Abstract
The core functional organization of the primate brain is remarkably conserved across the Order, but behavioral differences evident between species likely reflect derived modifications in the underlying neural processes. Here we performed the first study to directly compare visual recognition memory in two primate species - rhesus macaques and marmoset monkeys – on the same visual preferential looking task (VPLT) as a first step towards identifying similarities and differences in this cognitive process across the primate phylogeny. Preferences in looking behavior on the task were broadly similar between the species, with greater looking times for novel images compared to repeated images as well as a similarly strong preference for faces compared to other categories. Unexpectedly, we found large behavioral differences among the two species in looking behavior independent of image familiarity. Marmosets exhibited longer looking times, with greater variability compared to macaques, regardless of image content or familiarity. Perhaps most strikingly, marmosets shifted their gaze across the images more quickly, suggesting a different behavioral strategy when viewing images. While such differences limit the comparison of recognition memory across these closely related species, they point to interesting differences in the mechanisms underlying active vision that have significant implications for future neurobiological investigations with these two nonhuman primate species. Elucidating whether these patterns are reflective of species or broader phylogenetic differences – e.g. between New World and Old World monkeys – necessitates a broader sample of primate taxa from across the Order.
Introduction
Like all closely related species, primates are distinguished from other taxonomic groups by a unique assemblage of phenotypic characteristics, such as their behavioral repertoire. However, even within the taxa, meaningful differences in each species behavioral repertoire emerged over the course of their adaptive radiation. Though many other taxonomic groups exhibit a notable range of sophisticated cognitive behaviors (Bugnyar, 2013; Finn, Tregenza, & Norman, 2009; Heinrich, 2011; Marino et al., 2007), the breadth of primate cognition and its intersection with key sensory processes is routinely emphasized as amongst the most idiosyncratic characteristics of the Order (Burkart, Hrdy, & van Schaik, 2009; Koops, Visalberghi, & van Schaik, 2014; Miller et al., 2016; Platt, Seyfarth, & Cheney, 2016; Rosati, Santos, & Hare, 2010; Tomasello & Call, 1997; Whiten et al., 1999). Unlike most mammals, primates rely almost entirely on vision and audition to build representations of the sensory world (J. M. Allman, 1977; Kaas, 2010, 2013). For example, our capacity to rapidly visually distinguish between items in the world that are familiar and those that are novel is characteristic of primate memory (Eichenbaum, Yonelinas, & Ranganath, 2007), but there has been little effort to directly compare these processes between species in the Order. Systematic comparisons of key cognitive processes in primate behaviors offer a powerful opportunity to identify shared and idiosyncratic characteristics which, in turn, can inform predictions about differences in the supporting neural mechanisms (Brenowitz & Zakon, 2015; J. F. Mitchell & Leopold, 2015; Yartsev, 2017).
Primate neuroscience has been dominated by studies of rhesus macaques – an Old World monkey - for several decades; however, common marmosets – a New World monkey - have more recently emerged as a powerful complementary model organism (Kishi, Sato, Sasaki, & Okano, 2014; Miller, 2017; Miller et al., 2016; Okano et al., 2016). While these species share many of the core defining characteristics of primate brains and behavior, notable differences are also evident. Not only are marmosets significantly smaller in body and brain size, but are also, in contrast to macaques, entirely arboreal, endemic only to the forests of South America (Schiel & Souto, 2017). The marmoset behavioral repertoire also differs from macaques in a number of notable ways. Like humans, marmosets are amongst only a handful of primates that pair-bond (Fischer, 1993) and cooperatively care for their young (French, 1997; N. Solomon & French, 1997). These cooperative tendencies extend to other contexts, including food sharing (Brügger, Kappeler-Schmalzriedt, & Burkart, 2018), that are rare in other primates but, at the same time, consistent with the species’ prosocial tendencies thought to be integral to marmoset cognitive evolution (Burkart et al., 2009; Burkart & van Schaik, 2010). Furthermore, marmosets are the only species of monkey for which there is experimental evidence of imitation in adults (Bugnyar & Huber, 1997; Voelkl & Huber, 2000, 2007), a unique social learning mechanism commonly employed in humans. Such differences may not be surprising given that New World and Old World monkeys diverged from the human lineage ~ 35 and 25 mya, respectively. Although these simian groups are more closely related to each other than either is to humans (Springer, Meredith, Janecka, & Murphy, 2011), sufficient time has occurred to for each species to adapt their behavioral repertoire to their respective niches while still relying on the shared primate functional brain architecture (Chaplin, Yu, Soares, Gattass, & Rosa, 2013; Hung et al., 2015b; J. F. Mitchell & Leopold, 2015; S. G. Solomon & Rosa, 2014). Importantly, we expect any observed differences to be placed within the context of copious similarities owing to their shared evolutionary history as primates, particularly for core cognitive systems inherent to primate behaviors such as recognition memory.
Here we utilized a visual preferential looking task from previous studies of visual recognition memory in both macaques and humans (Crutcher et al., 2009; Jutras & Buffalo, 2010; Wilson & Goldman-Rakic, 1984) in order to directly compare visual recognition memory in these two closely related primate species. Whereas a previous comparative study showed broadly similar patterns of visual behavior across these species (Mitchell, Reynolds, & Miller, 2014), more detailed approaches are needed to better characterize a broader range of cognitive processes. By testing subjects in each species on an identical task with identical visual images, we were able to directly compare several dimensions of each species’ respective behavior – ranging from performance on the task to the fine details of eye movements - to identify points of similarity and important differences across these closely related primate species.
Materials & Methods
Subjects and Surgery
Three adult common marmosets (Callithrix jacchus) A, K, and L and three adult rhesus macaques (Macaca mulatta) I, P, and T served as subjects to measure recognition memory based on preferential looking. All experiments were approved by the Institutional Animal Care and Use Committees at their respective institutions (A, K, and L at the University of California, San Diego and I, P, and T at the University of Washington, Seattle). Surgical procedures for marmosets were as in (Nummela, Jovanovic, de la Mothe, & Miller, 2017; S. U. Nummela et al., 2017) with the following modifications: nylon screws were used to anchor the head post and C&M-Metabond was not applied. Surgical procedures for macaques were as in (Jutras & Buffalo, 2010).
Behavioral Tasks
Rhesus macaques were tested on the Visual Preferential Looking Task (VPLT) while head-restrained and seated in a primate chair. The monkey initiated each trial by fixating on a white cross (the fixation target, 1°) at the center of the computer screen. After maintaining fixation on this target for 1 s, the target disappeared and a square picture stimulus subtending 11° was presented. All stimuli were obtained from Flickr. A total of 3,000 stimuli were used in this study, each only presented two times (a novel presentation and a repeat presentation). Each stimulus disappeared when the monkey’s direction of gaze moved off the stimulus or after a maximum looking time of 5 s. A blank screen was displayed for 1 s between trials. The VPLT was given in 51 daily blocks of 6, 8, or 10 trials each, chosen pseudo-randomly, for a total of 400 trials each day. The first half of each block were novel trials, in which an image that the subject had never viewed was presented. The second half of each block consisted of repeat trials, in which the images from the novel trials were shuffled and then presented again. The median delay between successive presentations was 8.1 s. Reward was not delivered during blocks of the VPLT; however, five trials of the calibration task were presented between each block to give the monkey a chance to earn some reward and to verify calibration. The number of trials in each VPLT block was varied in order to prevent subjects from knowing when to expect the rewarded calibration trials.
For macaques, behavior was collected in a dimly illuminated room, 60 cm from a 19-inch CRT monitor. Eye movements were recorded using a noninvasive infrared eye-tracking system (ISCAN). Stimuli were presented using experimental control software (CORTEX, www.cortex.salk.edu). At the beginning of each recording session, the monkey performed a calibration task, which involved holding a touch-sensitive bar while fixating on a small (0.3°) gray point, presented on a dark background at various locations on the monitor. The monkey had to maintain fixation within a 3° window until the fixation point changed to an equiluminant yellow at a randomly chosen time between 500 ms and 1,100 ms after fixation onset. The subject was required to release the touch-sensitive bar within 500 ms of the color change for delivery of a drop of applesauce. During this task, the gain and offset of the oculomotor signals were adjusted so that the computed eye position matched targets that were a known distance from the central fixation point.
Likewise, marmosets performed the VPLT while head-restrained and seated in a primate chair. All behavior was collected in a chamber illuminated only by a 21-inch LED display (X2411z, BenQ), which had a dynamic range from 0.5 to 230 cd/m2, with luminance linearity verified by photometer. Background illuminance was 115 cd/m2. Eye calibration was performed by fixating detailed marmoset faces (1°), and then finely adjusted with a central fixation spot (0.3°) at the center of the visual display. The VPLT was identical except that fixation lasted only 0.2 – 0.4 s, the images subtended 10° of visual arc, and the interleaved calibration trials consisted of an array of up to 8 marmoset faces, with reward delivered for maintaining fixation on a face for over half a second. Eye position was acquired at 220 Hz using an Eye Tracker and Viewpoint software (Arrington Research), with eye position collected from infrared light reflected off of a dichroic mirror (part #64–472, Edmunds Optics). Eye calibration and the visual preferential looking task (VPLT) were controlled using a custom Matlab GUI on a Windows 7 machine with Intel i7 cpu, 8 GB RAM, and GeForce Ti graphics card, which was presented on a second display. The software sub-sampled eye position on-line at the display refresh rate of 120 Hz using the ViewPoint Matlab toolbox (Arrington) and presented visual stimuli using the PsychoPhysics Toolbox [41, 42, 43] (Brainard, 1997; Pelli, 1997). Frame timing was confirmed by monitoring a photodiode (SD200-12-22-041-ND, Digi-Key).
Both species were head-restrained and oriented to the center of the visual display for all behavioral tasks. Both marmosets and macaques typically completed 400 VPLT trials (200 images, each shown twice) with the exception of marmoset L, who completed behavioral sessions of 200 VPLT trials (100 images). This was done because marmoset L inspected images for longer periods of time than any other subject and we wanted to ensure as many complete behavioral sessions as possible.
Image Categorization
Images were sorted into 3 categories - Objects, Landscapes, and Faces - by one of the authors (S.U.N.) and lab technician (M.G.) using the following instructions: images with a distinct, non-biological object or objects in the foreground were to be marked as an Object, images with no distinct object or subject in the foreground were to be marked as Landscapes, and images with a human or animal face clearly visible were to be marked as faces. Not all images fit into these categories and only images that both sorters marked as clearly falling within each category were used for this analysis, resulting in 685 objects, 465 landscapes, and 516 faces. Author S.U.N. marked the spatial extent of each face using ellipses on images categorized to include faces for further analyses.
Saccade Identification
Saccades were identified using previously described methods (Hafed, Goffart, & Krauzlis, 2009), including 8°/s velocity criterion and 550°/s2 acceleration criteria for 1500 of the 3000 images. All saccades were manually inspected to confirm or modify the eye signal flagged as a saccade. Peak velocities and amplitudes were calculated to confirm all data conformed to the expected shape of the main sequence. Analyses were restricted to saccades 1° or greater, since the video-based eye tracking was unable to reliably detect microsaccades. All analyses were also performed using all identifiable saccades, which resulted in a higher frequency of saccades for all subjects, but resulted in the same, significant results across species.
Experimental Design and Statistical Analysis
Subjects were shown up to 200 images in a single VPLT behavioral session from a database of 3000 images, and all subjects completed at least 80% of this image database. Non-parametric sign tests compared looking times for VPLT trials that presented novel images (novel trials) compared to trials that presented the same image a short time later (repeat trials), within single behavioral sessions, or within subjects across all behavioral sessions; visual recognition produced shorter looking times for repeat trials. Brown-Forsythe tests compared the variance in VPLT looking times across subjects, with Holm-Sidak correction for multiple comparisons. This analysis was performed only on VPLT trials that did not reach the 5 s limit. Including those trials yields nearly identical results except that for novel VPLT trials, one comparison, marmoset L to macaque T, does not reach significance (p = 0.91). More detailed comparisons of subject behavior were performed for VPLT images that clearly fit into one of three categories (described above). 2-way ANOVA tested whether changes in looking time depended on subject species, category of image content, or an interaction between these groups. Differences between ANOVA groups were identified using Tukey’s tests, correcting for multiple comparisons. A non-parametric sign test was used to measure species differences in the proportion of looking time spent looking at faces in images categorized to include face content. Pearson’s correlations identified a relationship between novel trial looking times and the degree of visual recognition indicated by behavior (novel looking time minus repeat looking time). Saccadic eye movements were identified for half of our data set (1500 images). Median inter-saccade intervals were compared across subjects using non-parametric rank-sum tests, with Holm-Sidak correction for multiple comparisons.
Results
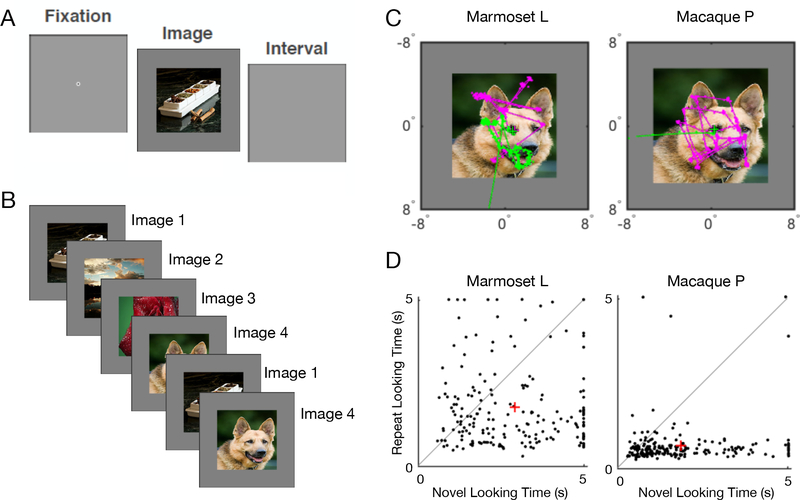
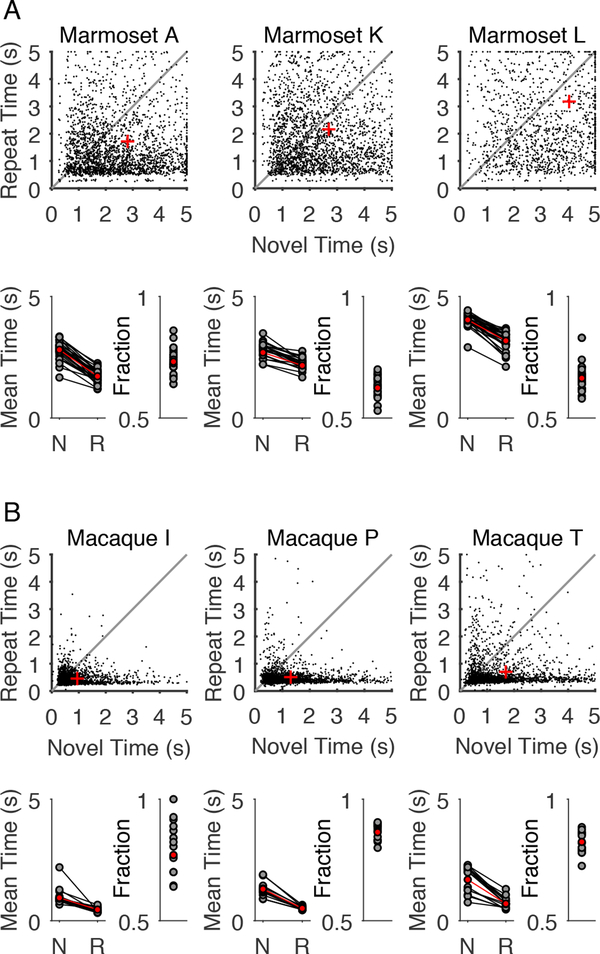
We compared the behavior of three marmoset and three macaque subjects performing a simple visual preferential looking task (VPLT). Briefly, each VPLT trial presents an image (Figure 1A), and the trial ends when the subject first looks away from the image or after 5 s has elapsed. The VPLT is organized in blocks of 6, 8, or 10 trials; the first half of the block consist of trials that display a completely novel image (novel trials). The rest of trials in the block display the same images from the novel trials, but in a randomized order (repeat trials). Figure 1B shows an example progression of an 8-trial block that started with 4 novel trials, followed by 2 repeat trials--the block would conclude after the remaining 2 images are repeated. Figure 1C shows example looking behavior from one marmoset and one macaque subject viewing an image. In this example, looking behavior during a novel trial is illustrated by the magenta eye traces and looking behavior for the repeat trial is illustrated by the green eye traces. In the novel trial, both subjects remained on the image for the entire 5 s. However, on the repeat trial both subjects looked away from the image before 5 s elapsed, ending the trial. Figure 1D summarizes both macaque and marmoset performance for 200 images in the behavioral sessions that included the trials illustrated in Figure 1C. In both cases, marmosets and macaques tended to look at novel images for longer periods of time compared to the same image repeated a short time later (sign tests: marmoset, sign = 147, 200 images, p < 0.0001; macaque, sign = 176, 200 images, p < 0.0001). However, the marmoset subject tended to look at images for greater periods of time than the macaque. Moreover, the marmoset exhibits more variability in looking times compared to the macaque subject. The trend observed in the sample behavioral sessions are representative of data for all subjects over many behavioral sessions. Figure 2 summarizes VPLT performance of marmoset (A) and macaque (B) subjects over all sessions. All subjects showed strong looking preferences for novel images compared to repeated images (sign tests: marmoset A, sign = 2271, 2735 images, p < 0.0001; marmoset K, sign = 1753, 2814 images, p < 0.0001; marmoset L, sign = 1996, 2990 images, p < 0.0001; macaque I, sign = 1567, 2027 images, p < 0.0001; macaque P, sign = 2054, 2400 images, p < 0.0001; macaque T, sign = 2251, 2731 images, p < 0.0001), Figure 2A,B top panels. This trend was observable in every individual behavioral session (Figure 2A,B bottom panels), but marmoset subjects had longer looking times for both novel and repeat image presentations as well as greater trial-to-trial variability. Importantly, both marmoset and macaque monkeys demonstrate the same qualitative looking preference for novel images, with shorter looking times for the repeated presentation demonstrating occurrence of visual recognition for some subset of the images.
Figure 1.
The visual preferential looking task and sample marmoset and macaque behavior. A - Trials began with a period of central fixation followed by presentation of an image. The subject could freely view the image until it looked away or 5 s had passed. A blank screen was displayed between consecutive trials. B - An example schematic of a sequence of trials shows 4 novel trials, in each of which a novel image was shown to the subject. Following the novel trials, those images are shuffled and displayed again in the same number of repeat trials--the first 2 are shown in this panel. Blocks could consist of 6, 8, or 10 trials, in which 3, 4, or 5 images are presented exactly twice. C - Sample looking behavior for one image from a marmoset (left) and a macaque subject (right). The eye position during the novel trial is indicated by the magenta traces and for the repeat trial by the green traces. D - Viewing times for a sample session of 200 unique images from a marmoset subject (left) and a macaque subject (right) with the time spent looking at each image indicated by a black dot. Mean viewing times for all novel presentations compared to repeats are indicated by red crosses.
Figure 2.
Marmosets and macaques look at novel images longer than recently viewed images. A - Above, looking times for every image are plotted by black points for three marmoset subjects. Points below the unity line are images with longer looking times for the novel presentation compared to the repeat presentation. The red cross indicates the average looking time for novel compared to repeat presentations. Below, looking times are summarized for each behavioral session by plotting the mean looking time for novel image presentations compared to repeat presentations, with the summary for all images plotted in red. Results of individual sessions are also summarized by plotting the fraction images with greater novel presentation looking times, with the results over all images plotted in red. In both cases, marmosets show a preference for looking at novel stimuli in every behavioral session. B - Looking preferences for three macaque subjects summarized using the same conventions as A.
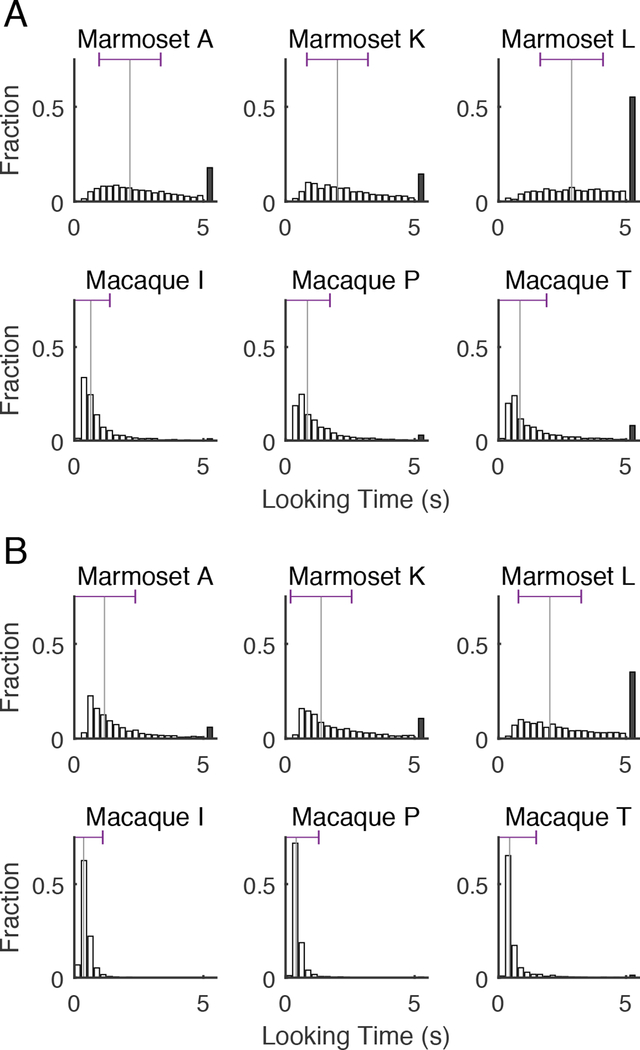
The greater variability for looking times in marmosets compared to macaques is more clearly observable in the distributions of looking times for each subject for both novel images (Figure 3A) and repeat images (Figure 3B). However, the shapes of looking time distributions in Figure 3 are difficult to quantify using a Gaussian standard deviation due to their asymmetric shape and the imposed 5 s time limit for each image. To quantify this variability, we applied a Brown-Forsythe transformation to the standard deviation of looking time for each subject, which is more robust to non-Gaussian distributions. This estimate of variance was calculated after removing trials that reached the maximum 5 s looking time in order to reduce the impact of that imposed ceiling on the looking time distributions. Notably, marmosets exhibited significantly greater variance in image looking times than macaques for all 9 possible subject comparisons between species (i.e. marmoset A to macaques I, P, and T; marmoset K to macaques I, P, and T; marmoset L to macaques I, P, and T) for repeat VPLT trials and for novel VPLT trials (Brown-Forsythe tests, p < 0.0001).
Figure 3.
Marmoset looking time varies more than macaque looking time. A - Comparison of distributions of looking times for marmosets (top) and macaques (bottom) for novel images. Histograms of looking times for each image are given, with looking times of 5 s (maximum time images were displayed) plotted as a black bar. Median is provided by vertical black lines, and the horizontal errorbar at the top of median provides the Brown-Forsythe standard deviation for each subject of all trials, excluding those with the maximum looking time. B - Comparison of distributions of looking times for marmosets and macaques for repeat images. Conventions are the same as A.
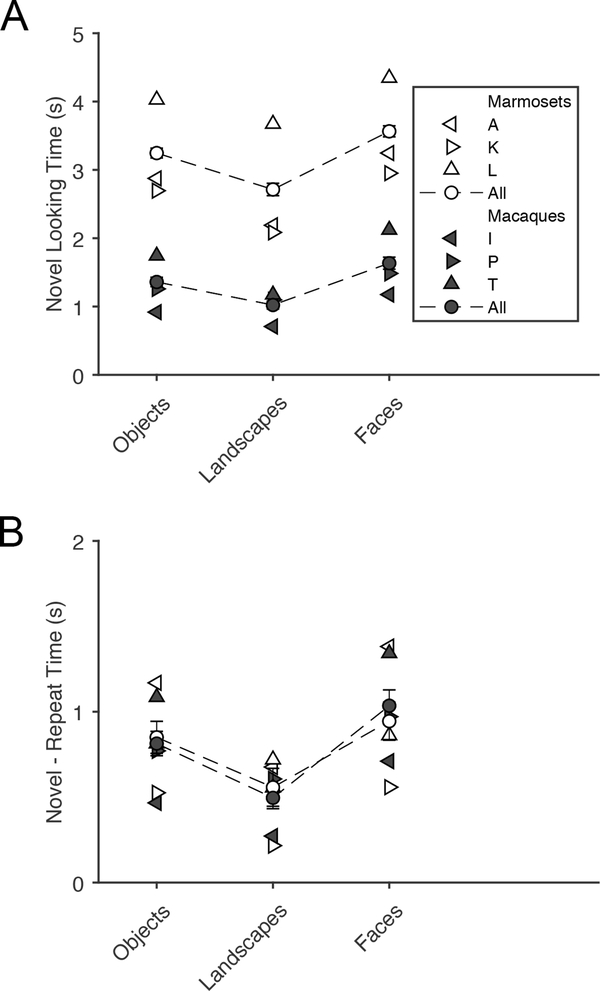
In order to search for deeper similarities in marmoset and macaque visual behaviors, we tested whether image content influenced this task by separately analyzing images that cleanly fit into categories of objects, landscapes, and faces (see Materials & Methods). Figure 4A shows looking times of every subject for each image category. 2-way ANOVA found looking time significantly depended on species and categories, but not on the interaction (species F(1,6684) = 2180, p < 0.0001; image category F(2,6684) = 236, p < 0.0001; interaction F(2,6684) = 2.10, p = 0.12). This resulted in two easily observable main effects. First, as previously observed, marmosets looked at images for longer periods of time than macaques. Second, faces resulted in the longest looking times for both species, followed by objects, with the shortest looking times reserved for landscapes with no distinct object or subject in the foreground (Tukey’s test, p < 0.0001 for all comparisons). We found the novel looking time was strongly correlated with the looking preference, (marmoset, r(2787) = 0.65, p < 0.0001; macaque, r(3898) = 0.90, p < 0.0001), resulting in the same pattern of results for looking preference as for novel looking time in Figure 4B. 2-way ANOVA found looking preference only depended on image content and not species (species F(1,6684) = 0.25, p = 0.62; image category F(2,6684) = 28.2, p < 0.0001; Tukey’s test: objects and landscapes, p < 0.0001; objects and faces p = 0.010; landscapes and faces, p < 0.0001).
Figure 4.
Novel image exploration and preference depends on image content. A - Mean viewing times of novel images for each subject, separated by whether the image contained objects, faces, or was a landscape with no distinct item in the foreground. Error bars for the species-wise averages are 95% confidence intervals. B - Mean novel preference (calculated by subtracting repeat looking times from novel looking times) for each subject, separated by image content. Error bars for the species-wise averages are 95% confidence intervals.
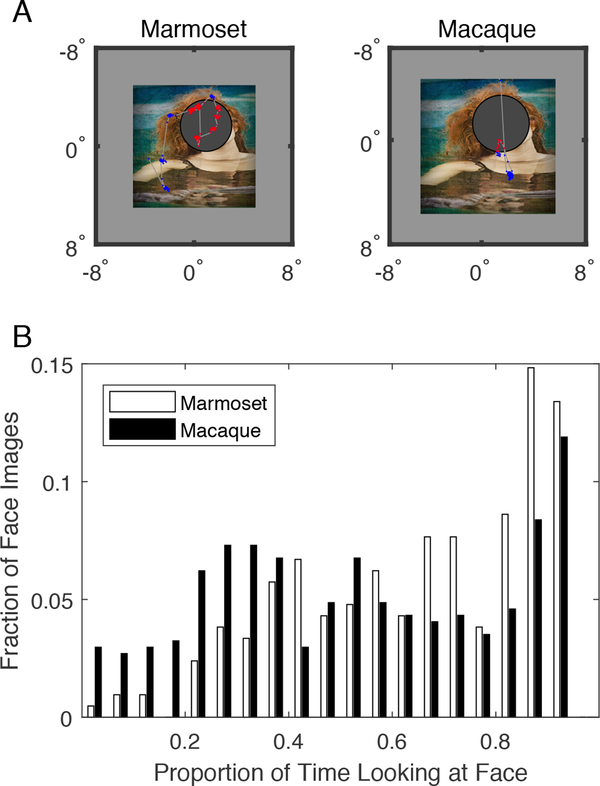
Because both species showed the greatest looking times and strongest looking preferences for faces, we performed an analysis to determine whether both species spent comparable amount of time looking at faces in these images compared to other parts of the image (Figure 5A). Figure 5B summarizes the distributions of time spent directly viewing the face parts of each image for marmosets (white bars) and macaques (black bars). This analysis was only performed for the initial, novel, image presentations. Whereas both species typically viewed face parts of images for a relatively large proportion of the total looking time (marmosets 79% median, macaques 73% median), marmosets had a significantly stronger proclivity to do so (sign test, sign = 117, 507 images, p < 0.01).
Figure 5.
Marmosets look directly at faces more often than macaques. (A)- An example image demonstrates how the proportion of time spent looking directly at faces was calculated, with the eye trace from a marmoset (left) and a macaque (right) subject. Faces in each image were marked by ellipses, and the proportion of time the eye was directed into the face region (red points) was divided by the proportion of time the eye was directed out of the face region (blue points). (B) - The distribution of the proportion of time looking directly at faces for marmosets (white bars) is shown compared to macaques (black bars). All subjects were pooled for clarity.
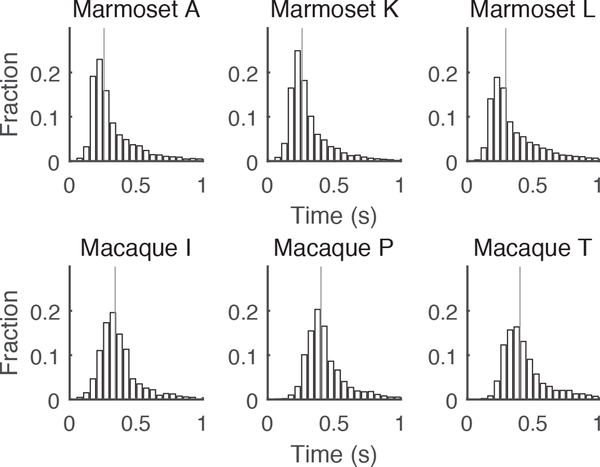
Because the largest observed differences between marmoset and macaque behavior were apparent in their looking behavior with regards to novel images, we examined the subjects’ saccadic eye movements to look for other differences in active vision. In particular, we focused on saccades that reoriented gaze by more than 1 degree of visual arc. Figure 6 compares the distributions of inter-saccade intervals for marmoset and macaque subjects. Strikingly, all marmosets showed significantly shorter intervals between such saccades, with medians of 259 ms for marmoset A, 267 ms for marmoset K, and 292 ms for marmoset L and medians of 342 ms for macaque I and 408 ms for macaques P and T. All marmosets had significantly shorter inter-saccade intervals than all macaque subjects (rank sum tests, p < 0.0001 after correction for multiple comparisons).
Figure 6.
Saccade frequency is greater for marmosets compared to macaques. Distributions of intervals between saccades greater than 1 degree in amplitude for the same set of 1500 novel images are provided for each marmoset (top) and macaque (bottom) subjects. Vertical black lines indicate the median inter-saccade interval.
Discussion
Here we directly compared marmoset and rhesus macaque monkey behavior on a visual preferential looking task to precisely identify similarities and differences in visual recognition memory of these primate species. Importantly, we found the same qualitative pattern of recognition memory exhibited by rhesus macaques (Jutras & Buffalo, 2010; Wilson & Goldman-Rakic, 1984) and humans (Crutcher et al., 2009) - the preference for looking at novel images compared to familiar images (i.e. the second presentation). Although not a complete primate phylogeny, the evidence that species from at least three primate families - apes, New World monkeys and Old World monkeys - exhibit notably similar patterns of recognition memory on this task is at least suggestive that the underlying processes may be conserved across all primates. To more fully test this hypothesis, however, additional species in these families, as well as prosimians, must be studied on the same task. In our comparison of rhesus macaques and marmosets, the greatest reduction in looking times occurred for faces followed by objects and then landscapes - the same order of looking times for image content when comparing across only novel image presentations. The interest in faces is consistent with its significant role in social signaling across primates (Freiwald, Duchaine, & Yovel, 2016; Hung et al., 2015a; Kanwisher, McDermott, & Chun, 1997; Tsao, Freiwald, Tootell, & Livingstone, 2006; Tsao, Moeller, & Freiwald, 2008; Taubert et al. 2017; Mosher et al., 2014). Data presented here suggest that attention to faces across both species of nonhuman primates may increase the likelihood that features of this social object are recognized over other salient objects and facilitate broader face recognition processes (Landi & Freiwald, 2017). Consistent with previous studies (Mitchell et al., 2014), these data suggest that marmosets and macaques exhibit broadly similar patterns of visual cognitive behavior. However, more detailed comparisons revealed significant differences across the species.
Further quantitative comparisons of recognition memory between marmosets and rhesus macaques were confounded by striking, unexpected differences in the active scanning of images that were independent of image familiarity. For ease of reader reference, these differences are summarized in Table 1. Most noticeably, marmosets exhibited longer looking times than rhesus macaques regardless of image content or familiarity. The increased looking times in marmosets were accompanied by an increase in the variability of looking times - apparent for both novel and familiar images - and complicates further direct comparisons of the degree of visual recognition between marmosets and rhesus macaques. We find that marmosets less consistently exhibited looking preference for novel images compared to macaques, but this could be due to either the greater variability in their looking behavior or to a lower frequency of recognition occurrence. These differences in visual behavior of marmosets and rhesus macaques limit further discussion of our results with regards to recognition memory, but they point to interesting differences in visual processing between the species.
Table 1.
Summary of subject behavior on the visual preferential looking task. The table provides a quick reference of several important metrics of macaque and marmoset behavior on the visual preferential looking task (VPLT). The first three columns report the median amount of time subjects spent looking at novel image presentations, repeat image presentations, and the difference between novel and repeat looking times, respectively. Columns 4 and 5 report the Brown-Forsythe standard deviations of looking time for novel and repeat image presentations. Column 6 reports the median inter-saccadic intervals (ISIs) during image presentations for novel images. Column 7 reports the number of image presentations, or trials, in a behavioral session.
| Median Looking Time (s) | St Dev (Brown-Forsythe) | Median ISI (s) | Trials per Session | ||||
|---|---|---|---|---|---|---|---|
| Marmoset | Novel | Repeat | Difference | Novel | Repeat | Novel | |
| A | 2.59 | 1.23 | 0.99 | 1.19 | 1.01 | 259 | 400 |
| K | 2.37 | 1.63 | 0.48 | 1.18 | 1.14 | 267 | 400 |
| L | 5 | 3.18 | 0.53 | 1.23 | 1.24 | 292 | 200 |
| Mean | 3.32 | 2.01 | 0.67 | 1.2 | 1.13 | 272 | |
| Macaque | |||||||
| I | 0.63 | 0.36 | 0.24 | 0.74 | 0.26 | 342 | 400 |
| P | 0.87 | 0.41 | 0.41 | 0.87 | 0.33 | 408 | 400 |
| T | 0.94 | 0.44 | 0.38 | 1.04 | 0.58 | 408 | 400 |
| Mean | 0.81 | 0.4 | 0.34 | 0.88 | 0.39 | 386 | |
| Species Diff | 2.51 | 1.61 | 0.32 | 0.74 | −114 | ||
Primate vision includes many facets of feature processing, selection, and control over eye movement. Visual processing in pre-cortical and cortical areas seems unlikely to cause the large behavioral differences we observed between rhesus macaques and marmosets, since both subjects share preferences for image content, including a strong preference for faces. In fact, deeper analysis demonstrated marmosets to have an even greater tendency to look at faces than macaques. The observed differences are better explained by differences in the active control of eye movements; specifically, we suggest that rhesus macaques and humans are more easily able to inhibit eye movement to distractors. Strong inhibition is necessary for macaque gaze aversion in order to suppress overt attention to salient facial features that also can signal aggression (R. A. Chance, 1967). Additionally, it is fast and routine to condition rhesus macaques to maintain fixation, even in the presence of many salient distractors (J. Allman, Miezin, & McGuinness, 1985; J. F. Mitchell, Sundberg, & Mitchell, 2009), yet it is difficult to train marmosets to maintain fixation, or smooth pursuit, for many seconds, even without salient distractors (J. Mitchell, Priebe, & Miller, 2015; J. Mitchell et al., 2014). We found that marmosets exhibited more frequent saccadic eye movements greater than 1 degree in amplitude, with median inter-saccade intervals of about 256 ms in marmoset compared to 375 ms in rhesus macaques. One specific mechanism that could cause such difference is a reduced suppression of alternative saccade plans in the frontal eye fields (Schall, Hanes, Thompson, & King, 1995) or superior colliculus (Krauzlis, Liston, & Carello, 2004), possibly due to weaker recurrent or lateral inhibition from the neurons active during fixation (D.P. Munoz & R.H. Wurtz, 1993; D. P. Munoz & R. H. Wurtz, 1993). Other possibilities include differences in connectivity between gaze control areas; e.g., one report indicated sparse or absent projections from supplementary eye fields to the superior colliculus in the marmoset (Collins, Lyon, & Kaas, 2005). Direct evidence for these hypotheses could be collected by applying standard electrophysiological techniques used in macaques to the marmoset frontal eye fields and superior colliculus during a simple stimulus discrimination task. Other possible explanations include differences in the circuitry underlying covert visual attention or visual selection, which is strongly associated with memory (Ballesteros, Reales, García, & Carrasco, 2006; Broadway, Hilimire, & Corballis, 2012). However, these mechanisms may be intertwined, as suppression of distractors may enhance recognition memory (Markant, Worden, & Amso, 2015) and some attributes of visual attention depend on gaze control structures (Moore & Fallah, 2004; Zénon & Krauzlis, 2012).
Here we found broad similarities in marmoset and rhesus macaque behavior, indicating the formation of recognition memories, with more reliable indications of memory for faces compared to objects or landscapes. However, we also observed substantive differences in their active vision that merits further investigation at the neurobiological level. Direct comparisons of behavior between primate species enable us to form key predictions about the nuanced relationship between differences in behavior and cognition across the Order and the supporting neural processes. The almost exclusive reliance on rhesus macaques for the past 30 years of research has significantly limited our capacity to leverage the power of phylogenetic analysis to explicate these issues. Comparatively studying marmosets and other closely related species in the taxa affords the unique opportunity to better understand many mechanisms most relatable to humans, such as those supporting social interaction, visual navigation, object recognition, decision-making, and memory.
Acknowledgements
This work was supported by grants from the NIH (R01 NS109294, R01 DC012087) to CTM, McKnight Foundation to JTW, and the Kavli Foundation to SUN. Maddie Heater collected data from marmoset L and Madeleine Gagne helped score image categories and identify saccadic eye movements.
Footnotes
We have no conflicts of interest
References
- Allman J, Miezin F, & McGuinness E (1985). Stimulus Specific Responses from Beyond the Classical Receptive Field: Neurophysiological Mechanisms for Local-Global Comparisons in Visual Neurons. Annual Review of Neuroscience, 8(1), 407–430. [DOI] [PubMed] [Google Scholar]
- Allman JM (1977). Evolution of the visual system in early primates . In Sprague JM & Epstein AM (Eds.), Progress in Psychobiology and Physiological Psychology (pp. 1–53). New York: Academic Press. [Google Scholar]
- Ballesteros S, Reales J, García E, & Carrasco M (2006). Selective attention affects implicit and explicit memory for familiar pictures at different delay conditions, Psicothema, 18, 88–99.. [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox, Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Brenowitz EA, & Zakon HH (2015). Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends in Neurosciences, 38(5), 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway J, Hilimire M, & Corballis P (2012). Orienting to external versus internal regions of space: Consequences of attending in advance versus after the fact, Psychophysiology, 49, 357–368. [DOI] [PubMed] [Google Scholar]
- Brügger RK, Kappeler-Schmalzriedt T, & Burkart JM (2018). Reverse audience effects on helping in cooperatively breeding marmoset monkeys. Biology Letters, 14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T (2013). Social cognition in ravens. Comparative Cognition and Behavior Reviews, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, & Huber L (1997). Push or pull: An experimental study of imitation in marmosets. Animal Behaviour, 54, 817–831. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Hrdy SB, & van Schaik CP (2009). Cooperative breeding and human cognitive evolution. . Evolutionary Anthropology, 18, 175–186. [Google Scholar]
- Burkart JM, & van Schaik CP (2010). Cognitive consequences of cooperative breeding in primates? Animal Cognition, 13, 1–19. [DOI] [PubMed] [Google Scholar]
- Chaplin T, Yu H, Soares J, Gattass R, & Rosa MGP (2013). A conserved pattern of differential expansion of cortical areaas in simian primates. Journal of Neuroscience, 18, 15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Lyon DC, & Kaas JH (2005). Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, 285A(1), 619–627. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, Calhoun-Haney R, Manzanares CM, Lah JJ, Levey AL, & Zola-Morgan S (2009). Eye tracking during a visual paired comparison task as a predictor of early dimentia. . American Journal of Alzheimers Disorders and other Dimensias, 24, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The Medial Temporal Lobe and Recognition Memory. Annual Review of Neuroscience, 30(1), 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JK, Tregenza T, & Norman MD (2009). Defensive tool use in a coconut-carrying octopus. Current Biology, 19, R1069–1070. [DOI] [PubMed] [Google Scholar]
- Fischer JM (1993). The metaphysics of death. Stanford: Stanford University Press. [Google Scholar]
- Freiwald W, Duchaine B, & Yovel G (2016). Face Processing Systems: From Neurons to Real-World Social Perception. Annual Review of Neuroscience, 39(1), 325–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA (1997). Proximate regulation of singular breeding in callitrichid primates In Solomon N & French JA (Eds.), Cooperative Breeding in Mammals. (pp. 34–75). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Hafed ZM, Goffart L, & Krauzlis RJ (2009). A Neural Mechanism for Microsaccade Generation in the Primate Superior Colliculus. Science, 323(5916), 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B (2011). Conflict, Cooperation and Cognition in the Common Raven. Advances in the Study of Behavior, 43, 189–238. [Google Scholar]
- Hung CC, Yen CC, Ciuchta JL, Papoti D, Bock NA, Leopold DA, et al. (2015a). Functional mapping of face-selective regions in the extrastriate visual cortex of the marmoset. J Neurosci, 35(3), 1160–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CC, Yen CC, Ciuchta JL, Papoti D, Bock NA, Leopold DA, et al. (2015b). Functional MRI of visual responses in the awake, behaving marmoset. Neuroimage, 120, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, & Buffalo EA (2010). Recognition memory signals in the macaque hippocampus. Proceedings of the National Academy of Sciences, 107, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH (2010). Sensory and motor systems in primates In Platt M & Ghazanfar AA (Eds.), Primate Neuroethology (pp. 177–200). New York, NY: Oxford University Press. [Google Scholar]
- Kaas JH (2013). The evolution of brains from early mammals to humans. Wiley Interdisciplinary Reviews in Cognitive Science, 4, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Sato K, Sasaki E, & Okano H (2014). Common marmoset as a new model animal for neuroscience research and genome editing technology. Development, Growth & Differentiation, 56(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Koops K, Visalberghi E, & van Schaik CP (2014). The ecology of primate material culture. Biology Letters, 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, & Carello CD (2004). Target selection and the superior colliculus: goals, choices and hypotheses. Vision Research, 44(12), 1445–1451. [DOI] [PubMed] [Google Scholar]
- Landi SM, & Freiwald WA (2017). Two areas for familiar face recognition in the primate brain. Science, 357(6351), 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L, Connor RC, Fordyce RE, Herman LM, Hof PR, Lefebvre L, et al. (2007). Cetaceans have complex brains for complex cognition. PLoS ONE, 5(5), e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Worden MS, & Amso D (2015). Not all attention orienting is created equal: Recognition memory is enhanced when attention orienting involves distractor suppression. Neurobiology of Learning and Memory, 120, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT (2017). Why Marmosets? . Developmental Neurobiology, 77, 237–243. [DOI] [PubMed] [Google Scholar]
- Miller CT, Freiwald W, Leopold DA, Mitchell JF, Silva AC, & Wang X (2016). Marmosets: A Neuroscientific Model of Human Social Behavior. Neuron, 90, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Priebe N, & Miller CT (2015). Motion dependence of smooth eye movements in the marmoset. Journal of Neurophysiology, 113, 3954–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Reynolds J, & Miller CT (2014). Active vision in marmosets: A model for visual neuroscience. Journal of Neuroscience, 34, 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, & Leopold DA (2015). The marmoset monkey as a model for visual neuroscience. Neuroscience Research, 93, 20–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, & Mitchell JH (2009). Spatial attention decorelates intrisic activity fluctuations in macaque area V4. Neuron, 63, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, Gothard KM (2014) Neurons in the monkey amygdala detect eye-contact during naturalistic social interactions. Current Biology, 24, 2459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, & Fallah M (2004). Microstimulation of the Frontal Eye Field and Its Effects on Covert Spatial Attention. Journal of Neurophysiology, 91(1) 152–162. [DOI] [PubMed] [Google Scholar]
- Munoz DP, & Wurtz RH (1993). Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. Journal of Neurophysiology, 70 (2), 559–575. [DOI] [PubMed] [Google Scholar]
- Munoz DP, & Wurtz RH (1993). Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. Journal of Neurophysiology, 70(2), 576–589. [DOI] [PubMed] [Google Scholar]
- Nummela S, Jovanovic V, de la Mothe LA, & Miller CT (2017). Social context-dependent activity in marmoset frontal cortex populations during natural conversations. . Journal of Neuroscience, 37, 7036–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela SU, Coop SH, Cloherty SL, Boisvert CJ, Leblanc M, & Mitchell JF (2017). Psychophysical measurement of marmoset acuity and myopia. Developmental Neurobiology, 77(3), 300–313. [DOI] [PubMed] [Google Scholar]
- Okano H, Sasaki E, Yamamori T, Iriki A, Shimogori T, Yamaguchi Y, et al. (2016). Brain/MINDS: A Japanese National Brain Project for Marmoset Neuroscience. Neuron, 92, 582–590. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision 10, 437–442. [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, & Cheney DL (2016). Adaptations for social cognition in the primate brain. . Philosophical Transactions of the Royal Society of London, 371, 20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance RA, M. (1967). Attention Structure as the Basis of Primate Rank Orders. Man 2(4), 503–518. [Google Scholar]
- Rosati A, Santos L, & Hare B (2010). Primate social cognition: Thirty years after Premack and Woodruff In Platt M & Ghazanfar AA (Eds.), Primate Neuroethology (pp. 117–142). Oxford, UK: Oxford University Press. [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, & King DJ (1995). Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. The Journal of Neuroscience, 15(10), 6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel N, & Souto A (2017). The common marmoset: An overview of its natural history, ecology and behavior. Developmental Neurobiology, 77, 244–262. [DOI] [PubMed] [Google Scholar]
- Solomon N, & French JA (1997). The study of mammalian cooperative breeding In Solomon N & French JA (Eds.), Cooperative Breeding in Mammals (pp. 1–10). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Solomon SG, & Rosa MGP (2014). A simpler primate brain: The visual system of the marmoset monkey. Frontiers in Neural Circuits, 8(96), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MS, Meredith RW, Janecka JE, & Murphy WJ (2011). The historical biogeography of Mammalia. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1577), 2478–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert J, Wardle SG, Flessert M, Leopold DA, & Ungerleider LG (2017) Face pareidolia in the rhesus monkey. Current Biology, 27 (16), P2505–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, & Call J (1997). Primate Cognition. Oxford: Oxford University Press. [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, & Livingstone MS (2006). A cortical region consisting entirely of face-selective cells. Science, 311, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, & Freiwald W (2008). Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences, 49, 19514–19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B, & Huber L (2000). True imitation in marmosets. Animal Behaviour, 60, 195–202. [DOI] [PubMed] [Google Scholar]
- Voelkl B, & Huber L (2007). Imitation as faithful copying of a novel technique in marmoset monkeys. PLoS ONE, 2(7), e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, et al. (1999). Cultures in chimpanzees. Nature, 399, 682–685. [DOI] [PubMed] [Google Scholar]
- Wilson FAW, & Goldman-Rakic PS (1984). Viewing preferences of rhesus monkeys related to memory for complex pictures, colours and faces. Behavioral and Brain Research, 60, 79–89. [DOI] [PubMed] [Google Scholar]
- Yartsev MM (2017). The emperor’s new wardrobe: Rebalancing diversity of animal models in neuroscience research. Science, 358(6362), 466. [DOI] [PubMed] [Google Scholar]
- Zénon A, & Krauzlis RJ (2012). Attention deficits without cortical neuronal deficits. Nature, 489, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]