Abstract
Worldwide, the prevalence of diabetes remains high. Studies have shown that iron deficiency anemia (IDA) is associated with increased levels of glycated hemoglobin A1c (HbA1c), but the mechanism remains unclear. Hematological changes, iron metabolism, study methodology, and other factors could affect the results of diagnostic investigations, leading to false results. Red blood cell turnover in the bone marrow and the quality and heterogeneity of erythrocytes may influence the rate of hemoglobin glycation. By changing the structure of hemoglobin and inducing peroxidation, iron deficiency accelerates glycation. This review aims to discuss the possible causes of the association between increased levels of HbA1c and IDA.
MeSH Keywords: Anemia, Iron-Deficiency; Diabetes Mellitus, Type 2; Health Care Evaluation Mechanisms
Background
Worldwide, the prevalence of type 2 diabetes mellitus remains high [1]. Type 2 diabetes results in a significant public health burden, reduces the quality of life and results in great suffering and economic burden to patients [2]. The measurement of glycated forms of hemoglobin are the products of a non-enzymatic reaction between hemoglobin and glucose and reflect the average plasma glucose from the previous two or three months [3]. Glycated hemoglobin A1c (HbA1c) is a major component of glycated hemoglobin and is widely used for screening, diagnosis, and monitoring of hyperglycemia. Compared with the oral glucose tolerance test (OGTT), which is the diagnostic gold standard [4], measurement of HbA1c levels is rapid and convenient and shows minimal effects from physiological and pharmacological conditions [5–8]. HbA1c levels also reflect the progression of diabetic microvascular lesions [9]. Therefore, HbA1c in diabetes mellitus has been widely studied [10].
In 2009, the American Diabetes Association (ADA) recommended that HbA1c ≥6.5% should be used as diagnostic criteria for diabetes [11], particularly when sustained [5,12,13]. However, physicians have argued that HbA1c results could be significantly affected by several factors, including the method of detection, other comorbidities, and patient medications and that HbA1c did not always reflect plasma glucose levels [3,14,15]. The recommendation from the ADA was that, ‘For conditions with abnormal red cell turnover, such as anemias from hemolysis and iron deficiency, the diagnosis of diabetes must employ glucose criteria exclusively’ [11].
However, reliance on the measurement of HbA1c alone in patients with diabetes mellitus remains controversial, and studies have shown that iron deficiency anemia (IDA) can also lead to falsely high values for HbA1c, but the mechanism for this remains unclear [16,17]. Therefore, this review aims to discuss the possible causes of the association between increased levels of HbA1c and IDA.
Overview: Glycated Hemoglobin A1c (HbA1c) and Iron Deficiency Anemia (IDA)
The types of hemoglobin found in adults include hemoglobin A (HbA) (95–98%), hemoglobin A2 (HbA2) (2–3%), and fetal hemoglobin (HbF) (1%) [18]. Also, HbA0, HbA1a1, HbA1a2, HbA1b, and HbA1c are subtypes of HbA that can be identified by electrophoresis. HbA1c represents 70–90% of HbA1, and is the glycated form of HbA1. HbA1c results from the glycosylation of the hemoglobin β chain N-terminal of proline [8], which reflects the plasma glucose concentration. The HbA1c concentration is influenced primarily by both hemoglobin and plasma glucose concentration. However, studies have shown that factors such as race, age, diet, medication and concomitant diseases may also alter HbA1c levels [18].
Iron deficiency is a pathophysiological state characterized by insufficient iron storage due to inadequate iron intake, impaired utilization, or excess loss. The most universally used diagnostic criteria for iron deficiency include the measurement of levels of ferritin, serum iron, and transferrin saturation. In addition to the biological activity, iron is an important component of hemoglobin. As iron deficiency worsens, hematopoiesis is impaired and leads to IDA. Worldwide, IDA accounts for one-third of cases of anemia [19], is recognized as the most common type of anemia, and is a serious public health issue, particularly in developing countries. Fatigue, tachycardia, pica, and growth retardation are typical signs and symptoms of IDA. Severe IDA leads to impairment of the nervous system and prevents cognitive development among infants and children [20–23]. Chinese healthcare services focus on early diagnosis and treatment of IDA, especially of children, adolescents, and women of reproductive age.
Current Research Findings
Epidemiological and clinical studies have shown that IDA causes an increase of HbA1c, irrespective of plasma glucose levels [8,16,17,24–28]. After collecting data of 6,666 women and 3,869 men from the US National Health and Nutrition Survey (1999–2006), Kim et al. [8] found that the HbA1c of women with iron deficiency without anemia (n=1,150) increased slightly (from <5.5% to ≥5.5%) independent of fasting glucose. Attard et al. [6] analyzed large data from the Chinese health and nutrition survey, which showed that men with iron deficiency alone or IDA had an increased relative risk of being diagnosed with prediabetes using HbA1c alone when compared with using both HbA1c and fasting blood glucose as the diagnostic criteria. However, studies have shown inconsistent results. A meta-analysis study showed that iron deficiency and IDA had no influence on the levels of HbA1c [29]. A study also showed that the HbA1c levels of non-diabetic elderly individuals [30] and Dutch children with type 1 diabetes [31] were unrelated to hemoglobin levels. Christy et al. [24] compared patients with glucose-controlled diabetes and IDA with a control group and found no association with ferritin or hemoglobin in either group.
Previously published studies have shown that the effect of iron deficiency and IDA on HbA1c levels occurred independently [6,32]. Attard et al. [6] studied the levels of HbA1c and fasting blood glucose as diagnostic criteria to evaluate the effect of iron deficiency and IDA on diabetes. The results showed that iron deficiency and IDA caused changes in HbA1c levels, which were not consistent with the actual disease state [6]. For example, women with iron deficiency had a lower relative risk (RR) of being diagnosed with diabetes using HbA1c compared with non-diabetics (RR ratio=0.52; 95% CI, 0.29–0.95%), and men with IDA had higher RR of being diagnosed with diabetes using both HbA1c and fasting blood glucose (RR ratio=2.38; 95% CI, 20–4.72%) [6]. The authors concluded that iron deficiency led to under-diagnosis, while IDA contributed to misdiagnosis [6]. Study investigators have reported that these findings were due to multifactorial reasons that included erythrocyte renewal, the altered conformation of hemoglobin, and glycation rate [17,25].
Anemia is characterized by a shorter erythrocyte life span, reduced hemoglobin concentration, and compensatory hyperplasia [33]. All these factors exert significant changes in the production of HbA1c. Iron deficiency has been reported to be independently associated with increased HbA1c, regardless of plasma glucose levels and the degree of anemia [8,26]. Studies have shown a significant reduction of HbA1c in non-diabetic patients with controlled plasma glucose levels after administration of iron supplements [34–36]. However, other studies have shown no correlation between markers of iron storage (ferritin) and increased HbA1c [24].
Authors of previously published studies have explored the important causes of the association between increased levels of HbA1c and IDA, and have suggested that the inconsistent findings might be due to the use of different methods for the measurement of HbA1c [7,37], and patient heterogeneity (Figure 1) [6]. For these reasons, some authors have recommended locally conducted clinical trials [6], while others have proposed large-scale global studies using standardized calibrated and verified measurement methods.
Figure 1.
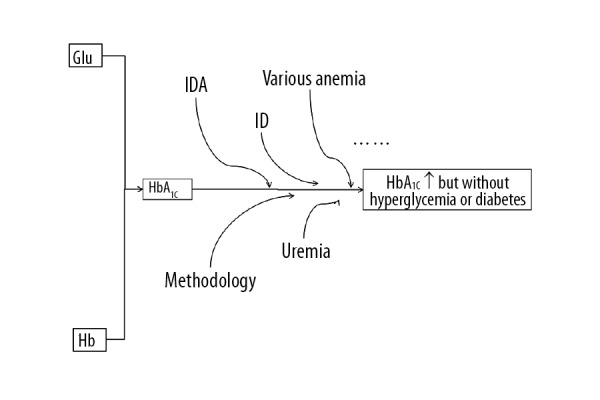
Current research studies on the association between increased levels of glycated hemoglobin A1c (HbA1c) and iron deficiency anemia (IDA).
Possible Causes of Increased Levels of HbA1c in IDA
Hemoglobin and the production of erythrocytes
The rate of formation of HbA1c is usually linear at a certain plasma glucose level [32], and the turnover rate of erythrocytes affects the level of HbA1c [33]. For circulating erythrocytes that remain in the plasma for a longer time, increased glycation can be expected to occur [32,34,38–40]. Wu et al. [41] used ion-exchange resin microparticles to determine the levels of glycated hemoglobin in patients with aplastic anemia. In four cases, glycated hemoglobin levels declined between 3–14 days after transfusion, and the authors inferred that this was because erythrocytes of patients with aplastic anemia had a low proportion of young erythrocytes which were unable to glycate [41]. The authors recommended that glycated hemoglobin could be regarded as an indicator of erythrocyte formation in aplastic anemia [41]. However, it is believed that transfusion has a greater impact on the overall status of erythrocytes, and this study did not allow enough time after transfusion to evaluate the actual levels of HbA1c [41]. However, previously published studies have shown that the average plasma glucose, glycated albumin, and fructosamine were more reliable than HbA1c in the setting of a changed lifespan of erythrocytes [42]. For example, patients with poorly-controlled diabetes with hereditary spherocytosis could have 379 mg/dl of plasma glucose with a HbA1c level of only 6.9%.
Sehrawat et al. [14] evaluated the diagnostic efficacy of HbA1c in patients with diabetes and liver cirrhosis with varying degrees of anemia. HbA1c was shown to be a less accurate monitoring test for glycemic control, and its sensitivity was higher in individuals with normal hemoglobin levels or with mild anemia (82.4%; 95% CI, 56.6–96.2%) when compared with patients who had moderate or severe anemia (72.2%; 95% CI, 46.5–90.3%) [14]. A similar trend was also found for specificity of the former group (96%; 95% CI, 79.7–99.9%) when compared with the latter one (86.9%; 95% CI, 71.9–95.6%), and area under the receiver operator characteristic curves were 0.9 (95% CI, 79–100%) and 0.8 (95% CI, 65–94%), respectively (P>0.05) [14]. These findings supported that the degree of anemia affected the levels of HbA1c [14].
In the study reported by Xia et al. [43], a patient with paroxysmal nocturnal hemoglobinuria showed a decline in HbA1c from more than 90 mmol/mol to 51 mmol/mol (normal range, 48–59 mmol/mol) with increased lactate dehydrogenase (LDH) levels and low haptoglobin levels, which indicated compensated hemolytic anemia. The results may have indicated suboptimal control of diabetes, with a random glucose of 11.0 mmol/L (normal range, 3.9–5.6 mmol/L), glycated albumin of 1.5% (normal range, 0.8–1.4%), fructosamine of 0.34 mmol/L (normal range, 0.19–0.27 mmol/L) [43]. These findings were most likely to have been due to reduced lifespan of erythrocytes caused by hemolysis [43]. Silva et al. [37] showed a negative correlation between HbA1c levels and hemoglobin, hematocrit, mean corpuscular volume (MCV), and ferritin (r=−0.557, r=−0.539, r=−0.488, r=−0.499) (P<0.01) using immunoturbidimetry, which were even weaker when high performance liquid chromatography (HPLC) was used (r=−0.272, r=−0.25, r=−0.273, r=−0.229) (P<0.05).
Rusak et al. [44] demonstrated a positive correlation between HbA1c levels and mean corpuscular volume (MCV) (r=0.31, P<0.01) and a negative correlation with mean corpuscular hemoglobin concentration (MCHC) (r=−0.33, P<0.001), respectively in children with type 1 diabetes. Simmons et al. [32] found that HbA1c of patients with a mean hemoglobin concentration (MCH) corresponded to the upper and lower reference limits of HbA1c and differed by about 5 mmol/mol. There was a gradient change from a mean HbA1c of 36 mmol/mol (95% CI, 34–38 mmol/mol) with MCHC ≤320 g/L to 30 mmol/mol (95% CI, 29–31 mmol/mol) and MCHC >370 g/L [32]. Studies conducted on non-diabetic patients with hemolytic anemia have shown that hemolysis shortened erythrocyte lifespan [42] and destroyed mature erythrocytes, leading to a reduction in HbA1c levels [45].
However, there have been controversial and differing study findings. Rajagopal et al. [28] reported that the HbA1c level increased as the severity of anemia increased. In 2018, Nakatani et al. [46] reported a case that studied glycemic control in a 41-year-old man who had dehydrated hereditary stomatocytosis with a PIEZO1 gene mutation associated with diabetes mellitus cirrhosis of the liver that was due to hemochromatosis. The estimated HbA1c was calculated from average glucose levels during continuous glucose monitoring to determine glycemic control, and glycated albumin levels were measured [46]. In this patient, HbA1c and glycated albumin were unreliable because of coexisting conditions [46].
Pernicious anemia, due to deficiency of vitamin B12, is characterized by secondary hemolysis [8]. The high concentrations of urea lead to the formation of carbamylated hemoglobin [47,48], which is a reaction that is similar to glycation. The isoelectric point of carbamylated hemoglobin is close to that of HbA1c [48]. Also, urea competes with glucose for the hemoglobin β chain N-terminal proline, reducing the concentration of HbA1c [49].
These studies support the impact of hematological factors on the measurement of HbA1c, which include the number of erythrocytes, their turnover, and lifespan. Although many clinical laboratories report HbA1c results as a percentage value, it is currently recommended that the absolute value is used (in mmol/mol) to minimize the effect of hemodilution [50]. Also, it is recommended that hematological studies are initially performed to provide a more precise clinical interpretation of the HbA1c results [32].
Heterogeneity of hemoglobin
The consequences of heterogeneity of HbA1c have previously been observed from discrepancies between the measured values and the predicted values of HbA1c [51]. Hemoglobin heterogeneity has been shown to affect HbA1c independently of plasma glucose [47]. In an epidemiological study of sickle cell anemia by Lacy et al. [52], patients with sickle cell anemia were found to have lower levels of HbA1c. The most common hemoglobin variants, or mutant forms of hemoglobin, were found to have altered charge in the hemoglobin molecule, including for HbS, HbC, HbD, and HbE [29]. Hemoglobin variants lead to inconsistent results in the different methods used for the measurement of HbA1c. For example, HbA1c significantly increases when methods based on charge separation are used, such as ion-exchange HPLC [49,53]. Otabe et al. [51] undertook a study to determine whether the discrepancy between values for HbA1c determined by HPLC and enzymatic measurements in patients with diabetes was clinically relevant. In a study that included 1,421 outpatients who underwent treatment for diabetes and follow-up, HbA1c values measured by HPLC were significantly higher compared with measurements by enzymatic assay [51]. The authors recommended that HbA1c values should be interpreted with consideration of the possible presence of hemoglobin variants [51].
Also, the kinetics of hemoglobin glycation variants may differ from those of normal hemoglobin, and reactive sites may be altered due to structural modifications, which limits the number of NH2 groups that react with glucose [54]. Studies have shown that HbC, HbE, and HbF have different glycation rates, with HbA being the slowest [54]. Higgins et al. [55] emphasized that the heterogeneity of glycated hemoglobin significantly affected the levels of HbA1c due to methodological factors that affect the immunoassays and chromatography assays, resulting in variation in glycation rate, and reduced erythrocyte survival as, for example, erythrocytes with HbS survive for 14–28 days. However, previously published studies have shown that heterozygous heterogeneity does not necessarily result in hemolytic anemia and does not affect glycosylation [56]. For example, Cavagnolli et al. [29] found no significant difference in HbA1c between HbS carriers and non-carriers in the non-diabetic population, which indicated that HbA and HbS had similar glycation rates. Despite instruments manufactured to detect hemoglobin variants [57], there are still some limitations, and clinicians should verify the methods and consider the presence of hemoglobin variants [51].
We believe that the hemoglobin variants affect the glycation process and that methods for the measurement of HbA1c may yield different results. In 2011, the World Health Organization (WHO) highlighted that, worldwide, hemoglobin heterogeneity affected HbA1c measurements [58]. Although hemoglobin variants have a relatively low prevalence, it is advised to exert caution when interpreting the clinical findings of HbA1c levels (Figure 2).
Figure 2.
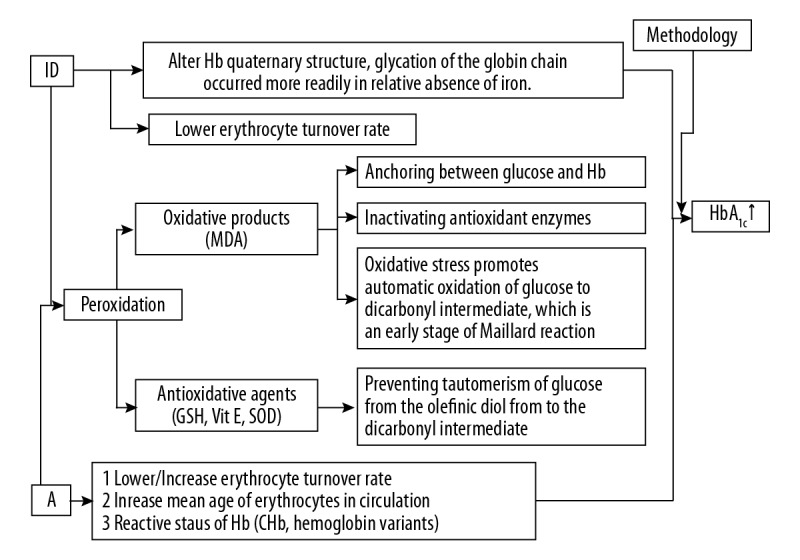
The possible causes of the association between increased levels of glycated hemoglobin A1c (HbA1c) and iron deficiency anemia (IDA).
Increased peroxidation induced by IDA
Iron deficiency promotes terminal proline glycation by changing the structure of hemoglobin [59], while lowering the erythrocyte turnover rate [17,25]. From the perspective of the pathogenesis of diabetes, iron deficiency may promote glycation by peroxidation. Peroxidation refers to the reaction in which reactive oxygen species cause damage. Oxygen radicals are unstable oxygen moieties that can react with molecules within cells. These active free radicals are involved in the destruction of pathogens, and the effects of immune reactions, including immune complexes. However, excess oxygen radicals will react with other molecules [60]. Iron deficiency can reduce the activity of iron-containing enzymes, which results in reduced antioxidant capacity [61].
Iron deficiency can induce peroxidation and accelerate glycation. By comparing 67 children with IDA and 31 controls from the Department of Pediatrics, Jawaharlal Nehru Medical College, Zaka-Ur-Rab et al. [62] found that biomarkers of oxidative stress, including malondialdehyde (MDA) were significantly increased (P<0.001), while the antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were significantly reduced in patients with IDA (P<0.001). Some of the children with IDA (n=35) received a daily conventional daily dose of iron (6 mg/kg), followed by a significant increase (P<0.001) in SOD, CAT, and GPx with a decline in MDA following iron supplementation (P<0.001) [62]. Sundaram [63] showed a significant correlation (r=0.6, P<0.01) in 22 patients with IDA between fructosamine and MDA using partial correlation analysis for the control of glucose. MDA and fructosamine levels decreased significantly in response to iron treatment (oral ferrous sulfate tablets, 200 mg) [63]. These findings supported the consensus view that lipid peroxides modulated the glycation reaction, and iron intervention was effective at this point [63].
Also, in support of these previous findings, Jain et al. [64] treated erythrocytes in vitro with increasing concentrations of a glucose solution mixed with hydrogen peroxide (H2O2), t-butyl hydroperoxide (TBH), and vitamin E to evaluate the degree of glycation. The levels of glycated hemoglobin increased and then decreased significantly in a dose-dependent manner after treatment with H2O2, TBH, and vitamin E, respectively [64]. The addition of MDA promoted the glycation rate, and the authors considered that MDA inactivated antioxidant enzymes with its aldehyde group acting as an anchor between glucose and hemoglobin [64]. The authors also hypothesized that oxidation promoted tautomerism, or intramolecular proton transfer, of glucose from the olefinic diol to the dicarbonyl intermediate, while antioxidants, such as glutathione, had the opposite effect [64]. Later, the same group modeled the glutathione-deficient erythrocyte [65] and used dithioerythritol (DTE)/dithiothreitol (DTT) as the glutathione supplement before the evaluation of glycated hemoglobin by both affinity and ion-exchange chromatography. The results showed a negative correlation between glutathione and glycated hemoglobin [65].
These findings indicate that peroxidation promotes glycation, and it is now recognized that the effect of iron deficiency on HbA1c levels is mediated through mechanisms that are linked to oxidative stress.
Methods Used to Measure HbA1c
Currently, there are up to 30 methods for the measurement of HbA1c, which can be classified into four types that include ion-exchange HPLC, affinity HPLC, immunoassay, and enzymatic methods [29]. Haliassos et al. [66] found no significant difference of HbA1c measured with ion-exchange HPLC and two immunoassay methods. Rai et al. [67] found no significant effect of from the type of method used to measure the HbA1c level. However, Shuichi et al. [51] showed that the HbA1c levels in 1421 patients with diabetes that were measured with HPLC were significantly higher when compared with those measured using enzymatic methods (P<0.0001). Little et al. [68] found only slight differences in the results between ion-exchange methods and HPLC and immunoassay. A meta-analysis reported by Cavagnolli et al. [29] showed that only certain methods were associated with a discrepancy in results and that charge-separation methods such as ion-exchange HPLC were more susceptible to variability when compared with other methods [49].
Goldstein et al. [69] showed that the levels of HbA1c measured by HPLC increased two hours after a standard breakfast, with the increase eliminated by incubating the erythrocytes in 0.9% saline at 37°C for 5 hours, a finding that was likely to have been due to labile HbA1c. However, reagents in newer enzymatic kits tend to eliminate this effect [24]. The approved International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) reference method for the measurement of HbA1c [56,70] includes the three steps of cleavage of hemoglobin into peptides, separation of glycated and non-glycated N-terminal hexapeptides by reversed-phase HPLC, and quantification of glycated hexapeptides by mass spectrometry or by capillary electrophoresis. The IFCC Working Group on HbA1c standardization came to the conclusion in a study conducted during 2001 to 2003 [71] that all three designated comparison methods used by the Japanese Diabetes Society (JDS)/Japanese Society of Clinical Chemistry (JSCC), the National Glycohemoglobin Standardization Program (NGSP) in the US and the Swedish standardization scheme generated significantly higher results than the IFCC reference method. Also, there were differences between the three methods of HPLC ion-exchange chromatography, ion-exchange HPLC, and the mono-S method (strong methylsulfonate cation-exchange on monobeads), which might be due to lack of specificity [71]. For example, the National Glycohemoglobin Standardization Program (NGSP) laboratories generated the highest HbA1c values because the peak contained a high proportion of non-HbA1c content, including HbF and minor hemoglobin forms, as well as carbamylated hemoglobin, which were not separated from the neighboring non-HbA1c peaks. Previously published studies have shown that it was possible to establish reliable numerical links between the results of the IFCC reference method and the designated comparison methods described by linear regression equations, which were based on strong correlations between IFCC values and results of the three methods.
We propose that diverse mechanisms explain the variability of results for HbA1c in specific cases of IDA. The standardization of glycated hemoglobin measurement in China is in progress [18], but this requires laboratory improvements and guidelines for interpretation. We recommend collaboration between clinical departments and laboratories to perform large-scale controlled studies.
Conclusions
The cutoff value of measurement of hemoglobin A1c (HbA1c) for the diagnosis of hyperglycemia associated with diabetes mellitus in patients with iron deficiency anemia (IDA) remains controversial. Misinterpretation may lead to misdiagnosis or under-diagnosis resulting in adverse consequences [46]. There is a lack of large-scale epidemiological studies based on age, population, and gender across countries and regions. Standardization of clinical laboratory methods for the measurement of HbA1c requires improvement. We believe that the impact of IDA on HbA1c is multifactorial and multidimensional and that further studies are needed to identify the key factors that affect HbA1c levels. Currently, there are no clear guidelines or verified calibrated methods to measure HbA1c, and we suggest that other clinical factors are recognized to affect the interpretation of HbA1c diagnostically, including IDA [24,69]. The interpretation of HbA1c based on information from hematological examination and iron metabolism indices may help to prevent misdiagnosis or under-diagnosis, and HbA1c should be evaluated carefully as a parameter of glycemic control in patients with IDA [24].
Footnotes
Source of support: The article was supported in part by grants from the National Science Foundation of China (No. 81501839, to Dr. Qi Zhou), Scientific and Technological 13th Five-Year Plan Project of Jilin Provincial Department of Education (No. JJKH20180214KJ, to Dr. Qi Zhou.), Jilin Province Health and Technology Innovation Development Program (No. 2017J071, to Dr. Jiancheng Xu), and the Jilin Science and Technology Development Program (No. 20170623092TC-09, to Dr. Jiancheng Xu; No. 20160101091JC, to Dr. Jiancheng Xu; No. 20150414039GH, to Dr. Jiancheng Xu; No. 20190304110YY to Dr. Jiancheng Xu), The First Hospital Translational Funding for Scientific and Technological Achievements (No. JDYYZH-1902002 to Dr. Jiancheng Xu) and the Norman Bethune Program of Jilin University (No. 2012223, to Dr. Jiancheng Xu
Conflict of interest
None.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomgarden ZT. A1C: Recommendations, debates, and questions. Diabetes Care. 2009;32:e141–47. doi: 10.2337/dc09-zb12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadelson J, Satapathy SK, Nair S. Glycated hemoglobin levels in patients with decompensated cirrhosis. Int J Endocrinol. 2016;2016 doi: 10.1155/2016/8390210. 8390210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard SM, Herring AH, Wang H, et al. Implications of iron deficiency/anemia on the classification of diabetes using HbA1c. Nutr Diabetes. 2015;5:e166. doi: 10.1038/nutd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Targher G. A laboratory standpoint on the role of hemoglobin A1c for the diagnosis of diabetes in childhood: More doubts than certainties? Pediatr Diabetes. 2011;12:183–86. doi: 10.1111/j.1399-5448.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010;33:780–85. doi: 10.2337/dc09-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merono T, Dauteuille C, Tetzlaff W, et al. Oxidative stress, HDL functionality and effects of intravenous iron administration in women with iron deficiency anemia. Clin Nutr. 2017;36:552–58. doi: 10.1016/j.clnu.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of medical care in diabetes – 2018. Diabetes Care. 2018;41:S13–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 14.Sehrawat T, Jindal A, Kohli P, et al. Utility and limitations of glycated hemoglobin (HbA1c) in patients with liver cirrhosis as compared with oral glucose tolerance test for diagnosis of diabetes. Diabetes Ther. 2018;9:243–51. doi: 10.1007/s13300-017-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Glycohemoglobin Standardization Program (NGSP) Factors that interfere with HbA1c test results. 2015. Available at [URL]: http://www.ngsp.org/factors.asp.
- 16.English E, Idris I, Smith G, et al. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: A systematic review. Diabetologia. 2015;58:1409–21. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad J, Rafat D. HbA1c and iron deficiency: A review. Diabetes Metab Syndr. 2013;7:118–22. doi: 10.1016/j.dsx.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Hanas R, John G International HBA1c Consensus Committee. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care. 2010;33:1903–4. doi: 10.2337/dc10-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) Worldwide prevalence of anaemia 1993–2005. 2008. Available at [URL]: https://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf.
- 20.Algarin C, Nelson CA, Peirano P, et al. Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Dev Med Child Neurol. 2013;55:453–58. doi: 10.1111/dmcn.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mojs E, Stanislawska-Kubiak M, Wojciak RW, et al. Reduced iron parameters and cognitive processes in children and adolescents with DM1 compared to those with standard parameters. J Investig Med. 2016;64:782–85. doi: 10.1136/jim-2015-000054. [DOI] [PubMed] [Google Scholar]
- 23.Soliman AT, De Sanctis V, Yassin M, Soliman N. Iron deficiency anemia and glucose metabolism. Acta Biomed. 2017;88:112–18. doi: 10.23750/abm.v88i1.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christy AL, Manjrekar PA, Babu RP, et al. Influence of iron deficiency anemia on hemoglobin A1c levels in diabetic individuals with controlled plasma glucose levels. Iran Biomed J. 2014;18:88–93. doi: 10.6091/ibj.1257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardikar PS, Joshi SM, Bhat DS, et al. Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care. 2012;35:797–802. doi: 10.2337/dc11-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford ES, Cowie CC, Li C, et al. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J Diabetes. 2011;3:67–73. doi: 10.1111/j.1753-0407.2010.00100.x. [DOI] [PubMed] [Google Scholar]
- 27.Son JI, Rhee SY, Woo JT, et al. Hemoglobin a1c may be an inadequate diagnostic tool for diabetes mellitus in anemic subjects. Diabetes Metab J. 2013;37:343–48. doi: 10.4093/dmj.2013.37.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopal L, Ganapathy S, Arunachalam S, et al. Does iron deficiency anaemia and its severity influence HbA1C level in non diabetics? An analysis of 150 cases. J Clin Diagn Res. 2017;11:EC13–15. doi: 10.7860/JCDR/2017/25183.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavagnolli G, Pimentel AL, Freitas PA, et al. Factors affecting A1C in non-diabetic individuals: Review and meta-analysis. Clin Chim Acta. 2015;445:107–14. doi: 10.1016/j.cca.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Grossman A, Gafter-Gvili A, Schmilovitz-Weiss H, et al. Association of glycated hemoglobin with hemoglobin levels in elderly nondiabetic subjects. Eur J Intern Med. 2016;36:32–35. doi: 10.1016/j.ejim.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Akkermans MD, Mieke Houdijk ECA, Bakker B, et al. Iron status and its association with HbA1c levels in Dutch children with diabetes mellitus type 1. Eur J Pediatr. 2018;177:603–10. doi: 10.1007/s00431-018-3104-3. [DOI] [PubMed] [Google Scholar]
- 32.Simmons D, Hlaing T. Interpretation of HbA1c: Association with mean cell volume and haemoglobin concentration. Diabet Med. 2014;31:1387–92. doi: 10.1111/dme.12518. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9–17. doi: 10.1111/j.1753-0407.2009.00009.x. [DOI] [PubMed] [Google Scholar]
- 34.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126–28. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 35.Tarim O, Kucukerdogan A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357–62. doi: 10.1046/j.1442-200x.1999.01083.x. [DOI] [PubMed] [Google Scholar]
- 36.Brooks AP, Metcalfe J, Day JL, Edwards MS. Iron deficiency and glycosylated haemoglobin A. Lancet. 1980;2:141. doi: 10.1016/s0140-6736(80)90019-7. [DOI] [PubMed] [Google Scholar]
- 37.Silva JF, Pimentel AL, Camargo JL. Effect of iron deficiency anaemia on HbA1c levels is dependent on the degree of anaemia. Clin Biochem. 2016;49:117–20. doi: 10.1016/j.clinbiochem.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Koga M, Morita S, Saito H, et al. Association of erythrocyte indices with glycated haemoglobin in pre-menopausal women. Diabet Med. 2007;24:843–47. doi: 10.1111/j.1464-5491.2007.02161.x. [DOI] [PubMed] [Google Scholar]
- 39.Sluiter WJ, van Essen LH, Reitsma WD, Doorenbos H. Glycosylated haemoglobin and iron deficiency. Lancet. 1980;2:531–32. doi: 10.1016/s0140-6736(80)91853-x. [DOI] [PubMed] [Google Scholar]
- 40.El-Agouza I, Abu Shahla A, Sirdah M. The effect of iron deficiency anaemia on the levels of haemoglobin subtypes: Possible consequences for clinical diagnosis. Clin Lab Haematol. 2002;24:285–89. doi: 10.1046/j.1365-2257.2002.00464.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Ding X, Dai J, et al. [Changes of glycosylated hemoglobin in various anemia diseases]. Modern Diagnosis and Treatment. 1991;2:228–30. [in Chinese] [Google Scholar]
- 42.O’Keeffe DT, Maraka S, Rizza RA. HbA1c in the evaluation of diabetes mellitus. JAMA. 2016;315:605–6. doi: 10.1001/jama.2015.16561. [DOI] [PubMed] [Google Scholar]
- 43.Xia D, McShine R, Berg AH. Misleading hemoglobin A1c levels in a patient with paroxysmal nocturnal hemoglobinuria. Am J Clin Pathol. 2014;142:261–65. doi: 10.1309/AJCPTK1HPR2KULJU. [DOI] [PubMed] [Google Scholar]
- 44.Rusak E, Rotarska-Mizera A, Adamczyk P, et al. Markers of anemia in children with type 1 diabetes. J Diabetes Res. 2018;2018 doi: 10.1155/2018/5184354. 5184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ditzel J, Kjaergaard JJ. Haemoglobin AIc concentrations after initial insulin treatment for newly discovered diabetes. Br Med J. 1978;1:741–42. doi: 10.1136/bmj.1.6115.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakatani R, Murata T, Usui T, et al. Importance of the average glucose level and estimated glycated hemoglobin in a diabetic patient with hereditary hemolytic anemia and liver cirrhosis. Intern Med. 2018;57:537–43. doi: 10.2169/internalmedicine.9135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks DB, Arnold M, Bakris GL, et al. Executive summary: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:793–98. doi: 10.1373/clinchem.2011.163634. [DOI] [PubMed] [Google Scholar]
- 48.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153–63. [PubMed] [Google Scholar]
- 49.Szymezak J, Lavalard E, Martin M, et al. Carbamylated hemoglobin remains a critical issue in HbA1c measurements. Clin Chem Lab Med. 2009;47:612–13. doi: 10.1515/CCLM.2009.136. [DOI] [PubMed] [Google Scholar]
- 50.Sinha N, Mishra TK, Singh T, Gupta N. Effect of iron deficiency anemia on hemoglobin A1c levels. Ann Lab Med. 2012;32:17–22. doi: 10.3343/alm.2012.32.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otabe S, Nakayama H, Ohki T, et al. Haemoglobin variants may cause significant differences in haemoglobin A1c as measured by high-performance liquid chromatography and enzymatic methods in diabetic patients: A cross-sectional study. Ann Clin Biochem. 2017;54:432–37. doi: 10.1177/0004563216664366. [DOI] [PubMed] [Google Scholar]
- 52.Lacy ME, Wellenius GA, Sumner AE, et al. Association of sickle cell trait with hemoglobin A1c in African Americans. JAMA. 2017;317:507–15. doi: 10.1001/jama.2016.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohlfing C, Hanson S, Weykamp C, et al. Effects of hemoglobin C, D, E and S traits on measurements of hemoglobin A1c by twelve methods. Clin Chim Acta. 2016;455:80–83. doi: 10.1016/j.cca.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee BS, Jayathilaka GD, Huang JS, et al. Analyses of in vitro nonenzymatic glycation of normal and variant hemoglobins by MALDI-TOF mass spectrometry. J Biomol Tech. 2011;22:90–94. [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: An interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104–6. doi: 10.1016/j.clinbiochem.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63–71. doi: 10.1016/j.cca.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koga M, Kurebayashi S, Murai J, et al. Degree of discrepancy between HbA1c and glycemia in variant hemoglobin is smaller when HbA1c is measured by new-type Arkray HPLC compared with old-type HPLC. Clin Biochem. 2014;47:123–25. doi: 10.1016/j.clinbiochem.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization (WHO) Guidelines Approved by the Guidelines Review Committee. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization; 2011. Available from [URL]: https://www.ncbi.nlm.nih.gov/books/NBK304267/ [PubMed] [Google Scholar]
- 59.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta. 1997;260:49–64. doi: 10.1016/s0009-8981(96)06508-4. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Liu B, Liu C. [Erythrocyte membrane lipid peroxidation damage and its mechanism]. Journal of Qingdao University Medical College. 2006;42:90–94. [in Chinese] [Google Scholar]
- 61.Zhang Y, Li Y, Zheng F. [Effects of iron deficiency on blood lipids and liver lipid peroxidation in rats]. Chinese Chronic Disease Prevention and Control. 2011;19:577–78. [in Chinese] [Google Scholar]
- 62.Zaka-Ur-Rab Z, Adnan M, et al. Effect of oral iron on markers of oxidative stress and antioxidant status in children with iron deficiency anaemia. J Clin Diagn Res. 2016;10:SC13–19. doi: 10.7860/JCDR/2016/23601.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sundaram RC, Selvaraj N, Vijayan G, et al. Increased plasma malondialdehyde and fructosamine in iron deficiency anemia: effect of treatment. Biomed Pharmacother. 2007;61:682–85. doi: 10.1016/j.biopha.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Jain SK, Palmer M. The effect of oxygen radicals metabolites and vitamin E on glycosylation of proteins. Free Radic Biol Med. 1997;22:593–96. doi: 10.1016/s0891-5849(96)00377-2. [DOI] [PubMed] [Google Scholar]
- 65.Jain SK. Glutathione and glucose-6-phosphate dehydrogenase deficiency can increase protein glycosylation. Free Radic Biol Med. 1998;24:197–201. doi: 10.1016/s0891-5849(97)00223-2. [DOI] [PubMed] [Google Scholar]
- 66.Haliassos A, Drakopoulos I, Katritsis D, et al. Measurement of glycated hemoglobin (HbA1c) with an automated POCT instrument in comparison with HPLC and automated immunochemistry method: Evaluation of the influence of hemoglobin variants. Clin Chem Lab Med. 2006;44:223–27. doi: 10.1515/CCLM.2006.041. [DOI] [PubMed] [Google Scholar]
- 67.Rai KB, Pattabiraman TN. Glycosylated haemoglobin levels in iron deficiency anaemia. Indian J Med Res. 1986;83:234–36. [PubMed] [Google Scholar]
- 68.Little RR, Rohlfing CL, Tennill AL, et al. Measurement of HbA(1c) in patients with chronic renal failure. Clin Chim Acta. 2013;418:73–76. doi: 10.1016/j.cca.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong JW, Ku CR, Noh JH, et al. Association between the presence of iron deficiency anemia and hemoglobin A1c in Korean adults: The 2011-2012 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2015;94:e825. doi: 10.1097/MD.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 71.Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: A method-comparison study. Clin Chem. 2004;50:166–74. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]


