Abstract
Rationale: Recent studies have demonstrated that extracellular vesicles (EVs) released during acute lung injury (ALI) were inflammatory.
Objectives: The current study was undertaken to test the role of EVs induced and released from severe Escherichia coli pneumonia (E. coli EVs) in the pathogenesis of ALI and to determine whether high-molecular-weight (HMW) hyaluronic acid (HA) administration would suppress lung injury from E. coli EVs or bacterial pneumonia.
Methods: E. coli EVs were collected from the perfusate of an ex vivo perfused human lung injured with intrabronchial E. coli bacteria for 6 hours by ultracentrifugation and then given intrabronchially or intravenously to naive human lungs. One hour later, HMW HA was instilled into the perfusate (n = 5–6). In separate experiments, HMW HA was given after E. coli bacterial pneumonia (n = 6–10). In vitro experiments were conducted to evaluate binding of EVs to HMW HA and uptake of EVs by human monocytes.
Measurements and Main Results: Administration of HMW HA ameliorated the impairment of alveolar fluid clearance, protein permeability, and acute inflammation from E. coli EVs or pneumonia and reduced total bacteria counts after E. coli pneumonia. HMW HA bound to E. coli EVs, inhibiting the uptake of EVs by human monocytes, an effect associated with reduced TNFα (tumor necrosis factor α) secretion. Surprisingly, HMW HA increased E. coli bacteria phagocytosis by monocytes.
Conclusions: EVs induced and released during severe bacterial pneumonia were inflammatory and induced ALI, and HMW HA administration was effective in inhibiting the uptake of EVs by target cells and decreasing lung injury from E. coli EVs or bacterial pneumonia.
Keywords: acute lung injury, extracellular vesicles, ex vivo perfused human lung, hyaluronic acid
At a Glance Commentary
Scientific Knowledge on the Subject
Extracellular vesicles released by living cells may have significant inflammatory properties, contributing to lung injury during severe bacterial pneumonia.
What This Study Adds to the Field
We have discovered that high-molecular-weight hyaluronic acid may suppress inflammation by binding to extracellular vesicles released during severe bacterial pneumonia.
Acute respiratory distress syndrome (ARDS) is a devastating clinical condition common in patients with respiratory failure in the ICU. It is associated with high mortality rates and long-term physical and psychological dysfunctions among survivors (1–3). Despite intense research into the pathophysiology, there are no specific pharmacological therapies (4).
Most eukaryotic cells release small anuclear membrane-bound vesicles into the extracellular environment in either physiological or pathophysiological conditions, often called extracellular vesicles (EVs) by the International Society for Extracellular Vesicles (5). Through their cargo containing bioactive molecules such as proteins, mRNAs, and microRNAs and their interaction with target cells, EVs are recognized as important mediators of cellular communication (6–9). The role of EVs in the pathogenesis of acute lung injury (ALI) or ARDS has generated considerable interest.
Hyaluronan or hyaluronic acid (HA) is synthesized as a high-molecular-weight (HMW) (>1,000 kD) nonsulfated glycosaminoglycan composed of repeating polymeric disaccharides of d-glucuronic acid and N-acetyl-d-glucosamine. In the lung, it is located mainly in the peribronchial and perialveolar space and is one of the chief components of the extracellular matrix and critical for maintaining the normal structure of the alveolar air–blood barrier and homeostasis (10). In multiple lung diseases including ALI, asthma, or chronic obstructive pulmonary disease, HA undergoes degradation by lysosomal hyaluronidases, reactive oxygen and nitrogen species, and inflammatory mediators (11). The degradation products, low-molecular-weight HA (<500 kD), can decrease endothelial cell barrier function, stimulate angiogenesis, and induce inflammation (12). Surprisingly, primarily because of its molecular size, HMW HA has the opposite properties of low-molecular-weight (LMW) HA. Therefore, many investigators have focused on the therapeutic use of exogenous administration of HMW HA in lung diseases. HA influences cell behavior through binding to various cell surface receptors such as CD44 (cluster of differentiation 44), TLR2 (toll-like receptor 2) and TLR4, HABP2 (hyaluronan-binding protein 2), or RHAMM (receptor for hyaluronan-mediated motility) (13). CD44 is commonly expressed on innate and adaptive immune cells such as macrophages, neutrophils, etc., and EVs released by these immune cells, which may be critical for trafficking.
In the current study, we initially examined the biological effects of EVs induced and released into the perfusate during Escherichia coli bacterial pneumonia to initiate inflammation and lung protein permeability in a naive perfused human lung. We next studied the therapeutic effects of HMW HA administration, instilled into the perfusate, on lung injury from E. coli–induced EVs. We hypothesized that HA may bind to CD44, commonly expressed on the surface of EVs, and prevent the uptake of EVs by target cells, ameliorating lung injury. Last, we studied the therapeutic effects of HMW HA in E. coli bacterial pneumonia to suppress inflammation, pulmonary edema, and, potentially, bacterial growth.
Methods
Ex Vivo Perfused Human Lung Preparation
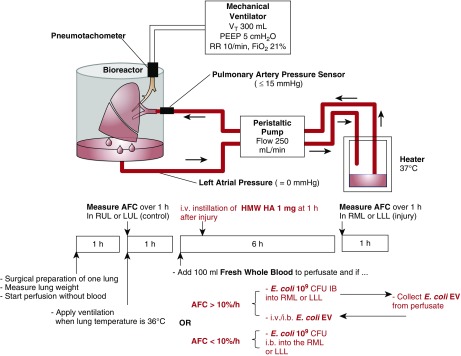
A detailed description of the ex vivo perfused human lung preparation and the experimental protocols are described in the online supplement (14, 15) (Figure 1). Briefly, either the right or left human lung was slowly perfused with crystalloid solution containing 5% bovine albumin fraction V (MP Biomedicals LLC) and fresh human whole blood and ventilated. Alveolar fluid clearance (AFC) was then measured in the upper lobe. If AFC > 10%/h, 109 cfu of E. coli bacteria (K1 strain) was instilled into the middle or lower lobe. After 6 hours of perfusion, the perfusate was collected to isolate EVs (E. coli EVs) by ultracentrifugation. In naive human lungs, E. coli EVs collected from 400 ml of perfusate were given intravenously or into the middle or lower lobe intrabronchially. As therapy, 1 mg HMW HA (LifeCore, Inc.) was instilled into the perfusate 1 hour after injury. In separate experiments, marginal human lungs with AFC < 10% and injured further with 109 cfu of i.b. E. coli bacteria were treated with 1 mg HMW HA.
Figure 1.
Schematic of the ex vivo perfused human lung. Human lungs rejected for clinical transplantation were perfused with Dulbecco’s modified Eagle medium containing 5% albumin with 100 ml fresh human whole blood at a rate of 250 ml/min. Once rewarmed to 37°C, the lung was ventilated using a Vt of 300 ml with 5 cm H2O of positive end-expiratory pressure and respiratory rate 10 breaths/min in room air. For lungs with alveolar fluid clearance (AFC) > 10%, 109 cfu of Escherichia coli K1 strain was instilled to the middle or lower lobe. At 6 hours, extracellular vesicles (EVs) were isolated from the perfusate (E. coli EVs). In separate naive human lungs with AFC > 10%/h, E. coli EVs collected from 400 ml of perfusate were given intrabronchially or intravenously to induce lung injury. In separate experiments with lungs with AFC < 10%, 109 cfu E. coli was instilled into the middle or lower lung lobes to induce severe bacterial pneumonia. For all treatment groups, 1 mg of HMW HA was administered through the pulmonary artery 1 hour after injury. HA = hyaluronic acid; HMW = high-molecular-weight; LLL = left lower lung lobe; LUL = left upper lung lobe; PEEP = positive end-expiratory pressure; RML = right middle lung lobe; RR = respiratory rate; RUL = right upper lung lobe.
Statistical Analysis
Results are expressed as mean ± SD if the data were normally distributed and median with interquartile range (IQR) if not. Comparisons between two groups were made using unpaired t test if the data were normally distributed or Mann-Whitney test if not. Comparisons between more than two groups were made using an ANOVA using the Bonferroni’s correction for multiple-comparison testing if the data were normally distributed or Dunn’s test after Kruskal-Wallis analysis if not. All statistical analysis was performed using GraphPad Prism software.
Results
HMW HA Treatment Improved AFC Rate and Decreased Lung Weight Gain after Injury by E. coli EV Instillation
The demographic and clinical data for human lungs used for experiments with E. coli EVs are listed in Table 1.
Table 1.
Donor Demographic and Clinical Data for Experiments with Escherichia coli EVs and HMW HA
| Control (n = 17) | i.b. E. coli EVs (n = 6) | i.v. E. coli EVs (n = 5) | i.v. E. coli EVs + i.v. HMW HA (n = 6) | |
|---|---|---|---|---|
| Age, yr | 55 ± 16 | 65 ± 6 | 49 ± 16 | 50 ± 19 |
| Male sex, n (%) | 11 (65%) | 2 (33%) | 4 (80%) | 5 (83%) |
| PaO2/FiO2, mm Hg, median (IQR) | 267 (221–308) | 296 (218–339) | 255 (182–271) | 280 (205–306) |
| Cdyn, ml/cm H2O | 40 ± 12 | 37 ± 14 | 36 ± 10 | 49 ± 9 |
| Murray LIS | 1.5 ± 0.6 | 1.4 ± 0.8 | 1.8 ± 0.5 | 1.3 ± 0.5 |
| Ischemic time, h:min | 26:41 ± 8:36 | 22:53 ± 5:06 | 24:43 ± 11:20 | 29:36 ± 6:45 |
Definition of abbreviations: Cdyn = dynamic compliance; EVs = extracellular vesicles; HA = hyaluronic acid; HMW = high-molecular-weight; IQR = interquartile range; LIS = lung injury score.
Data are presented as mean ± SD unless otherwise indicated. No significant differences in PaO2/FiO2 ratio, lung compliance, or lung injury score prior to organ harvest or total ischemic time were observed among groups.
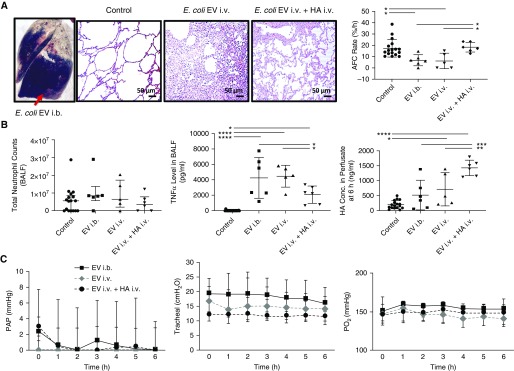
Representative histological images from the middle or lower lung lobe after injury with E. coli EVs showed an increase in lung edema and cellularity. The lung lobes treated with intravenous (i.v.) HMW HA 1 hour after E. coli EV i.v. instillation showed a reduction in inflammatory cell infiltration and septal thickening. AFC significantly decreased from 17.3 ± 7.7% to 6.7 ± 4.9% or 6.1 ± 6.8% after E. coli EV i.b. or i.v. instillation, respectively. Administration of HMW HA significantly restored AFC rate to 18.3 ± 4.3% (Figure 2A). Total lung weight increased by 574 ± 152 g or 636 ± 65 g with E. coli EV i.b. or i.v. administration, respectively, after 6 hours of perfusion. Administration of HMW HA numerically decreased total lung weight gain by 14% (547 ± 120 g) compared with injury induced by i.v. E. coli EV, although not statistically significant.
Figure 2.
Effect of high-molecular-weight (HMW) hyaluronic acid (HA) on lung injury induced by Escherichia coli extracellular vesicle (EV) instillation. Administration of HMW HA as therapy restored alveolar fluid clearance (AFC) rate and reduced inflammation in the injured alveolus. (A) Gross human lung image of extravasation of Evan’s blue into the injured alveolus after intrabronchial instillation of E. coli EVs. Representative histopathological images of lung tissue slice from control, intravenous (i.v.) E. coli EV, and i.v. E. coli EV plus i.v. HMW HA groups with hemasolin and eosin staining. Instillation of E. coli EVs intrabronchially or intravenously significantly reduced AFC rate, which was restored with HMW HA administration. (Data are mean ± SD, n = 5–6 per treatment group, and *P < 0.05 by ANOVA [Bonferroni].) (B) Although administration of HMW HA decreased median absolute neutrophils counts, there was no statistical difference. (Data are median with interquartile range, n = 5–6 per treatment group.) Instillation of E. coli EVs intrabronchially or intravenously significantly increased levels of TNFα (tumor necrosis factor α) and total HA, presumably low-molecular-weight HA, in the injured alveolus and perfusate, respectively. Administration of HMW HA reduced TNFα in the injured alveolus and further increased total HA in the perfusate. (Data are mean ± SD, n = 5–6 per treatment group, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA [Bonferroni].) (C) There were no consistent effects on pulmonary artery pressure, airway pressure, or perfusate Po2 levels over 6 hours or between individual groups. (Data are median with interquartile range, n = 5–6 per treatment group.) P values and confidence intervals are shown in Table E1 in the online supplement. BALF = BAL fluid; PAP = pulmonary artery pressure.
HMW HA Reduced Inflammation Induced by E. coli EV Instillation
Although median neutrophil counts decreased by 46% with HMW HA administration compared with injury, there were no significant differences in total white blood cell and neutrophil counts in the BAL fluid (BALF) among the groups (6.5 × 106 median neutrophil count with IQR 2.1 × 106–17.3 × 106 for i.v. EVs, 3.6 × 106 median neutrophil count with IQR 0.0 × 106–7.9 × 106 for i.v. EVs + i.v. HA). TNFα (tumor necrosis factor α) levels in the BALF significantly increased >200× after i.b. or i.v. administration of E. coli EVs compared with control lung lobes. Administration of HMW HA significantly reduced the increase in TNFα levels in the BALF by >50% (4,245 ± 2,635 pg/ml for i.b. EVs or 4,465 ± 1,424 pg/ml for i.v. EVs vs. 2,067 ± 1,129 pg/ml for i.v. EVs + i.v. HA) (Figure 2B).
Instillation of i.v. E. coli EVs significantly increased the release of endogenous HA, predominantly LMW HA (16), into the perfusate. Administration of HMW HA further raised total HA concentration in the perfusate (519 ± 495 ng/ml for i.b. EVs or 714 ± 558 ng/ml for i.v. EVs vs. 1,431 ± 260 ng/ml for i.v. EVs + HA) (Figure 2B). Surprisingly, administration of HMW HA also increased BALF levels of total HA by 42% compared with i.v. EVs; however, it was not statistically significant.
During 6 hours of perfusion, there were no differences in pulmonary artery pressure, airway pressure, or Po2 levels among the groups (Figure 2C).
Characterization of E. coli EVs
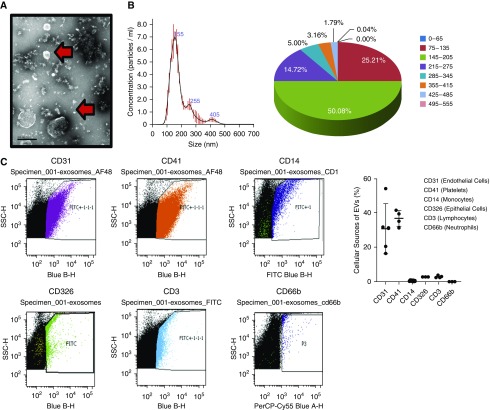
Using electron microscopy, E. coli EVs appeared as spheroids between 50 and 200 nm in size (Figure 3A). The mean E. coli EV size was 155 nm as analyzed with NanoSight NS300 (Malvern, Inc.) (Figure 3B). About 50% of the EVs were 145–205 nm, and 25% of the EVs were 75–135 nm. Total protein concentration and RNA content of E. coli EVs derived from 400 ml of perfusate were 23 ± 5 μg and 2,038 ± 111 ng, respectively (n = 4).
Figure 3.
Characterization of extracellular vesicles (EVs) released during Escherichia coli pneumonia in the ex vivo perfused human lung. (A) Plasma EVs appeared as small membrane-bound vesicles by electron microscopy (scale bar, 200 nm). The arrows highlight the different sizes of EVs found. (B) NanoSight analyses revealed that >50% of the EVs were ∼145–205 nm in size with a mean EV size of 155 nm. (C) By flow cytometry, EVs isolated from the perfusate 6 hours after injury were predominantly from endothelial cells and platelets. In addition, 27 ± 6% expressed CD44, a marker of microvesicles, and 59 ± 20% expressed CD9, a marker of exosomes, at 6 hours. Data are expressed as mean ± SD, n = 3–4. The Blue B-H axes relate to detection with a 525/50-nm bandpass filter when excited with a blue laser (488 nm). The Blue A-H axis relates to detection with a 695/40-nm bandpass filter when excited with a blue laser (488 nm). FITC = fluorescein isothiocyanate; PerCP = peridinin-chlorophyll proteins; SSC = side scatter.
By flow cytometry, 44 ± 7% and 27 ± 6% of EVs as a percentage of total PKH26-labeled EVs were CD44 positive, labeled for microvesicles, at 3 and 6 hours, respectively, and 59 ± 20% were CD9 positive, labeled for exosomes, at 6 hours (n = 4). At 6 hours, most EVs were released from endothelial cells (CD31+, 31 ± 15%) and platelets (CD41+, 37 ± 5%). Other cellular sources included monocytes (CD14+, 0.5 ± 0.2%), epithelial cells (CD326, 2.8 ± 0.04%), and lymphocytes (CD3+, 3.0 ± 0.7%). We were unable to detect EVs released from neutrophils using the CD66b+ Ab (Figure 3C).
HMW HA Binding to E. coli EVs through CD44 Expressed on the Surface of the EVs
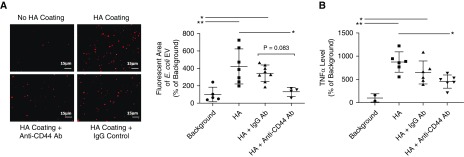
We compared the attachment of E. coli EVs prelabeled with PKH26 to glass slides or glass slides precoated with HMW HA. We also confirmed the results by measuring total TNFα levels on the glass slides as a surrogate marker of the intra-EV content of the cytokine. E. coli EVs significantly attached to HMW HA, which was inhibited with an anti-CD44 blocking antibody as compared with IgG control (Figure 4).
Figure 4.
Binding of Escherichia coli extracellular vesicles (EVs) to high-molecular-weight (HMW) hyaluronic acid (HA). (A and B) PKH26-labeled E. coli EVs significantly bound to HMW HA plated on glass slides compared with control (scale bars, 15 μm), which was inhibited with coincubation with anti-CD44 antibody (Ab) by fluorescence (A) and total TNFα (tumor necrosis factor α) levels in EVs (B). Total TNFα levels in EVs were used as a surrogate measure of EV attachment to the HA. (Data are mean ± SD, *P < 0.05 and **P < 0.01 by ANOVA [Bonferroni], n = 4–8.) P values and confidence intervals are shown in Table E1.
Binding of E. coli EVs to HMW HA Inhibited the Proinflammatory Effect of E. coli EVs
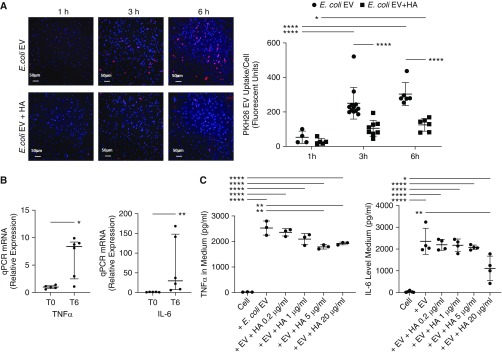
To determine if EVs transferred RNA to target cells, we initially measured mRNAs for TNFα and IL-6 in the vesicles using quantitative PCR. We found that EVs collected at 6 hours had 8× the level of TNFα and 30× the level of IL-6 mRNA compared with EVs at 0 hours (Figure 5). We then studied the uptake of EVs by human blood monocytes ex vivo to understand the mechanisms underlying the inflammatory effect of the vesicles. The uptake of E. coli EVs by monocytes progressively increased over time. However, HMW HA administration significantly reduced the uptake of E. coli EVs, which was associated with reduced TNFα and IL-6 levels in the medium (Figure 5).
Figure 5.
Update of Escherichia coli extracellular vesicles (EVs) by human monocytes. (A) The normal uptake of PKH26-labeled E. coli EVs by human monocytes was inhibited by coincubation with high-molecular-weight (HMW) hyaluronic acid (HA) by fluorescent microscopy as quantified by the average fluorescence intensity of PKH26 for each cell (scale bars, 50 μm). (B) Compared with EVs released from healthy lungs, E. coli EVs contained significantly higher mRNA levels of the inflammatory cytokines TNFα (tumor necrosis factor α) and IL-6 at 6 hours. (C) Coincubation of human monocytes with HMW HA decreased the release of TNFα and IL-6 caused by E. coli EVs by 29% and 53%, respectively, by human monocytes. Data are mean ± SD for PKH26 E. coli EV uptake and TNFα and IL-6 levels. Data are median with interquartile range for PCR data. *P < 0.05, **P < 0.01, and ****P < 0.0001 by ANOVA (Bonferroni) in C, ANOVA with Sidak’s multiple comparison test in A, and Mann-Whitney test in B; n = 3–12. P values and confidence intervals are shown in Table E1. qPCR = quantitative PCR.
HMW HA Administration Improved AFC and Decreased Total Lung Weight Gain after E. coli Pneumonia
Lungs with AFC < 10% were given i.b. E. coli bacteria to replicate nosocomial pneumonia in ICU patients with baseline lung injury. Their demographic, clinical data, and ischemia time for donor lungs are listed in Table 2.
Table 2.
Donor Demographic and Clinical Data for Experiments with Escherichia coli Bacterial Pneumonia and HMW HA
| Control (n = 16) | E. coli Pneumonia (n = 10) | E. coli Pneumonia + i.v. HMW HA (n = 6) | |
|---|---|---|---|
| Age, yr | 45 ± 15 | 46 ± 12 | 45 ± 20 |
| Male sex, n (%) | 7 (41%) | 5 (50%) | 2 (29%) |
| PaO2/FiO2, mm Hg, median (IQR) | 188 (148–268) | 178 (148–294) | 193 (175–253) |
| Cdyn, ml/cm H2O | 40 ± 18 | 40 ± 19 | 41 ± 16 |
| Murray LIS | 1.7 ± 0.6 | 1.7 ± 0.7 | 1.8 ± 0.3 |
| Ischemic time, h:min | 32: 58 ± 4:47 | 33: 58 ± 5:31 | 31: 16 ± 3:42 |
Definition of abbreviations: Cdyn = dynamic compliance; HA = hyaluronic acid; HMW = high-molecular-weight; IQR = interquartile range; LIS = lung injury score.
Data are presented as mean ± SD unless otherwise indicated. No significant differences in PaO2/FiO2 ratio, lung compliance, or lung injury score prior to organ harvest or total ischemic time were observed among groups.
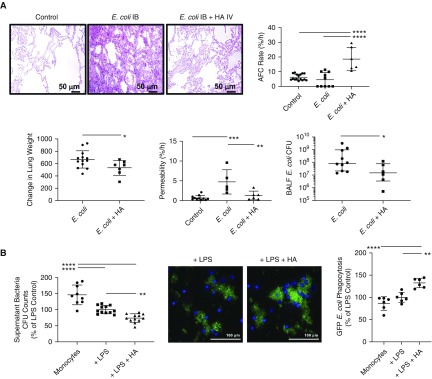
By histology, i.b. instillation of E. coli bacteria resulted in interalveolar septal thickening, edema, and cellularity, which was largely reduced with HMW HA administration. HMW HA also significantly increased AFC rate compared with E. coli-injured lung lobes (4.4 ± 4.9% with E. coli injury alone compared with 18.6 ± 8.0% with HA administration), which was associated with reduced lung weight gain (666 g ± 140 g after E. coli injury compared with 532 g ± 121 g with HA administration) and decreased lung protein permeability (Figure 6A).
Figure 6.
Therapeutic effects of high-molecular-weight hyaluronic acid (HMW HA) in Escherichia coli pneumonia. Intrabronchial instillation of E. coli bacteria caused severe lung injury, which was ameliorated with administration of HMW HA as therapy. (A) HMW HA reduced cellularity, interstitial thickening, and edema by hemasolin and eosin staining, restored alveolar fluid clearance (AFC) rate, and reduced lung protein permeability and pulmonary edema. HMW HA also reduced total E. coli cfu counts in the injured alveolus and increased the phagocytosis of bacteria by human blood monocytes in vitro. Data are mean ± SD for AFC rate, change in lung weight, and permeability, and data are median with interquartile range for BALF cfu counts, n = 6–10 per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA (Bonferroni) or Mann-Whitney test. (B) Coincubation of human monocytes with HMW HA decreased bacterial cfu counts in the supernatant and increased GFP-labeled E. coli bacteria phagocytosis as measured by fluorescence. Data are mean ± SD, n = 5–12. **P < 0.01 and ****P < 0.0001 by ANOVA (Bonferroni). P values and confidence intervals are shown in Table E1. BALF = BAL fluid.
HMW HA Administration Improved Bacterial Clearance
We measured bacteria cfu counts in the BALF after E. coli bacteria instillation with or without HMW HA treatment. HMW HA administration significantly decreased total E. coli cfu counts (median 8.6 × 108 cfu/ml with IQR 2.1 × 107–10.0 × 108 cfu/ml after E. coli injury compared with 1.5 × 107 cfu/ml with IQR 3.5 × 106–7.9 × 107 cfu/ml with HA administration). To confirm the effect of HMW HA on bacterial clearance, we added HMW HA to human blood monocytes cultured ex vivo injured with LPS and GFP-labeled E. coli bacteria. HMW HA administration significantly reduced E. coli bacteria cfu levels in the medium and increased bacteria phagocytosis by monocytes (Figure 6B).
Effect of HMW HA in Lung Inflammation
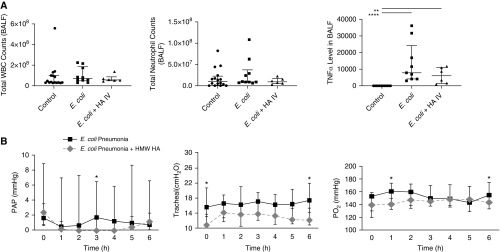
HMW HA treatment numerically decreased TNFα levels in the BALF by 23% (median 7,865 pg/ml with IQR 4,123–24,275 for E. coli pneumonia vs. median 6,064 with IQR 1,473–11,131 for E. coli pneumonia + HMW HA). There were no significant differences in white blood cells and neutrophil counts in BALF among the groups (Figure 7A).
Figure 7.
Effect of Escherichia coli pneumonia with or without high-molecular-weight hyaluronic acid (HMW HA) administration on inflammation and pulmonary arterial pressure, airway pressure, and Po2 levels. (A) There was no significant effect of HMW HA on total white blood cell (WBC) and neutrophil counts. However, HMW HA administration reduced the median level of TNFα (tumor necrosis factor α) in the BAL fluid (BALF) by 23% induced by E. coli pneumonia, although it was not statistically significant. Data are median with interquartile range, n = 6–10 per treatment group. *P < 0.05, **P < 0.01, and ****P < 0.0001 by Kruskal-Wallis with Dunn’s correction. P values and confidence intervals are shown in Table E1. (B) Although at individual time points there were statistically significant differences between groups, there appeared to be no overall effect of HMW HA administration on pulmonary arterial pressure, airway pressure, or Po2 levels over time. Data are median with interquartile range, n = 6–10 per treatment group, P is significant versus corresponding group at each time point by Mann-Whitney test. PAP = pulmonary artery pressure.
Although at individual time points there were statistically significant differences between groups, there appeared to be no overall effect of HMW HA administration on pulmonary arterial pressure, airway pressure, or Po2 levels over time (Figure 7B).
Administration of HMW HA Increased Total Concentration of HA in the Injured Alveolus and Perfusate
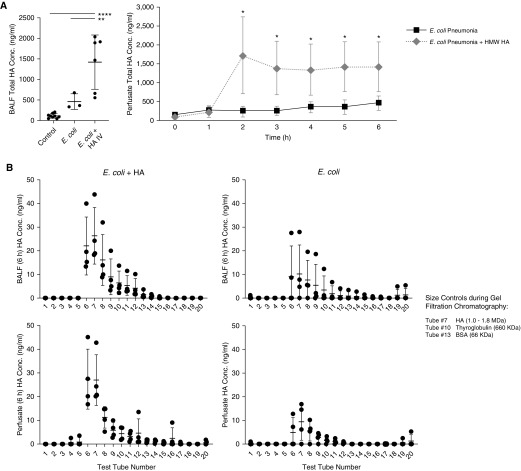
Similar to E. coli EVs, E. coli bacteria i.b. instillation significantly increased endogenous HA concentration in both the injured alveolus and perfusate. HMW HA treatment further increased total HA concentration ∼3× more in the injured alveolus or perfusate over 6 hours (Figure 8A). In the treatment group, the majority of HA in both the injured alveolus and perfusate were HMW (>1,000 kD) in size despite 6 hours of perfusion. Even in the injured group, endogenous levels of HMW HA were elevated, suggesting some potential for repair and recovery (Figure 8B).
Figure 8.
Effect of Escherichia coli pneumonia with or without high-molecular-weight hyaluronic acid (HMW HA) administration on total HA concentration in the injured alveolus and perfusate. (A) Administration of HMW HA dramatically increased total HA levels in both the injured alveolus and perfusate over time. Data are mean ± SD, n = 3–6. For BALF HA levels, *P < 0.05, **P < 0.01, and ****P < 0.0001 by ANOVA (Bonferroni). For perfusate serial data, P is significant versus corresponding group at each specific time point by Student’s t test. P values and confidence intervals are shown in Table E1. (B) In the treatment group, the majority of HA in both the injured alveolus and perfusate were HMW (>1,000 kD) in size despite 6 hours of perfusion. Even in the injured group, endogenous levels of HMW HA were elevated, suggesting some attempt at repair and recovery, although lower when compared with the treatment group. Size distribution of HA was separated out by gel filtration chromatography (Figure E1), and the concentration measured by ELISA. Data are mean ± SD, n = 3. BALF = BAL fluid; BSA = bovine serum albumin; Conc. = concentration.
Discussion
The main findings in this study can be summarized as follows: 1) Instillation of EVs induced and released during E. coli pneumonia given intrabronchially or intravenously initiated severe lung injury in a naive ex vivo perfused human lung (Figure 2). 2) Administration of HMW HA into the perfusate as therapy significantly restored AFC and reduced inflammation after ALI induced by E. coli EVs (Figure 2). 3) The predominant cellular sources of E. coli EVs identified were from endothelial cells and platelets, although the contributions from immune cells cannot be disregarded due to the majority of EVs being exosomes (Figure 3). 4) Binding of HMW HA to E. coli EVs in vitro inhibited the uptake of the vesicles by human blood monocytes in part through a CD44-dependent mechanism, reducing the release of TNFα and IL-6 by injured monocytes (Figures 4 and 5). 5) Administration of HMW HA was effective in restoring AFC and reducing lung weight gain, inflammation, and protein permeability in an ex vivo perfused human lung injured with E. coli bacterial pneumonia (Figure 6). 6) Despite administration of i.v. HMW HA, total HA levels in the BALF in E. coli pneumonia increased significantly, suggesting that HMW HA was drawn to the injured alveolus. Analyses of HA in the BALF and perfusate demonstrated that HA were predominantly HMW in size after 6 hours of perfusion (Figures 7 and 8). 7) And, perhaps more significantly, HMW HA administration reduced total bacterial cfu counts in the BALF and increased the phagocytosis of E. coli bacteria by human monocytes (Figure 6).
To our knowledge, this is the first demonstration of the critical role of EVs in the inflammatory response during severe bacterial pneumonia in a human model of ALI. EVs isolated from 400 ml of perfusate, approximately one-fourth of the perfusate volume, were enough to decrease AFC and increase inflammation and pulmonary edema in a naive perfused human lung (Figure 2). The result was similar to the study by Li and colleagues that found that administration of microparticles derived from blood in LPS-induced ALI in rats if given to a naive animal either intrabronchially or intravenously could induce ALI (17).
EVs are a heterogeneous group and can be broadly classified as exosomes, microvesicles, and apoptotic bodies according to their size, biogenesis, and secretion mechanisms (18). Exosomes with a diameter of 20–200 nm originate from the release of intraluminal vesicles (19, 20), while microvesicles with a diameter of 200–1,000 nm are generated from outward budding and fission of the plasma membrane (21). For E. coli EVs, both exosomes and microvesicles were found using electron microscopy. The roles of exosomes or microvesicles or even apoptotic bodies independently in the pathogenesis of lung injury constitute new pathways that need further study. EVs can be formed and released by multiple cells under various stimuli during normal physiological or pathological conditions. In healthy humans, circulating EVs were mainly derived from platelets and, to a lesser extent, from endothelial cells (22). While in ALI, EVs derived from epithelial cells, lymphocytes, red blood cells, monocytes, or macrophages increased (23, 24). Several investigators found that EVs derived from endothelial cells were important markers of lung vascular injury in ventilator-induced lung injury, and i.v. instillation of stimulated endothelial cell–derived EVs could induce ALI (25, 26). Soni and colleagues found that alveolar macrophage-derived microvesicles played an important role in initiating ALI (23). In addition, multiple exogenous triggers can induce the release of EVs; for example, even the storage conditions of plasma like temperature or propyl gallate supplementation can lead to differences in the production of platelet-derived EVs (27, 28). In the current study, we were unable to detect significant levels of EVs derived from immune cells at 6 hours (Figure 3). However, the cellular sources of EVs often cannot be identified by flow cytometry because the EVs are commonly derived from intracellular vesicles or exosomes which contain no identifiable cellular marker.
EVs can exert their biological effect according to their interaction with target cells through possible transfer of various bioactive molecules including mRNA, microRNA, proteins, and organelles. In this study, the RNA content of the released EVs for TNFα or IL-6 increased in expression after E. coli pneumonia injury (Figure 5). The transfer of these mRNAs to target cells may be in part responsible for the inflammatory injury that developed with E. coli EVs.
HA can promote or inhibit inflammation and injury based primarily on its size. As a major component of the endothelial glycocalyx, located on the luminal side of the endothelium in all vessels (29), hyaluronan plays a critical role in limiting protein permeability (30). LMW HA activates specific HA-binding proteins to promote disruption of endothelial cell–cell contacts, while exogenous administration of HMW HA can promote vascular integrity (31). HMW HA can also exert antiinflammatory and immunosuppressive effects, while LMW HA can stimulate gene expression and synthesis of proinflammatory cytokines (32). Therefore, potential therapeutic application of HMW HA for sterile ALI has attracted considerable interest. Previous publications demonstrated that HMW HA administration enhanced endothelial cell barrier properties, reducing protein permeability and the influx of immune cells in LPS or ventilator-induced lung injury (33, 34). Singleton and colleagues found that intravenous administration of HMW HA 4 hours after LPS-induced ALI improved endothelial permeability (33) via CD44, sphingosine 1 phosphate, Akt and Rac signaling (35). In the current study, we also found that instillation of HMW HA into the perfusate ameliorated lung injury induced by E. coli EVs in an ex vivo perfused human lung and inhibited E. coli EV–induced inflammation in cultured human blood monocytes (Figures 2 and 5).
However, the mechanisms underlying the therapeutic effects remain largely unknown. Most of the focus in the literature has been on the repair of damaged vascular endothelium by HMW HA (33). In the current study, we hypothesized that binding of HMW HA to inflammatory EVs, thus inhibiting the uptake of EVs by target cells, may contribute to its protective effect. The biological rationale was based on binding of HMW HA to CD44, a major transmembrane glycoprotein commonly expressed on immune cells and its released EVs. In a previous study, we found that CD44 expressed on stem cell microvesicles was critical for the uptake of the microvesicles by target cells and its therapeutic effect (15, 36). In the current study, we found that binding of E. coli EVs by HMW HA, which was dependent in part on CD44, inhibited the uptake of EVs by human blood monocytes and reduced inflammation induced by E. coli EVs (Figures 4 and 5). Endogenous LMW HA fragments may also interact with CD44, but the effect it induces in target cells may be different than those caused by HMW HA. Studies are ongoing to determine if the differential effect of LMW HA from HMW HA is due to receptor avidity (13) or clustering (37) or due to inhibition of internalization of the EVs into the target cells.
The protective effects of HMW HA in E. coli EV–induced lung injury raised the possibility for the therapeutic use of HMW HA in severe pneumonia and/or sepsis, the most common causes of ARDS. To address this hypothesis, we instilled E. coli bacteria into marginal human lungs with an AFC < 10% to induce a severe bacterial pneumonia. Similar to the effect of HA on injury induced by E. coli EVs, administration of HMW HA restored and improved AFC and decreased lung protein permeability. Unexpectedly, we found that the administration of HMW HA also decreased total bacterial cfu levels in the injured alveolus (Figure 6). There are several possible explanations for the antimicrobial effect of HMW HA: 1) As shown in the current study, HMW HA may enhance bacteria phagocytosis by immune cells, possibly through activation of CD44, which is involved in phagocytosis (38, 39). 2) HMW HA may interfere with bacterial adhesion to a cellular substrate (40), as adhesion to oral and airway cells is necessary for the first step of microbial colonization and pathogenesis. Currently, several new biomaterials composed with HMW HA are undergoing clinical investigation to prevent the formation of biofilms and the adhesion of bacteria (41, 42). 3) HMW HA appears to have bacteriostatic properties (43, 44). 4) And last, the beneficial effect of HA on endothelial barrier properties may prevent translocation of bacteria (45).
There are some limitations to the current study: 1) lack of an intact lymphatic system in our ex vivo perfused human lung for lung interstitial fluid clearance, which may be relevant if AFC is measured over longer periods; 2) lack of other immune organs such as the spleen or liver which may participate in injury and/or repair and is required for HA metabolism (46); 3) a short duration of lung injury, which limits assessment of whether the effects of HA can be sustained; 4) and lack of study on the contributions of EVs released from bacteria itself on ALI.
In conclusion, EVs isolated from human lungs with E. coli bacterial pneumonia induced ALI in naive human lungs, emphasizing the potential pathogenetic importance of EVs, generated in a localized pneumonia, for compounding ALI and suggesting that studies of EVs in patients at risk or with ARDS are needed to understand the pathogenesis of ARDS more completely. And for the first time in a human model of ARDS, exogenous administration of HMW HA was found to bind to these inflammatory EVs, inhibiting uptake by target cells and decreasing inflammation and injury and enhancing the antimicrobial activity of immune cells. Based on these new findings, further investigations of HMW HA are warranted for the treatment of ARDS and/or sepsis.
Supplementary Material
Footnotes
Supported by NIH NHLBI grants HL 113022 (J.-W.L.) and HL 140026 and HL 134828 (M.A.M.).
Author Contributions: A.L.: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. J.-H.P., X.Z., S.S., Y.N., J.-H.L., H.K., and Q.H.: collection and assembly of data, and data analysis and interpretation. M.A.M.: financial support and data analysis and interpretation. J.-W.L.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2296OC on August 7, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Biehl M, Kashyap R, Ahmed AH, Reriani MK, Ofoma UR, Wilson GA, et al. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study. Crit Care. 2015;19:356. doi: 10.1186/s13054-015-1062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy C, Withrow J, Hunter M, Liu Y, Tang YL, Fulzele S, et al. Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Aspects Med. 2018;60:123–128. doi: 10.1016/j.mam.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv LL, Wu WJ, Feng Y, Li ZL, Tang TT, Liu BC. Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J Cell Mol Med. 2018;22:728–737. doi: 10.1111/jcmm.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tofaris GK. A critical assessment of exosomes in the pathogenesis and stratification of Parkinson’s disease. J Parkinsons Dis. 2017;7:569–576. doi: 10.3233/JPD-171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindoso RS, Collino F, Vieyra A. Extracellular vesicles as regulators of tumor fate: crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investig. 2017;4:75. doi: 10.21037/sci.2017.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 2010;293:982–985. doi: 10.1002/ar.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev. 2016;97:186–203. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011;301:L137–L147. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 14.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA.Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs Am J Physiol Lung Cell Mol Physiol 2007293L52–L59.17351061 [Google Scholar]
- 15.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito AJ, Bhatraju PK, Stapleton RD, Wurfel MM, Mikacenic C. Hyaluronic acid is associated with organ dysfunction in acute respiratory distress syndrome. Crit Care. 2017;21:304. doi: 10.1186/s13054-017-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Meng X, Liang X, Gao Y, Cai S. Administration of microparticles from blood of the lipopolysaccharide-treated rats serves to induce pathologic changes of acute respiratory distress syndrome. Exp Biol Med (Maywood) 2015;240:1735–1741. doi: 10.1177/1535370215591830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 20.Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 2016;16:859–871. doi: 10.1517/14712598.2016.1170804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaar V, Romana M, Tripette J, Broquere C, Huisse MG, Hue O, et al. Effect of strenuous physical exercise on circulating cell-derived microparticles. Clin Hemorheol Microcirc. 2011;47:15–25. doi: 10.3233/CH-2010-1361. [DOI] [PubMed] [Google Scholar]
- 23.Soni S, Wilson MR, O’Dea KP, Yoshida M, Katbeh U, Woods SJ, et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020–1029. doi: 10.1136/thoraxjnl-2015-208032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L364–L381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]
- 25.Buesing KL, Densmore JC, Kaul S, Pritchard KA, Jr, Jarzembowski JA, Gourlay DM, et al. Endothelial microparticles induce inflammation in acute lung injury. J Surg Res. 2011;166:32–39. doi: 10.1016/j.jss.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letsiou E, Sammani S, Zhang W, Zhou T, Quijada H, Moreno-Vinasco L, et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol. 2015;52:193–204. doi: 10.1165/rcmb.2013-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao HY, Matsubayashi H, Bonderman DP, Bonderman PW, Reid T, Miraglia CC, et al. Generation of annexin V-positive platelets and shedding of microparticles with stimulus-dependent procoagulant activity during storage of platelets at 4 degrees C. Transfusion. 2000;40:420–427. doi: 10.1046/j.1537-2995.2000.40040420.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Mody M, Herst R, Sher G, Freedman J. Flow cytometric analysis of platelet function in stored platelet concentrates. Transfus Sci. 1999;20:129–139. doi: 10.1016/s0955-3886(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler-Jones CP, Farrar CE, Pitsillides AA. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Curr Opin Investig Drugs. 2010;11:997–1006. [PubMed] [Google Scholar]
- 30.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singleton PA. Hyaluronan regulation of endothelial barrier function in cancer. Adv Cancer Res. 2014;123:191–209. doi: 10.1016/B978-0-12-800092-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albano GD, Bonanno A, Cavalieri L, Ingrassia E, Di Sano C, Siena L, et al. Effect of high, medium, and low molecular weight hyaluronan on inflammation and oxidative stress in an in vitro model of human nasal epithelial cells. Mediators Inflamm. 2016;2016:8727289. doi: 10.1155/2016/8727289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, et al. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639–L651. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, et al. High-molecular-weight hyaluronan: a possible new treatment for sepsis-induced lung injury. A preclinical study in mechanically ventilated rats. Crit Care. 2008;12:R102. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 36.Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 38.Fu Q, Wei Z, Xiao P, Chen Y, Liu X. CD44 enhances macrophage phagocytosis and plays a protective role in Streptococcus equi subsp. zooepidemicus infection. Vet Microbiol. 2017;198:121–126. doi: 10.1016/j.vetmic.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Amash A, Wang L, Wang Y, Bhakta V, Fairn GD, Hou M, et al. CD44 antibody inhibition of macrophage phagocytosis targets Fcγ receptor- and complement receptor 3-dependent mechanisms. J Immunol. 2016;196:3331–3340. doi: 10.4049/jimmunol.1502198. [DOI] [PubMed] [Google Scholar]
- 40.Drago L, Cappelletti L, De Vecchi E, Pignataro L, Torretta S, Mattina R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS. 2014;122:1013–1019. doi: 10.1111/apm.12254. [DOI] [PubMed] [Google Scholar]
- 41.Michalska-Sionkowska M, Kaczmarek B, Walczak M, Sionkowska A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater Sci Eng C. 2018;86:103–108. doi: 10.1016/j.msec.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Romanò CL, De Vecchi E, Bortolin M, Morelli I, Drago L. Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. J Bone Jt Infect. 2017;2:63–72. doi: 10.7150/jbji.17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW. Bacteriostatic effects of hyaluronic acid. J Periodontol. 1999;70:370–374. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 44.Carlson GA, Dragoo JL, Samimi B, Bruckner DA, Bernard GW, Hedrick M, et al. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun. 2004;321:472–478. doi: 10.1016/j.bbrc.2004.06.165. [DOI] [PubMed] [Google Scholar]
- 45.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 46.Fraser JR, Laurent TC, Pertoft H, Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981;200:415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.