.I., White P.C. The human gene for 11 beta-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J. Biol. Chem. 1991;266:16653–16658. http://www.ncbi.nlm.nih.gov/pubmed/1885595 [PubMed] [Google Scholar]
- 235.Stewart P.M., Whorwood C.B., Mason J.I. Type 2 11β-hydroxysteroid dehydrogenase in foetal and adult life. J. Steroid Biochem. Mol. Biol. 1995;55:465–471. doi: 10.1016/0960-0760(95)00195-6. [DOI] [PubMed] [Google Scholar]
- 236.Zumoff B., Bradlow H.L., Levin J., Fukushima D.K. Influence of thyroid function on the in vivo cortisol ⇌ cortisone equilibrium in man. J. Steroid Biochem. 1983;18:437–440. doi: 10.1016/0022-4731(83)90062-6. [DOI] [PubMed] [Google Scholar]
- 237.Rogers S.L., Hughes B.A., Jones C.A., Freedman L., Smart K., Taylor N., Stewart P.M., Shackleton C.H.L., Krone N.P., Blissett J., Tomlinson J.W. Diminished 11beta-hydroxysteroid dehydrogenase type 2 activity is associated with decreased weight and weight gain across the first year of life. J Clin Endocrinol Metab. 2014;99:E821–E831. doi: 10.1210/jc.2013-3254. [DOI] [PubMed] [Google Scholar]
- 238.Lavery G.G., Idkowiak J., Sherlock M., Bujalska I., Ride J.P., Saqib K., Hartmann M.F., Hughes B., Wudy S.A., De Schepper J., Arlt W., Krone N., Shackleton C.H., Walker E.A., Stewart P.M., De Schepper J. Novel H6PDH mutations in two girls with premature adrenarche: “apparent” and “true” CRD can be differentiated by urinary steroid profiling. Eur J Endocrinol. 2013;168:K19–K26. doi: 10.1530/EJE-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Weitzman E.D., Czeisler C.A., Zimmerman J.C., Moore-Ede M.C. Biological rhythms in man: relationship of sleep-wake, cortisol, growth hormone, and temperature during temporal isolation. Adv. Biochem. Psychopharmacol. 1981;28:475–499. http://www.ncbi.nlm.nih.gov/pubmed/7010946 [PubMed] [Google Scholar]
- 240.Weitzman E.D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T.F., Hellman L. Twenty-four hour pattern of the episodic seretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 241.Chan S., Debono M. Review: replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010;1:129–138. doi: 10.1177/2042018810380214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smyth J.M., Ockenfels M.C., Gorin A.A., Catley D., Porter L.S., Kirschbaum C., Hellhammer D.H., Stone A.A. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 243.Whitaker M.J., Debono M., Huatan H., Merke D.P., Arlt W., Ross R.J. An oral multiparticulate, modified-release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin. Endocrinol. (Oxf). 2014;80:554–561. doi: 10.1111/cen.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Prete A., Yan Q., Al-Tarrah K., Akturk H.K., Prokop L.J., Alahdab F., Foster M.A., Lord J.M., Karavitaki N., Wass J.A., Murad M.H., Arlt W., Bancos I. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin. Endocrinol. (Oxf). 2018;89:554–567. doi: 10.1111/cen.13820. [DOI] [PubMed] [Google Scholar]
- 245.Daughaday W.H. Binding of corticosteroids by plasma proteins. IV. The electrophoretic demonstration of corticosteroid binding globulin. J. Clin. Invest. 1958;37:519–523. doi: 10.1172/JCI103633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Siiteri P.K., Murai J.T., Raymoure W.J., Kuhn R.W., Hammond G.L., Nisker J.A. The serum transport of steroid hormones. Proc. 1981 Laurentian Horm. Conf. 1982:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- 247.Meulenberg P., Hofman J. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin. Chem. 1990;36:70–75. http://clinchem.aaccjnls.org/content/36/1/70.short [PubMed] [Google Scholar]
- 248.Jung C., Ho J.T., Torpy D.J., Rogers A., Doogue M., Lewis J.G., Czajko R.J., Inder W.J. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J. Clin. Endocrinol. Metab. 2011;96:1533–1540. doi: 10.1210/jc.2010-2395. [DOI] [PubMed] [Google Scholar]
- 249.Lebbe M., Arlt W. What is the best diagnostic and therapeutic management strategy for an addison patient during pregnancy? Clin. Endocrinol. (Oxf). 2013;78:497–502. doi: 10.1111/cen.12097. [DOI] [PubMed] [Google Scholar]
- 250.Allolio B., Hoffmann J., Linton E.A., Winkelmann W., Kusche M., Schulte H.M. Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clin. Endocrinol. (Oxf) 1990;33:279–289. doi: 10.1111/j.1365-2265.1990.tb00492.x. http://www.ncbi.nlm.nih.gov/pubmed/2225483 [DOI] [PubMed] [Google Scholar]
- 251.Leon Bradlow H., Zumoff B., Gallagher T.F., Hellman L. Tetrahydrocortisol metabolism in man. Steroids. 1968;12:303–308. doi: 10.1016/0039-128x(68)90023-8. [DOI] [PubMed] [Google Scholar]
- 252.Zumoff B., Bradlow H.L., Gallagher T.F., Hellman L. Metabolism of tetrahydrocortisone in health and disease. J. Clin. Endocrinol. Metab. 1968;28:1330–1334. doi: 10.1210/jcem-28-9-1330. [DOI] [PubMed] [Google Scholar]
- 253.Shackleton C.H.L.H., Roitman E., Monder C., Bradlow H.L.L. Gas chromatographic and mass spectrometric analysis of urinary acidic metabolites of cortisol. Steroids. 1980;36:289–298. doi: 10.1016/0039-128x(80)90003-3. [DOI] [PubMed] [Google Scholar]
- 254.Tokarz J., Mindnich R., Norton W., Möller G., Hrabé de Angelis M., Adamski J. Discovery of a novel enzyme mediating glucocorticoid catabolism in fish: 20beta-hydroxysteroid dehydrogenase type 2. Mol. Cell. Endocrinol. 2012;349:202–213. doi: 10.1016/j.mce.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 255.Morgan R.A., Beck K.R., Nixon M., Homer N.Z.M., Crawford A.A., Melchers D., Houtman R., Meijer O.C., Stomby A., Anderson A.J., Upreti R., Stimson R.H., Olsson T., Michoel T., Cohain A., Ruusalepp A., Schadt E.E., Björkegren J.L.M., Andrew R., Kenyon C.J., Hadoke P.W.F., Odermatt A., Keen J.A., Walker B.R. Carbonyl reductase 1 catalyzes 20β-reduction of glucocorticoids, modulating receptor activation and metabolic complications of obesity. Sci. Rep. 2017;7:10633. doi: 10.1038/s41598-017-10410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Eisenschmid B., Heilmann P., Oelkers W., Rejaibi R., Schöneshöfer M. 20-dihydroisomers of cortisol and cortisone in human urine: excretion rates under different physiological conditions. J. Clin. Chem. Clin. Biochem. 1987;25:345–349. doi: 10.1515/cclm.1987.25.6.345. http://www.ncbi.nlm.nih.gov/pubmed/3625132 [DOI] [PubMed] [Google Scholar]
- 257.Burstein S., Savard K., Dorfman R.I. The in vivo metabolism of hydrocortisone. Endocrinology. 1953;52:88–97. doi: 10.1210/endo-53-1-88. https://academic.oup.com/endo/article-abstract/53/1/88/2759520 [DOI] [PubMed] [Google Scholar]
- 258.Shackleton C.H.L., Neres M.S., Hughes B.A., Stewart P.M., Kater C.E. 17-Hydroxylase/C17, 20-lyase (CYP17) is not the enzyme responsible for side-chain cleavage of cortisol and its metabolites. Steroids. 2008;73:652–656. doi: 10.1016/j.steroids.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 259.Ged C., Rouillon J.M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br. J. Clin. Pharmacol. 1989;28:373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. http://www.ncbi.nlm.nih.gov/pubmed/2590599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Joellenbeck L., Qian Z., Zarba A., Groopman J.D. Urinary 6 beta-hydroxycortisol/cortisol ratios measured by high-performance liquid chromatography for use as a biomarker for the human cytochrome P-450 3A4. Cancer Epidemiol. Biomarkers Prev. 2018;1:567–572. http://www.ncbi.nlm.nih.gov/pubmed/1302569 (n.d.) [PubMed] [Google Scholar]
- 261.Lenders J.W.M., Williams T.A., Reincke M., Gomez-Sanchez C.E. 18-oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids? Eur. J. Endocrinol. 2018;178:R1–R9. doi: 10.1530/EJE-17-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gomez-Sanchez C.E., Gomez-Sanchez E.P., Smith J.S., Ferris M.W., Foecking M.F. Receptor binding and biological activity of 18 oxocortisol. Endocrinology. 1985;116:6–10. doi: 10.1210/endo-116-1-6. [DOI] [PubMed] [Google Scholar]
- 263.Geller D.S., Zhang J., Wisgerhof M.V., Shackleton C., Kashgarian M., Lifton R.P. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lifton R.P., Dluhy R.G., Powers M., Rich G.M., Cook S., Ulick S., Lalouel J.-M. A chimaeric llβ-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 265.Pascoe L., Curnow K.M., Slutsker L., Connell J.M., Speiser P.W., New M.I., White P.C. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Choi M., Scholl U.I., Yue P., Björklund P., Zhao B., Nelson-Williams C., Ji W., Cho Y., Patel A., Men C.J., Lolis E., Wisgerhof M.V., Geller D.S., Mane S., Hellman P., Westin G., Åkerström G., Wang W., Carling T., Lifton R.P. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rich G.M., Ulick S., Cook S., Wang J.Z., Lifton R.P., Dluhy R.G. Glucocorticoid-remediable aldosteronism in a large kindred: clinical spectrum and diagnosis using a characteristic biochemical phenotype. Ann. Intern. Med. 1992;116:813. doi: 10.7326/0003-4819-116-10-813. [DOI] [PubMed] [Google Scholar]
- 268.Litchfield W.R., New M.I., Coolidge C., Lifton R.P., Dluhy R.G. Evaluation of the dexamethasone suppression test for the diagnosis of glucocorticoid-remediable aldosteronism 1 J . Clin. Endocrinol. Metab. 1997;82:3570–3573. doi: 10.1210/jcem.82.11.4381. [DOI] [PubMed] [Google Scholar]
- 269.Mosso L., Gómez-Sánchez C.E., Foecking M.F., Fardella C. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertens. (Dallas, Tex. 1979) 2001;38:688–691. doi: 10.1161/01.hyp.38.3.688. http://www.ncbi.nlm.nih.gov/pubmed/11566957 [DOI] [PubMed] [Google Scholar]
- 270.Litchfield W.R. Glucocorticoid-remediable aldosteronism. Curr Opin Endocrinol Diabetes. 1996;3:265–267. https://ci.nii.ac.jp/naid/10009565225/ [Google Scholar]
- 271.Nakamura Y., Satoh F., Morimoto R., Kudo M., Takase K., Gomez-Sanchez C.E., Honma S., Okuyama M., Yamashita K., Rainey W.E., Sasano H., Ito S. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J. Clin. Endocrinol. Metab. 2011;96:E1272–E1278. doi: 10.1210/jc.2010-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Eisenhofer G., Dekkers T., Peitzsch M., Dietz A.S., Bidlingmaier M., Treitl M., Williams T.A., Bornstein S.R., Haase M., Rump L.C., Willenberg H.S., Beuschlein F., Deinum J., Lenders J.W.M., Reincke M. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin. Chem. 2016;62:514–524. doi: 10.1373/clinchem.2015.251199. [DOI] [PubMed] [Google Scholar]
- 273.Palermo M., Gomez-Sanchez C., Roitman E., Shackleton C.H.L. Quantitation of cortisol and related 3-oxo-4-ene steroids in urine using gas chromatography/mass spectrometry with stable isotope-labeled internal standards. Steroids. 1996;61:583–589. doi: 10.1016/s0039-128x(96)00118-9. [DOI] [PubMed] [Google Scholar]
- 274.Marcos J., Renau N., Casals G., Segura J., Ventura R., Pozo O.J. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal. Chim. Acta. 2014;812:92–104. doi: 10.1016/j.aca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 275.Raff H., Auchus R.J., Findling J.W., Nieman L.K. Urine Free cortisol in the diagnosis of cushing’s syndrome: Is It worth doing and, If so, how? J. Clin. Endocrinol. Metab. 2015;100:395–397. doi: 10.1210/jc.2014-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Labrie F., Luu-The V., Labrie C., Bélanger A., Simard J., Lin S.-X.X., Pelletier G., Bélanger A., Simard J., Lin S.-X.X., Pelletier G., Bélanger A., Simard J., Lin S.-X.X., Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr. Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 277.Burger H.G. Androgen production in women. Fertil. Steril. 2002;77(Suppl 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 278.Morley J.E., Perry H.M.I.I.I. Androgens and women at the menopause and beyond. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2003;58A:409–416. doi: 10.1093/gerona/58.5.m409. [DOI] [PubMed] [Google Scholar]
- 279.Hammer F., Subtil S., Lux P., Maser-Gluth C., Stewart P.M., Allolio B., Arlt W. No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 2005;90:3600–3605. doi: 10.1210/jc.2004-2386. [DOI] [PubMed] [Google Scholar]
- 280.Miller W.L. Steroidogenesis: unanswered questions. Trends Endocrinol. Metab. 2017;28:771–793. doi: 10.1016/j.tem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 281.Labrie F., Bélanger A., Pelletier G., Martel C., Archer D.F., Utian W.H. Science of intracrinology in postmenopausal women. Menopause. 2017;24:702–712. doi: 10.1097/GME.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 282.Laughlin G.A., Barrett-Connor E., Kritz-Silverstein D., von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo study 1. J. Clin. Endocrinol. Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 283.Massafra C., De Felice C., Agnusdei D.P., Gioia D., Bagnoli F. Androgens and osteocalcin during the menstrual cycle. J. Clin. Endocrinol. Metab. 1999;84:971–974. doi: 10.1210/jcem.84.3.5512. [DOI] [PubMed] [Google Scholar]
- 284.Abraham G.E. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. Endocrinol. Metab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- 285.Anderson D.C. Sex‐hormone binding-globulin. Clin. Endocrinol. (Oxf). 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 286.Mendel C.M. The Free hormone hypothesis: a physiologically based mathematical model. Endocr. Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 287.Hammond G.L. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J. Endocrinol. 2016;230:R13–R25. doi: 10.1530/JOE-16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Turcu A.F., Nanba T., Auchus R.J., Rise The. Fall, and resurrection of 11-oxygenated androgens in human physiology and disease. Horm. Res. Paediatr. 2018;89:284–291. doi: 10.1159/000486036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nanba A.T., Rege J., Ren J., Auchus R.J., Rainey W.E., Turcu A.F. 11-oxygenated C19 steroids Do not decline with age in women. J. Clin. Endocrinol. Metab. 2019;104(7):2615–2622. doi: 10.1210/jc.2018-02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sten T., Kurkela M., Kuuranne T., Leinonen A., Finel M. UDP-glucuronosyltransferases in conjugation of 5alpha- and 5beta-androstane steroids. Drug Metab. Dispos. 2009;37:2221–2227. doi: 10.1124/dmd.109.029231. [DOI] [PubMed] [Google Scholar]
- 291.Sten T., Bichlmaier I., Kuuranne T., Leinonen A., Yli-Kauhaluoma J., Finel M. UDP-glucuronosyltransferases (UGTs) 2B7 and UGT2B17 display converse specificity in testosterone and epitestosterone glucuronidation, whereas UGT2A1 conjugates both androgens similarly. Drug Metab. Dispos. 2009;37:417–423. doi: 10.1124/dmd.108.024844. [DOI] [PubMed] [Google Scholar]
- 292.Tchernof A., Labrie F., Bélanger A., Prud’homme D., Bouchard C., Tremblay A., Nadeau A., Després J.-P. Androstane-3α,17β-diol glucuronide as a steroid correlate of visceral obesity in men. J. Clin. Endocrinol. Metab. 1997;82:1528–1534. doi: 10.1210/jcem.82.5.3924. [DOI] [PubMed] [Google Scholar]
- 293.Tchernof A., Lévesque E., Beaulieu M., Couture P., Després J.P., Hum D.W., Bélanger A. Expression of the androgen metabolizing enzyme UGT2B15 in adipose tissue and relative expression measurement using a competitive RT-PCR method. Clin. Endocrinol. (Oxf) 1999;50:637–642. doi: 10.1046/j.1365-2265.1999.00709.x. http://www.ncbi.nlm.nih.gov/pubmed/10468930 [DOI] [PubMed] [Google Scholar]
- 294.Strott C.A. Steroid sulfotransferases. Endocr. Rev. 1996;17:670–697. doi: 10.1210/edrv-17-6-670. [DOI] [PubMed] [Google Scholar]
- 295.Chang H.-J., Shi R., Rehse P., Lin S.-X. Identifying androsterone (ADT) as a cognate substrate for human dehydroepiandrosterone sulfotransferase (DHEA-ST) important for steroid homeostasis. J. Biol. Chem. 2004;279:2689–2696. doi: 10.1074/jbc.M310446200. [DOI] [PubMed] [Google Scholar]
- 296.Janne O., Vihko R. Undefined 1970, neutral steroids in urine during pregnancy. I. Isolation and identification of dihydroxylated C19 and C21 steroid disulphates. Acta Endocrinol. (Copenh) 1970;65:50–68.. [PubMed] [Google Scholar]
- 297.Laatikainen T., Peltonen J., Nylander P. Determination of estriol, estriol sulfate, progesterone and neutral steroid mono- and disulfates in umbilical cord blood plasma. Steroids. 1973;21:347–359. doi: 10.1016/0039-128x(73)90029-9. [DOI] [PubMed] [Google Scholar]
- 298.Meng L.-J., Sjövall J. Method for combined analysis of profiles of conjugated progesterone metabolites and bile acids in serum and urine of pregnant women. J. Chromatogr. B Biomed. Sci. Appl. 1997;688:11–26. doi: 10.1016/s0378-4347(97)88051-6. [DOI] [PubMed] [Google Scholar]
- 299.Torimoto N., Ishii I., Toyama K.-I., Hata M., Tanaka K., Shimomura H., Nakamura H., Ariyoshi N., Ohmori S., Kitada M. Helices F-G are important for the Substrate specificities of CYP3A7. Drug Metab. Dispos. 2006;35:484–492. doi: 10.1124/dmd.106.011304. [DOI] [PubMed] [Google Scholar]
- 300.du Toit T., Swart A.C. Inefficient UGT-conjugation of adrenal 11β-hydroxyandrostenedione metabolites highlights C11-oxy C19 steroids as the predominant androgens in prostate cancer. Mol. Cell. Endocrinol. 2018;461:265–276. doi: 10.1016/j.mce.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 301.Towobola O.A., Crilly R.C., Oakey R.E. Oestrone sulphate in plasma from postmenopausal women and the effects of oestrogen and androgen therapy. Clin. Endocrinol. (Oxf) 1980;13:461–471. doi: 10.1111/j.1365-2265.1980.tb03412.x. http://www.ncbi.nlm.nih.gov/pubmed/6261992 [DOI] [PubMed] [Google Scholar]
- 302.Simpson E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 303.Simpson E.R., Misso M., Hewitt K.N., Hill R.A., Boon W.C., Jones M.E., Kovacic A., Zhou J., Clyne C.D. Estrogen—the Good, the Bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 304.Simpson E., Rubin G., Clyne C., Robertson K., O’Donnell L., Jones M., Davis S. The role of local estrogen Biosynthesis in males and females. Trends Endocrinol. Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 305.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015;145:133–138. doi: 10.1016/j.jsbmb.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 306.Stricker R., Eberhart R., Chevailler M.-C., Quinn F.A., Bischof P., Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the abbott ARCHITECT analyzer. Clin. Chem. Lab. Med. 2006;44:883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 307.Rothman M.S., Carlson N.E., Xu M., Wang C., Swerdloff R., Lee P., Goh V.H.H., Ridgway E.C., Wierman M.E. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography–tandem mass spectrometry. Steroids. 2011;76:177–182. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fuhrman B.J., Xu X., Falk R.T., Dallal C.M., Veenstra T.D., Keefer L.K., Graubard B.I., Brinton L.A., Ziegler R.G., Gierach G.L. Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid Chromatography–Tandem mass spectrometry. Cancer Epidemiol. Prev. Biomarkers. 2014;23:2649–2657. doi: 10.1158/1055-9965.EPI-14-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Muir M., Romalo G., Wolf L., Elger W., Schweikert H.-U. Estrone sulfate Is a Major source of local estrogen formation in human bone. J. Clin. Endocrinol. Metab. 2004;89:4685–4692. doi: 10.1210/jc.2004-0049. [DOI] [PubMed] [Google Scholar]
- 310.Reed M.J., Purohit A., Woo L.W.L., Newman S.P., Potter B.V.L. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 311.Peltoketo H., Luu-The V., Simard J., Adamski J. 17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; Nomenclature and main characteristics of the 17HSD/KSR enzymes. J. Mol. Endocrinol. 1999;23:1–11. doi: 10.1677/jme.0.0230001. [DOI] [PubMed] [Google Scholar]
- 312.Miettinen M.M., Mustonen M.V., Poutanen M.H., Isomaa V.V., Vihko R.K. Human 17 beta-hydroxysteroid dehydrogenase type 1 and type 2 isoenzymes have opposite activities in cultured cells and characteristic cell- and tissue-specific expression. Biochem. J. 1996;314:839–845. doi: 10.1042/bj3140839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Labrie F., Luu-The V., Lin S.-X., Claude L., Simard J., Breton R., Bélanger A. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 314.Thomas M.P., Potter B.V.L. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013;137:27–49. doi: 10.1016/j.jsbmb.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Huang Z., Guengerich F.P., Kaminsky L.S. 16Alpha-hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis. 1998;19:867–872. doi: 10.1093/carcin/19.5.867. [DOI] [PubMed] [Google Scholar]
- 316.Cribb A.E., Knight M.J., Dryer D., Guernsey J., Hender K., Tesch M., Saleh T.M. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol. Biomarkers Prev. 2006;15:551–558. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 317.Badawi A.F., Cavalieri E.L., Rogan E.G. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 318.Henderson B.E., Ponder B.A.J., Ross R.K. Oxford University Press; USA: 2003. Hormones, Genes, and Cancer. [Google Scholar]
- 319.Yamazaki H., Shaw P.M., Guengerich F.P., Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem. Res. Toxicol. 1998;11:659–665. doi: 10.1021/tx970217f. [DOI] [PubMed] [Google Scholar]
- 320.Hanna I.H., Dawling S., Roodi N., Guengerich F.P., Parl F.F. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000;60:3440–3444. http://www.ncbi.nlm.nih.gov/pubmed/10910054 [PubMed] [Google Scholar]
- 321.Nishida C.R., Everett S., Ortiz de Montellano P.R. Specificity determinants of CYP1B1 estradiol hydroxylation. Mol. Pharmacol. 2013;84:451–458. doi: 10.1124/mol.113.087700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tsuchiya Y., Nakajima M., Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 323.Zhu B., Conney A.H. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 324.Lee A.J., Kosh J.W., Conney A.H., Zhu B.T. Characterization of the NADPH-dependent metabolism of 17-estradiol to multiple metabolites by human liver microsomes and selectively expressed human cytochrome P450 3A4 and 3A5. J Pharmacol Exp Ther. 2001 http://jpet.aspetjournals.org [PubMed] [Google Scholar]
- 325.Tikkanen M.J. Urinary excretion of estriol conjugates in normal pregnancy. J. Steroid Biochem. 1973;4:57–63. doi: 10.1016/0022-4731(73)90080-0. [DOI] [PubMed] [Google Scholar]
- 326.Musey P.I., Collins D.C., Preedy J.R.K. Separation of estrogen conjugates by high pressure liquid chromatography. Steroids. 1978;31:583–592. doi: 10.1016/0039-128x(78)90039-9. [DOI] [PubMed] [Google Scholar]
- 327.Wang L.-Q., James M.O. Sulfotransferase 2A1 forms estradiol-17-sulfate and celecoxib switches the dominant product from estradiol-3-sulfate to estradiol-17-sulfate. J. Steroid Biochem. Mol. Biol. 2005;96:367–374. doi: 10.1016/j.jsbmb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 328.Zhang H., Varlamova O., Vargas F.M., Falany C.N., Leyh T.S., Varmalova O. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J. Biol. Chem. 1998;273:10888–10892. doi: 10.1074/jbc.273.18.10888. [DOI] [PubMed] [Google Scholar]
- 329.Falany C.N., Krasnykh V., Falany J.L. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J. Steroid Biochem. Mol. Biol. 1995;52:529–539. doi: 10.1016/0960-0760(95)00015-r. [DOI] [PubMed] [Google Scholar]
- 330.Kallionpää R.A., Järvinen E., Finel M. Glucuronidation of estrone and 16α-hydroxyestrone by human UGT enzymes: the key roles of UGT1A10 and UGT2B7. J. Steroid Biochem. Mol. Biol. 2015;154:104–111. doi: 10.1016/j.jsbmb.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 331.Sneitz N., Vahermo M., Mosorin J., Laakkonen L., Poirier D., Finel M. Regiospecificity and stereospecificity of human UDP-glucuronosyltransferases in the glucuronidation of estriol, 16-epiestriol, 17-epiestriol, and 13-epiestradiol. Drug Metab. Dispos. 2013;41:582–591. doi: 10.1124/dmd.112.049072. [DOI] [PubMed] [Google Scholar]
- 332.Lépine J., Bernard O., Plante M., Têtu B., Pelletier G., Labrie F., Bélanger A., Guillemette C. Specificity and regioselectivity of the conjugation of estradiol, estrone, and their catecholestrogen and methoxyestrogen metabolites by human uridine diphospho-glucuronosyltransferases expressed in endometrium. J. Clin. Endocrinol. Metab. 2004;89:5222–5232. doi: 10.1210/jc.2004-0331. [DOI] [PubMed] [Google Scholar]
- 333.Stanczyk F.Z., Henzl M.R. Use of the name “Progestin”. Contraception. 2001;64:1–2. doi: 10.1016/s0010-7824(01)00222-0. [DOI] [PubMed] [Google Scholar]
- 334.Strott C.A., Yoshimi T., Lipsett M.B. Plasma progesterone and 17-hydroxyprogesterone in normal men and children with congenital adrenal hyperplasia. J. Clin. Invest. 1969;48:930–939. doi: 10.1172/JCI106052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Benassayag C., Souski I., Mignot T.-M., Robert B., Hassid J., Duc-Goiran P., Mondon F., Rebourcet R., Dehennin L., Nunez E.-A., Ferré F. Corticosteroid-binding globulin Status at the fetomaternal interface during human term Pregnancy1. Biol. Reprod. 2001;64:812–821. doi: 10.1095/biolreprod64.3.812. [DOI] [PubMed] [Google Scholar]
- 336.Wiswell J.G., Samuels L.T. The metabolism of progesterone by liver tissue in vitro. J. Biol. Chem. 1953;201:155–160. http://www.ncbi.nlm.nih.gov/pubmed/13044784 [PubMed] [Google Scholar]
- 337.Stanczyk F.Z. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 338.Chantilis S., Dombroski R., Shackleton C.H., Casey M.L., MacDonald P.C. Metabolism of 5 alpha-dihydroprogesterone in women and men: 3 beta- and 3 alpha-,6 alpha-dihydroxy-5 alpha-pregnan-20-ones are major urinary metabolites. J. Clin. Endocrinol. Metab. 1996;81:3644–3649. doi: 10.1210/jcem.81.10.8855816. [DOI] [PubMed] [Google Scholar]
- 339.Shackleton C.H.L. Profiling steroid hormones and urinary steroids. J Chromatogr. 1986;379:91–156. doi: 10.1016/s0378-4347(00)80683-0. [DOI] [PubMed] [Google Scholar]
- 340.Shackleton C.H. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J. Steroid Biochem. Mol. Biol. 1993;45:127–140. doi: 10.1016/0960-0760(93)90132-g. http://www.ncbi.nlm.nih.gov/pubmed/8481337 [DOI] [PubMed] [Google Scholar]
- 341.Shackleton C., Pozo O.J., Marcos J. GC/MS in recent years has defined the Normal and clinically disordered steroidome: will It Soon Be surpassed by LC/Tandem MS in this role? J. Endocr. Soc. 2018;2:974–996. doi: 10.1210/js.2018-00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Storbeck K.-H., Gilligan L., Jenkinson C., Baranowski E.S., Quanson J.L., Arlt W., Taylor A.E. The utility of ultra-high performance supercritical fluid chromatography–tandem mass spectrometry (UHPSFC-MS/MS) for clinically relevant steroid analysis. J. Chromatogr. B. 2018;1085:36–41. doi: 10.1016/j.jchromb.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 343.Auchus R.J. Steroid assays and endocrinology: Best practices for basic scientists. Endocrinology. 2014;155:2049–2051. doi: 10.1210/en.2014-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hines J.M., Bancos I., Bancos C., Singh R.D., Avula A.V., Young W.F., Grebe S.K., Singh R.J. High-Resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin. Chem. 2017;63:1824–1835. doi: 10.1373/clinchem.2017.271106. [DOI] [PubMed] [Google Scholar]
- 345.Kulle A., Krone N., Holterhus P.M., Schuler G., Greaves R.F., Juul A., De Rijke Y.B., Hartmann M.F., Saba A., Hiort O., Wudy S.A. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of Eu COST action BM 1303 “DSDnet”. Eur. J. Endocrinol. 2017;176(5) doi: 10.1530/EJE-16-0953. P1–P9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wudy S.A., Schuler G., Sánchez-Guijo A., Hartmann M.F. The art of measuring steroids: principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018;179:88–103. doi: 10.1016/j.jsbmb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 347.Liere P., Schumacher M. Mass spectrometric analysis of steroids: all that glitters is not gold. Expert Rev. Endocrinol. Metab. 2015;6651:3–6. doi: 10.1586/17446651.2015.1063997. [DOI] [PubMed] [Google Scholar]
- 348.Arlt W., Biehl M., Taylor A.E., Hahner S., Libe R., Hughes B.A., Schneider P., Smith D.J., Stiekema H., Krone N., Libé R., Hughes B.A., Schneider P., Smith D.J., Stiekema H., Krone N., Porfiri E., Opocher G., Bertherat J., Mantero F., Allolio B., Terzolo M., Nightingale P., Shackleton C.H.L., Bertagna X., Fassnacht M., Stewart P.M. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011;96:3775–3784. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]