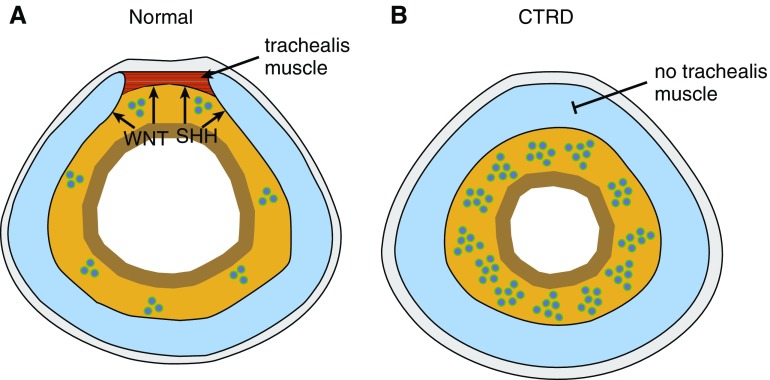
The trachea provides a direct conduit from the respiratory system to the external environment. In mammals, the trachea is surrounded by cartilaginous rings that perform several duties, including providing structural support and keeping the trachea patent. The development of the trachea has been explored using genetic mouse models that reveal the importance of multiple paracrine signaling pathways, such as the SHH (sonic hedgehog) and WNT (Wingless-related integration site) pathways (1–4). In humans, tracheal defects are common in the pediatric patient population and include defects such as tracheomalacia and the rarer disease, complete tracheal ring deformity (CTRD) (5, 6). In CTRD, the cartilaginous rings surrounding the trachea are complete and lack a dorsal gap filled in with the trachealis muscle. As with other orphan diseases, the pathogenic mechanisms of CTRD have eluded investigators given the paucity of tissue and representative animal models of these diseases. As such, it is frequently unclear whether disease-causing mutations in humans follow molecular pathways similar to those discovered in genetic mouse models. In this issue of the Journal, Sinner and colleagues (pp. 1267–1281) uncover multiple human genetic lesions that lead to CTRD, including mutations in genes within the SHH pathway and mutations in multiple genes within the WNT pathway, including ROR2 (receptor tyrosine kinase–like orphan receptor 2) (7) (Figure 1). This study highlights the close relationship between mouse models of respiratory development and human congenital tracheal abnormalities, and it provides new target genes and pathways that play an important role in tracheal development in humans.
Figure 1.
Disruption in SHH or WNT signaling leads to complete tracheal ring deformity. (A) Normal tracheal development results in incomplete cartilage rings surrounding the trachea with the trachealis muscle on the dorsal side. (B) Complete tracheal ring deformity (CTRD) resulting from mutations in genes within the WNT and SHH pathways generates complete cartilage rings and an absence of the trachealis muscle.
The trachea initially develops from the anterior foregut. Subsequently, mesenchymal–epithelial cross-talk sets up a ventral–dorsal gradient of WNT and BMP (bone morphogenetic protein) signaling to promote the proper specification, patterning, and separation of the trachea and esophagus, respectively (1, 2, 8, 9). Along with WNT and BMP signaling, SHH signaling has been show to play important roles in separation of the trachea and esophagus, as well as in promoting proper differentiation of mesenchymal derivatives in the trachea and other regions within the respiratory system (3, 4, 10). After separation occurs, the cartilaginous progenitors within the trachea are denoted by expression of the transcription factor SOX9, which is both a marker and functional regulator of cartilage development (11). Defects in the development of these progenitors can lead to failure to form the tracheal cartilaginous rings surrounding the trachea. Sinner and colleagues performed a trio analysis with whole-exome sequencing of patients with CTRD along with their parents to identify novel mutations (7). Remarkably, many of the mutations implicated either the WNT or SHH pathway. These included both heritable and spontaneous mutations. The mutations in the WNT pathway, including ROR2 and LRRC7 (leucine rich repeat containing 7), involve both β-catenin–dependent (LRRC7) and β-catenin–independent (ROR2) pathways. De novo mutations were found in SHH, and compound heterozygous mutations were observed in HSPG2, an extracellular heparin sulfate proteoglycan that is known to modulate SHH and other paracrine pathways (12).
Previous studies in mice have demonstrated important roles for WNT and SHH in the development of multiple tissues within the respiratory system, including the trachea. Loss of SHH also leads to failure of tracheal cartilage formation (10). As SHH is expressed exclusively within the developing respiratory endoderm, this suggests that SHH acts in a paracrine manner to drive cartilage formation, possibly by regulating the differentiation of SOX9+ progenitors. This is consistent with the authors’ finding of heterozygous gene variants in the downstream transcriptional effectors of SHH signaling (GLI1 [GLI family zinc finger 1], GLI2, and GLI3) in several patients with CTRD. The role of WNT signaling in tracheal cartilage formation was directly tested by the authors through gene deletion of the essential component of WNT ligand secretion, Wls (Wntless). Loss of Wls in the developing respiratory endoderm resulted in loss of tracheal cartilage formation and a reduction in SOX9+ cartilage progenitors. This correlated with a general loss of WNT signaling throughout the lung, as noted by decreased expression of the WNT target gene Axin2, and decreased WNT reporter activity. Although the authors found that multiple aspects of WNT signaling were disrupted, including downregulation of the ligand Wnt5a, there are additional important ligands expressed in the developing lung endoderm (e.g., Wnt7b) that may contribute to the phenotype (13). The finding that loss of Wls led to the almost complete loss of cartilage formation in the trachea as compared with mutations in human WLS exhibiting complete cartilaginous ring phenotype, indicates differences either in the role of WNT signaling in human tracheal development or differences in how the human mutations affect WNT signaling versus a complete loss due to deletion of Wls.
One of the more interesting findings of this study is that many of the patients with CTRD also had associated cardiac defects, including defects in pulmonary artery development. Both the heart and lung develop in a coordinated fashion to establish proper functioning of the cardiopulmonary system. Previous work has demonstrated a common cardiopulmonary progenitor (CPP) that can generate mesenchymal derivatives in both the developing lungs and heart (14). CPPs express the WNT2 ligand and their function is regulated by SHH activity. It is tempting to speculate that the defects in SHH and WNT signaling that lead to CTRD may involve defective CPP development in humans.
Although there are many signaling pathways that play critical roles in embryonic morphogenesis, some pathways, such as the WNT and SHH signaling pathways, appear to play a central role in many tissues, indicating that they act as hubs of cell–cell interactions during tissue development. Moreover, both the WNT and SHH signaling pathways are active during normal adult respiratory homeostasis and play important roles in repair and regeneration of multiple cell types in the respiratory tract (15, 16). Such a reiterative use of a core set of signaling pathways throughout the lifespan of mammals underscores the impact these pathways have on both genome stability and the inherent plasticity that is necessary to rapidly engage specific progenitor cells in the adult after injury.
The findings in this report will help advance our understanding of both the genetic causes of tracheal defects in humans and the cell–cell interactions that are important for mesenchymal development in the airways. Such interactions have become a hallmark of organ development as well as repair and regeneration in the respiratory tract and other tissues. Providing valuable human correlates in this area of research will both support and enhance model organism studies, which remain a necessary workhorse of mechanistic science.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201907-1285ED on July 10, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci USA. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 4.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 5.Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005;128:3391–3397. doi: 10.1378/chest.128.5.3391. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox LJ, Hart CK, de Alarcon A, Schweiger C, Peddireddy NS, Tabangin M, et al. Unrepaired complete tracheal rings: natural history and management considerations. Otolaryngol Head Neck Surg. 2018;158:729–735. doi: 10.1177/0194599817751889. [DOI] [PubMed] [Google Scholar]
- 7.Sinner DI, Carey B, Zgherea D, Kaufman KM, Leesman L, Wood RE, et al. Complete tracheal ring deformity: a translational genomics approach to pathogenesis. Am J Respir Crit Care Med. 2019;200:1267–1281. doi: 10.1164/rccm.201809-1626OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239:514–526. doi: 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 12.Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, DeGueme AM, Dhir R, et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol Cancer. 2006;5:9. doi: 10.1186/1476-4598-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 14.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526:578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.