Abstract
Traumatic brain injury (TBI) contributes to almost one third of all trauma-related deaths, and those that survive often suffer from long-term physical and cognitive deficits. Ciclosporin (cyclosporine, cyclosporin A) has shown promising neuroprotective properties in pre-clinical TBI models. The Copenhagen Head Injury Ciclosporin (CHIC) study was initiated to establish the safety profile and pharmacokinetics of ciclosporin in patients with severe TBI, using a novel parenteral lipid emulsion formulation. Exploratory pharmacodynamic study measures included microdialysis in brain parenchyma and protein biomarkers of brain injury in the cerebrospinal fluid (CSF). Sixteen adult patients with severe TBI (Glasgow Coma Scale 4–8) were included, and all patients received an initial loading dose of 2.5 mg/kg followed by a continuous infusion for 5 days. The first 10 patients received an infusion dosage of 5 mg/kg/day whereas the subsequent 6 patients received 10 mg/kg/day. No mortality was registered within the study duration, and the distribution of adverse events was similar between the two treatment groups. Pharmacokinetic analysis of CSF confirmed dose-dependent brain exposure. Between- and within-patient variability in blood concentrations was limited, whereas CSF concentrations were more variable. The four biomarkers, glial fibrillary acidic protein, neurofilament light, tau, and ubiquitin carboxy-terminal hydrolase L1, showed consistent trends to decrease during the 5-day treatment period, whereas the samples taken on the days after the treatment period showed higher values in the majority of patients. In conclusion, ciclosporin, as administered in this study, is safe and well tolerated. The study confirmed that ciclosporin is able to pass the blood–brain barrier in a TBI population and provided an initial biomarker-based signal of efficacy.
Keywords: biomarkers, ciclosporin, NeuroSTAT®, pharmacokinetics, traumatic brain injury
Introduction
Traumatic brain injury (TBI) contributes to almost one third of all trauma-related deaths. Surviving patients often suffer from lifelong physical and cognitive deficits, with devastating consequences for the patients and their families.1,2 One year after being hospitalized for TBI, almost half of the patients have a disability related to the initial injury.3 TBI also puts a strain on society as a whole, attributed to the required long-term medical care and rehabilitation, incurring a financial burden on the global economy that has been estimated to be approximately $400 billion annually.4
From a pathophysiological perspective, TBI may be considered as a biphasic injury, consisting of a primary structural injury, followed by a secondary injury cascade that may continue for days or weeks after the primary insult. The opening of the mitochondrial permeability transition pore (mPTP) has been suggested to be an important pathophysiological mechanism associated with the secondary injury cascade.5–7 Under physiological conditions, the mPTP is closed, but as a result of the injury, the mitochondria may undergo permeability transition with detrimental effects to the cell.8–11 Ciclosporin (cyclosporine, cyclosporin A) has shown promising neuroprotective properties in more than 20 independent experimental in vivo studies of TBI.12–14 Ciclosporin is thought to possess neuroprotective properties through inhibition of the cyclophilin D–dependent activation of the mPTP and limiting the secondary injury cascade.6,7,15–18
Three clinical phase IIa studies have already been completed with ciclosporin in TBI, indicating safety in this patient population.19–23 Patients who received ciclosporin at doses up to 5 mg/kg/day within 8–12 h of injury had a similar mortality rate and adverse events as in the placebo group. The number of patients studied was too small to exclude the possibility of adverse events attributed to ciclosporin administration. In one of the studies, there was a trend toward improved outcome in the higher dosage cohorts as compared to the low, subtherapeutic dosage cohorts assessed at 6 months, using the Glasgow Outcome Scale-Extended (GOSE-E).20–23
NeuroSTAT® is a novel lipid emulsion of ciclosporin not containing the potentially harmful Kolliphor® EL solubilizer (previously named Cremophor® EL).24 This novel formulation has also been shown to be safe in other patient populations.25,26 Further, it was recently shown, in a porcine contusion injury model, that NeuroSTAT is able to reduce the volume of parenchymal injury by 35%, as well as improve markers of neuronal injury and metabolism.27
Based on the promising pre-clinical and clinical data, a phase IIa safety and pharmacokinetics (PK) study of NeuroSTAT in severe TBI patients was initiated. The primary objective was to establish safety and characterize the PK profile of two dosing regimens of ciclosporin in severe TBI patients. The present study uses a higher dosing group and longer duration of treatment than in previous trials. As exploratory pharmacodynamic study objectives, focusing on the secondary injury cascade, microdialysis in brain parenchyma and protein biomarkers of brain injury in the cerebrospinal fluid (CSF) were measured.
Methods
Study design and conduct
The Copenhagen Head Injury Ciclosporin (CHIC) study was an open-label, uncontrolled, single-center, phase II study to investigate the PK and safety of two dose levels of NeuroSTAT in patients with severe TBI. The study was conducted in accord with applicable regulatory requirements, the principles of International Council for Harmonisation–E6 Good Clinical Practice, and the Declaration of Helsinki. Patients included in this study were temporarily incapacitated because of impaired consciousness, and the informed consent process was designed accordingly based on local regulations for emergency research. Consent was obtained for study patients in accord with this procedure.
Permits were obtained from the national ethics committee and the regulatory authority (Danish Medicinal Agency). The study was registered with EudraCT number 2012-000756-34 and at ClinicalTrials.gov as NCT01825044. The study was performed at the Department of Neurosurgery, Rigshospitalet, Copenhagen University Hospital, Denmark between June 2013 and May 2017. The study had planned to include 20 patients, but was closed after inclusion of total 16 patients because of slow recruitment. The study started with the lower dose level for the first 10 patients and then was completed with 6 patients at the higher dose. Safety oversight of the study was under the direction of an independent data safety monitoring board that approved dose escalation to the higher dose level.
Study patients and procedures
Pre-screening of potential study patients was conducted at the neuro-intensive care unit (neuro-ICU). After obtaining informed consent, 16 patients were screened and subsequently enrolled after clinical stabilization (Fig. 1). Thereafter, patients received a 2.5-mg/kg bolus dose infusion of ciclosporin, followed by either 5 or 10 mg/kg/day of ciclosporin as a continuous infusion for 5 days. The study procedures included 3 additional days of study-specific monitoring in the neuro-ICU after the termination of the study treatment. After an additional 30 days, a follow-up phone call was made to the patient, or the patient's nursing staff, checking patient status and potential serious (SAEs) adverse events (AEs).
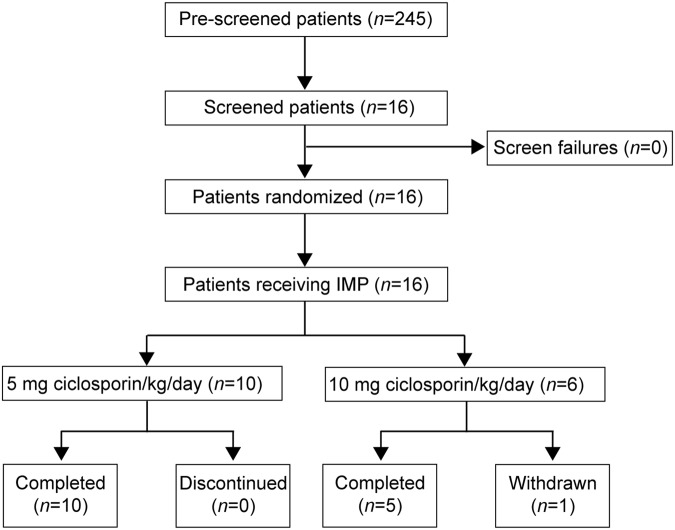
FIG. 1.
Patient disposition. A total of 16 patients were screened. All 16 patients received the investigational medicinal product (IMP). Ten patients received ciclosporin at a dose of 5 mg/kg/day, and 6 patients received ciclosporin at a dose of 10 mg/kg/day for 5 days after an initial loading dose of 2.5 mg/kg. Study treatment was discontinued prematurely in 1 patient in the 10-mg/kg/day cohort, but study measurements continued throughout the study according to the investigational plan.
The studied patient population included patients with non-penetrating severe TBI with a Glasgow Coma Scale (GCS) score of 4–8. Only patients who required admission to the ICU and had a clinical indication for the placement of an external ventricular drainage (EVD) were eligible for enrollment in the study. Patients with bilaterally fixed dilated pupils, spinal cord injury, or pure epidural hematoma were not included in the study. A medical history of hepatic or renal disease were also among the exclusion criteria. Inclusion and exclusion criteria are listed in Table 1.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion criteria |
| • Male or female patients, age between 18 and 75 years, inclusive |
| • Requirement for intensive care unit admission and clinical indication for external ventricular drainage and intracranial pressure monitoring |
| • Evidence of non-penetrating severe traumatic brain injury (TBI), confirmed by history and abnormalities consistent with a non-penetrating trauma on computerized tomography scan upon admission |
| • Clinical examination with post-resuscitation Glasgow Coma Scale of 4–8, inclusive |
| Exclusion criteria |
| • Bilaterally fixed dilated pupils |
| • Penetrating TBI |
| • Spinal cord injury |
| • Pure epidural hematoma |
| • Currently developed, known, or a medical history of renal disorder, significant renal failure, or high-risk renal failure |
| • Known or a medical history of hepatic disease |
| • Ongoing pre-injury therapy with any of these drugs: rosuvastatin, tacrolimus, Hypericum perforatum (St. John's wort; a herbal dietary supplement), stiripentol, aliskiren, bosentan, diltiazem, verapamil, and antiepileptics |
| • Participation in other clinical trials |
| • Any significant disease or disorder, including abnormal laboratory tests, which, in the opinion of the investigator, may either put the patient at risk because of participation in the study or may influence the results of the study |
Study endpoints
The primary study objective was to establish safety and characterize the PK profile of two dosing regimens of ciclosporin in a severe TBI population. The primary endpoints were related to PK parameters based on ciclosporin levels in blood, as well as specific safety endpoints that included markers of renal and liver function, intracranial pressure (ICP) monitoring, assessment of infections, and AEs. The secondary endpoints were related to PK parameters based on ciclosporin levels in CSF, as well as further evaluation of safety.
The safety variables included markers of renal function based on blood samples (plasma creatinine, plasma cystatin c and blood urea nitrogen), markers of hepatic function based on blood samples (prothrombin time, aspartate transaminase [AST], alanine transaminase [ALT], and bilirubin), assessment of infections, intracranial pressure, electrocardiogram abnormalities, safety biomarkers for renal function in urine, vital signs, and physical findings (mean arterial pressure, heart rate, body temperature, cerebral perfusion pressure, GCS, pupil size, and pupil response).
The study also included exploratory efficacy endpoints with assessment of biomarkers of brain injury, measured using microdialysis in the more and less traumatized side, brain tissue oxygen measured through a Licox® catheter, as well as CSF biomarkers of brain injury; glial fibrillary acidic protein (GFAP), neurofilament light (NF-L), tau, and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1). Microdialysis was measured using the M Dialysis ISCUSflex Microdialysis Analyzer (M Dialysis AB, Johanneshov, Sweden). CSF biomarkers were analyzed using a Quanterix SIMOA Neurology 4-plex assay (Quanterix Corporation, Billerica, MA).28
Statistical analyses
The details of the statistical analyses were predefined in a detailed statistical analysis plan.
The PK analysis was described using a population PK approach performed in the non-linear mixed-effect modeling software, NONMEM (Version 7.3; ICON, Dublin, Ireland). The software R (R Foundation for Statistical Computing, Vienna, Austria) was used for goodness-of-fit analyses, model evaluation, and computation of Cav,ss and t1/2 based on results from NONMEM. The remaining data were analyzed and presented using the SAS software (SAS Institute Inc., Cary, NC).
The CSF biomarker data were defined as an exploratory endpoint in the statistical analysis plan without pre-defining the method of statistical analysis. The analysis was, because of the small sample size, performed using the non-parametric Wilcoxon signed-rank test to compare the slopes during (48–120 h after infusion start) and post-treatment (120–168 h after infusion start).
Results
Patient characteristics
Characteristics of the enrolled patients at baseline are outlined in Table 2. The 5-mg group had a median GCS of 6 (range, 4–7), and the higher dose had a median GCS of 5 (range, 4–7). The TBI phenotype had the following distribution: 7 patients had a combination of acute subdural hematoma (ASDH) and contusional injury, 4 patients were judged to have predominantly diffuse axonal injury (DAI) with no mass lesion, 2 patients had contusional injury, 2 patients had a combination of contusional injury and traumatic subarachnoid hemorrhage (TSAH), and 1 patient had ASDH. Overall, 75% of the patients were men. Mean age was 34.5 years (range, 19–58) in the 5-mg/kg/day group and 37.3 years (range, 19–55) in the 10-mg/kg/day group. Mean weight was 79.3 kg (range, 60–105) in the 5-mg/kg/day group and 78.6 kg (range, 60–100) in the 10-mg/kg/day group.
Table 2.
Patient Baseline Characteristics
| Patient identifier | Treatment arm (mg) | Sex/age | Weight | TBI phenotype | More affected hemisphere | Craniectomy (Y/N) | GCS | Injury to first dose (hours) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | M/31 | 80 | ASDH, contusion | Left | Y | 6 | 37 |
| 2 | 5 | M/21 | 90 | ASDH, contusion | Left | Y | 4 | 53 |
| 3 | 5 | F/43 | 68 | ASDH | Right | N | 4 | 44 |
| 4 | 5 | F/19 | 60 | DAI | Right | N | 6 | 43 |
| 5 | 5 | F/58 | 70 | Contusion | Left | Y | 6 | 35 |
| 6 | 5 | M/51 | 80 | DAI | Right | N | 6 | 54 |
| 7 | 5 | M/28 | 105 | ASDH, contusion | Left | Y | 6 | — |
| 8 | 5 | M/49 | 90 | ASDH, contusion | Left | Y | 6 | 37 |
| 9 | 5 | M/23 | 75 | Contusion, TSAH | Left | N | 7 | 26 |
| 10 | 5 | M/22 | 75 | ASDH, contusion | Right | Y | 6 | 21 |
| 11 | 10 | F/46 | 60 | ASDH, contusion | Left | Y | 7 | 22 |
| 12 | 10 | M/47 | 85 | Contusion, TSAH | Right | N | 5 | 28 |
| 13 | 10 | M/55 | 70 | Contusion | Left | Y | 6 | 20 |
| 14 | 10 | M/23 | 80 | DAI | Left | N | 4 | 21 |
| 15 | 10 | M/34 | 100 | DAI | Right | N | 5 | 64 |
| 16 | 10 | M/19 | 76.5 | ASDH, contusion | Right | Y | 4 | 36 |
Treatment arm: ciclosporin/kg/day.
TBI, traumatic brain injury; ASDH, acute subdural hematoma; DAI, diffuse axonal injury; TSAH, traumatic subarachnoid hemorrhage; GCS, Glasgow Coma Scale.
Safety
No deaths were registered in the study. This is a patient category that is prone to AEs as part of their natural history, and a total of 8 patients (80%) in the 5-mg/kg/day group and 6 patients (100%) in the 10-mg/kg/day group reported at least one AE before the start of investigational medical product (IMP) administration. All patients in both dose groups had at least one AE at some point during the study. Compared to the 5-mg/kg/day group, a higher percentage of patients in the 10-mg/kg/day group had at least one AE assessed as related to study drug (83% vs. 40%).
Twenty-one AEs that occurred after initiation of IMP administration were categorized as related to the medical product: 8 in the 5-mg/kg/day group and 13 in the 10-mg/kg/day group. All AEs that occurred during the treatment/follow-up period in the 5-mg/kg/day group were graded as mild or moderate. Seven unique AEs that occurred during the treatment/follow-up period in the 10-mg/kg/day group were graded as severe. The most frequent unique AEs (those occurring in at least 25% of patients) were constipation, impaired gastric emptying, decrease in blood phosphorus, pneumonia, decrease in blood zinc, oliguria, increase in cystatin C, increase in ICP, increase in blood bilirubin, increase in blood urea, and abnormal pupillary light reflex tests.
Blood creatinine increase and cystatin C increase were recorded as AEs in patients in both dose groups. Three events of increased blood creatinine and five events of increased cystatin C were assessed as possibly related to study drug. Graphical analysis of safety laboratory parameters showed no clear differences between the dose groups, with the notable exception of plasma bilirubin, which showed reversible time- and dose-dependent elevation during study drug administration. Hyperbilirubinemia was more common and more prominent during IMP administration in the 10-mg/kg/day group compared to the 5-mg/kg/day group. Safety laboratory values presented are in Table 3. There were five events of oliguria, two of which were assessed as possibly related to study drug. All events of oliguria occurred in patients in the 10-mg/kg/day group. No clear time trends were observed in mean values for body temperature and systolic and diastolic blood pressure, and no obvious differences in vital signs were observed between the dose groups. Further, ciclosporin did not seem to have an impact on ICP.
Table 3.
Safety Laboratory Values
| Visit day | ALT median (min, max) | AST median (min, max) | Bilirubin median (min, max) | INR median (min, max) | Creatinine median (min, max) | BUN median (min, max) |
|---|---|---|---|---|---|---|
| Ciclosporin 5 mg/kg/day | ||||||
| Baseline | 89.0 (14, 252) | 143.0 (27, 264) | 8.0 (3, 14) | 1.15 (1.0, 1.5) | 62.0 (52, 84) | 2.70 (2.1, 4.6) |
| Treatment day 1 | 41.0 (16, 215) | 42.0 (24, 190) | 11.0 (5, 34) | 1.30 (1.1, 1.5) | 68.0 (49, 119) | 3.10 (1.5, 5.2) |
| Treatment day 2 | 36.0 (15, 261) | 31.5 (24, 245) | 14.5 (4, 31) | 1.20 (1.1, 1.3) | 69.5 (53, 163) | 4.05 (2.5, 7.4) |
| Treatment day 3 | 46.5 (17, 224) | 35.5 (17, 184) | 16.0 (4, 48) | 1.20 (1.1, 1.3) | 70.5 (56, 215) | 5.40 (3.3, 9.0) |
| Treatment day 4 | 48.5 (22, 171) | 51.5 (25, 151) | 20.0 (3, 42) | 1.20 (1.1, 1.4) | 70.5 (54, 277) | 6.95 (3.8, 13.1) |
| Treatment day 5 | 67.0 (24, 165) | 73.0 (37, 123) | 15.0 (4, 42) | 1.10 (1.0, 1.2) | 67.5 (53, 258) | 8.65 (5.3, 18.1) |
| Monitoring day 1 | 61.5 (26, 145) | 55.5 (32, 134) | 13.5 (3, 35) | 1.10 (1.0, 1.1) | 65.0 (50, 250) | 9.40 (6.6, 21.4) |
| Monitoring day 2 | 64.0 (30, 183) | 66.5 (35, 162) | 10.0 (3, 23) | 1.00 (1.0, 1.2) | 54.5 (45, 186) | 7.40 (4.6, 16.7) |
| Monitoring day 3 | 69.0 (28, 159) | 54.5 (26, 163) | 8.0 (4, 19) | 1.00 (0.9, 1.1) | 53.5 (37, 108) | 5.95 (4.3, 9.4) |
| Ciclosporin 10 mg/kg/day | ||||||
| Baseline | 32.0 (18, 83) | 50.0 (22, 89) | 4.5 (3, 22) | 1.15 (1.0, 1.5) | 69.0 (38, 108) | 4.10 (2.0, 10.9) |
| Treatment day 1 | 28.0 (17, 47) | 37.0 (32, 75) | 11.5 (7, 24) | 1.15 (1.1, 1.4) | 72.0 (54, 100) | 3.80 (2.5, 7.4) |
| Treatment day 2 | 26.0 (15, 43) | 43.0 (30, 76) | 17.5 (8, 36) | 1.15 (1.0, 1.6) | 80.0 (61, 104) | 5.10 (2.8, 9.5) |
| Treatment day 3 | 41.0 (15, 63) | 64.5 (42, 109) | 23.5 (9, 101) | 1.25 (1.0, 1.5) | 80.5 (52, 192) | 8.00 (5.4, 9.9) |
| Treatment day 4 | 43.0 (21, 81) | 46.0 (28, 99) | 51.0 (8, 106) | 1.20 (0.9, 1.4) | 104.5 (51, 296) | 11.00 (6.9, 12.3) |
| Treatment day 5 | 35.0 (21, 78) | 58.0 (20, 128) | 47.0 (6, 117) | 1.20 (0.9, 1.3) | 92.0 (47, 267) | 11.90 (7.2, 15.9) |
| Monitoring day 1 | 34.0 (16, 78) | 50.0 (16, 79) | 55.0 (4, 70) | 1.10 (0.9, 1.3) | 105.0 (39, 424) | 15.95 (7.4, 24.6) |
| Monitoring day 2 | 58.0 (26, 110) | 64.0 (33, 124) | 24.5 (3, 36) | 1.10 (1.0, 1.2) | 90.0 (40, 327) | 13.60 (7.2, 22.6) |
| Monitoring day 3 | 66.5 (23, 128) | 56.0 (29, 147) | 16.5 (4, 28) | 1.00 (1.0, 1.0) | 65.5 (38, 350) | 10.20 (6.6, 27.2) |
ALT, alanine transaminase; AST, aspartate transaminase; INR, international normalized ratio; BUN, blood urea nitrogen.
One patient in the 10-mg/kg/day group suffered from acute renal tubular necrosis (ATN) 3 days after initiation of treatment, which was determined to be an SAE. The patient developed a septic shock simultaneously with the ATN. A pneumonia was diagnosed and Escherichia coli was later verified in sputum cultures. Administration of the IMP was discontinued; the patient was treated with acute hemodialysis and eventually recovered. The concurrent sepsis was judged to be the most likely cause of the ATN. The biochemical signs of ATN followed the increasing C-reactive protein, and not ciclosporin concentration. However, a link to ciclosporin infusion could not be excluded.
Pharmacokinetic parameters
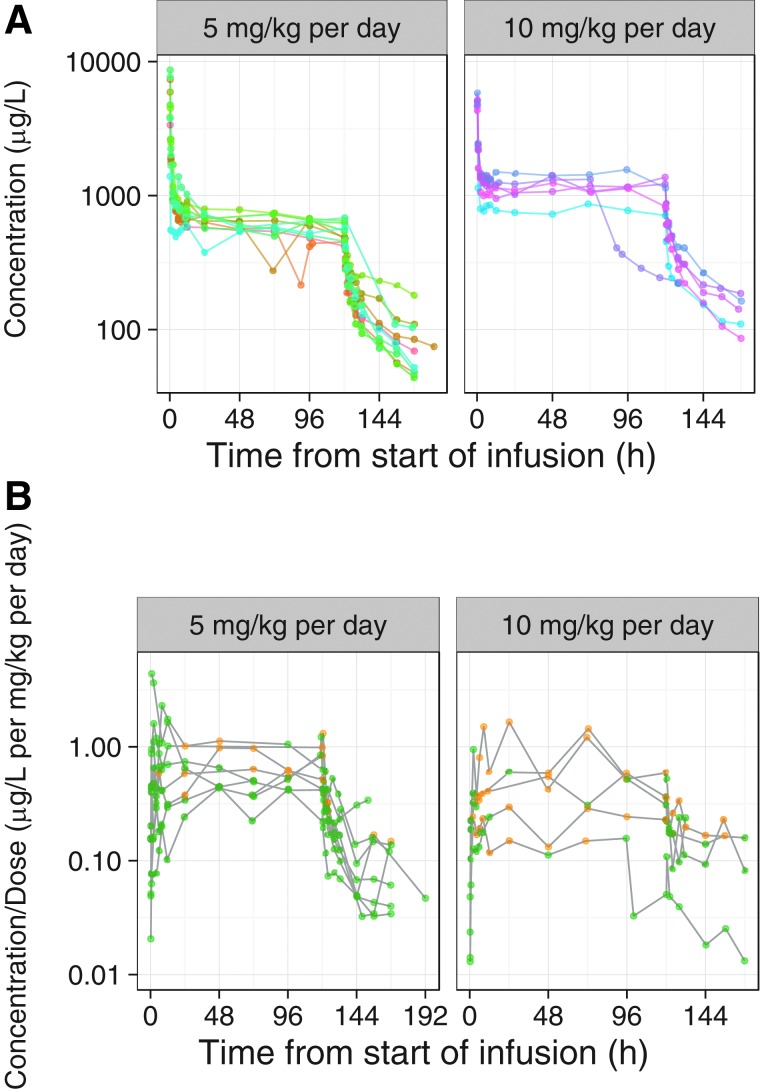
The graphical analysis of blood ciclosporin concentration time profiles illustrates that all patients had detectable ciclosporin concentrations. The concentrations decreased rapidly after completing the intravenous (i.v.) bolus dose and relatively stable concentrations were obtained from about 24 h after the initiation of treatment until the end of the infusion (Fig. 2A). Between- and within-patient variability in blood ciclosporin concentrations was limited. The ciclosporin concentrations decreased in a multi-exponential manner after the end of the infusion, and there appeared to be substantial between-patient variability in the rate of the elimination of ciclosporin. There were no signs of deviations from dose proportionality and no clear differences between patients with DAI (no mass lesion) and patients with the other TBI phenotypes.
FIG. 2.
Observed (A) blood and (B) CSF concentrations of ciclosporin over time, stratified by the dose group. Each point represents the data for one patient and the points are connected with lines. In (A) the points and lines are colored by patient for blood ciclosporin concentrations. CSF samples with suspected blood contamination were discarded from analysis. In (B) the points and lines colored green represent clear CSF samples and those in yellow represent samples with minor blood contamination. Data sets are shown on semi-logarithmic scales. CSF, cerebrospinal fluid.
The graphical analysis of CSF ciclosporin concentration-time profiles illustrates that all patients had detectable ciclosporin concentrations. There was substantial between-patient variability in the time to Cmax (maximum drug concentration) that is, Tmax (time to maximum concentration following drug administration), where Cmax was observed at the end of the bolus dose in 1 patient and within the first few hours after the bolus dose in other patients, and in some patients, the CSF concentration increased for several days (Fig. 2B). CSF Tmax was, in general, much later than the end of the bolus infusion, possibly indicating a slow absorption into the CSF. In general, the CSF ciclosporin concentrations were more variable than blood concentrations. However, in those patients with relatively low random noise in the data and a sufficient number of CSF observations, it appears as if the PK profiles in blood and CSF were similar after the constant infusion was stopped. This is an indication of formation-rate–limited PK in CSF, meaning that the terminal t½ (elimination half-life) in CSF is determined by the t½ in blood.
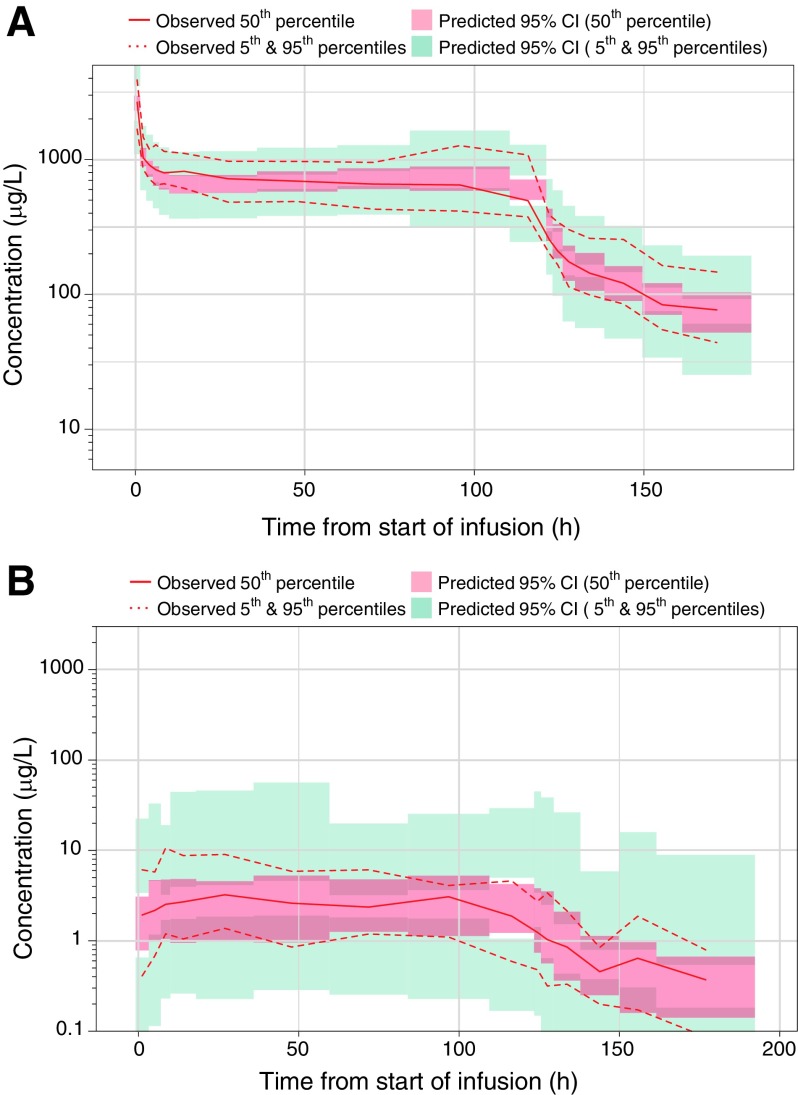
We developed population PK models to describe the PK characteristics of ciclosporin in blood (Fig. 3A) and CSF (Fig. 3B), and based on these models, individual PK variables for the patients were derived. The PK characteristics of ciclosporin in blood were well described by a three-compartment disposition model with first-order elimination from the central compartment. The means of the individual predicted Cav,ss (steady-state drug concentration) values were 665 and 1320 ng/mL in the 5- and 10-mg/kg/day groups, respectively, indicating a proportional dose to Cav,ss relationship in blood. Mean AUC0-t (area under the concentration curve) was 82.5 mg·h/L in the 5-mg/kg/day group and 146 mg·h/L in the 10-mg/kg/day group. Values for t½, CL (total clearance), Cmax, and Tmax were similar for both dose groups. Overall mean values for all patients were 44.4 h for t½, 24.4 L/h for CL, and 4600 ng/mL for Cmax. Median (range) Tmax for all patients was 0.258 h (0.250–0.417; Table 4).
FIG. 3.
Visual predictive check of ciclosporin concentrations, for the pharmacokinetic model in blood (A) and CSF (B). The population pharmacokinetic model for ciclosporin in blood was a three-compartment model with first-order elimination from the central compartment. The model for ciclosporin in CSF was a one-compartment model with first-order absorption and first-order elimination from the CSF compartment. The ciclosporin concentrations are displayed versus time after dose, on semilogarithmic scales. The solid and dashed red lines represent the median and 5th and 95th percentiles of the observations. The ciclosporin concentrations were normalized by the dose to allow evaluation of both the 5- and 10-mg/kg dose arms in this figure; the shaded red and blue areas represent the 95% confidence interval of the median and 5th and 95th percentiles predicted by the model. CI, confidence interval; CSF, cerebrospinal fluid.
Table 4.
PK Parameters for Ciclosporin in Blood and CSF
| Blood | CSF | |||
|---|---|---|---|---|
| 5 mg ciclosporin/kg/day | 10 mg ciclosporin/kg/day | 5 mg ciclosporin/kg/day | 10 mg ciclosporin/kg/day | |
| CL (L/h) | ||||
| Mean (SD) | 24.3 (5.82) | 24.6(4.52) | 6.40 (7.38) | 5.71 (2.06) |
| Cav,ss (ng/mL) | ||||
| Mean (SD) | 665 (117) | 1 320 (279) | 2.50 (0.670) | 3.54 (2.07) |
| Cmax (ng/mL) | ||||
| Mean (SD) | 4 420 (2 350) | 4 930 (511) | 6.32 (5.94) | 6.30 (6.59) |
| Tmax (h) | ||||
| Median (min, max) | 0.250 (0.250, 0.417) | 0.308 (0.250, 0.417) | 8.02 (0.883, 121) | 24.2 (2.33, 95.8) |
| AUC0-t (mg·h/L) | ||||
| Mean (SD) | 82.5 (12.6) | 146 (29.2) | 0.301 (0.0371) | 0.431 (0.254) |
| t½ (h) | ||||
| Mean (SD) | 43.5 (11.3) | 46.0 (8.06) | 43.2 (11.6) | 45.7 (8.83) |
PK, pharmacokinetics; CSF, cerebrospinal fluid; CL, total clearance; SD, standard deviation; Cav,ss, steady-state drug concentration; Cmax, maximum drug concentration; Tmax, time to maximum concentration after drug administration; AUC0-t, area under the concentration curve; t½, elimination half-life.
After accounting for the ciclosporin concentrations in blood, we established a numerically stable population PK model for ciclosporin in CSF. The final population PK model is a one-compartment model with first-order absorption and first-order elimination from the CSF compartment. The means of the individual predicted Cav,ss,CSF values were 2.50 and 3.54 ng/mL in the 5- and 10-mg/kg/day groups, respectively. After excluding the Cav,ss,CSF value for the patient with an outlying value, the mean steady-state concentration in the 10-mg/kg/day group was 4.74 ng/mL, which suggests a proportional dose to Cav,ss,CSF relationship in CSF. Mean AUC0-t,CSF was 0.301 mg·h/L in the 5-mg/kg/day group and 0.431 mg·h/L in the 10-mg/kg/day group. Median (range) Tmax was 8.02 h (0.883–121) in the 5-mg/kg/day group and 24.2 h (2.33–95.80) in the 10-mg/kg/day group. Values for t½, CLCSF, and Cmax were similar for both dose groups. Overall mean values for all patients were 44.1 h for t½, 6.15 L/h for CLCSF and 6.31 ng/mL for Cmax (Table 4).
Cerebral microdialysis
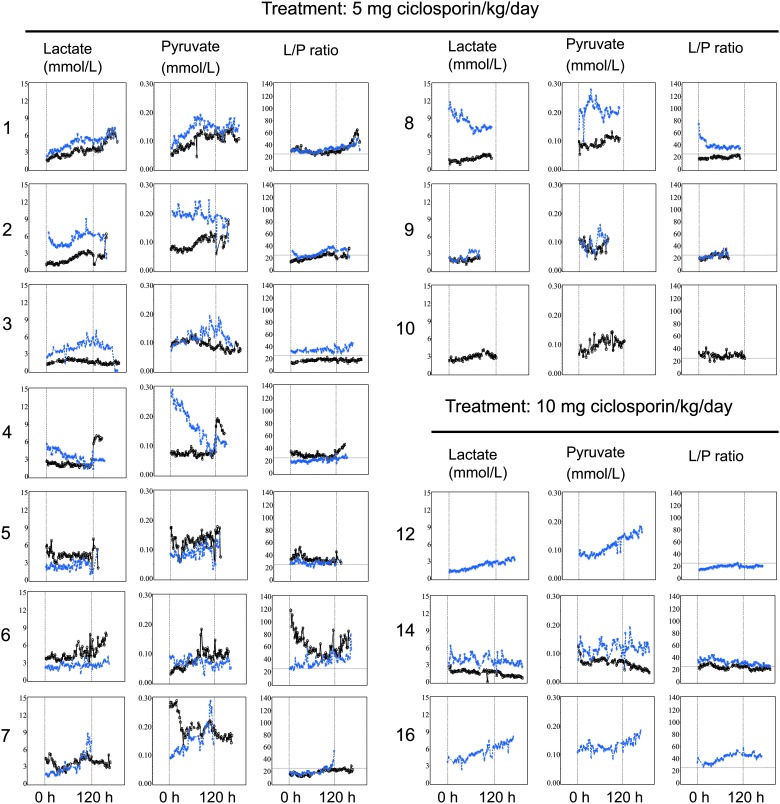
Cerebral microdialysis was measured in all 10 patients in the 5-mg/kg/day group. In the 10-mg/kg/day group, only 3 of 6 patients had microdialysis measurements. The majority of patients had prolonged periods of elevated lactate/pyruvate ratios on one or both sides. There were potential signs of a positive shift in the lactate/pyruvate ratio related to the start or stop of study drug administration in some individuals (Fig. 4). Two patients showed reductions (improvements) in lactate/pyruvate ratio after initiation of study treatment, and 4 patients showed a trend of increased lactate/pyruvate ratio after the termination of study treatment. At the group level, no differences between the low- and high-dose cohorts were observed. No conclusions could be drawn from comparing the more affected hemisphere with the less badly affected hemisphere.
FIG. 4.
Brain microdialysate levels of lactate, pyruvate, and the lactate/pyruvate (L/P) ratio over time. Individual traces are shown for each patient for the predicted better (black marks) and worse (blue marks) hemisphere, as judged by the investigators. Dashed vertical lines indicate the start and stop of ciclosporin infusion. Lactate/pyruvate ratio values above 25 (horizontal dashed line) have been associated with worse outcome. For patient 4 in better hemisphere and patient 7 in worse hemisphere, probes were suspected to be incorrectly placed by CT imaging. CT, computed tomography.
Cerebrospinal fluid biomarkers
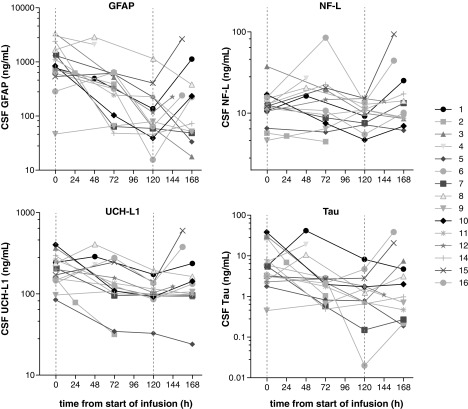
The concentrations of GFAP–astroglia injury, NF-L–axonal injury, tau–axonal injury and neurodegeneration, and UCH-L1–neuronal cell body injury were increased compared to control samples at the analyzed time points in all TBI patients (data not shown). There were large individual variations in the absolute levels and kinetics of the biomarkers of brain injury, but all four showed consistent trends to decrease during the 5-day treatment period whereas the samples taken on the days after the treatment period ended showed higher values in the majority of patients (Fig. 5). On an overall group level, the mean slopes for all four biomarkers were declining during the ciclosporin infusion period, but increased 24–48 h after end of treatment. These breaks in trends were statistically significant for all markers (Table 5).
FIG. 5.
Temporal profile of brain injury biomarkers in CSF. Individual levels of glial fibrillary acidic protein (GFAP), neurofilament light (NF-L), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), and tau (ng/ml) are depicted. CSF samples were drawn at pre-dose, during the continuous ciclosporin infusion, and after treatment had ended at indicated time points from start of ciclosporin administration. Dashed vertical lines indicate the start and stop of infusion. CSF, cerebrospinal fluid.
Table 5.
Biomarker Statistics
| Variable | Measure | Slope during infusion | Slope post-infusion | p value |
|---|---|---|---|---|
| GFAP | Mean | −5.80 | 5.84 | |
| Median | −4.69 | 0.55 | 0.0061 | |
| UCH-L1 | Mean | −1.02 | 1.73 | |
| Median | −0.54 | 0.13 | 0.0017 | |
| NF-L | Mean | −0.17 | 0.27 | |
| Median | −0.10 | 0.04 | 0.0171 | |
| Tau | Mean | −0.05 | 0.11 | |
| Median | −0.001 | 0.004 | 0.0266 |
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxy-terminal hydrolase L1; NF-L, neurofilament light.
Discussion
This study indicates that ciclosporin administered continuously for 5 days, using a novel lipid emulsion formulation, is safe and well tolerated in severe TBI patients. Further, CSF concentrations confirm a dose-dependent brain exposure. All patients had detectable concentrations of ciclosporin in blood and CSF. The analyses suggest dose proportionality in both blood and CSF. No clear differences according to TBI phenotype were observed when patients with isolated diffuse injuries were compared to patients with focal injuries. Whereas between- and within-patient variability in blood ciclosporin concentrations was limited, CSF ciclosporin concentrations were more variable. Patients with relatively low random noise in the data appeared to have similar PK profiles in blood and CSF after the constant infusion was stopped. This is an indication of formation-rate–limited PK in CSF, meaning that the terminal half-life in CSF is determined by the half-life in blood. Our data are in line with previous PK data with ciclosporin in this patient population.20–22
The bilirubin increase in the 10-mg/kg/day NeuroSTAT dose group in our present study appears to be more pronounced than what has been reported from the previous studies using doses of other ciclosporin formulations of up to 5 mg/kg. Apart from the bilirubin increase, the overall safety profile of NeuroSTAT was found to be similar to that found in the two previous studies of ciclosporin in patients with TBI. The clearance in blood in the current study, 24.4 L/h, is in line with the reported values in the two other studies conducted with ciclosporin in TBI patients and higher than the reported clearance in healthy volunteers. In the phase I bioequivalence study referred to in the introduction, in which the PK of NeuroSTAT and Sandimmun® (also Sandimmune®, an intravenous ciclosporin formulation marketed by Novartis Pharmaceuticals) administered as a 4-h infusion at a dose of 5 mg/kg was compared, clearance in blood was 17.3 and 15.5 L/h for NeuroSTAT and Sandimmun, respectively.24 The terminal half-life in the current study is substantial longer than what has been reported in the two TBI studies mentioned above. However, several differences between these studies make a direct comparison of these values difficult. The current study included more frequent sampling during the elimination phase, had a more sensitive bioanalytical assay for the CSF analyses, and used a more refined statistical method for analysis of the PK variables. Our study shows that the half-life of ciclosporin in blood is longer in patients with severe TBI patient than in healthy volunteers.24 Similarly to the current study, the dose-escalation study reported AUC and Cmax values that appeared to be dose proportional in the studied dose range, with very good proportionality between the 1.25- and 2.5-mg/kg groups.23
Ciclosporin has been used extensively in transplant medicine for more than three decades. This mechanism of action is related to its inhibitory effect on calcineurin and not mPTP. The PK and safety profile is well described in this patient population. The oral loading doses for immunosuppression are in the range of 10–15 mg/kg bodyweight. The i.v. loading dose recommended is one third of the oral dose, or 3 to 5 mg/kg injected i.v. over 2–6 h. In clinical studies of ciclosporin used in conjunction with chemotherapeutic agents, cancer patients tolerated a 4- to 6-mg/kg i.v. loading dose over 2 h, followed by a continuous infusion at 10–18 mg/kg/day for 60–72 h.29,30 TBI patients have so far safely received 2.5-mg/kg ciclosporin loading plus 5 mg/kg/day for 72-h infusion (mean blood concentration, 461 ng/mL).20 This study evaluated a longer duration of infusion compared to previous TBI studies, 5 days, for 5-mg/kg daily dose as well as 10 mg/kg/day, which resulted in dose-proportional, steady-state blood concentrations of 665 and 1320 ng/mL, respectively.
Ciclosporin is a substrate for cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp). Drug interactions mainly occur when ciclosporin is coadministered with either inhibitors or inducers of CYP3A4 or P-gp. Severe TBI patients often require a number of pharmacological interventions. In the present study, 9 patients (of 10) in the 5-mg/kg/day group and 4 (of 6) in the 10-mg/kg/day group received erythromycin, and 1 patient in the 5-mg/kg/day group received fluconazole, both of which are known to be able to increase ciclosporine concentrations because of CYP3A4 and P-gp inhibition, respectively. Four patients in the 5-mg/kg/day group and 3 in the 10-mg/kg/day group received ciprofloxacin, and 3 patients in the 10-mg/kg/day group received vancomycin, both of which can potentiate renal dysfunction. These medications may have influenced the PK parameters and AE profile in the study.
Poor drug penetration into the brain has been emphasized as one reason why drugs with pre-clinical promise fail to translate into clinical efficacy in pivotal trials.31,32 Blood–brain barrier (BBB) penetration of ciclosporin is dose dependent. At a sufficiently high blood concentration of ciclosporin, the P-gp transporter will be saturated and ciclosporin will thus inhibit the efflux of itself, and steady state with the brain can be reached.20,33,34 The biomechanical forces associated with TBI may alter the BBB, and the penetration of ciclosporin, through its effects on the microvasculature and possible microhemorrhages with subsequent BBB disruption. In this study, it was verified that ciclosporin is able to cross the BBB in the present patient population when administered according to the studied dosing regimens.
NeuroSTAT is a novel lipid emulsion of ciclosporin not containing the potentially harmful Kolliphor EL solubilizer (previously named Cremophor EL).24 The Sandimmun ciclosporin formulation contains Kolliphor EL as a carrier medium. Many drugs that previously contained Kolliphor EL in the formulation are now only available as lipid emulsions.35,36 The novel lipid emulsion of ciclosporin has been shown to be safe in other patient populations and in a clinical phase I bioequivalence study where NeuroSTAT was compared to Sandimmun and was shown to cause fewer adverse reactions.24 Using the same novel lipid emulsion of ciclosporin as used in this study, it has previously been shown that a single 2.5-mg/kg rapid bolus, which achieved peak levels in excess of 6000 ng/mL (reached 1 min after infusion), did not produce any nephrotoxicity in a vulnerable patient population undergoing percutaneous coronary interventions in the context of acute coronary syndrome.25,37 When the novel lipid emulsion of ciclosporin was infused before cardiopulmonary bypass surgery, a transient increase in creatinine and cystatin c was observed.26
Given that the primary objectives of the study were safety and PK, a prolonged time between trauma and administration of drug was allowed. For the exploratory efficacy measures, this study design constitutes a major limitation. In a pre-clinical study evaluating the therapeutic window in rodents, it was concluded that administration of drug within 8 h after trauma provides neuroprotection. Earlier treatment within the first 3 h was even more protective.38 The therapeutic window is a challenge that has to be taken into account when designing future clinical trials. A second major limitation is that the present study only included a small cohort, and TBI based solely on GCS is inherently a heterogeneous diagnosis, composing of various injury mechanisms and phenotypes. Patients in the higher dose group had a slightly higher mean GCS score at baseline than patients in the lower dose group, and this could have implications related to the outcome metrics of the two treatment groups. The requirement of a clinical indication for use of an EVD to allow for the CSF sampling for PK analyses may also have introduced a selection bias of the study population. A third limitation of the explorative efficacy data was the lack of a matching control group.
Regarding the explorative microdialysis analyses, lactate/pyruvate ratio, measured by microdialysis, is of special interest. Increased lactate/pyruvate ratio is a marker of metabolic dysfunction caused by, for example, mitochondrial dysfunction. Based on the observed pattern of lactate/pyruvate ratios, there is a tentative indication in some of the study patients that ciclosporin may have a positive effect on regional mitochondrial function in the present study population. At the group level, no differences between the low- and high-dose groups were observed.
The selected biomarkers measured in CSF, reflecting various aspects of neuronal, including axonal, and astroglial injury, have all shown promise as fluid-based biomarkers of brain injury.39–41 More specifically, NF-L levels in serum have been shown to be predictive of long-term clinical outcome, measured as GOSE at 12 months, after TBI.39 If ciclosporin attenuates brain injury, it is also reasonable to believe that the absolute levels and temporal profile of these biomarkers may be altered.
The temporal profile of the injury biomarkers in this study does not follow previously published data. For example, NF-L has been described to continuously rise in both serum and CSF for at least 10–12 days after severe TBI.39,42 The decreasing levels observed in this study may indicate that the secondary brain injury can be attenuated by ciclosporin treatment for several days after the initial trauma. This is further supported by the significant breaks in trends noted after the end of treatment. This is not only an interesting signal of efficacy, but also substantiates the feasibility of using these biomarkers as outcome metrics in future proof-of-concept trials. These findings are intriguing, but need to be confirmed in a prospective, placebo-controlled setting.
In conclusion, ciclosporin administration to severe TBI patients is safe under careful monitoring of renal function. Further, ciclosporin is able to pass the BBB in this patient population with the studied dosing regimen. Therapeutic development for TBI is challenging, and there is a long history of failed trials. The safety and efficacy of NeuroSTAT (ciclosporin) needs to be further studied in TBI patients in double-blinded, randomized, placebo-controlled studies, and given the solid pre-clinical data indicating effect in TBI and the established safety profile, a larger phase IIb trial focusing on efficacy is warranted. This planned trial will make use of the development of standardized injury classifications and validation of novel endpoints that are being addressed by ongoing, large, multi-center studies, such as CENTER-TBI, TRACK-TBI and TBI Endpoints Development (TED).43
Acknowledgments
The authors thank the study nurses, Pia Breum and Helene Ravnkilde, and all the employees at the Department of Neurosurgery, Rigshospitalet, Copenhagen, Denmark, as well as Associate Professor Peter Reinstrup and Professor Hans Friberg of the Data Safety Monitoring Board, and Helena Lindén Petersson, former study manager at NeuroVive Pharmaceutical AB, that contributed to this study. The authors also thank Professor Andrew Maas and the European Brain Injury Consortium for original input to the study design, to Mats Magnusson at Pharmetheus AB, Annika Syrén and Ola Malmsten at I-Mind Consulting AB, and Fabian Söderdahl and Johan Bring at Statisticon AB for PK and data analyses. Finally, the authors also especially acknowledge the late Professor Bertil Romner, who initiated the study.
Author Disclosure Statement
The study was sponsored by NeuroVive Pharmaceutical AB, a public company developing pharmaceuticals for the treatment of traumatic brain injury and owns the intellectual property for NeuroSTAT. As indicated by their affiliation, listed authors have received salary support from NeuroVive Pharmaceutical AB. Magnus J. Hansson, Marcus F. Keep, and Eskil Elmér have equity interest in NeuroVive. Carl-Henrik Nordström has received consultancy remuneration from NeuroVive.
References
- 1. Jorge R.E., Robinson R.G., Moser D., Tateno A., Crespo-Facorro B., and Arndt S. (2004). Major depression following traumatic brain injury. Arch. Gen. Psychiatry 61, 42–50 [DOI] [PubMed] [Google Scholar]
- 2. Vasterling J.J., Brailey K., Proctor S.P., Kane R., Heeren T., and Franz M. (2012). Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br. J. Psychiatry 201, 186–192 [DOI] [PubMed] [Google Scholar]
- 3. Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 4. Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Buki A., Chesnut R.M., Citerio G., Coburn M., Cooper D.J., Crowder A.T., Czeiter E., Czosnyka M., Diaz-Arrastia R., Dreier J.P., Duhaime A.C., Ercole A., van Essen T.A., Feigin V.L., Gao G., Giacino J., Gonzalez-Lara L.E., Gruen R.L., Gupta D., Hartings J.A., Hill S., Jiang J.Y., Ketharanathan N., Kompanje E.J.O., Lanyon L., Laureys S., Lecky F., Levin H., Lingsma H.F., Maegele M., Majdan M., Manley G., Marsteller J., Mascia L., McFadyen C., Mondello S., Newcombe V., Palotie A., Parizel P.M., Peul W., Piercy J., Polinder S., Puybasset L., Rasmussen T.E., Rossaint R., Smielewski P., Soderberg J., Stanworth S.J., Stein M.B., von Steinbuchel N., Stewart W., Steyerberg E.W., Stocchetti N., Synnot A., Te Ao B., Tenovuo O., Theadom A., Tibboel D., Videtta W., Wang K.K.W., Williams W.H., Wilson L., andYaffe K.; InTBIR Participants and Investigators. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 [DOI] [PubMed] [Google Scholar]
- 5. Crompton M. (1999). The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341, Pt. 2, 233–249 [PMC free article] [PubMed] [Google Scholar]
- 6. Hansson M.J., Mansson R., Mattiasson G., Ohlsson J., Karlsson J., Keep M.F., and Elmer E. (2004). Brain-derived respiring mitochondria exhibit homogeneous, complete and cyclosporin-sensitive permeability transition. J. Neurochem. 89, 715–729 [DOI] [PubMed] [Google Scholar]
- 7. Hansson M.J., Mansson R., Morota S., Uchino H., Kallur T., Sumi T., Ishii N., Shimazu M., Keep M.F., Jegorov A., and Elmer E. (2008). Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 45, 284–294 [DOI] [PubMed] [Google Scholar]
- 8. Lemasters J.J., Nieminen A.L., Qian T., Trost L.C., Elmore S.P., Nishimura Y., Crowe R.A., Cascio W.E., Bradham C.A., Brenner D.A., and Herman B. (1998). The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366, 177–196 [DOI] [PubMed] [Google Scholar]
- 9. Lemasters J.J. (1999). V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am. J. Physiol. 276, G1–G6 [DOI] [PubMed] [Google Scholar]
- 10. Lemasters J.J., Qian T., Bradham C.A., Brenner D.A., Cascio W.E., Trost L.C., Nishimura Y., Nieminen A.L., and Herman B. (1999). Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J. Bioenerg. Biomembr. 31, 305–319 [DOI] [PubMed] [Google Scholar]
- 11. Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., and Springer J.E. (2005). Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 79, 231–239 [DOI] [PubMed] [Google Scholar]
- 12. Sullivan P.G., Thompson M.B., and Scheff S.W. (1999). Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 13. Sullivan P.G., Thompson M., and Scheff S.W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161, 631–637 [DOI] [PubMed] [Google Scholar]
- 14. Vink R., and Van Den Heuvel C. (2004). Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin. Investig. Drugs 13, 1263–1274 [DOI] [PubMed] [Google Scholar]
- 15. Snyder S.H., Sabatini D.M., Lai M.M., Steiner J.P., Hamilton G.S., and Suzdak P.D. (1998). Neural actions of immunophilin ligands. Trends Pharmacol. Sci. 19, 21–26 [DOI] [PubMed] [Google Scholar]
- 16. Woodfield K., Ruck A., Brdiczka D., and Halestrap A.P. (1998). Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 336, Pt. 2, 287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waldmeier P.C., Feldtrauer J.J., Qian T., and Lemasters J.J. (2002). Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 62, 22–29 [DOI] [PubMed] [Google Scholar]
- 18. Hansson M.J., Persson T., Friberg H., Keep M.F., Rees A., Wieloch T., and Elmer E. (2003). Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 960, 99–111 [DOI] [PubMed] [Google Scholar]
- 19. Aminmansour B., Fard S.A., Habibabadi M.R., Moein P., Norouzi R., and Naderan M. (2014). The efficacy of cyclosporine-A on diffuse axonal injury after traumatic brain injury. Adv. Biomed. Res. 3, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatton J., Rosbolt B., Empey P., Kryscio R., and Young B. (2008). Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 109, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazzeo A.T., Alves O.L., Gilman C.B., Hayes R.L., Tolias C., Niki Kunene K., and Ross Bullock M. (2008). Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir. (Wien) 150, 1019–1031; discussion, 1031 [DOI] [PubMed] [Google Scholar]
- 22. Mazzeo A.T., Brophy G.M., Gilman C.B., Alves O.L., Robles J.R., Hayes R.L., Povlishock J.T., and Bullock M.R. (2009). Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J. Neurotrauma 26, 2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Empey P.E., McNamara P.J., Young B., Rosbolt M.B., and Hatton J. (2006). Cyclosporin A disposition following acute traumatic brain injury. J. Neurotrauma 23, 109–116 [DOI] [PubMed] [Google Scholar]
- 24. Ehinger K.H., Hansson M.J., Sjovall F., and Elmer E. (2013). Bioequivalence and tolerability assessment of a novel intravenous ciclosporin lipid emulsion compared to branded ciclosporin in Cremophor (R) EL. Clin. Drug Investig. 33, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cung T.T., Morel O., Cayla G., Rioufol G., Garcia-Dorado D., Angoulvant D., Bonnefoy-Cudraz E., Guerin P., Elbaz M., Delarche N., Coste P., Vanzetto G., Metge M., Aupetit J.F., Jouve B., Motreff P., Tron C., Labeque J.N., Steg P.G., Cottin Y., Range G., Clerc J., Claeys M.J., Coussement P., Prunier F., Moulin F., Roth O., Belle L., Dubois P., Barragan P., Gilard M., Piot C., Colin P., De Poli F., Morice M.C., Ider O., Dubois-Rande J.L., Unterseeh T., Le Breton H., Beard T., Blanchard D., Grollier G., Malquarti V., Staat P., Sudre A., Elmer E., Hansson M.J., Bergerot C., Boussaha I., Jossan C., Derumeaux G., Mewton N., and Ovize M. (2015). Cyclosporine before PCI in patients with acute myocardial infarction. N. Engl. J. Med. 373, 1021–1031 [DOI] [PubMed] [Google Scholar]
- 26. Ederoth P., Dardashti A., Grins E., Bronden B., Metzsch C., Erdling A., Nozohoor S., Mokhtari A., Hansson M.J., Elmer E., Algotsson L., Jovinge S., and Bjursten H. (2018). Cyclosporine before coronary artery bypass grafting does not prevent postoperative decreases in renal function: a randomized clinical trial. Anesthesiology 128, 710–717 [DOI] [PubMed] [Google Scholar]
- 27. Karlsson M., Pukenas B., Chawla S., Ehinger J.K., Plyler R., Stolow M., Gabello M., Hugerth M., Elmer E., Hansson M.J., Margulies S., and Kilbaugh T.J. (2018). Neuroprotective effects of cyclosporine in a porcine pre-clinical trial of focal traumatic brain injury. J. Neurotrauma. July 24. doi: 10.1089/neu.2018.5706. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rissin D.M., Kan C.W., Campbell T.G., Howes S.C., Fournier D.R., Song L., Piech T., Patel P.P., Chang L., Rivnak A.J., Ferrell E.P., Randall J.D., Provuncher G.K., Walt D.R., and Duffy D.C. (2010). Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yahanda A.M., Alder K.M., Fisher G.A., Brophy N.A., Halsey J., Hardy R.I., Gosland M.P., Lum B.L., and Sikic B.I. (1992). Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J. Clin. Oncol. 10, 1624–1634 [DOI] [PubMed] [Google Scholar]
- 30. List A.F., Kopecky K.J., Willman C.L., Head D.R., Persons D.L., Slovak M.L., Dorr R., Karanes C., Hynes H.E., Doroshow J.H., Shurafa M., and Appelbaum F.R. (2001). Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood 98, 3212–3220 [DOI] [PubMed] [Google Scholar]
- 31. Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., and Barsan W.G. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B., and Yurkewicz L. (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redell J.B., Zhao J., and Dash P.K. (2007). Acutely increased cyclophilin a expression after brain injury: a role in blood-brain barrier function and tissue preservation. J. Neurosci. Res. 85, 1980–1988 [DOI] [PubMed] [Google Scholar]
- 34. Schinkel A.H., Wagenaar E., Mol C.A., and van Deemter L. (1996). P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97, 2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker M.T., and Naguib M. (2005). Propofol: the challenges of formulation. Anesthesiology 103, 860–876 [DOI] [PubMed] [Google Scholar]
- 36. Trapani G., Altomare C., Liso G., Sanna E., and Biggio G. (2000). Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr. Med. Chem. 7, 249–271 [DOI] [PubMed] [Google Scholar]
- 37. Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N., Elbelghiti R., Cung T.T., Bonnefoy E., Angoulvant D., Macia C., Raczka F., Sportouch C., Gahide G., Finet G., Andre-Fouet X., Revel D., Kirkorian G., Monassier J.P., Derumeaux G., and Ovize M. (2008). Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 359, 473–481 [DOI] [PubMed] [Google Scholar]
- 38. Sullivan P.G., Sebastian A.H., and Hall E.D. (2011). Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J. Neurotrauma 28, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shahim P., Gren M., Liman V., Andreasson U., Norgren N., Tegner Y., Mattsson N., Andreasen N., Ost M., Zetterberg H., Nellgard B., and Blennow K. (2016). Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papa L., Brophy G.M., Welch R.D., Lewis L.M., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D.I., Silvestri S., Giordano P., Weber K.D., Hill-Pryor C., and Hack D.C. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubenstein R., Chang B., Davies P., Wagner A.K., Robertson C.S., and Wang K.K. (2015). A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids. J. Neurotrauma 32, 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al Nimer F., Thelin E., Nystrom H., Dring A.M., Svenningsson A., Piehl F., Nelson D.W., and Bellander B.M. (2015). Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS One 10, e0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bodien Y.G., McCrea M., Dikmen S., Temkin N., Boase K., Machamer J., Taylor S.R., Sherer M., Levin H., Kramer J.H., Corrigan J.D., McAllister T.W., Whyte J., Manley G.T., and Giacino J.T.; TRACK-TBI Investigators. (2018). Optimizing outcome assessment in multicenter tbi trials: perspectives from TRACK-TBI and the TBI Endpoints Development Initiative. J. Head Trauma Rehabil. 33, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]