Abstract
Rationale: Population studies suggest improved sepsis outcomes with statins, but the results of randomized controlled trials in patients with sepsis and organ dysfunction in critical care settings have broadly been negative. In vitro data suggest that statins modulate age-related neutrophil functions, improving neutrophil responses to infection, but only in older patients and at high doses.
Objectives: To determine if high-dose simvastatin improves neutrophil functions and is safe and tolerated in hospitalized older adults with community-acquired pneumonia with sepsis (CAP + S) not admitted to critical care.
Methods: We conducted a randomized, double-blind, placebo-controlled pilot study of simvastatin 80 mg or placebo for 7 days for patients with CAP + S aged 55 years or older admitted to a secondary care hospital. The Day 4 primary endpoint was change in neutrophil extracellular trap formation (NETosis). Day 4 secondary endpoints included neutrophil chemotaxis, safety and tolerability, Sequential Organ Failure Assessment score, mortality, readmission, and markers of tissue degradation/inflammation.
Measurements and Main Results: Four days of simvastatin adjuvant therapy in patients with CAP + S was associated with improvements in systemic neutrophil function (NETosis and chemotaxis), a reduction in systemic neutrophil elastase burden, and improved Sequential Organ Failure Assessment scores compared with placebo. A post hoc analysis demonstrated that simvastatin therapy was associated with improved hospitalization-free survival compared with placebo. Simvastatin was well tolerated in this elderly and multimorbid patient group with common coprescription of macrolide antibiotics.
Conclusions: This pilot study supports high-dose simvastatin as an adjuvant therapy for CAP + S in an older and milder disease cohort than assessed previously. A definitive multicenter study is now warranted in this population to assess the likelihood of benefit and harm.
Clinical trial registered with EudraCT (2012-00343-29).
Keywords: innate immunity, sepsis, pneumonia, statin, elderly care
At a Glance Commentary
Scientific Knowledge on the Subject
Mortality of pneumonia-associated sepsis in the elderly remains high, with evidence of altered neutrophil responses. There has been interest in the adjuvant use of statins to improve outcomes, but results of trials in unselected patients with sepsis in critical care units have been broadly negative.
What This Study Adds to the Field
This pilot randomized controlled double-blind clinical trial demonstrates simvastatin-associated improvements in neutrophil functions and clinical outcomes when used at a high dose in elderly patients admitted to the hospital with pneumonia-associated sepsis who do not require critical care unit support. These data suggest that the patient population, dose, and timing of the statin intervention are crucial and that a larger multicenter clinical trial is now needed to test the potential for benefit in this vulnerable population.
Pneumonia is the leading infectious cause of death globally (1). Community-acquired pneumonia (CAP) with sepsis (CAP + S) is common in the elderly and associated with high mortality, and elderly survivors experience increased long-term morbidity, readmission, and a reduced quality of life (2). Most CAP + S cases are not caused by multiresistant bacteria, even in vulnerable adults (3). Given global concerns about antibiotic usage, new strategies are needed to improve patient outcomes.
Bacterial infections such as CAP require a rapid but proportionate neutrophil response, and neutrophils maintain an exquisite, environment-dependent balance between aggression or quiescence and mortality and morbidity seen with either inappropriately exaggerated or inhibited responses (4). In sepsis, both heightened and reduced neutrophil functions are linked to mortality. For example, reduced neutrophil extracellular trap formation (NETosis) upon admission (potentially representing an inadequate response) and increased NETosis on Day 7 of the admission (potentially representing an overly aggressive, sustained proinflammatory response) are associated with poorer patient outcomes (5, 6).
Host age impacts neutrophil functions. Neutrophils isolated in vitro from older adults without an acute infection display reduced neutrophil migratory accuracy (7), phagocytosis (8), and NETosis (9), although degranulation appears to be increased (7). Our previous data suggest that during infection in older adults, some but not all functions are blunted further. Migratory accuracy is reduced during respiratory infections, which progresses to migratory failure during sepsis, remaining impaired 6 weeks after the initial infective event (10). Phagocytosis of Escherichia coli (11) and NETosis in response to PMA (phorbol myristate acetate) (5) appears preserved, and reactive oxygen species generation may increase (12). A reduced ability to target bacteria with enhanced degranulation would potentially increase bacterial invasion, local inflammation, and bystander tissue damage, which is in keeping with the higher burden of severe sepsis and end-organ damage experienced by the elderly (13).
There is great interest in using 3-hydroxy-3-methyl-glutaryl–coenzyme A reductase inhibitors (“statins”) to improve the immune response to infections, but studies have shown discordant results. In vitro and in a proof-of-concept clinical trial, simvastatin improved the migratory accuracy of neutrophils from older people at therapeutically relevant concentrations (10, 14). High-dose simvastatin increased NETosis in vitro (15), whereas simvastatin reduced NETosis in vivo in a murine model (16). In observational studies and during milder sepsis events, statins have been associated with improved patient outcomes (17). However, most studies of sepsis in critical care units have shown no benefit (18), although a post hoc analysis identified a group of responders (19). It seems likely that the recipient, dose of statin, and timing of the intervention are key. Of note, simvastatin (C25H38O5) is a prodrug activated by first-pass hepatic metabolism to a hydroxyacid metabolite (C25H40O6) (20). Researchers in most in vitro studies do not explicitly state whether they used the prodrug but activated it or used the active drug, and therefore discordant results may represent off-target effects. Current human studies suggest positive outcomes will be limited to older patients receiving high-dose statins early during a less severe infection event (17, 21) or those receiving statins before the infective insult (22, 23).
Statins have been prescribed in critically ill patients (24), but some patients experience serious side effects (25). Concerns have been raised about interactions between statins and medications that inhibit the drug-metabolizing enzyme cytochrome P450 3A4 (26), including clarithromycin, commonly prescribed for patients with CAP. However, a recent study found only a small increase in the relative risk of adverse events during coprescription (27).
We hypothesized that simvastatin added to the standard treatment for older patients with CAP + S treated outside a critical care setting would improve neutrophil responses to infection (NETosis and migratory accuracy) and would be well tolerated and safe in this elderly population. This proof-of-concept experimental medicine study had three aims:
-
1.
To determine if treatment with simvastatin altered neutrophil function in older patients with CAP + S
-
2.
To determine the safety and tolerability of high-dose simvastatin in CAP + S for 7 days
-
3.
To ascertain if simvastatin had effects on both clinical and laboratory outcomes relevant to CAP + S
Methods
Study Design
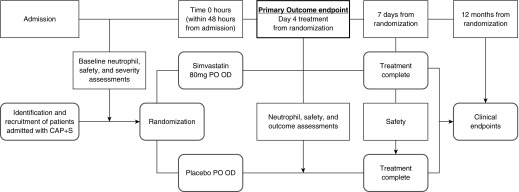
The trial schedule is shown in Figure 1. Patients were eligible if they fulfilled the criteria described in Table 1. The British Thoracic Society guidelines for CAP require patients to have at least three (of cough, sputum production, breathlessness, pleuritic chest pain, hemoptysis, fever, and headache) signs that are consistent with pneumonia on chest auscultation with a chest X-ray of consolidative changes for which there is no other clinical explanation (28). Patients also had to meet the 2012 Surviving Sepsis Campaign guidelines (29). This research was started before the Sepsis-3 definitions (Third International Consensus Definitions for Sepsis and Septic Shock) of 2016. Sepsis and pneumonia criteria had to be met within the same 24-hour period in patients enrolled within 48 hours of admission to the hospital.
Figure 1.
The study schedule. Patients with community-acquired pneumonia and sepsis (CAP + S) were identified and recruited within 48 hours of admission. Baseline information (clinical assessment, symptoms, blood samples for neutrophil isolation, clinical studies [including cholesterol, renal function, liver function, a full blood count, clotting status, and creatine kinase], and inflammatory studies) was obtained just before randomization. After randomization, patients were prescribed simvastatin 80 mg or placebo once daily for 7 days. Medications were dispensed by ward nursing staff, and tablet ingestion was noted in an electronic prescribing log. On Day 4 (after the ingestion of the fourth tablet), clinical and safety data were collected alongside blood tests for neutrophil studies, safety information, and inflammatory studies (as described above). These assessments were repeated 7 days after the ingestion of the first tablet (after seven doses of the tablet) if patients remained as inpatients. Patients and their general practitioners were contacted, and electronic health records were analyzed at 12 months after recruitment to gather data on readmission to the hospital and survival. OD = once daily; PO = by mouth.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥55 yr | Age <55 yr |
| Patients with a diagnosis of community-acquired pneumonia per British Thoracic Society definition | More than 48 h from admission at time of consent |
| Meet the criteria for sepsis on the basis of the 2012 Surviving Sepsis Campaign guidelines | Current or recent high-dose statin use within 1 mo (defined as simvastatin >40 mg or atorvastatin 20 mg or rosuvastatin 10 mg) |
| Known prior myositis or a family history of muscular disorders | |
| Creatine kinase >10 times upper limit of normal* | |
| Transaminases (ALT/AST) >8 times upper limit of normal | |
| Patients with severe renal impairment (creatinine clearance <30 ml/min) not receiving renal replacement therapy | |
| Patients currently receiving ongoing and sustained treatment with any of the following: itraconazole, ketoconazole, posaconazole, voriconazole, telithromycin, HIV protease inhibitors (e.g., nelfinavir, boceprevir, and telaprevir), nefazodone, cyclosporine, danazol, gemfibrozil, fusidic acid, amiodarone, verapamil, diltiazem, and fibric acid derivatives (except fenofibrate) | |
| Known HIV or hepatitis B or C virus infection | |
| Contraindication to enteral drug administration (either orally or nasogastric; e.g., patients with mechanical bowel obstruction) | |
| Patient declines consent, relative or advocate assent declined, or professional consultee assent declined | |
| Treatment withdrawal imminent within 24 h |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Upper limit of normal of creatine kinase is 195 IU/L.
Eligible patients were included after written informed consent or assent was obtained from their personal legal representatives or professional legal representatives. Retrospective consent was obtained when possible. The randomization sequence was predetermined by Sharp Clinical Services (UDG Healthcare plc) and was designed to provide a 1:1 randomization pattern. Standard treatments for pneumonia (including antibiotics and fluids) were administered as directed by the medical team, and these are listed in Table 1. Data for the primary endpoint were collected within a 5-hour window after the fourth simvastatin or placebo drug administration, and this is referred to as “Day 4.” Throughout the study, participants and researchers were blinded to treatment until all study assays were complete and 1-year survival data were collected.
Endpoints
The primary outcome was change in NETosis at Day 4 compared with Day 0. The following were secondary outcomes:
-
1.
Change in neutrophil migratory accuracy at Day 4 compared with Day 0
-
2.
Safety and tolerability of simvastatin
-
3.
Change in extracellular matrix degradation at Day 4 compared with Day 0
-
4.
Mortality and readmissions at 30, 180, and 365 days
-
5.
Sequential Organ Failure Assessment (SOFA) score
-
6.
Critical care unit admissions
-
7.
Critical care unit and hospital lengths of stay
In detail, the laboratory comparisons planned a priori were change in function (Day 4 minus Day 0) and NETosis after stimulation with formyl-methionyl-leucyl-phenylalanine (fMLP), migration toward IL8 (CXCL8), and measurement of systemic NE (neutrophil elastase) activity as a surrogate marker for extracellular matrix degradation, as defined in the statistical analysis plan. The SOFA score is a well-validated scoring system used to determine the extent of organ failure. Six individual organ systems are assessed (respiratory, cardiovascular, liver, kidney, neurological, and hematological), with a score of 0 to 4 given for each organ with a maximum score of 24. Higher SOFA scores indicate a greater burden of organ failure.
Exploratory and Post Hoc Analyses
Changes in inflammatory cytokines and neutrophil/lymphocyte ratios (NLRs) formed exploratory endpoints. Recently, the Sepsis-3 (30) definitions were published. A post hoc analysis of the key neutrophil functions between the placebo and simvastatin groups was performed, classifying recruited patients by the presence or absence of SOFA score greater than or equal to 2 in the presence of presumed/confirmed infection.
In addition, a composite secondary endpoint combining readmissions and survival at 180 days and 1 year, termed “hospitalization-free survival,” was calculated as part of a post hoc analysis. This patient-centered outcome (31, 32) was included on the basis of discussion with patient and public work groups (see online supplement). Deaths were confirmed at 180 and 365 days after enrollment. Hospitalizations in the same period were assessed through electronic patient records. Time in days to the first readmission or death was compared between groups.
Neutrophil Studies
Full methods of all neutrophil studies are detailed in the online supplement, including validation of 100 nM fMLP to generate NETosis in a CAP + S population. Neutrophils were isolated from whole blood as described previously (33). The neutrophils (>97% viable by exclusion of trypan blue and >95% pure) were resuspended in buffer (RPMI 1640 medium; Sigma-Aldrich) containing 0.15% bovine serum albumin (Sigma-Aldrich).
NETosis
NETosis was induced in freshly isolated CAP + S neutrophils stimulated with fMLP 100 nM to represent a pathologically relevant stimulus and 25 nM PMA, used as a positive control, or vehicle control (RPMI 1640 supplemented with 2 nM l-glutamine, 100 U/ml streptomycin, and 100 μg/ml penicillin) (Sigma-Aldrich for all), as described previously (9). Validation for fMLP as a stimulus in patients with CAP + S is reported in the online supplement, and these studies also informed power calculations.
Neutrophil migration
Migration was assessed using an Insall chamber (Weber Scientific International Ltd.) as previously described (34).
Neutrophil apoptosis
The percentage of apoptotic neutrophils was determined using the binding of annexin–fluorescein isothiocyanate (Thermo Fisher Scientific) protein to the cell surface, as previously described (9) and as presented in the online supplement.
Neutrophil elastase activity
Aα-Val360 is an NE-specific fibrinogen degradation product and therefore a surrogate marker of NE activity in vivo. Aα-Val360 was measured in plasma using a highly specific ELISA-based assay as described previously (35).
White cell response and CRP
Differential white cell count and CRP (C-reactive protein) were measured by the National Health Service laboratories and reported as a standard clinical assessment. With these measurements, changes in total white cell count and NLR from Day 0 to Day 4 were compared.
Systemic cytokines
Plasma cytokines were measured using the Luminex multianalyte assay kit (Bio-Techne) as per the manufacturer’s instructions and compared from Day 0 to Day 4.
Statistical Analysis
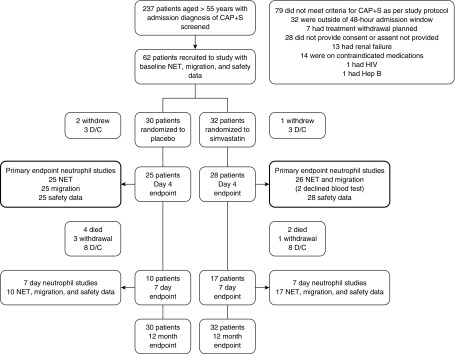
Statistical analyses were performed using PASW Statistics version 18.0 software (SPSS Inc.). Nonparametric tests were used throughout. The assessments of neutrophil functions (NETosis and chemotaxis) and NE activity were performed by calculating the change in these functions between Day 4 and baseline to provide a measure of the treatment effect in each group. Power calculations are provided below. All statistical tests are listed for each comparison and were two sided, with P < 0.05 accepted as statistically significant. All clinical outcomes, including safety monitoring, side effects, and adverse event reporting, were based on intention to treat. For SOFA scores and nonclinical outcomes, the change from Day 0 to Day 4 was compared in primary and secondary endpoints, so those without data for Day 4 were excluded (see Figure 2). Reported P values are not adjusted for multiple comparisons, but all results are reported.
Figure 2.
Modified Consolidated Standards of Reporting Trials diagram of trial endpoints. A total of 237 patients were screened with community-acquired pneumonia and sepsis (CAP + S), but 28 did not provide consent or assent, 111 did not meet inclusion criteria, and 36 met exclusion criteria. Sixty-two patients were recruited to the study, and all were followed for clinical endpoints at 12 months using an intention-to-treat analysis with consent even if they withdrew from the study. Patients who did not complete simvastatin or placebo therapy to Day 4 endpoints or then to Day 7 endpoints because of study withdrawal or hospital discharge were not included in neutrophil functional studies, because the study compared changes in function from baseline in a paired analysis. D/C = discharged because of clinical recovery; NET = neutrophil extracellular trap.
Power calculations
Our preliminary studies suggested that mean NETosis from systemic, isolated neutrophils from patients with CAP upon admission was 7,500 AU (SD, 2,432 AU). Eighteen patients in each arm would be needed to change NETosis by 5,000 AU from Day 0 to Day 4 with a power of 0.8 (P = 0.05), and 23 patients would be needed in each arm for 0.9 power. In vitro assays with neutrophils from patients with CAP + S suggested that simvastatin incubation would reduce fMLP-induced NETosis from a mean 5,298.8 AU (SD, 1,283 AU) to 4,173.6 AU (SD, 1,055 AU), suggesting that 18 patients in each arm would provide 0.8 power and 24 patients in each arm would provide 0.9 power (P = 0.05) (see Figure E1 in the online supplement). Mean neutrophil migration in older patients with CAP was suppressed to 0.8 μm/min (SD, 0.5 μm/min) versus 1 μm/min (SD, 0.4 μm/min) in age-matched control neutrophils. Assuming that statin treatment in vivo had a magnitude of effect similar to that in vitro on elderly patient neutrophils (+87% accuracy), then 16 patients in each arm would be needed to increase migratory accuracy by 0.7 μm/min from Day 0 to Day 4 with a power of 0.8 (P = 0.05). To allow for dropouts, we aimed to recruit at least 30 patients in each arm of this proof-of-concept study (therefore, a minimum of 60 participants in total).
Results
This study was a single-center, randomized, double-blind, placebo-controlled trial. The initial protocol, registered with the European Clinical Trials Database (EudraCT identifier 2012-003343-29), required amendment, as published elsewhere (36); however, a further amendment was undertaken after the start of recruitment. An abridged version of the final protocol is available in the online supplement. The trial and all amendments were approved by the Yorkshire and the Humber Research Ethics Committee (REC 12/YH/0375), and patients with CAP + S were randomized to receive simvastatin 80 mg or placebo (both manufactured by Sharp Clinical Services Ltd.) once daily for 7 days or until hospital discharge, whichever was sooner. Patients were followed for 12 months to assess readmission rates and mortality. The trial was ended once recruitment targets were met.
In total, 62 patients were recruited from one center (Queen Elizabeth Hospital Birmingham, Birmingham, UK), with clinical and safety data analyzed on an intention-to-treat basis between November 2013 and January 2016 and 1-year follow-up completed in January 2017. A modified Consolidated Standards of Reporting Trials diagram is shown in Figure 2. Table 2 describes patient demographics. There were no differences in baseline demographics between patients in the intervention or placebo group. Comorbidities are described in Table E1.
Table 2.
Baseline Demographics of Patients in Placebo and Simvastatin Groups
| Statin (n = 32) | Placebo (n = 30) | P Value | |
|---|---|---|---|
| Age, yr | 78.1 (70–88) | 83.8 (68–90) | 0.283 |
| Sex, M, n (%)* | 19 (59%) | 16 (53%) | 0.616 |
| Comorbidities, n (%) | 0.215 | ||
| 0 | 0 (0%) | 1 (3%) | |
| 1 | 16 (50%) | 8 (27%) | |
| 2 | 8 (25%) | 9 (30%) | |
| 3 or more | 8 (25%) | 12 (40%) | |
| Severe sepsis, n (%)* | 18 (56%) | 18 (60%) | 0.80 |
| Sepsis-3 positive (27 of 62), n (%) | 16 (50%) | 11 (37%) | 0.317 |
| CURB-65 score | 1 (1–2) | 2 (1–2) | 0.312 |
| Patients receiving low-dose statins before admission, n (%)* | 5 (16%) | 6 (20%) | 0.745 |
| C-reactive protein, mg/ml | 139 (52–240) | 156 (43–267) | 0.809 |
| Lactate, mmol/L | 2.0 (1.2–2.8) | 2.1 (1.3–2.9) | 0.662 |
| Creatinine, μmol/L | 79.5 (66.5–105) | 82.5 (70.5–92.3) | 0.746 |
| Creatine kinase, IU/L | 85.5 (46.8–203) | 62.5 (37–163) | 0.129 |
| Bilirubin, μmol/L | 8 (6–14.3) | 10 (6–16) | 0.311 |
| ALT, μmol/L | 19 (13–24.8) | 14.5 (13–27.3) | 0.310 |
| Total cholesterol, mmol/L | 3 (2.5–3.8) | 3.3 (2.6–4.5) | 0.555 |
| SOFA score | 2 (1–3) | 1 (0–3) | 0.129 |
| Antibiotics at admission, n (%)* | |||
| Cotreatment with amoxicillin/clavulanic acid/clarithromycin | 21 (66%) | 17 (57%) | 0.407 |
| Piperacillin/tazobactam | 8 (25%) | 11 (37%) | 0.642 |
| Other | 3 (9%) | 2 (7%) | 0.51 |
Definition of abbreviations: ALT = alanine aminotransferase; CURB-65 = confusion, urea, respiratory rate, blood pressure, and age 65 or over; Sepsis-3 = Third International Consensus Definitions for Sepsis and Septic Shock; SOFA = Sequential Organ Failure Assessment.
Sepsis-3 positive is defined as those patients who would be classified as having sepsis using the 2015 Sepsis-3 criteria (30). Values represent the median (interquartile range) with P values derived from a Mann-Whitney U test, unless noted otherwise. See Table E1 for more details on comorbidities. Normal ranges are as follows: C-reactive protein <10 mg/ml, lactate 0–1 mmol/L, creatinine 45–110 μmol/L, creatine kinase 5–195 IU/L, bilirubin <17 μmol/L, ALT <47 μmol/L, and total cholesterol <6.2 mmol/L.
Absolute values and P values derived from Fisher’s exact test.
Primary Outcome: Neutrophil NETosis Is Reduced in Patients Receiving Simvastatin
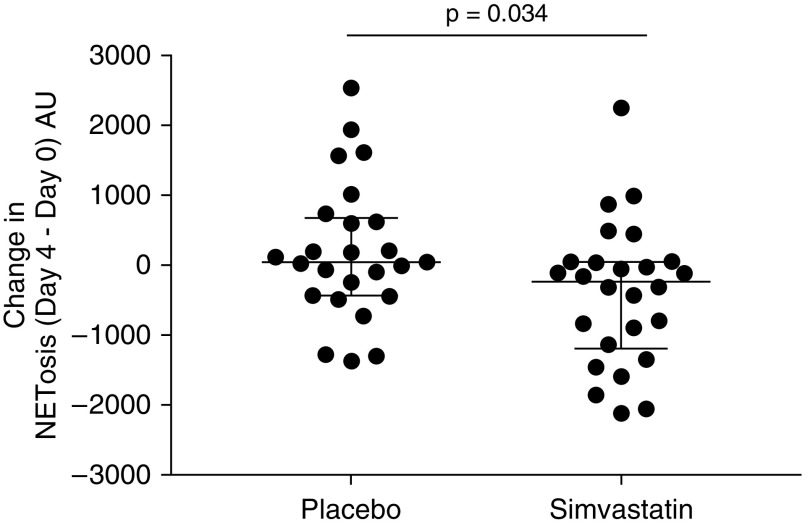
There were no differences in Day 0 fMLP-associated NETosis between the two groups (median [interquartile range (IQR)], simvastatin 405.5 [−32.1 to 883.8] AU vs. placebo 488.8 [34.8 to 805.3] AU; P = 0.64 by Mann-Whitney U test). Simvastatin treatment was associated with a significant change (reduction) in fMLP-induced NETosis compared with placebo (median [IQR] change in NETosis [Day 4 − Day 0], simvastatin −230.0 [−1,187.0 to 53.7] AU vs. placebo 46.2 [−430.8 to 679.8] AU; nominal P = 0.034 by Mann-Whitney U test). See Figures 3 and E2 for NETosis values on Day 0 and Day 4 for both groups.
Figure 3.
Change in neutrophil extracellular trap formation (NETosis) in patients with community-acquired pneumonia and sepsis (Day 4 minus Day 0). Patients with community-acquired pneumonia and sepsis received simvastatin 80 mg or placebo as well as standard medical treatment. Neutrophils were stimulated with formyl-methionyl-leucyl-phenylalanine (100 nM), and after 3 hours, NET formation was measured, both at Day 0 and at Day 4. Each dot represents one patient. NET data are displayed as Day 4 formyl-methionyl-leucyl-phenylalanine–induced reduced NETosis minus Day 0 NETosis (therefore, the change in NETosis), measured in arbitrary units. The lines show the median and interquartile range. Patients receiving simvastatin had a greater reduction in NETosis at Day 4 than patients receiving placebo (P = 0.034 by Mann-Whitney U test).
There were no differences in PMA-induced NETosis at baseline between the two groups (median [IQR], 48,446.2 [37,616.7 to 55,489.0] AU for simvastatin vs. 45,295.1 [37,971.2 to 52,841.6] AU for placebo; P = 0.50 by Mann-Whitney U test). There were no differences in the changes in PMA-induced NETosis between Day 4 and Day 0 between the two groups (−3,333.6 [−11,332.5 to 8,091.9] AU for simvastatin vs. −2,990.4 [−10,322.8 to 7,790.3] AU for placebo; P = 0.10 by Mann-Whitney U test). This confirmed that oral simvastatin reduced NETosis after in vitro activation with a pathologically relevant trigger, with studies suggesting that NETosis can differ between a physiological/pathological and a nonphysiological/nonpathological (PMA) trigger (37).
Neutrophil Migratory Accuracy Improves in Patients Receiving Simvastatin
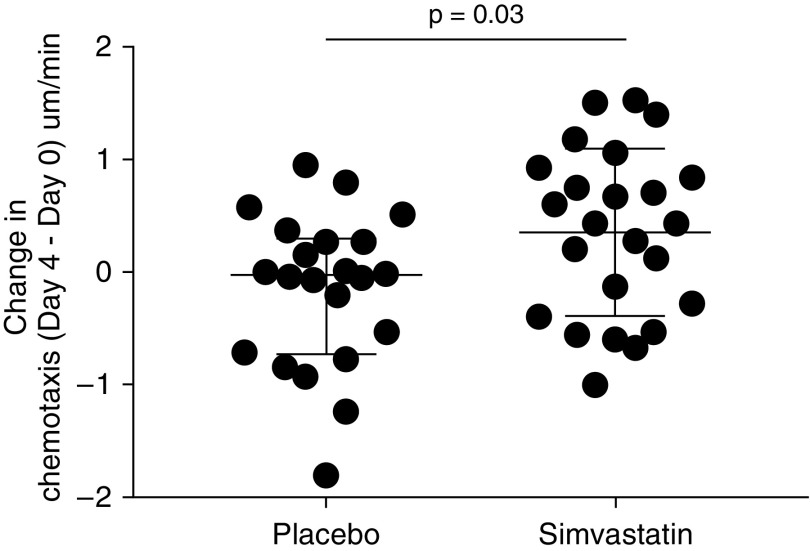
There were no differences in Day 0 chemotaxis toward CXCL8 at baseline between the two groups (median [IQR], 0.23 [−0.01 to 0.74] μm/min for simvastatin vs. 0.24 [−0.17 to 0.68] μm/min for placebo; P = 0.52 by Mann-Whitney U test). Simvastatin was associated with a greater change (improvement) in migratory accuracy than placebo (median [IQR] change in chemotaxis [Day 4 − Day 0], 0.36 [−0.43 to 0.86] μm/min for simvastatin vs. −0.04 [−0.74 to 0.32] μm/min for placebo; nominal P = 0.033 by Mann-Whitney U test). See Figures 4 and E3 for chemotaxis values on Day 0 and Day 4 for each group.
Figure 4.
Change in chemotaxis in patients with community-acquired pneumonia and sepsis (Day 4 minus Day 0). Patients with community-acquired pneumonia and sepsis received simvastatin 80 mg or placebo as well as standard medical treatment. Neutrophils migrated to CXCL8 (100 nM) isolated both at Day 0 and at Day 4. Each dot represents one patient. Lines are median and interquartile range. Chemotaxis data are measured in μm/min. Compared with placebo, simvastatin treatment was associated with a greater change in the accuracy of neutrophil migration (improvement) from Day 0 to Day 4 (P = 0.03 by Mann-Whitney U test).
Neutrophil Apoptosis Does Not Change with Simvastatin Treatment
There were no differences in the percentage of early or late apoptotic cells on Day 0 or Day 4 between patients in either group (see online supplement).
Systemic Neutrophil Elastase Activity Falls in Patients Receiving Simvastatin
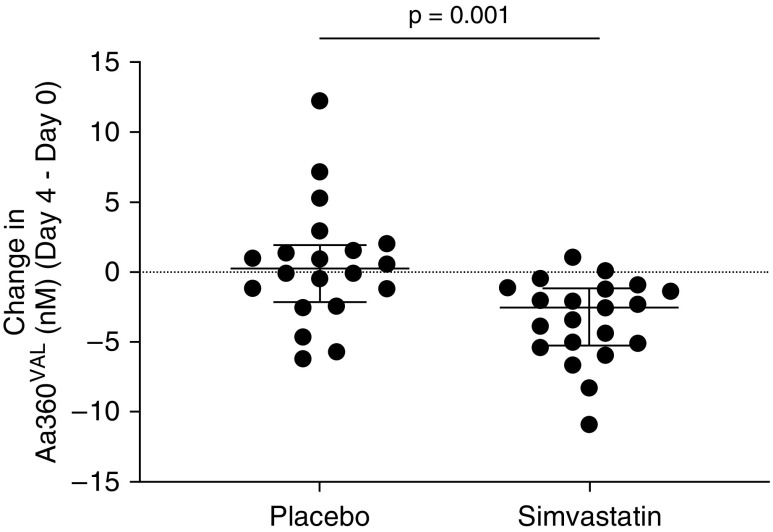
Matched samples were available in 21 patients in the simvastatin arm and 20 patients in the placebo arm. Baseline characteristics did not differ between this subcohort and the main group. There were no differences in plasma Aα360VAL at Day 0 between patients receiving simvastatin or placebo (median [IQR], 13.3 [9.6 to 14.8] nM for simvastatin vs. 13.6 [9.7 to 17.0] nM for placebo; P = 0.56 by Mann-Whitney U test). Simvastatin was associated with a greater change in Aα360VAL (reduction) between Day 4 and Day 0 than placebo (median [IQR] change, −2.55 [−5.23 to −1.15] nM for simvastatin vs. 0.25 [−2.13 to 1.916] nM for placebo; nominal P = 0.001 by Mann-Whitney U test) (see Figures 5 and E4 for actual data points on each day).
Figure 5.
Change in systemic neutrophil elastase activity in patients with community-acquired pneumonia and sepsis (Day 4 minus Day 0). Patients with community-acquired pneumonia and sepsis received simvastatin 80 mg or placebo as well as standard medical treatment. Plasma Aα360VAL was measured. Each dot represents one patient. The lines are the median and interquartile range. Compared with placebo, simvastatin treatment was associated with a significant reduction in systemic neutrophil elastase activity (Day 4 minus Day 0; P = 0.001 by Mann-Whitney U test).
Simvastatin Is Not Associated with Changes in the Neutrophil: NLR
The NLR is considered a biomarker of adverse outcome in sepsis and may be reduced by statin therapy (38). For both the simvastatin- and placebo-treated groups, there was a decrease in the NLR from Day 0 to Day 4, assessed from full blood counts (median [IQR] NLR, 10.3 [6.7 to 23.4] on Day 0 vs. 5.9 [3.4 to 8.2] on Day 4 for simvastatin; nominal P = 0.0001; 16.4 [11.0 to 24.7] on Day 0 vs. 7.5 [4.7 to 11.8] on Day 4 for placebo; nominal P < 0.0001; Wilcoxon test for both). There were no differences in the change of the NLR between Day 0 and Day 4 between the placebo or simvastatin group (P = 0.26 by Mann-Whitney U test).
Simvastatin Was Not Associated with Changes in CRP or Other Measured Systemic Cytokines
There were no differences in Day 0 or Day 4 CRP or systemic cytokine concentrations between the two groups. Furthermore, there were no differences in the change between Day 0 and Day 4 CRP or systemic cytokine concentrations between the groups (see online supplement).
Tolerability and Safety Data
Simvastatin 80 mg was well tolerated, with no serious adverse reactions and only one adverse reaction (myalgia). Biochemical safety monitoring did not show any change between Day 0 and Day 4 or between Day 0 and Day 7 on treatment, apart from cholesterol, which was lower in patients receiving simvastatin at Day 4 (see Table 3). No patients met Hy’s law (drug-induced liver injury, defined as ALT [alanine aminotransferase] elevation greater than three times the upper limit of normal [ULN] and/or total bilirubin elevation of greater than two times the ULN [39]), and no patients demonstrated rises in ALT above the ULN. No patients demonstrated a creatine kinase rise above the ULN. Furthermore, no rises in either ALT or creatine kinase were seen in patients receiving simvastatin who were also receiving clarithromycin. No patients showed signs of rhabdomyolysis (symptoms of muscle pain, muscle weakness, and discolored urine or decreased urination in the presence of high or increasing creatine kinase and myoglobin concentrations in the urine). Electronic hospital records demonstrated good compliance with the study drugs, with only three patients missing one dose each of the study drug/placebo (two for placebo and one for simvastatin).
Table 3.
Biochemical Safety Data
| Day 4 Values |
Day 7 Values |
|||||
|---|---|---|---|---|---|---|
| Statin (n = 32) | Placebo (n = 30) | P Value | Statin (n = 17) | Placebo (n = 10) | P Value | |
| C-reactive protein, mg/ml | 86 (35–198) | 59 (27–116) | 0.248 | 55 (11–82) | 26 (11–43) | 0.561 |
| Creatinine, μmol/L | 65 (62–95) | 66 (50–83) | 0.552 | 66 (57–83) | 68 (54–85) | 0.975 |
| Creatine kinase, IU/L | 90 (57–132) | 41 (28–116) | 0.061 | 42 (33–130) | 59 (22–91) | 0.187 |
| Bilirubin, μmol/L | 7 (6–10) | 8 (6–14.5) | 0.618 | 8 (5–12) | 7 (5–11) | 0.716 |
| ALT, μmol/L | 22 (16–31.5) | 21.5 (14–34.3) | 0.404 | 29.5 (16–59) | 25 (17–38) | 0.662 |
| Total cholesterol, mmol/L | 3.1 (2.4–3.7) | 4.0 (3.6–4.5) | 0.006 | N/A | N/A | |
Definition of abbreviations: ALT = alanine aminotransferase; N/A = not available.
Data were analyzed on an intention-to-treat basis. Values are represented as median (interquartile range) with P values derived by Mann-Whitney U test. Normal ranges are as follows: C-reactive protein <10 mg/ml, lactate 0–1 mmol/L, creatinine 45–110 μmol/L, creatine kinase 5–195 IU/L, bilirubin <17 μmol/L, ALT <47 μmol/L, and total cholesterol <6.2 mmol/L. Cholesterol was measured on Day 0 and Day 4 only.
Clinical Outcomes
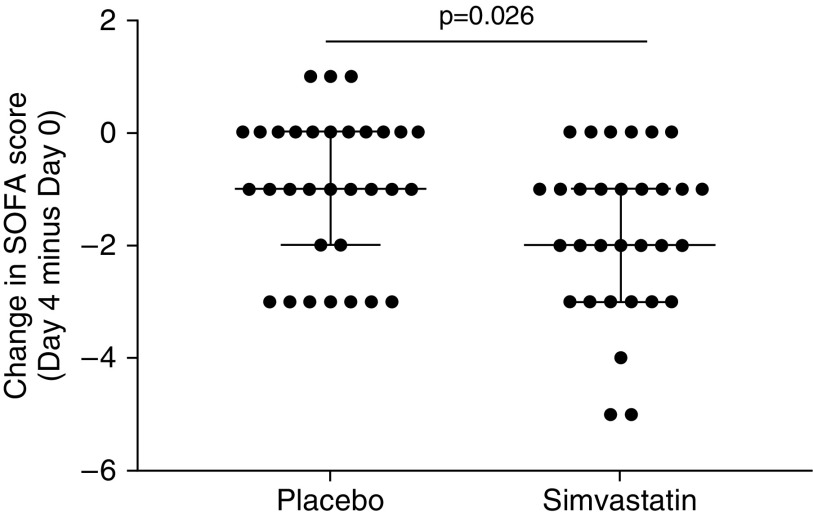
SOFA scores were generally low, as expected in this ward-based cohort of patients with CAP. There was a greater change (reduction) in SOFA score (Day 4 − Day 0; median [IQR]) in patients receiving simvastatin (−2 [−3 to −1]) than in those receiving placebo (−1 [−2 to 0]) (nominal P < 0.026 by Mann-Whitney U test; see Figure 6). All patients treated with simvastatin showed a reduction in SOFA score, whereas three patients in the placebo group showed an increase in SOFA score.
Figure 6.
Change in Sequential Organ Failure Assessment (SOFA) score in patients with community-acquired pneumonia and sepsis from Day 0 to Day 4. Patients with community-acquired pneumonia and sepsis received simvastatin 80 mg or placebo as well as standard medical treatment. SOFA score was calculated. Each dot represents one patient. The lines are the median and interquartile range. Compared with placebo, simvastatin treatment was associated with a greater change (reduction) in SOFA score (Day 4 minus Day 0; P = 0.026 by Mann-Whitney U test).
There were no differences in hospital length of stay or readmissions between groups. Placebo-treated patients had higher mortality at 30, 180, and 365 days, but this did not reach significance using Fisher’s exact test (see Table 4). In survivors, up to 1 year after discharge, there was no difference in the time without readmission to the hospital in the first year (median [IQR], 365 [103 to 365] d for simvastatin; 172 [69 to 365] d for placebo; P = 0.33 by Mann-Whitney U test).
Table 4.
Clinical Outcomes
| Simvastatin (n = 32) | Placebo (n = 30) | P Value | |
|---|---|---|---|
| Change in SOFA score, median (IQR) | −1.5 (−3 to −1) | −1 (−2 to 0) | 0.040 |
| Length of stay, d, median (IQR) | 9.5 (5 to 21) | 9 (6 to 14) | 0.578 |
| Number of patients readmitted within 180 d, n (%) | 9 (28%) | 13 (43%) | 0.290 |
| Number of patients readmitted within 365 d, n (%) | 11 (34%) | 13 (43%) | 0.601 |
| 30-d mortality, n (%) | 2 (6%) | 6 (20%) | 0.141 |
| Cumulative 90-d mortality, n (%) | 4 (13%) | 8 (27%) | 0.206 |
| Cumulative 180-d mortality, n (%) | 5 (16%) | 10 (33%) | 0.141 |
| Cumulative 1-yr mortality, n (%) | 7 (22%) | 13 (43%) | 0.103 |
Definition of abbreviations: IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Length of stay, readmission (over 365 d), and all-cause mortality for all patients were analyzed on an intention-to-treat basis. Mortality is all-cause and cumulative, with differences assessed using Fisher’s exact test. Length of stay is expressed as the median in days with IQR, and differences were compared using the Mann-Whitney U test.
Severity of Infection according to Sepsis-3 Criteria
Patients’ severity of infection was stratified in a post hoc analysis according to Sepsis-3 definitions, and neutrophil functions of NETosis and chemotaxis were assessed. A total of 27 (44%) patients met the Sepsis-3 definition (SOFA score ≥2), and the simvastatin (n = 16) and placebo (n = 11) groups showed no differences in patient characteristics between the two groups (see Table E2). None of the patients recruited to the trial had septic shock, but two were moved to critical care during their hospital stay, neither of whom met the Sepsis-3 definition of septic shock.
There was no difference in NETosis at Day 0 in those patients who met the definition of sepsis according to the Sepsis-3 definition (Sepsis-3 +ve) and those who did not meet the criteria for sepsis 3 (Sepsis-3 −ve) (fMLP, Sepsis-3 +ve 326.3 [−145.5 to 716.0] AU vs. Sepsis-3 −ve 488.8 [93.3 to 1,016.1] AU; P = 0.138 by Mann-Whitney U test). We observed no difference in the change of fMLP NETosis between Day 0 and Day 4 in Sepsis-3 +ve patients when we compared those who received simvastatin with those who received placebo (median [IQR] change, −215.0 [−1,049.0 to 673.2] AU for simvastatin vs. −10.0 [−867.2 to 818.9] AU for placebo; P = 0.601 by Mann-Whitney U test).
There was a trend toward reduced chemotaxis on Day 0 in Sepsis-3 +ve versus Sepsis-3 −ve patients (−0.035 [−0.2 to 0.4] μm/min for Sepsis-3 +ve vs. 0.23 [−0.3 to 0.8] μm/min for Sepsis-3 −ve; P = 0.07 by Mann-Whitney U test). We observed no difference in the change in chemotaxis between Day 0 and Day 4 in Sepsis-3 +ve patients when we compared those who received simvastatin or placebo (median [IQR] change, 0.43 [0.06 to 0.98] μm/min for simvastatin; P = 0.15; 0.08 [−0.7 to 0.8] μm/min for placebo; P = 0.2; Wilcoxon test for both).
Our a priori hypothesis was that Sepsis-3 −ve patients would have a greater neutrophil chemotactic response to simvastatin. There was a trend in this direction (Sepsis-3 −ve improvement n = 12 [80%] vs. no improvement n = 3 [20%]; Sepsis-3 +ve improvement n = 4 [44.4%] vs. no improvement n = 5 [55.6%]), but this did not reach significance (P = 0.09 by two-tailed Fisher’s exact test).
Post Hoc Composite Clinical Endpoint: Hospitalization-Free Survival
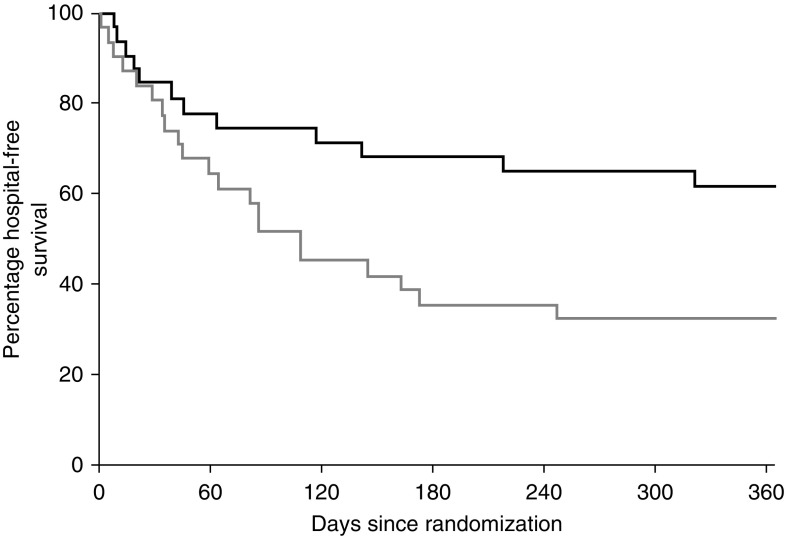
Analysis of readmission and survival as a composite endpoint demonstrated a significant increase in hospitalization-free survival at both 180 days (odds ratio, 0.45; 95% confidence interval, 0.22 to 0.93; nominal P = 0.03) and 365 days (odds ratio, 0.45; 95% confidence interval, 0.22 to 0.90; nominal P = 0.03) for those patients in the simvastatin group compared with the placebo group (see Figure 7).
Figure 7.
Kaplan-Meier curves for hospitalization-free survival at 365 days in an intention-to-treat analysis. The black line represents the simvastatin group, and the gray line represents patients who received placebo. The 365-day hospitalization-free survival rate demonstrates an odds ratio of 0.45 (95% confidence interval, 0.22–0.90; P = 0.03).
Discussion
To our knowledge, this proof-of-concept study provides the first report of a therapeutic intervention impacting neutrophil functions and clinical endpoints in older patients with CAP + S. At Day 4, simvastatin was associated with reductions in neutrophil NETosis (after physiological stimulation) and systemic NE activity, improved neutrophil migrational accuracy, and a small reduction in SOFA score. Furthermore, high-dose simvastatin was safe and tolerated with the coprescription of clarithromycin. Although mortality and time to readmission or readmission rates were not different between groups, a post hoc analysis suggested that simvastatin treatment was associated with increased hospitalization-free survival, an endpoint highlighted as important by patients and included in clinical trials (40).
Pneumonia hospitalizations are predicted to double by 2040 (41), and the World Health Organization has called for new strategies for treating infectious diseases (42). This proof-of-concept study suggests that modifying neutrophil function may improve outcomes during acute infection, but it also highlights the complex, stimulus-specific, and dynamic nature of neutrophil responses in sepsis.
In the present study, we interpret a reduction in NETosis and NE activity and an improvement in neutrophil migratory accuracy at Day 4 as positive, antiinflammatory events. The optimal neutrophil response to infection should be proportionate to the degree of threat and will vary during the course of the illness, from infection onset (when responses should be proinflammatory and bactericidal) to recovery (when these proinflammatory responses should diminish). Sustained NETosis is associated with endovascular damage and coagulopathy in human and murine sepsis (43, 44). Reduced migratory accuracy has been associated with poor patient outcomes and increased tissue damage due to obligate proteolysis (34, 45). Fibrinogen degradation is a marker of systemic NE activity, and NE has pleiotropic proinflammatory effects that may drive tissue injury (46).
CAP bacterial eradication is commonly seen within approximately 3 days with appropriate treatment (47, 48), and negative outcomes are rarely associated with antibiotic failure but are associated with dysregulated immune responses. We propose that inappropriately sustained proinflammatory responses at Day 4 should be considered pathological. There is some evidence to support this. For example, sustained NETosis is implicated in end organ damage in sepsis (49). In this study, we aimed to normalize functions to their “optimal” state at Day 4, which we assumed to be a less inflammatory state than at Day 0.
The pleiotropic effects of statins have led to studies in sepsis and acute respiratory distress syndrome, but evidence of efficacy is controversial. In most cases, results of phase 3 trials have been negative in patients recruited from critical care units (50), although recently a group of patients with “hyperinflammatory” acute respiratory distress syndrome showed benefit (19), supporting our data that simvastatin has an “antiinflammatory” effect in vivo. Most previous studies included a wide range of patients and diseases, and many used “low” statin doses. We and others have described how the patient group (older), severity of the host insult (milder), dose of statin (higher), and timing of the intervention (early) are likely to impact the effectiveness of the therapy (21). Concordantly, a recent meta-analysis (51) suggested that statins reduced mortality after nonsevere pneumonia but not after severe pneumonia. Our study did not identify changes in systemic proinflammatory mediators but was not powered for this endpoint (which was considered exploratory); therefore, further studies could assess whether a greater statin response is seen in patients with a more proinflammatory systemic readout, as recently reported (19).
Our patient cohort was elderly and had significant medical comorbidities, but these were well matched between groups. The Kaplan-Meier curves for hospitalization-free survival diverged from approximately 20 days after enrollment, suggesting that simvastatin had a long-term beneficial effect, but the mechanism for this is unclear, and further studies are needed to explore this. However, statins are known to have multiple epigenetic effects on a wide number of cell types even after short-term exposure (52), and statins can affect immune cell signaling, gene transcription, epigenetic modification, and metabolism in both mature and immature progenitor cells (53). It is possible that the long-term effects of a short course of simvastatin might be through neutrophil progenitors, but this is speculation.
The present study has important limitations. The study was not powered for any of the clinical endpoints included, so these results must be interpreted with caution. We could not explore differences in patients’ responses to simvastatin, which might inform who would gain the most clinical benefit from adjuvant therapy, and further trials should focus on this. Although simvastatin was considered safe and well tolerated in this group, the study was not powered to assess differences in creatine kinase or liver dysfunction, and a larger study is needed to assess safety (as well as efficacy) further. This study assessed ALT only for liver dysfunction, but this has been found to be sufficient in a recent study of statins (54). Not all neutrophil functions could be studied, nor did we assess the mechanism of effect of simvastatin on neutrophil functions. Furthermore, the effect of simvastatin on other immune cells requires clarification. The understanding of neutrophil responses, including NETosis, is evolving. In many studies, PMA is used as the gold standard for maximal NET generation; however, we chose fMLP because this represents a physiologically relevant stimulus. We demonstrate that fMLP is sufficient to cause NETosis in systemic neutrophils from the inflammatory environment of sepsis, and there are studies highlighting important differences in NETosis triggered by biologically relevant stimuli from that triggered by PMA (37). The assay for NETosis only included live-cell imaging in a proportion of patients, and therefore our indirectly measured assay, although included in many publications, should be considered a limitation. Also, the change in NETosis seen in this study between Day 0 and Day 4 was less than predicted in our a priori power calculations. It is difficult to judge what the minimally important effect size is for a cellular function, and this may be dependent on how much NETosis is required to contain bacteria in a specific setting. However, although the actual change in NETosis was relatively small, it is notable that a number of cellular functions seemed to change in a manner that might be associated with less tissue damage to the host at Day 4 (improved neutrophil directional migration, a reduced signal of proteinase activity, and reduced NETosis at Day 4).
Despite these limitations, this trial is of importance. This study highlights that it is possible to conduct a randomized controlled trial in elderly and acutely unwell patients outside a critical care setting. The trial also is the first to demonstrate improvements in neutrophil functions that are impeded with age and infection and to link these to clinical benefit. The study provides further clarity on the potential role of simvastatin during infections, including which patients (older), when (early and mild), at what dose (high), and for how long (7 d). It also provides reassurance that high-dose simvastatin is safe. There is now a pressing need for a multicenter trial in a ward-based cohort of patients with pneumonia and to consider a similar approach in other infections. These trials should clarify both efficacy (using both mortality and readmission, which appeared of particular importance to patients) and risk, and they should also try to determine whether the effect on neutrophil function is indeed a critical function of response or whether other cellular mechanisms are equally important.
Supplementary Material
Acknowledgments
Acknowledgment
This study was conducted with the assistance of the National Institute for Health Research Clinical Research Facility, Birmingham, United Kingdom. The authors thank Professor Robert Stockley for the use of the Aα360VAL assay.
Footnotes
Supported by the British Lung Foundation and the Medical Research Council.
Author Contributions: E.S. and D.R.T. designed the study, oversaw regulatory approvals, undertook patient recruitment, and analyzed data. E.S. wrote the manuscript. J.M.P. helped with study design, recruited patients, undertook laboratory assays, and analyzed data. F.G., D.P., R.Y.M., R.C.A.D., S.T.L., and P.A.H. recruited patients. H.G., G.M.W., J.H., P. Newby, and A.S. undertook laboratory assays. A.T.H. chaired the independent trial ethics and safety committee. P. Nightingale was the trial statistician. All authors commented on and approved the final version of the manuscript.
Data sharing statement: Data for this trial are available upon request to the corresponding author. All requests will be considered by the trial steering committee, including the ethical use of data. Deidentified participant data, including patient demographics, outcomes, and cellular studies, will be shared on request after publication of this paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2328OC on June 17, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangen MJJ, Huijts SM, Bonten MJM, de Wit GA. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17:208. doi: 10.1186/s12879-017-2302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klapdor B, Ewig S, Schaberg T, Rohde G, Pletz MW, Schütte H, et al. CAPNETZ study group. Presentation, etiology and outcome of pneumonia in younger nursing home residents. J Infect. 2012;65:32–38. doi: 10.1016/j.jinf.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Sapey E, Stockley RA. Red, amber and green: the role of the lung in de-priming active systemic neutrophils. Thorax. 2014;69:606–608. doi: 10.1136/thoraxjnl-2014-205438. [DOI] [PubMed] [Google Scholar]
- 5.Patel JM, Sapey E, Parekh D, Scott A, Dosanjh D, Gao F, et al. Sepsis induces a dysregulated neutrophil phenotype that is associated with increased mortality. Mediators Inflamm. 2018;2018:4065362. doi: 10.1155/2018/4065362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delabranche X, Stiel L, Severac F, Galoisy AC, Mauvieux L, Zobairi F, et al. Evidence of NETosis in septic shock-induced disseminated intravascular coagulation. Shock. 2017;47:313–317. doi: 10.1097/SHK.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 7.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher SK, Chahal H, Lord JM. The effect of age on neutrophil function: loss of CD16 associated with reduced phagocytic capacity. Mech Ageing Dev. 2001;122:20. [Google Scholar]
- 9.Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13:690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapey E, Patel JM, Greenwood HL, Walton GM, Hazeldine J, Sadhra C, et al. Pulmonary infections in the elderly lead to impaired neutrophil targeting, which is improved by simvastatin. Am J Respir Crit Care Med. 2017;196:1325–1336. doi: 10.1164/rccm.201704-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demaret J, Venet F, Friggeri A, Cazalis MA, Plassais J, Jallades L, et al. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J Leukoc Biol. 2015;98:1081–1090. doi: 10.1189/jlb.4A0415-168RR. [DOI] [PubMed] [Google Scholar]
- 12.Martins PS, Brunialti MKC, Martos LSW, Machado FR, Assunçao MS, Blecher S, et al. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis. Crit Care. 2008;12:R25. doi: 10.1186/cc6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 14.Walton GM, Stockley JA, Griffiths D, Sadhra CS, Purvis T, Sapey E. Repurposing treatments to enhance innate immunity: can statins improve neutrophil functions and clinical outcomes in COPD? J Clin Med. 2016;5:E89. doi: 10.3390/jcm5100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ghoul WM, Kim MS, Fazal N, Azim AC, Ali A. Evidence for simvastatin anti-inflammatory actions based on quantitative analyses of NETosis and other inflammation/oxidation markers. Results Immunol. 2014;4:14–22. doi: 10.1016/j.rinim.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel JM, Snaith C, Thickett D, Linhortova L, Melody T, Hawkey P, et al. Atorvastatin for preventing the progression of sepsis to severe sepsis (ASEPSIS Trial): a randomised, double-blind, controlled trial (ISRCTN64637517) [abstract] Crit Care. 2011;15(Suppl 1):P268. [Google Scholar]
- 18.Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. Am J Med. 2015;128:410–417, e1. doi: 10.1016/j.amjmed.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 19.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Irish Critical Care Trials Group. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waller DG, Sampson AP. Lipid disorders. In: Waller DG, Sampson AP, editors. Medical pharmacology and therapeutics. 5th ed. Edinburgh, UK: Elsevier; 2018. pp. 547–557. [Google Scholar]
- 21.Patel JM, Thickett DR, Gao F, Sapey E. Statins for sepsis: distinguishing signal from the noise when designing clinical trials. Am J Respir Crit Care Med. 2013;188:874. doi: 10.1164/rccm.201302-0392LE. [DOI] [PubMed] [Google Scholar]
- 22.Smit J, López-Cortés LE, Thomsen RW, Schønheyder HC, Nielsen H, Frøslev T, et al. Statin use and risk of community-acquired Staphylococcus aureus bacteremia: a population-based case-control study. Mayo Clin Proc. 2017;92:1469–1478. doi: 10.1016/j.mayocp.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Shyamsundar M, McAuley DF, Shields MO, MacSweeney R, Duffy MJ, Johnston JR, et al. Effect of simvastatin on physiological and biological outcomes in patients undergoing esophagectomy: a randomized placebo-controlled trial. Ann Surg. 2014;259:26–31. doi: 10.1097/SLA.0b013e31829d686b. [DOI] [PubMed] [Google Scholar]
- 24.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. HARP-2 Investigators; Irish Critical Care Trials Group. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 25.Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian working group consensus conference. Can J Cardiol. 2011;27:635–662. doi: 10.1016/j.cjca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Trieu J, Emmett L, Perera C, Thanakrishnan K, Van Der Wall H. Rhabdomyolysis resulting from interaction of simvastatin and clarithromycin demonstrated by Tc-99m MDP scintigraphy. Clin Nucl Med. 2004;29:803–804. doi: 10.1097/00003072-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Li DQ, Kim R, McArthur E, Fleet JL, Bailey DG, Juurlink D, et al. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ. 2015;187:174–180. doi: 10.1503/cmaj.140950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy ML, Le Jeune I, Woodhead MA, Macfarlane JT, Lim WS British Thoracic Society Community Acquired Pneumonia in Adults Guideline Group. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19:21–27. doi: 10.4104/pcrj.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. International Surviving Sepsis Campaign Guidelines Committee American Association of Critical-Care Nurses American College of Chest Physicians American College of Emergency Physicians Canadian Critical Care Society European Society of Clinical Microbiology and Infectious Diseases European Society of Intensive Care Medicine European Respiratory Society International Sepsis Forum Japanese Association for Acute Medicine Japanese Society of Intensive Care Medicine Society of Critical Care Medicine Society of Hospital Medicine Surgical Infection Society World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 Crit Care Med 200836296–327.18158437 [Google Scholar]
- 30.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myles PS, Shulman MA, Heritier S, Wallace S, McIlroy DR, McCluskey S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open. 2017;7:e015828. doi: 10.1136/bmjopen-2017-015828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brzostek T, Van de Werf F, Scheys I, Lesaffre E, Dubiel J, De Geest H. Prediction of event-free survival after hospital discharge in acute myocardial infarction treated with tissue-plasminogen activator. Acta Cardiol. 1994;49:9–24. [PubMed] [Google Scholar]
- 33.Mikami M, Llewellyn-Jones CG, Bayley D, Hill SL, Stockley RA. The chemotactic activity of sputum from patients with bronchiectasis. Am J Respir Crit Care Med. 1998;157:723–728. doi: 10.1164/ajrccm.157.3.9606120. [DOI] [PubMed] [Google Scholar]
- 34.Sapey E, Stockley JA, Greenwood H, Ahmad A, Bayley D, Lord JM, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1176–1186. doi: 10.1164/rccm.201008-1285OC. [DOI] [PubMed] [Google Scholar]
- 35.Carter RI, Mumford RA, Treonze KM, Finke PE, Davies P, Si Q, et al. The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax. 2011;66:686–691. doi: 10.1136/thx.2010.154690. [DOI] [PubMed] [Google Scholar]
- 36.Greenwood H, Patel J, Mahida R, Wang Q, Parekh D, Dancer RC, et al. Simvastatin to modify neutrophil function in older patients with septic pneumonia (SNOOPI): study protocol for a randomised placebo-controlled trial. Trials. 2014;15:332. doi: 10.1186/1745-6215-15-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Linden M, Westerlaken GHA, van der Vlist M, van Montfrans J, Meyaard L. Differential signalling and kinetics of neutrophil extracellular trap release revealed by quantitative live imaging. Sci Rep. 2017;7:6529. doi: 10.1038/s41598-017-06901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akin F, Ayça B, Köse N, Sahin I, Akin MN, Canbek TD, et al. Effect of atorvastatin on hematologic parameters in patients with hypercholesterolemia. Angiology. 2013;64:621–625. doi: 10.1177/0003319713479154. [DOI] [PubMed] [Google Scholar]
- 39.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 40.Ko EJ, Kim BH, Jeong HY, Soe SU, Yang DH, Lee SY. Serum 25-hydroxyvitamin D as a predictor of hospitalization-free survival in predialysis and dialysis patients with chronic kidney disease: a single-center prospective observational analysis. Kidney Res Clin Pract. 2016;35:22–28. doi: 10.1016/j.krcp.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205:1589–1592. doi: 10.1093/infdis/jis240. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Antimicrobial resistance. 2018 Feb 15 [accessed 2018 Jun 21]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- 43.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 45.Butler KL, Ambravaneswaran V, Agrawal N, Bilodeau M, Toner M, Tompkins RG, et al. Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One. 2010;5:e11921. doi: 10.1371/journal.pone.0011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bieth JG. Elastases: catalytic and biological properties. In: Mecham RP, editor. Regulation of matrix accumulation (Biology of Extracellular Matrix series). Orlando, FL: Academic Press; 1986. pp. 217–320. [Google Scholar]
- 47.Ruiz M, Arosio C, Salman P, Bauer TT, Torres A. Diagnosis of pneumonia and monitoring of infection eradication. Drugs. 2000;60:1289–1302. doi: 10.2165/00003495-200060060-00004. [DOI] [PubMed] [Google Scholar]
- 48.el Moussaoui R, de Borgie CA, van den Broek P, Hustinx WN, Bresser P, van den Berk GE, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355. doi: 10.1136/bmj.332.7554.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czaikoski PG, Mota JMSC, Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansur A, Steinau M, Popov AF, Ghadimi M, Beissbarth T, Bauer M, et al. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC Med. 2015;13:128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia M, Huang W, Li L, Xu Z, Wu L. Statins reduce mortality after non-severe but not after severe pneumonia: a systematic review and meta-analysis. J Pharm Pharm Sci. 2015;18:286–302. doi: 10.18433/j34307. [DOI] [PubMed] [Google Scholar]
- 52.Allen SC, Mamotte CDS. Pleiotropic and adverse effects of statins-do epigenetics play a role? J Pharmacol Exp Ther. 2017;362:319–326. doi: 10.1124/jpet.117.242081. [DOI] [PubMed] [Google Scholar]
- 53.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154:69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homer K, Robson J, Solaiman S, Davis A, Khan SZ, McCoy D, et al. Reducing liver function tests for statin monitoring: an observational comparison of two clinical commissioning groups. Br J Gen Pract. 2017;67:e194–e200. doi: 10.3399/bjgp17X689365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.