Abstract
Purpose:
Contemporary treatment for metastatic hormone sensitive prostate cancer (mHSPC) includes androgen deprivation therapy (ADT) plus abiraterone or docetaxel. While these intensified regimens have improved efficacy, they are also associated with increased cost and toxicities. Not all men with mHSPC may be candidates for these intensified regimens, yet there are no clinical models or biomarkers used to optimize treatment selection. Herein, we hypothesized that longer time from prior definitive therapy (DT), either radical prostatectomy, definitive radiotherapy, or both, to onset of metastatic disease is associated with improved survival outcomes in men with newly diagnosed mHSPC.
Methods:
This multicenter retrospective study included men initiating systemic therapy with ADT for new mHSPC. Kaplan-Meier and COX proportional hazard models assessed time to metastatic castration-resistant prostate cancer (mCRPC) and overall survival (OS) by receipt of prior DT.
Results:
Of the 253 men with new mHSPC, 115 (45%) had received prior DT. In a multivariate analysis, increasing years from DT to the start of ADT was an independent predictor of time to mCRPC (per year: hazard ratio 0.91 95% confidence interval 0.84–0.99, P = 0.020) and improved OS (per year: hazard ratio 0.87, 95% confidence interval 0.74–0.99, P = 0.0025) in patients with new mHSPC, and may assist with risk stratification in these patients at time of mHSPC.
Conclusion:
Time from DT to start of ADT is an independent predictor of time to mCRPC and OS in men with new mHSPC, and may assist with risk stratification of these patients for systemic therapy selection.
Keywords: Predictive biomarker, mHSPC, Definitive therapy, Radical prostatectomy, Radiotherapy
1. Introduction
Over the past 3 years, systemic treatment for metastatic hormone sensitive prostate cancer (mHSPC) has rapidly changed with the additions of docetaxel or abiraterone acetate to androgen deprivation therapy (ADT) [1-4]. Currently, National Comprehensive Cancer Network guidelines recommend ADT plus docetaxel, ADT plus abiraterone acetate, or ADT alone for systemic treatment of mHSPC [5]. Additionally, there are many ongoing registration trials testing novel androgen axis inhibitors alone or in combination with docetaxel in men with mHSPC. While these intensified regimens are more efficacious than standard ADT, they are also associated with increased cost and toxicities, so not all men with mHSPC are candidates for intensified upfront treatment. Furthermore, data from SWOG9346 trial suggests that there is a subset of men with mHSPC who have excellent overall survival (OS) with standard continuous ADT (~7 years), and may not require such aggressive upfront treatment [6,7]. Currently, patient/physician preference and disease characteristics guide selection between these regimens. However, clinical models or biomarkers predictive of response to ADT are needed to optimize treatment selection for men with mHSPC.
To date, there is limited literature on whether men with de novo metastatic prostate cancer vs. those with new metastatic prostate cancer who received “prior” definitive therapy (DT) have different survival outcomes and response to systemic therapy, and if the time from prior DT to onset of mHSPC correlates with survival outcomes and response to systemic therapy. An early report demonstrated that prior radical prostatectomy (RP) in men with newly diagnosed mHSPC was associated with a significant decrease in the risk of death (hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.53–0.89) relative to those who did not undergo earlier prostatectomy [8]. While this study suggests that DT prior to onset of metastatic disease could be a prognostic factor at time of mHSPC, there are no subsequent studies that have addressed this question.
Herein, we hypothesized that longer time from prior DT, either RP, definitive radiotherapy, or both, to onset of metastatic disease will be associated with improved survival outcomes in men with newly diagnosed mHSPC.
2. Patients and methods
2.1. Patient selection
Eligible patient data were retrospectively extracted from 6 different institutional databases. Between 2007 and 2013, men starting ADT for their newly diagnosed mHSPC were eligible for inclusion. Newly diagnosed mHSPC was defined by radiographic evidence of mHSPC. The index date was defined as the date of starting ADT for mHSPC. Patients were stratified based on whether or not they received prior DT (defined as having received RP, definitive radiation, or both). Patients who received ADT in the localized disease setting were excluded from the study. The study was conducted according to the guidelines of the Institutional Review Board of each participating center.
2.2. Clinical data and primary outcome collection
The clinical variables collected included demographics and disease characteristics including: Gleason score, visceral metastasis, baseline prostate-specific antigen (PSA), baseline testosterone at the time of ADT initiation and at 6 to 7 months after ADT, time to onset of metastatic castration-resistant prostate cancer (mCRPC), and OS. The clinical variables used in the analysis are shown in Table 1. Per Prostate Cancer Working Group 2 criteria, mCRPC was defined as those with a rising PSA level or clinical/radiographic progression in the presence of castrate level of testosterone and continuous ADT.
Table 1.
Demographics and clinical characteristics.
| Variable | Definitive therapy |
No definitive therapy |
P value |
|---|---|---|---|
| Total patients, n (%) | 115 (45) | 138 (55) | |
| Median age at initiation of ADT (IQR) | 68 (63–76) | 67.5 (62–75) | 0.81 |
| Race | |||
| Black/African American | 6 (5) | 21 (15) | 0.025 |
| Other | 96 (83) | 100 (72) | |
| Unknown | 13 (11) | 17 (12) | |
| Gleason score | |||
| Median, (IQR) | 7 (7–9) | 8 (7–9) | <0.0001 |
| Missing, n (%) | 9 (8) | 22 (16) | |
| ECOG Performance Status at initiation of ADT, n (%) | |||
| 0 | 62 (54) | 60 (44) | 0.25 |
| 1 | 23 (20) | 38 (28) | |
| 2 | 3 (3) | 9 (7) | |
| 3 | 1 (1) | 1 (1) | |
| Presence of visceral mets at dx, | |||
| n (%) | 6 (5) | 13 (9) | 0.18 |
| Missing, n (%) | 9 (8) | 15 (11) | |
| PSA at initiation of ADT | |||
| Median, (IQR) | 15 (6–44) | 75 (18–392) | <0.0001 |
| Missing, n (%) | 8 (7) | 3 (2) | |
| Testosterone at initiation of ADT | |||
| Median, (IQR) | 296 (173–458) | 213 (8–406) | 0.031 |
| Missing, n (%) | 41 (36) | 63 (55) | |
| PSA 6–7 mo after ADT | |||
| Median, (IQR) | 0.2 (0.1–1.1) | 1.1 (0.11–4.2) | 0.004 |
| Missing, n (%) | 49 (43) | 55 (48) | |
| Testosterone 6–7 m after ADT | |||
| Median, (IQR) | 7.5 (3–20) | 10 (3–20) | 0.57 |
| Missing, n (%) | 73 (64) | 103 (75) | |
| Type of DT received | |||
| Radical prostatectomy | 64 (56%) | ||
| Definitive radiation | 35 (30%) | ||
| Prostatectomy + radiation | 16 (14%) | ||
ADT = androgen deprivation therapy; DT = definitive therapy.
2.3. Statistical analysis
Kaplan-Meier and Cox proportional hazard methods with stratified log-rank tests were used to assess the time to mCRPC and OS in patients with or without prior DT. Men who did not develop mCRPC during the study period were censored in the survival analysis. Univariate and multivariate analyses were performed using the Kaplan-Meier and Cox proportional hazard methods. Correlation between the duration of time between DT and the initiation of ADT was also assessed. Variables significant in univariate survival analysis were included in the final COX models. COX models were adjusted for race and age at initiation of ADT. Significance level was set at P < 0.05.
3. Results
3.1. Impact of prior DT on survival outcomes
Two hundred and fifty-three men with newly diagnosed mHSPC were included in this multi-institutional, retrospective analysis. Baseline variables of eligible patients are reported in Table 1. Fifty-seven percent of men in the cohort developed mCRPC (n = 145). Patients were stratified based on whether they received prior DT for local disease. Prior DT was performed in 115 men (45%). The patient characteristics in the prior DT vs. no DT group were as follows: age at initiation of ADT was 68 years vs. 67.5 years, P = 0.81; and Gleason score 7 vs. 8, P < 0.0001; Eastern Cooperative Oncology Group (ECOG) 0 54% vs. 44.6%, P = 0.25, respectively.
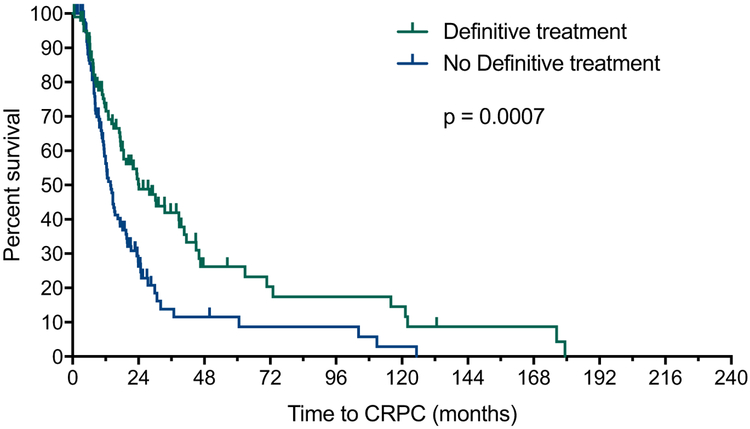
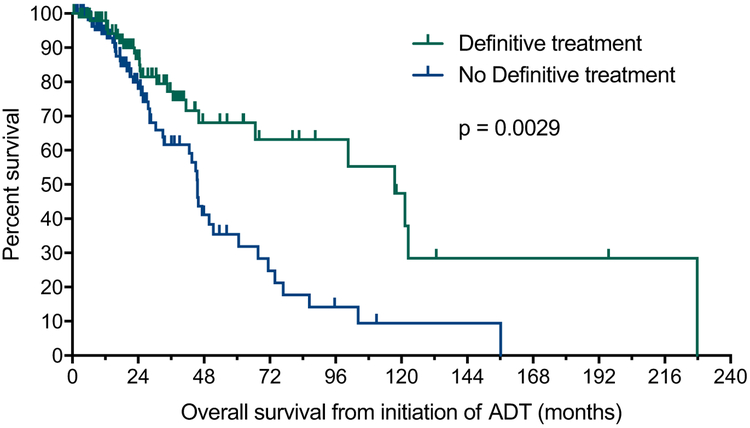
In a univariate analysis, prior DT was associated with improved time to mCRPC (24 vs. 13.8 months, HR 0.56; 95% CI 0.40–0.78; P = 0.0007, Fig. 1A) and OS (117.5 vs. 45.6 months; HR 0.47; 95% CI 0.28–0.77; P = 0.0029; Fig. 1B). When combined with other prognostic factors in a multivariate analysis, a strong trend was observed for time to mCRPC (HR 0.68; 95% CI 0.45–1.02; P = 0.063) and OS (HR 0.57; 95% CI 0.28–1.13; P = 0.11) (Table 2).
Fig. 1A.
Effect of prior definitive therapy for localized prostate cancer on time to mCRPC for men with newly diagnosed mHSPC. mCRPC = metastatic castration-resistant prostate cancer; mHSPC = metastatic hormone sensitive prostate cancer.
Fig. 1B.
Effect of prior definitive therapy for localized prostate cancer on time to OS for men with newly diagnosed mHSPC. mHSPC = metastatic hormone sensitive prostate cancer; OS = overall survival.
Table 2.
Effect of definitive therapy and baseline characteristics on time to mCRPC and OS.
| Variable | Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| CRPC |
OS |
CRPC |
OS |
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at initiation of ADT, continuous | 0.99 (0.98–1.01) | 0.55 | 1.06 (1.03–1.08) | <0.0001 | 0.99 (0.98–1.01) | 0.55 | 1.05 (1.02–1.08) | 0.0011 |
| Race (Black/African American vs. other) | 1.02 (0.62–1.60) | 0.38 | 1.62 (0.87–2.86) | 0.13 | 0.58 (0.32–0.99) | 0.084 | 0.87 (0.42–1.72) | 0.78 |
| ECOG | ||||||||
| 0 | ref | 0.015 | ref | <0.0001 | ref | 0.045 | ref | 0.012 |
| 1 | 1.31 (0.90–1.88) | 2.11 (0.122–3.59) | 1.19 (0.76–1.83) | 1.42 (0.75–2.64) | ||||
| 2 | 3.02 (1.26–6.12) | 10.03 (3.33–24.71) | 2.81 (1.11–6.17) | 6.62 (1.87–20.12) | ||||
| 3 | 15.72 (0.86–79.06) | 30.96 (1.66–170.68) | 18.01 (0.91–116.63) | 23.53 (0.98–259.14) | ||||
| Gleason score | ||||||||
| 5 | ref | 0.017 | ref | 0.38 | ref | 0.021 | ref | 0.63 |
| 6 | 6.61 (0.80–54.55) | 3.77 (0.43–32.96) | 6.66 (0.78–56.55) | 2.70 (0.29–25.28) | ||||
| 7 | 6.80 (092–49.95) | 3.16 (0.41–24.30) | 7.65 (1.02–57.41) | 3.75 (0.46–30.41) | ||||
| 8 | 7.71 (1.05–56.97) | 4.61 (0.60–35.31) | 7.65 (1.00–58.39) | 3.62 (0.44–29.75) | ||||
| 9 | 9.36 (1.27–68.84) | 4.94 (0.65–37.53) | 9.04 (1.20–68.41) | 3.99 (0.50–31.81) | ||||
| 10 | 17.42 (2.00–151.44) | 3.46 (0.21–56.71) | 25.07 (2.72–231.26) | 10.26 (0.56–187.26) | ||||
| PSA at initiation of ADT (log, continuous) | 1.51 (1.23–1.83) | <0.0001 | 1.50 (1.13–1.97) | 0.0044 | 1.40 (1.11–1.77) | 0.0045 | 1.11 (0.80–1.54) | 0.52 |
| Definitive Treatment (Y vs. N) | 0.56 (0.40–0.78) | 0.0007 | 0.47 (0.28–0.77) | 0.0029 | 0.68 (0.45–1.02) | 0.063 | 0.57 (0.28–1.13) | 0.11 |
ADT = androgen deprivation therapy; CI = confidence interval; CRPC = castration-resistant prostate cancer; HR = hazard ratio; OS = overall survival.
3.2. Association of time from DT to ADT initiation
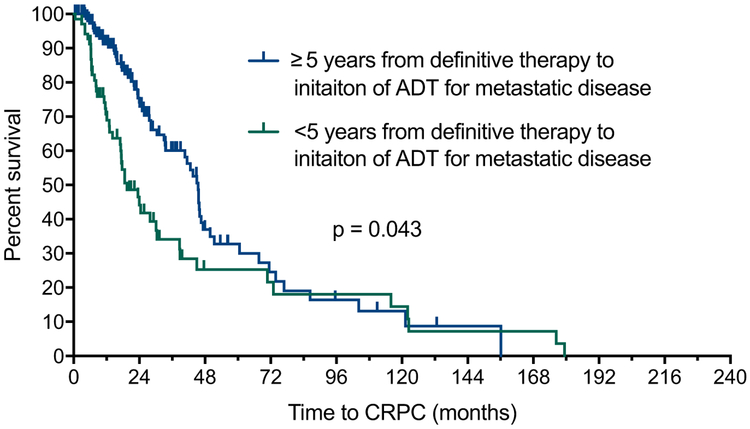
A total of 112 men with new mHSPC initiating ADT after prior DT were eligible for inclusion to assess the duration of time from DT for localized prostate cancer to ADT initiation for newly diagnosed mHSPC. The patient characteristics were as follows, median age, 68 years; Gleason score, 7; median PSA, 14 ng/ml; performance status, 0; and median time from DT to start of ADT, 54 months. In the univariate analysis, increasing years from DT to start of ADT was associated with longer time to mCRPC (Per year: HR 0.94; 95% CI 0.89–0.99; P = 0.027). When time from DT to start of ADT was stratified by <5 years and ≥5 years, patients with <5 years between DT and initiation of ADT had shorter time to mCRPC (HR 1.78, 95% CI 1.04–3.17, P = 0.043, Fig. 2). After adjustment for Gleason score, log PSA, ECOG performance status, age at initiation of ADT, and race in multivariate COX models, increasing years from DT to the start of ADT remained an independent predictor of time to mCRPC (per year: HR 0.91 95% CI 0.84–0.99, P = 0.020) and improved OS (per year: HR 0.87, 95% CI 0.74–0.99, P = 0.0025) in patients with new mHSPC.
Fig. 2.
Effect of duration of time from definitive therapy to initiation of ADT (<5 years vs. ≥5 years) on time to mCRPC. ADT = androgen deprivation therapy; mCRPC = metastatic castration-resistant prostate cancer
4. Discussion
Systemic treatment for mHSPC is rapidly evolving. There are a number of prognostic, clinical biomarkers available for metastatic prostate cancer (Gleason score, lactate dehydrogenase (LDH), alkaline phosphatase, albumin, hemoglobin (HGB), and PSA kinetics), but there are no pretreatment, predictive clinical factors, or biomarkers currently used to risk stratify patients to optimize treatment selection [9]. In this study, we found that time from DT to start of ADT is an “independent” predictor of time to mCRPC and OS in men with new mHSPC. Furthermore, when time from DT to initiation of ADT was stratified by <5 years and ≥5 years, it remained an independent predictor of time to mCRPC. While the majority of patients with mHSPC appear to benefit from docetaxel or abiraterone acetate, there are a subset of men who are frail, older, or have medical comorbidities that make ADT alone the preferred regimen. If validated, time from DT to start of ADT could help physicians personalize intensity of up-front treatment for mHSPC. Specifically, men with ≥5 years between DT and start of ADT may be candidates for less intense up-front treatment of their mHSPC. As a clinical biomarker, time from DT to start of ADT is attractive because it does not require additional data or costly testing. Furthermore, urologic oncologists are familiar with a similar biomarker used for metastatic renal cell carcinoma (mRCC). In mRCC, time from diagnosis to systemic treatment (of less than 1 year) is one of the 6 clinical risk factors used in the International Metastatic Renal Cell Carcinoma Database Consortium risk score, and some of the recent approvals for treatment of mRCC were based on the International Metastatic Renal Cell Carcinoma Database Consortium risk categorization [10].
To date, only 1 study has investigated the effect of prior DT on survival outcomes in mHSPC [8]. In that secondary analysis from SWOG 8894, 148 men with newly diagnosed mHSPC had previously received RP for localized disease, and those men had improved OS compared to men did not have prior RP (HR 0.68, 95% CI 0.53–0.89, P = 0.004). However, 219 men had received definitive radiotherapy, and these men experienced a nonsignificant trend towards inferior OS (HR 1.22, 95% CI 0.93–1.59, P = 0.15). In our multi-institutional study, the analysis was not separated by type of DT, yet when controlling for confounding variables, prior DT showed a nonsignificant trend towards improved time to mCRPC and OS. This significance of this observation is limited by our exclusion of men who received ADT for localized prostate cancer, as this likely removed some men with high-risk localized prostate cancer from the DT group. However, our findings are consistent with a similar study in men with mCRPC [11]. In that retrospective, pooled analysis of 1,238 men from 9 clinical trials, prior RP did not improve OS (HR 1.03, 95% CI 0.90–1.09, P = 0.65). To be clear, our study was not designed to investigate whether men with de novo mHSPC benefit from receiving DT “after” being diagnosed with metastatic disease. This is a separate question that is currently being investigated in the soon to be activated SWOG-1802 trial.
Due to its multi-institutional design, our study is limited by potential patient selection and treatment selection bias across the different institutions. Our limited cohort size prevented us from performing a subgroup analysis by type of DT or volume of disease. We also did not add time from DT to initiation of ADT to other known prognostic clinical variables for mHSPC, such as PSA nadir at 7 months. To limit confounding variables, we excluded men who received ADT for localized prostate cancer. This exclusion criterion likely removed some men with high-risk localized prostate cancer who received radiation plus ADT from the DT group. Finally, these patients were all treated prior to docetaxel and abiraterone acetate becoming standard of care for mHSPC.
5. Conclusion
In conclusion, in men with newly diagnosed mHSPC, time from DT for localized prostate cancer to initiation of ADT is an independent predictor of time to mCRPC and OS. If this hypothesis-generating data can be validated independently, time from DT to start of ADT for new mHSPC may assist with risk stratification and systemic therapy selection in these men.
Acknowledgments
Funding: No funding supported this manuscript.
Footnotes
Conflict of interest
AWH, DMG, and ABA have no conflicts-of-interest to disclose. Conflict-of-interest disclosure: NA reports consultancy to Pfizer, Novartis, Merck, Genentech, Eisai, Exelixis, Clovis, EMD Serono, BMS, Astra Zeneca, Foundation One, Astellas, Ely Lilly, Bayer, Argos, Medivation, Clovis, and Nektar; and research funding to my institution on my behalf from Active Biotech, Astra Zeneca, Bavarian Nordic, BMS, Calithera, Celldex, Eisai, Exelixis, Genetech, Glaxosmithkline, Immunomedics, Janssen, Medivation, Merck, New link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Sanofi, Takeda, and Tracon. HHC reports research funding from Janssen, Inovio, Clovis, Astellas, Medivation, and Sanofi. UV reports consultancy to Pfizer, Novartis, Genentech, Exelixis, BMS and Bayer and research support from Astellas, BMS and Exelixis Inc. ERK reports research support from Astellas, BMS and Pfizer.
References
- [1].Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352. [DOI] [PubMed] [Google Scholar]
- [4].James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw 2016;14:19. [DOI] [PubMed] [Google Scholar]
- [6].Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 2006;24:3984. [DOI] [PubMed] [Google Scholar]
- [7].Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thompson IM, Tangen C, Basler J, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol 2002;168:1008. [DOI] [PubMed] [Google Scholar]
- [9].Terada N, Akamatsu S, Kobayashi T, et al. Prognostic and predictive biomarkers in prostate cancer: latest evidence and clinical implications. Ther Adv Med Oncol 2017;9:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293. [DOI] [PubMed] [Google Scholar]
- [11].Halabi S, Vogelzang NJ, Ou SS, et al. The impact of prior radical prostatectomy in men with metastatic castration recurrent prostate cancer: a pooled analysis of 9 cancer and leukemia group b trials. J Urol 2007;177:531. [DOI] [PubMed] [Google Scholar]