Abstract
BACKGROUND:
Filanesib is a kinesin spindle protein inhibitor that has demonstrated encouraging activity in patients with recurrent/refractory multiple myeloma. Preclinical synergy with bortezomib was the rationale for the current phase 1 study.
METHODS:
The current study was a multicenter study with an initial dose-escalation phase to determine the maximum tolerated dose of 2 schedules of filanesib plus bortezomib with and without dexamethasone, followed by a dose-expansion phase.
RESULTS:
With the addition of prophylactic filgastrim, the maximum planned dose was attained: 1.3 mg/m2/day of bortezomib plus 40 mg of dexamethasone on days 1, 8, and 15 of a 28-day cycle, with filanesib given intravenously either at a dose of 1.5 mg/m2/day (schedule 1: days 1, 2, 15, and 16) or 3 mg/m2/day (schedule 2: days 1 and 15). The most common adverse events (assessed for severity using version 4.0 of the National Cancer Institute Common Terminology Criteria for Adverse Events) were transient, noncumulative neutropenia and thrombocytopenia with grade 3/4 events reported in 44% (16% in cycle 1 with filgastrim) and 29% of patients, respectively. A low (≤11%) overall rate of nonhematological grade 3/4 toxicity was observed. With a median of 3 prior lines of therapy and 56% of patients with disease that was refractory to proteasome inhibitors, the overall response rate was 20% (55 patients), and was 29% in 14 patients with proteasome inhibitors-refractory disease receiving filanesib at a dose of ≥1.25 mg/m2 (duration of response, 5.2 to ≥21.2 months).
CONCLUSIONS:
The current phase 1 study established a dosing schedule for the combination of these agents that demonstrated a favorable safety profile with a low incidence of nonhematologic toxicity and manageable hematologic toxicity. The combination of filanesib, bortezomib, and dexamethasone appears to have durable activity in patients with recurrent/refractory multiple myeloma.
Keywords: bortezomib, dexamethasone, filanesib, multiple myeloma, neoplasm recurrence, proteasome inhibitors, recurrent/refractory
INTRODUCTION
Despite improvements in the survival of patients with multiple myeloma (MM) over the last decade with the introduction of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs), patients eventually develop disease recurrence.1,2 An emerging treatment strategy to combat acquired drug resistance and induce durable responses is the incorporation of novel drugs with unique mechanisms of action. Kinesin spindle proteins (KSPs) are critical for normal mitosis. KSP inhibition results in the formation of a monopolar spindle, causing mitotic arrest and apoptosis, particularly in cells that exhibit rapid, sustained depletion of Mcl-1 (myeloid cell leukemia 1), an antiapoptotic member of the Bcl-2 family.3 Because MM cells are Mcl-1-dependent, KSP inhibition represents a novel therapeutic approach in patients with MM.4,5
Filanesib (ARRY-520) is a highly selective, first-in-class targeted KSP inhibitor. Due to its novel mechanism of action, filanesib is expected to overcome PI and/or IMiD resistance. In addition, no additive peripheral neuropathy is expected, due to the absence of KSP expression in neurons. Single-agent filanesib already has demonstrated efficacy and safety in patients with recurrent/refractory MM (RRMM) (unpublished data).
In preclinical models, the combination of bortezomib and filanesib has demonstrated additive apoptosis, and filanesib appears to have significant antitumor activity in bortezomib-resistant MM cell lines.6 Furthermore, MM cells in mitotic arrest also are rendered more sensitive to dexamethasone; therefore, the addition of dexamethasone is hypothesized to enhance filanesib activity.3,7 Bortezomib in combination with dexamethasone was the first PI approved for the treatment of RRMM.8 Therefore, the current phase 1 study was designed to establish the maximum tolerated dose (MTD) of filanesib in combination with bortezomib and dexamethasone in patients with RRMM.
MATERIALS AND METHODS
The current study was a phase 1 multicenter study with an initial dose-escalation phase to determine the MTD of 2 schedules of filanesib plus bortezomib with and without dexamethasone, followed by a dose-expansion phase. Additional objectives in the dose-escalation phase were to obtain preliminary estimates of efficacy and possible biomarkers to predict response. Because data analyses currently are ongoing in the expansion cohorts, the current study focused on the completed dose-escalation phase.
Patients
Patients aged ≥18 years with measurable RRMM or plasma cell leukemia were eligible for participation in the current study. Patients had received ≥2 prior regimens including a PI (eg, bortezomib, carfilzomib) and an IMiD (eg, thalidomide, lenalidomide), with disease progression (PD) during or after the last prior regimen. Patients with PI-refractory disease were eligible. Patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate liver function, serum creatinine ≤2.5 mg/dL or calculated creatinine clearance ≥50 mL/minute, a neutrophil count ≥1.5 × 109/L, and a platelet count ≥75 × 109/L (or ≥50 × 109/L if bone marrow contained ≥50% plasma cells) without transfusion or growth factor support for 2 weeks before screening. Key exclusion criteria included primary amyloidosis and any stem cell transplantation performed within 3 months before initiating the study drug.
The current study was conducted in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines and all applicable regulatory requirements. The study was approved by the Institutional Review Boards of all participating centers, and patients provided written informed consent. This study was registered at www.ClinicalTrials.gov with identifier .
Treatment Schedules
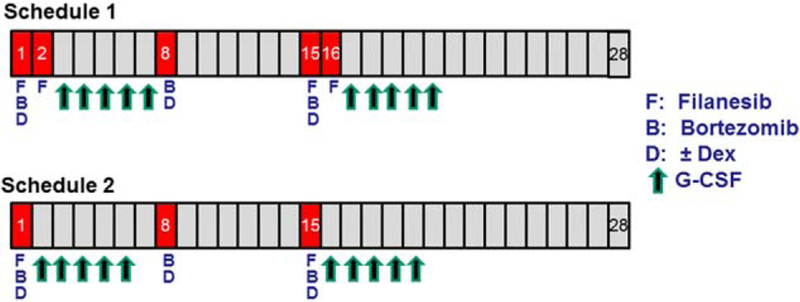
Dose escalations were conducted using 2 filanesib treatment schedules within 28-day cycles (Fig. 1).
Figure 1.
Treatment schedules. DEX indicates dexamethasone; G-CSF, granulocyte colony-stimulating factor.
Schedule 1 first determined the MTD of filanesib administered on days 1, 2, 15, and 16 in combination with bortezomib on days 1, 8, and 15, followed by a second dose escalation starting at 1 dose level below the established MTD of the combination with the addition of dexamethasone on the same dosing days as bortezomib. Filanesib was administered at escalating doses starting at 0.5 mg/m2/day as a 1-hour intravenous (iv) infusion and bortezomib was administered at a dose of 1.0 or 1.3 mg/m2/day according to the assigned dose level. Bortezomib administration was initially iv, but the protocol was amended to allow for subcutaneous (SC) dosing in accordance with current clinical practice.9 Dexamethasone was administered orally at a standard dose of 40 mg/day (20 mg/day for patients aged ≥75 years).
Schedule 2 determined the MTD of filanesib administered on days 1 and 15 plus bortezomib and dexamethasone on days 1, 8, and 15. A weekly bortezomib schedule was used primarily so that all drugs would be given on both day 1 and day 15, thereby allowing for optimal in vivo synergy.
Due to the exacerbated neutropenia observed in the first 2 dosing cohorts, the protocol was amended to add prophylactic filgastrim. Patients received or self-administered concurrent filgastrim as a daily SC injection for a total of 5 to 7 days after each filanesib dosing. Varicella zoster virus prophylaxis was prescribed as per standard of care, and gram-negative antibiotic prophylaxis was prescribed if patients were neutropenic.
Patients received study drug(s) in continuous cycles until unacceptable toxicity or PD occurred.
Determination of the MTD
A standard 3+3 design was used to determine the MTD, defined as the dose below that which resulted in dose-limiting toxicities (DLTs) in ≥33% of patients. A DLT was defined as an adverse event (AE) in cycle 1 that was considered related to the study drug(s) and met any of the following criteria: grade 4 neutropenia of >7 days; febrile neutropenia; grade 4 thrombocytopenia of >7 days and not responding to platelet transfusions; any thrombocytopenia associated with grade ≥3 bleeding attributed to the study drug(s); any grade 3 or 4 nonhematologic AE except nausea, vomiting, or diarrhea in the absence of prophylaxis; or any treatment-related AE delaying the day 15 dose or initiation of cycle 2 by ≥2 weeks.
The first patient in every cohort was followed for ≥8 days before subsequent patients were enrolled. Data from the first 28-day cycle for all patients in a cohort were reviewed for safety to inform subsequent dose-escalation decisions. Patients not completing cycle 1 for reasons other than toxicity were considered unevaluable for assessment of DLTs and were replaced.
At the investigator’s discretion, patients experiencing a DLT were permitted to be treated at a lower dose level, and patients at lower dose levels were permitted to escalate to higher tolerated doses, including the addition of dexamethasone once it was deemed tolerated.
Assessments
Safety was assessed by AEs, DLTs, laboratory tests, and electrocardiograms. AEs were assessed for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0)10 and coded using the Medical Dictionary for Regulatory Activities (version 13.0).
Investigators used the International Myeloma Working Group response criteria to determine the overall response rate (ORR) in patients who were evaluable for response.11 Other preliminary efficacy analyses included duration of response (DOR), defined as the time from first disease assessment of partial response (PR) or better to the date of first PD, death, or disease recurrence; and the time on study (ToS), defined as the time from the first dose of the study drugs to the date of study termination. Post hoc analyses included the rates of minimal response (MR), the clinical benefit rate (CBR) (ie, responses of ≥MR), and the disease control rate (DCR) (ie, responses of ≥stable disease of ≥8 weeks in duration).
Statistical Analysis
Approximately 30 and 15 evaluable patients, respectively, were anticipated to be enrolled on schedules 1 and 2. Safety data were summarized using descriptive statistics. Patients who received at least 1 dose of filanesib were evaluable for safety. Patients were evaluable for response if they received at least 1 dose of filanesib and had a postbaseline disease assessment or were discontinued from the study due to PD, intolerable toxicity, or death before the first assessment. Time-to-event analyses were estimated using the Kaplan-Meier method. No formal comparisons were planned or performed.
RESULTS
Patient Population
A total of 55 patients with MM were enrolled between December 2010 and October 2013 at 6 centers in the United States and all patients were evaluable for safety and efficacy. At the time of data cutoff (April 30, 2015), 3 patients were continuing to receive study treatment.
Demographics are described in detail in Table 1. The median age of the patients was 63 years (range, 31–79 years) and patients were heavily pretreated, with a median of 3 prior lines (range, 1–9 prior lines) of therapy, including 89% with a prior stem cell transplantation. Nearly all patients had received prior bortezomib (95%) and lenalidomide (98%), with 51% and 75%, respectively, having disease that was refractory to each agent, and 35% having disease that was refractory to both agents. In addition, 16% received prior carfilzomib, all of whom had disease that was refractory to the agent. In total, 56% of patients had disease that was refractory to a PI (bortezomib and/or carfilzomib). Finally, 13% of patients had disease that was refractory to pomalidomide. A total of 42% of patients had disease that was refractory to a PI and an IMiD. Twelve patients (22%) had high-risk molecular findings.
TABLE 1.
Demographic and Baseline Disease Characteristics and Prior Therapies (All Patients)
| Characteristic | Dose-Escalation Phase Total N = 55 |
|---|---|
| Sex | |
| Male, no. (%) | 30 (55) |
| Female, no. (%) | 25 (45) |
| Race, no. (%) | |
| White | 36 (65) |
| Black/African American | 14 (25) |
| Other | 5 (9) |
| Median age (range), y | 63 (31–79) |
| Median y since diagnosis (range) | 5.1 (1.5–12.0) |
| Ig subtype at diagnosis, no. (%) | |
| IgG | 34 (62) |
| IgA | 9 (16) |
| IgM | 1 (2) |
| Light chain only | 11 (20) |
| Light chain at diagnosis, no. (%) | |
| Kappa | 34 (62) |
| Lambda | 21 (38) |
| ISS stage at diagnosis, no. (%) | |
| I | 9 (16) |
| II | 16 (29) |
| III | 12 (22) |
| Missing | 18 (33) |
| ECOG performance status, no. (%) | |
| 0 | 19 (35) |
| 1 | 36 (65) |
| Creatinine clearance, mL/minute | |
| >90 | 24 (44) |
| 60–89 | 17 (31) |
| 30–59 | 13 (24) |
| 15–29 | 1 (2) |
| High-risk cytogenetics, no. (%)a | |
| Yes | 12 (22) |
| No | 41 (75) |
| Missing/unknown | 2 (4) |
| Prior lines of therapy, median (range) | 3 (1–9) |
| Prior proteasome inhibitor | 55 (100) |
| Recurrent | 23 (42) |
| Refractory | 31 (56) |
| Unknown | 1 (2) |
| Prior bortezomib | 52 (95) |
| Recurrent | 23 (42) |
| Refractory | 28 (51) |
| Unknown | 1 (2) |
| Prior carfilzomib | 9 (16) |
| Refractory | 9 (16) |
| Prior IMiD | 55 (100) |
| Recurrent | 12 (22) |
| Refractory | 42 (76) |
| Prior lenalidomide | 54 (98) |
| Recurrent | 12 (22) |
| Refractory | 41 (75) |
| Unknown | 1 (2) |
| Prior pomalidomide | 7 (13) |
| Refractory | 7 (13) |
| Prior corticosteroid | 55 (100) |
| Prior alkylator | 51 (93) |
| Prior stem cell transplantation | 49 (89) |
| Prior anthracycline | 22 (40) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Ig, immunoglobulin; IMiD, immunomodulatory agent; ISS, International Staging System.
Defined as ≥1of the following: del(17p), t(14;16), or 1q21 gain.
Determination of MTD and Recommended Phase 2 Dose
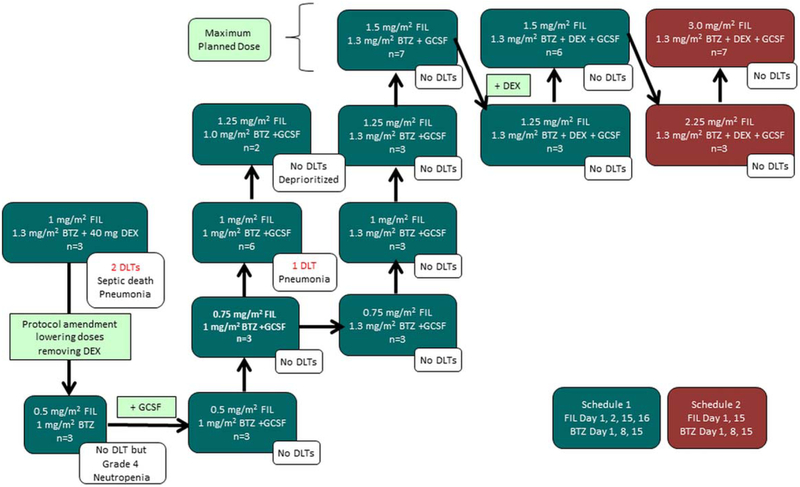
The schedule of events is outlined in Figure 2. On schedule 1, patients initially received a dose of 1.0 mg/m2 of filanesib (without prophylactic filgastrim) plus 1.3 mg/m2 of bortezomib and 40 mg of dexamethasone. Two DLTs occurred at this dose level (pneumonia and pseudomonal sepsis in 3 evaluable patients), and therefore the doses of bortezomib and filanesib were reduced and dexamethasone omitted until an MTD of bortezomib plus filanesib could be determined. Because these toxicities were unexpected based on experience with the single-agent filanesib or bortezomib doses (even in combination with dexamethasone), the rationale for these modifications was to maximally protect the safety of the patients.
Figure 2.
Dose-escalation schedules. BTZ indicates bortezomib; DEX, dexamethasone; DLT, dose-limiting toxicity; FIL, filanesib; GCSF, granulocyte colony-stimulating factor.
The next dose level investigated 0.5 mg/m2 of filanesib plus 1.0 mg/m2 of bortezomib (3 evaluable patients). Although no DLTs were observed, neutropenia still was exacerbated; therefore, prophylactic filgastrim was added in a subsequent cohort testing, starting at the same dose level. The bortezomib dose was progressively escalated to1.3 mg/m2 and filanesib to 1.5 mg/m2, which were the maximum planned levels because these were the recommended single-agent doses, respectively, of each agent.8,12 Dexamethasone was added back into the regimen and no DLTs were observed through the final dose level, which included the recommended single-agent doses of all 3 agents and was determined to be the MTD of schedule 1.
Dose escalation on schedule 2 was initiated with a filanesib dose of 2.25 mg/m2 that was escalated to 3.0 mg/m2 (both with filgastrim), neither of which elicited DLTs. Because the dose intensity of this regimen was equivalent to the recommended single-agent doses for each study drug, no further dose escalation was explored and this dose level was declared the MTD of schedule 2.
Three of 55 evaluable patients (5%) experienced DLTs (Fig. 2). The onset of all DLTs occurred within the first 3 weeks of treatment initiation and lasted for 2 to 7 days.
Treatment Exposure and Safety
Patients received a median of 4 cycles of filanesib (range, 1 to ≥41 cycles), with a median of 3.7 months on treatment (range, 0.4 to ≥38.3 months), and 23 patients (42%) received treatment for >6 months. The primary reasons for treatment discontinuation for 52 patients (95%) as of the data cutoff date were PD (39 patients [71%]), toxicity (5 patients [9%]), investigator decision (5 patients [9%]), withdrawal of consent (2 patients [4%]), and patient death (1 patient [2%]).
The majority of patients (98%) reported at least 1 nonhematologic AE on study, the most common of which were diarrhea (47%), upper respiratory tract infection (42%), cough (27%), and fatigue and pyrexia (25% each). Grade 3/4 nonhematologic AEs (Table 2) were reported for 67% of patients (all but 6 patients reported grade 3/4 events that were assessed as related to study treatment). Pneumonia and elevated pancreatic enzymes were the most common nonhematologic grade ≥3 events, although their incidence was low (7% and 11%, respectively). Two of the 4 events of pneumonia met DLT criteria.
TABLE 2.
Treatment-Emergent Nonhematologic Grade ≥3 AEs Occurring in >1 Patient in All Cycles (Safety Population)
| MedDRA-Preferred Term Total | Schedule 1, <1.25 mg/m2 FIL or BTZ = 1.0 N = 26 | Schedule 1, ≥1.25 mg/m2 FIL and BTZ = 1.3 N = 19 | Schedule 2 N = 10 | Total Treated N = 55 |
|---|---|---|---|---|
| Total no. of patients with any grade ≥3 AE (%) | 18 (69) | 12 (63) | 7 (70) | 37 (67) |
| Lipase increased | 0 (0) | 4 (21) | 2 (20) | 6 (11) |
| Blood amylase increased | 1 (4) | 2 (11) | 1 (10) | 4 (7) |
| Pneumonia | 3 (12) | 0 (0) | 1 (10) | 4 (7) |
| Cholecystitis | 1 (4) | 0 (0) | 1 (10) | 2 (4) |
| Fall | 1 (4) | 0 (0) | 1 (10) | 2 (4) |
| Hyperuricemia | 2 (8) | 0 (0) | 0 (0) | 2 (4) |
| Hyponatremia | 2 (8) | 0 (0) | 0 (0) | 2 (4) |
Abbreviations: AE, adverse event; BTZ, bortezomib; FIL, filanesib; MedDRA, Medical Dictionary for Regulatory Activities (version 13.0).
Approximately 45% of patients experienced grade 3/4 neutropenia and 29% experienced grade 3/4 thrombocytopenia (Table 3). The incidence of neutropenia was highest (67%) in the 2 dose cohorts that did not include filgastrim prophylaxis. Of 55 patients treated, 49 were in cohorts in which filgastrim was mandated, and in these cohorts grade 3/4 neutropenia occurred in only 8 patients (16%) during cycle 1. Of these 49 patients, 29 and 20 patients, respectively, initiated with 5 and 7 days of prophylactic filgastrim. The number of days of filgastrim remained the same throughout the study in 25 patients (51%), whereas 18 patients (37%) were able to decrease the number of days, 4 (8%) could have their number of days temporarily be decreased, and only 2 patients (4%) required an increase in the number of days. The majority of cases of cytopenia were reversible and did not appear to be cumulative. By the time of the next scheduled weekly visit, the vast majority of patients had recovered to a neutrophil count of ≥1.5 × 109.
TABLE 3.
Hematologic Events Grade ≥3 Occurring in >1 Patient (Safety Population)
| Hematologic Events (Laboratory Values) Grade ≥3, No. (%) | Schedule 1, <1.25 mg/m2 FIL or BTZ = 1.0 N = 26 | Schedule 1, ≥1.25 mg/m2 FIL and BTZ = 1.3 N = 19 | Schedule 2 N = 10 | Total Treated N = 55 |
|---|---|---|---|---|
| Leukopenia | ||||
| Cycle 1 | 7 (27) | 1 (5) | 1 (10) | 9 (16) |
| All cycles | 7 (27) | 7 (37) | 5 (50) | 19 (35)a |
| Neutropenia | ||||
| Cycle 1 | 6 (23) | 4 (21) | 2 (20) | 12 (22) |
| All cycles | 8 (31) | 9 (48) | 7 (70) | 24 (44)a |
| Thrombocytopenia | ||||
| Cycle 1 | 6 (23) | 3 (16) | 2 (20) | 11 (20) |
| All cycles | 7 (27) | 5 (26) | 4 (40) | 16 (29) |
| Anemia | ||||
| Cycle 1 | 6 (23) | 3 (16) | 2 (20) | 11 (20) |
| All cycles | 9 (35) | 3 (16) | 4 (40) | 16 (29) |
Abbreviations: BTZ, bortezomib; FIL, filanesib.
In patients from cohorts in which prophylactic filgastrim was mandated (49 patients), the incidence of leukopenia grade ≥3 was 31% and that of neutropenia was 41%. The incidence of both leukopenia and neutropenia in the 6 patients treated in the 2 cohorts without mandatory filgastrim was 67% for each (all of which were grade 4).
Throughout the study, approximately one-half of the patients had some delay in dosing during study treatment; in 36% of patients, this was due to an AE for both filanesib and bortezomib. Only 25% and 35% of patients, respectively, needed a dose reduction for filanesib or bortezomib, related to an AE in 24% and 33%, respectively, for filanesib and bortezomib (Table 4). Dose reductions did not correlate with dose or schedule. Only 5 patients discontinued protocol treatment due to AEs (bronchitis in 1 patient, neutropenia and cytopenia in 1 patient, thrombocytopenia in 1 patient, diarrhea in 1 patient, and blurred vision in 1 patient). The majority of AEs resulting in dose reductions or discontinuation resolved after dose modification. All patients had a baseline serum creatinine ≤2.5 mg/dL, and 14 patients (25%) had a creatinine clearance of <60 mL/minute with no increase in toxicity. There also was no difference in toxicity noted among patients receiving iv versus SC bortezomib.
TABLE 4.
Dose Modifications
| Modification Type | Schedule 1, <1.25 mg/m2 FIL or BTZ = 1.0 N = 26 | Schedule 1, ≥1.25 mg/m2 FIL and BTZ = 1.3 N = 19 | Schedule 2 N = 10 | Total Treated N = 55 |
|---|---|---|---|---|
| Total patients with any dose modification for FIL | 13 (50) | 13 (68) | 6 (60) | 32 (58) |
| Delay | 12 (46) | 11 (58) | 5 (50) | 28 (51) |
| AE | 8 (31) | 7 (37) | 5 (50) | 20 (36) |
| Other | 6 (23) | 10 (53) | 3 (30) | 19 (35) |
| Reduced | 6 (23) | 6 (32) | 2 (20) | 14 (25) |
| AE | 6 (23) | 5 (26) | 2 (20) | 13 (24) |
| Other | 1 (4) | 2 (11) | 0 (0) | 3 (5) |
| Total patients with any dose modification for BTZ | 17 (65) | 13 (68) | 7 (70) | 37 (67) |
| Delay | 12 (46) | 11 (58) | 5 (50) | 28 (51) |
| AE | 8 (31) | 7 (37) | 5 (50) | 20 (36) |
| Other | 6 (23) | 10 (53) | 3 (30) | 19 (35) |
| Reduced | 8 (31) | 7 (37) | 4 (40) | 19 (35) |
| AE | 7 (27) | 7 (37) | 4 (40) | 18 (33) |
| Other | 1 (4) | 1 (5) | 1 (10) | 3 (5) |
Abbreviations: AE, adverse event; BTZ, bortezomib; FIL filanesib.
Deaths attributable to AEs on study or within 30 days of the last filanesib dose occurred in 2 patients (4%), both of whom were treated under schedule 1 without filgastrim prophylaxis. The cause of death was pneumococcal meningitis, assessed as not related to study treatment, for a patient treated with 0.5 mg/m2 of filanesib plus 1.0 mg/m2 of bortezomib. The second patient was treated with 1.0 mg/m2 of filanesib plus 1.3 mg/m2 of bortezomib plus 40 mg of dexamethasone and died on study day 13 from pseudomonal sepsis (a DLT), which was assessed as being related to filanesib.
Peripheral neuropathy was only reported for 5 patients (9%), occurring at varying doses of filanesib, but all occurring at 1.3 mg/m2 of bortezomib, with 3 of the 5 patients receiving bortezomib iv and 2 with baseline grade 1 peripheral neuropathy.
Efficacy
Including patients at all dose levels in the current phase 1 study, the ORR was 20%, the CBR was 33%, and the DCR was 65% (Table 5). In the 12 patients with high-risk cytogenetics at baseline, the ORR was 25% and the DCR was 58%. Ten of the 11 responses (including 4 without any concomitant dexamethasone) were observed among the 29 patients treated with ≥1.25 mg/m2 of filanesib (on either schedule) and 1.3 mg/m2 of weekly bortezomib (ORR, 31%). Within this subset of therapeutic dosing of filanesib, an ORR of 40% and a DCR of 87% were observed among the 15 patients whose disease was previously sensitive to PIs. The median DOR was 17.2 months. It is interesting to note that within the same dosing subset, in the 14 patients with disease previously refractory to PIs, there were responses noted in 4 of 14 patients (29%): 2 with a very good PR and 1 PR in schedule 1 and another PR in schedule 2, and a DCR of 64% was observed. The doses of filanesib/bortezomib/dexamethasone for the responders were 1.5 mg/m2/1.3 mg/m2/40 mg (1 patient), 1.5 mg/m2/1.3 mg/m2/none (2 patients), and 3 mg/m2/1.3 mg/m2/40 mg (1 patient), respectively. The DOR for these 4 patients was 7.9 months, ≥21.2 months, 12.3 months, and 5.2 months, respectively.
TABLE 5.
Clinical Response Among the Response-Evaluable Population
| Schedule 1, <1.25 mg/m2 FIL or BTZ = 1.0 N = 26 | Schedule 1, ≥1.25 mg/m2 FIL and BTZ = 1.3 N = 19 | Schedule 2 N = 10 | Total Treated N = 55 | |
|---|---|---|---|---|
| Best disease response Stringent CR | 0 (0) | 1 (5) | 0 (0) | 1 (2) |
| Very good PR | 0 (0) | 4 (21) | 1 (10) | 5 (9) |
| PR | 1 (4) | 3 (16) | 1 (10) | 5 (9) |
| MR | 2 (8) | 3 (16) | 2 (20) | 7 (13) |
| SD | 18 (69) | 9 (47) | 7 (70) | 34 (62) |
| SD ≥8 wk | 11 (42) | 3 (16) | 4 (40) | 18 (33) |
| PD | 6 (23) | 2 (11) | 1 (10) | 9 (16) |
| NE | 1 (4) | 0 (0) | 0 (0) | 1 (2) |
| ORR (≥ PR) | 1 (4) | 8 (42) | 2 (20) | 11 (20) |
| Median time on study (95% CI), mo | NR (NR-NR) | 20.9 (6.9–30) | 16.6 (7.4–13.9) | 16.7 (6.9-NR) |
| CBR (≥MR) | 3 (11) | 11 (58) | 4 (40) | 18 (33) |
| Median time on study (95% CI), mo | 14.5 (6.6-NR) | 13.0 (6.9–30) | 13.0 (7.4–14.5) | 13.4 (9.9–16.9) |
| DCR (≥SD[>8 wk]) | 14 (54) | 14 (74) | 8 (80) | 36 (65) |
| Median time on study (95% CI), mo | 5.4 (3.0–14.5) | 12.8 (6.9–25.1) | 8.5 (4.2–13.9) | 9.3 (6.9–13.0) |
Abbreviations: 95% CI, 95% confidence interval; BTZ, bortezomib; CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; FIL, filanesib; MR, minimal response; NE, not evaluable; NR, not reached; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
The median ToS for the 14 patients with PI-refractory disease was 4.7 months (schedule 1) and 8.0 months (schedule 2). Among the 7 patients who were dual refractory to pomalidomide and a PI, 1 patient (14%) achieved a PR (DOR of 5.2 months; ToS, 7.4 months) and another patient achieved MR, for a CBR of 29%. The median ToS in this subset was 5.6 months. Among the 11 patients with disease that was refractory to a PI and IMiD, the ORR was 27% and the CBR was 36%. The median DOR in the 5 patients with PI-refractory disease (3 of whom did not receive dexamethasone) was 10.1 months.
Patients who responded to treatment generally attained a response within the first cycle of treatment. The median time to first response was 1.0 months (range, 0.7–33.9 months) (Table 6). Responses were durable, with a median of 14.1 months (range, ≥3.8 to ≥24.6 months). Seven of 11 responders (64%) maintained a response for >6 months and 5 of 11 responders remained in response at the time of data cutoff.
TABLE 6.
Time-to-Event Endpoints in the Response-Evaluable Population
| Parameter | Statistic | Schedule 1 <1.25 mg/m2 FIL or BTZ = 1.0 N = 26 | Schedule 1, ≥1.25 mg/m2 FIL and BTZ = 1.3 N = 19 | Schedule 2 N = 10 | Total Treate N = 55 |
|---|---|---|---|---|---|
| Time on study, mo | No. | 26 | 19 | 10 | 55 |
| Median | 3.1 | 9.9 | 8.0 | 5.6 | |
| 95% CI | 2.3–4.0 | 3.7–14.4 | 1.9–12.2 | 3.2–8.5 | |
| Minimum-maximum | 0.4–38.3a | 1.3–30.0 | 1.9–14.5 | 0.4–38.3a | |
| Time to first response (≥PR), mo | No. | 1 | 8 | 2 | 11 |
| Median | 33.9 | 1.3 | 0.7 | 1.0 | |
| 95% CI | NR | 0.7–6.6 | NR | 0.7–6.6 | |
| Minimum-maximum | 33.9–33.9 | 0.7–10.6 | 0.7–0.7 | 0.7–33.9 | |
| Duration of response (≥PR), mo | No. | 1 | 8 | 2 | 11 |
| Median | NR | 17.2 | 8.7 | 14.1 | |
| 95% CI | NR | 7.9-NR | 5.2–12.2 | 5.2-NR | |
| Minimum-maximum | 3.8a–3.8a | 4.9a–24.6a | 5.2–12.2 | 3.8a–24.6a |
Abbreviations: 95% CI, 95% confidence interval; BTZ, bortezomib; FIL, filanesib; NR, not reached; PR, partial response.
Indicates censoring.
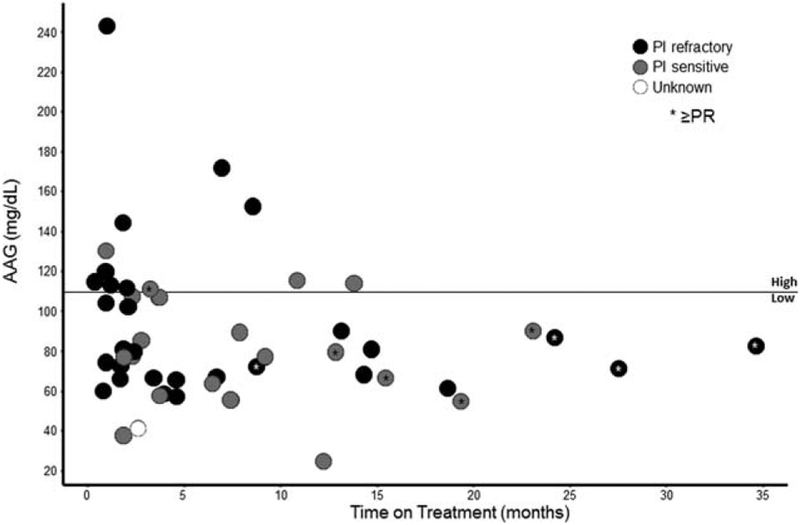
It has been shown that lower levels of alpha 1-acid glycoprotein (AAG), an acute-phase reactant binding filanesib in the serum, may be a useful biomarker with which to predict response to filanesib, perhaps due to greater levels of unbound fraction of filanesib and therefore higher therapeutic exposure to the drug.13 Therefore, its role was explored further in the current study. As shown in Figure 3 and Table 7, using an AAG cutoff of 110 mg/dL, there did appear to be a trend toward patients with lower AAG levels remaining on study longer than those with higher AAG levels, including patients with PI-refractory disease.
Figure 3.
Time on treatment by baseline alpha 1-acid glycoprotein (AAG) value among the population evaluable for response. PI indicates proteasome inhibitor; PR, partial response.
TABLE 7.
Time on Study
| Group | No. | Time on Study Median (Minimum-Maximum), Months |
|---|---|---|
| All patients | 55 | 5.6 (0.4–38.3a) |
| Bortezomib refractory | 28 | 3.0 (0.4–38.3a) |
| PI refractory | 31 | 3.7 (0.4–38.3a) |
| PI sensitive | 23 | 6.9 (1.2–26.7a) |
| Low AAG (≤110 mg/dL) | 40 | 5.7 (0.9–38.3a) |
| High AAG (>110 mg/dL) | 12 | 2.6 (0.4–14.5) |
| Low AAG and PI refractory | 22 | 4.8 (0.9–38.3a) |
| High AAG and PI refractory | 8 | 2.3 (0.4–8.5) |
| Low AAG and PI sensitive | 17 | 6.9 (1.9–26.7a) |
| High AAG and PI sensitive | 4 | 9.5 (1.2–14.5) |
Abbreviations: AAG, alpha 1-acid glycoprotein; PI, proteasome inhibitor.
Indicates censoring.
DISCUSSION
Filanesib is a first-in-class KSP inhibitor with a novel mechanism of action that has demonstrated preclinical synergy with both bortezomib and dexamethasone. The current phase 1 study established a dosing schedule for the combination of these agents that demonstrated a favorable safety profile with a low incidence of nonhematologic toxicity and peripheral neuropathy. Similar to the antimyeloma effect of filanesib, neutropenia may be a pharmacodynamic effect of the drug because both neutrophils and MM cells are Mcl-1-dependent.14 However, after filgastrim was incorporated, hematologic toxicities were rapidly reversible and noncumulative (neutropenia, which was largely of brief duration and without associated infections, and thrombocytopenia), and very few dose reductions were required.
Encouraging activity was observed in this heavily pretreated population, with a median of 3 lines of prior therapy, including an impressive median DOR of 14.1 months. Furthermore, in patients with PI-refractory disease, the median ToS of 4.7 months and 8 months, respectively, in schedules 1 and 2; an ORR of 29%; and a median DOR of 10.1 months compare quite favorably with recently approved agents in patients with RRMM such as carfilzomib, pomalidomide, and daratumumab. The current study also represents the third clinical trial demonstrating the possible use of AAG as a biomarker with which to enrich a population of patients with myeloma for possible response to filanesib.
FUNDING SUPPORT
Funded by Array BioPharma Inc. Biostatistics support was provided by Roger Aitchison (Array BioPharma Inc). Statistical programming was performed by Jennifer Regensburger (Array BioPharma Inc). Lindsey Lozano, a medical writer supported by funding from Array BioPharma Inc, provided drafts and editorial assistance to the authors during the preparation of this article.
We thank our patients, their families, and the Multiple Myeloma Research Consortium.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Ajai Chari has received research funding from and acted as a member of the Advisory Board for Millennium/Takeda, Celgene, Array BioPharma Inc, Novartis Pharmaceuticals, and Amgen-Onyx; received research funding from and acted as a paid consultant for Janssen Pharmaceuticals; received research funding from Pharmacyclics; and acted as a paid consultant for Bristol-Myers Squibb for work performed outside of the current study. Myo Htut has previously acted as a paid consultant for Millennium/Takeda for work performed outside of the current study. Jeffrey A. Zonder previously acted as a paid member of the Advisory Board for Array Bio-Pharma Inc and as a paid consultant for Bristol-Myers Squibb and Janssen; receives research support and acts as a member of the Advisory Board for Celgene; acts as a member of the Advisory Board for Takeda, Amgen, Prothena Corporation, and Seattle Genetics; and acts as a member of the Data Safety and Monitoring Board for Pharmacyclics. Andrzej J. Jakubowiak currently or has previously acted as a paid consultant for Millennium-Takeda, Amgen-Onyx, Bristol-Myers Squib, Celgene, Janssen, Karyopharm Therapeutics, Sanofi-Aventis, and SkylineDx for work performed outside of the current study. Joan B. Levy is a member of the Multiple Myeloma Research Foundation. Steven M. Burt is compensated by Celgene Corporation as part of the Speakers Bureaus. Brian J. Tunquist is a current employee of Array BioPharma Inc and has 2 patents currently pending (United States Patent and Trademark Office Application 2015/0231117 [ARRY-520 for use in treating cancer in a patient with low AAG] and United States Patent and Trademark Office Application 2010/0099697 [Method of Treatment Using Inhibitors of Mitosis]). Brandi W. Hilder, Selena A. Rush, DuncanH. Walker, and Mieke Ptaszynski are current or previous employees of Array BioPharma Inc; Selena A. Rush, Duncan H. Walker, and Mieke Ptaszynski hold stock options in Array BioPharma Inc. Jonathan L. Kaufman has received a grant from Merck; a grant and personal fees from Amgen-Onyx, Novartis Pharmaceuticals, and Celgene; and acted as a paid consultant for Millennium/Takeda, Janssen Pharmaceuticals, Spectrum, Incyte, and Pharmacyclics for work performed outside of the current study.
REFERENCES
- 1.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79: 867–874. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Lee JH, Lahuerta JJ, et al. ; International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther. 2010;9:2046–2056. [DOI] [PubMed] [Google Scholar]
- 4.Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. [DOI] [PubMed] [Google Scholar]
- 5.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. [DOI] [PubMed] [Google Scholar]
- 6.Woessner R, Tunquist B, Walker D, Rana S, Orlowski R, Kuhn D. Combination of the KSP inhibitor ARRY-520 with bortezomib causes sustained tumor regressions and significantly increased time to regrowth in bortezomib sensitive and resistant models of multiple myeloma. Presented in abstract form at: American Association for Cancer Research (AACR) 102nd Annual Meeting; April 2–6, 2011; Orlando, FL: Abstract 2550. [Google Scholar]
- 7.Heidari N, Hicks MA, Harada H. GX15–070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell Death Dis. 2010;1:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, et al. ; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed October 13, 2014.
- 11.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah JJ, Zonder JA, Cohen AD, et al. A phase 1/2 trial of the KSP inhibitor ARRY-520 in relapsed/refractory multiple myeloma [abstract]. Blood. 2010;116(21). Abstract 1959. [Google Scholar]
- 13.Tunquist B, Brown K, Hingorani G, et al. Alpha 1-acid glycoprotein (AAG) is a potential patient selection biomarker for improved clinical activity of ARRY-520 in relapsed and refractory multiple myeloma (MM). Presented in abstract form at: 18th Congress of the EHA; June 13–16, 2013; Stockholm, Sweden: Abstract P786. [Google Scholar]
- 14.Murphy MP, Caraher E. Mcl-1 is vital for neutrophil survival. Immunol Res. 2015;62:225–233. [DOI] [PubMed] [Google Scholar]